Abstract
Obstructive sleep apnea (OSA) is associated with metabolic disorders such as insulin resistance and liver fat accumulation. However, the specific mediating role of liver-related metabolic indicators in this association has not been fully studied. The purpose of this study was to investigate the relationship between Metabolic Score for Insulin Resistance (METS-IR) and OSA, focusing on the mediating effects of liver fat percentage (PLF) and hepatic steatosis index (HSI). Understanding these mechanisms may provide insights into targeted interventions for OSA. A total of 12,655 participants from the National Health and Nutrition Examination Survey (NHANES) were included in this analysis. Obstructive sleep apnea (OSA) was assessed using the NHANES questionnaire. Weighted multivariate logistic regression was employed to assess the relationship between METS-IR and OSA, with a mediation model constructed to explore the mediating roles of key liver and metabolic markers, including PLF, HSI, SII and OBS. Among 12,655 subjects, 31.04% had OSA. METS-IR was closely related to the increased risk of OSA, and the highest quartile group of METS-IR had a significantly increased risk of OSA (OR = 2.36, 95% CI 1.73–3.23). Mediating effect analysis showed that PLF and HSI mediated 6.95% and 17.87% of the effects, respectively, while systemic immunity-inflammation index (SII) and oxidative balance score (OBS) had no significant mediating effect. METS-IR is an important predictor of OSA risk, primarily mediated by hepatic lipid accumulation. Addressing insulin resistance and hepatic metabolic health is crucial for the effective management of OSA and provides valuable guidance for clinical risk assessment in susceptible populations.
Similar content being viewed by others
Introduction
Obstructive sleep apnea syndrome (OSA) is a prevalent sleep disorder characterized by repeated partial or complete blockage of the upper airway during sleep, leading to intermittent breathing interruptions and hypoxemia1. These recurring episodes of apnea not only severely disrupt the patient’s nighttime sleep quality, but also lead to excessive daytime sleepiness, cognitive impairment, and a significant reduction in overall quality of life2. More critically, OSA is closely linked to a range of metabolic disorders and cardiovascular diseases, including type 2 diabetes, hypertension, coronary artery disease, and stroke3,4. In recent years, the global rise in obesity rates has contributed to a rapid increase in OSA prevalence, posing significant challenges for public health5.
Although a large number of studies have revealed the correlation between OSA and cardiovascular and metabolic diseases, there is still a lack of in-depth understanding of its potential metabolic mechanisms6. Insulin resistance is considered to be one of the core mechanisms linking OSA and metabolic syndrome. Insulin resistance is not only closely related to metabolic syndrome, but also interacts with pathological processes such as visceral fat accumulation, chronic inflammation and oxidative stress, which may play a key role in the pathogenesis of OSA7. The excessive activation of sympathetic nerve and oxidative stress induced by intermittent hypoxemia in OSA patients are important causes of insulin resistance, and insulin resistance further aggravates metabolic disorders.
In recent years, the metabolic insulin resistance score (METS-IR), as a new metabolic index, can effectively evaluate the individual’s insulin resistance8. METS-IR combines a variety of metabolic-related parameters, such as waist circumference, triglyceride (TG) and fasting blood glucose, serving as a reliable alternative indicator of metabolic syndrome and metabolic dysfunction. In view of the important role of insulin resistance in the pathogenesis of OSA, METS-IR provides a new perspective to understand how metabolic disorders affect OSA.
Hepatic steatosis involves abnormal liver fat accumulation, is often linked to obesity, insulin resistance, and metabolic syndrome. Percentage of liver fat (PLF) and hepatic steatosis index (HSI) are key markers for assessing liver fat buildup and functional impairment9,10. In OSA patients, liver fat accumulation is common and correlates with heightened insulin resistance and metabolic disturbances11. Over time, excessive liver fat can lead to non-alcoholic fatty liver disease (NAFLD), further disrupting metabolic balance and elevating the risk of cardiovascular events12. Liver dysfunction may also exacerbate OSA by worsening insulin resistance and systemic inflammation.
Systemic inflammation plays a significant role in the metabolic consequences of OSA. OSA often triggers sympathetic overactivation, driving a chronic inflammatory state. The systemic immunity-inflammation index (SII), based on neutrophil, platelet, and lymphocyte ratios, measures systemic inflammation. Elevated SII in OSA patients suggests ongoing low-grade inflammation, which not only aggravates insulin resistance but may also directly contribute to OSA progression by affecting the respiratory system’s inflammatory state13.
Oxidative stress arises when the body’s antioxidant defenses are overwhelmed by free radicals, resulting in cell damage14. OSA-induced intermittent hypoxia increases oxidative stress, particularly in the cardiovascular and respiratory systems15. This stress impairs endothelial function, promoting vascular sclerosis and increasing the risk of cardiovascular events16. Elevated oxidative balance score (OBS) in OSA patients indicate persistent oxidative stress, highlighting its role as another key mechanism linking OSA to metabolic dysfunction17,18.
Based on this background, the purpose of this study is to systematically evaluate the association between METS-IR and OSA by analyzing large-scale data from the National Health and Nutrition Survey (NHANES), and to focus on the mediating role of PLF, HSI, SII and OBS in this association. However, it is important to note that while metabolic markers offer methods for evaluating participants, they cannot detect OSA in its early stages. By revealing the mediating effects of these metabolic markers, this study provides a new clinical perspective for the early identification and intervention of OSA.
Methods
Study design and participants
The National Health and Nutrition Examination Survey (NHANES) is an ongoing national program designed to gather comprehensive data regarding the dietary patterns and overall health of the U.S. population. Before beginning data collection, participants provided written informed consent, and all study procedures received approval from the ethical review board of the National Center for Health Statistics. Additional details about the NHANES program are available on its official website.
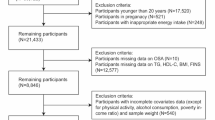
This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES) from 2005–2008 and 2015–2020, including participants aged 20 years and older (n = 29,763). Participants with missing data required to calculate the Metabolic Score for Insulin Resistance (METS-IR) and the four mediators—percentage of liver fat (PLF), hepatic steatosis index (HSI), systemic immunity-inflammation index (SII) and oxidative balance score (OBS) were excluded (n = 17,105). Additionally, data from participants with missing or incomplete information required for an obstructive sleep apnea syndrome (OSA) diagnosis were excluded from the analysis (n = 3). The final sample size included in the analysis was 12,655 participants (Fig. S1).
Definitions of the exposure variable, mediating variable and outcome variable
The exposure variable in this study is the Metabolic Score for Insulin Resistance (METS-IR), which serves as a predictor for insulin resistance and metabolic health. Data for calculating METS-IR were obtained from the NHANES database. The formula used is: \({\text{METS - IR}} = \frac{{Ln\left( {2 \times FPG + TG} \right)}}{{Ln\left( {BMI \times HDL - C} \right)}}\), where FPG stands for fasting plasma glucose, TG stands for triglycerides, BMI is the body mass index, and HDL-C refers to high-density lipoprotein cholesterol8.
The mediating variables in this study were percentage of liver fat (PLF), hepatic steatosis index (HSI), systemic immunity-inflammation index (SII) and oxidative balance score (OBS). These markers are linked to liver fat accumulation, inflammation, and oxidative stress, which are key metabolic processes associated with OSA. The formulas for calculating each of these mediators are as follows:
AST stands for aspartate aminotransferase (a liver enzyme indicating liver damage when elevated), ALT stands for alanine aminotransferase (another enzyme indicating liver injury), and insulin represents fasting insulin levels. This formula incorporates metabolic syndrome and type 2 diabetes as binary variables (yes/no)9.
BMI is body mass index, and ALT and AST are the liver enzymes. Diabetes and female sex are included as binary variables19,20.
The OBS combines 16 dietary components and four lifestyle factors, with higher scores indicating greater antioxidant exposure. Tobacco use was assessed via the cotinine test, and alcohol consumption was categorized into nondrinkers, nonheavy drinkers, and heavy drinkers, with scores of 2, 1 and 0, respectively. Antioxidants were scored higher in upper tertiles, while pro-oxidants were inversely scored17.
In accordance with earlier studies, the outcome variable OSA is diagnosed when a person answers ‘yes’ to at least one of the following three NHANES questions21: (1) feeling excessively sleepy during the day despite getting at least 7 h of sleep per night, as reported 16–30 times; (2) experiencing episodes of gasping, snorting, or stopping their breath on three or more occasions per week; (3) snoring on three or more occasions every week.
Potential covariates
The self-reported sociodemographic characteristics included age, sex (male/female), race/ethnicity (non-Hispanic White, Mexican American, non-Hispanic Black, other Hispanic, or other Race/multiple Races), education level (less than high school, completed high school, or more than high school), marital status (married/living with a partner, never married, widowed, divorced, or separated) and family poverty index ratio (PIR, a ratio of family income to the poverty threshold). BMI was calculated as weight (kg) divided by height (m) squared. Physical activity was assessed by asking participants to report the types of physical activities they engaged in regularly, categorized into vigorous physical activities (e.g., running, playing basketball) and moderate physical activities (e.g., brisk walking, swimming, cycling). Alcohol consumption and smoking status were also treated as categorical variables.
The participants’ alcohol consumption status was categorized into five groups: never (fewer than 12 drinks in a lifetime), former (12 or more drinks in a lifetime but none in the previous year), mild (1 + drinks/day for women, 2 + drinks/day for men), moderate (2 + drinks/day for women, 3 + drinks/day for men), and heavy (5 + drinks/day for women, 4 + drinks/day for men). This categorization was based on the number of alcoholic beverages consumed per day, with the following thresholds applied: ≥ 2 drinks/day for women and ≥ 3 drinks/day for men, binge drinking ≥ 2 days/month, and heavy drinking (≥ 3 drinks/day for women and ≥ 4 drinks/day for men, or binge drinking ≥ 5 days/month). The participants were divided into three smoking categories: never smokers (smoked fewer than 100 cigarettes), former smokers (smoked at least 100 cigarettes but not currently smoking), and current smokers (smoked at least 100 cigarettes and currently smoking daily or some days). The definition of metabolic syndrome (MetS) was in accordance with the updated National Cholesterol Education Program/Adult Treatment Panel III criteria for Americans22. A history of physician-diagnosed hypertension, a measured average systolic blood pressure of at least 140 mmHg, a measured average diastolic blood pressure of at least 90 mmHg, or a history of antihypertensive medication use were all considered indicators of hypertension23. A self-reported diagnosis of diabetes, a fasting plasma glucose level ≥ 7.0 mmol/L, glycosylated hemoglobin (HbA1c) ≥ 6.5%, and/or the usage of anti-diabetic medication were all considered indicators of diabetes24.
All missing values in this study were handled using appropriate imputation methods: continuous variables were imputed with mean values, while categorical variables were assigned dummy variables24. The confounding variables and their detailed definitions are collated in Table S1 for reference. The missing covariates of study participants are summarized in Table S2.
Statistical analyses
The statistical analyses for this study were performed using the R statistical computing environment (version 4.4.0), with the “NhanesR” package (details provided in Table S3). All analyses accounted for the complex, multistage survey design of NHANES by applying sample weights, stratification, and clustering to ensure the results are representative of the U.S. population. Baseline characteristics were summarized using weighted means and 95% confidence intervals (CI) for continuous variables, while categorical variables were expressed as weighted frequencies and percentages. Continuous variables were analyzed using weighted t-tests or Wilcoxon rank-sum tests, depending on the distribution of the data, which was assessed using the Shapiro–Wilk test for normality. Categorical variables were compared using weighted chi-square tests or Fisher’s exact tests, where appropriate. Statistical significance was set at p < 0.05 for all analyses.
The associations between METS-IR and OSA were investigated using multivariate logistic regression across four distinct models to provide a comprehensive assessment of their relationship. The crude model was unadjusted for covariates, while Model 1 adjusted for age, sex, and race/ethnicity. Model 2 further included education level, marital status, physical activity, smoking status, and alcohol consumption, addressing lifestyle factors that may confound the association. Model 3 incorporated additional controls for the poverty-to-income ratio (PIR), Body Mass Index (BMI), metabolic syndrome, diabetes, and hypertension, thereby accounting for critical physiological and socioeconomic variables. This methodological framework enhances the reliability of our findings by enabling the simultaneous control of multiple independent variables and yielding odds ratios for binary outcomes like OSA. By thoroughly adjusting for known confounders, we reinforce our conclusions regarding the relationship between METS-IR and OSA, thereby contributing valuable insights into the factors influencing OSA risk. A further assessment of the heterogeneity between METS-IR and OSA was conducted through subgroup analysis, which included the following variables: age, sex, race/ethnicity, smoking status, alcohol consumption, marital status, physical activity, metabolic syndrome, diabetes and hypertension.
The indirect effect of METS-IR on the relationship between METS-IR and OSA was further explored through mediation analysis. Using causal mediation analysis, the indirect and direct effects were evaluated to determine the proportion of the total effect of METS-IR on OSA that could be explained by four mediators: PLF, HSI, SII and OBS. The indirect effect quantified how much of the association between METS-IR and OSA was mediated by these markers, while the direct effect measured the remaining effect not attributed to mediators. The non-parametric bootstrap re-sampling method was employed to estimate confidence intervals and significance levels for the mediation and direct effects, using 1000 bootstrap iterations. The proportion of mediation for each variable was calculated to identify the pathways through which METS-IR influences OSA.
Results
Baseline characteristics
The weighted baseline characteristics of the participants in the study are shown in Table 1. A total of 12,655 participants were included in the analysis, with a mean age of 48.08 years (95% CI 47.47, 48.68). Among them, 49.43% were male and 50.57% were female. The majority of participants identified as non-Hispanic white (66.39%), followed by non-Hispanic black (10.80%), Mexican American (8.52%), other Hispanic origin (5.84%), and other or multi-racial groups (8.45%).
In total, 31.04% of the participants were categorized as having OSA. Across the four METS-IR groups, all variables showed statistical significance. Compared to participants in the lower METS-IR group, those in the highest quartile (Q4) were more likely to be male, older, and non-Hispanic white. They also tended to have higher PLF, HSI, SII and BMI levels, were more likely to be never-smokers or mild drinkers, and had lower levels of physical activity, poverty income ratio (PIR) and OBS. Additionally, these participants exhibited higher educational attainment, were more often widowed, divorced, separated, or never married, and had a greater prevalence of metabolic syndrome and hypertension.
Association between METS-IR and OSA
In the restricted cubic spline (RCS) analysis, METS-IR was positively associated with OSA (Fig. 1). Table 2 presents the associations between METS-IR and the risk of obstructive sleep apnea (OSA) across different models. Whether confounding factors were adjusted or not, METS-IR consistently showed a significant positive correlation with OSA in all participants. In the multivariate regression analysis, METS-IR was divided into quartiles, using the Q1 group as the reference to assess the relationship with OSA.
Restricted cubic spline of the relationship between the METS-IR and the OSA. Age, sex, race/ethnicity, education level, marital status, physical activity, smoking status, alcohol status, the ratio of family income to poverty, body mass index, metabolic syndrome, diabetes and hypertension were adjusted for. The solid line represents the line of best fit, and the pale pink area represents the 95% confidence interval.
After adjusting for age, gender, race, education level, marital status, physical activity, smoking, drinking status, PIR, BMI, metabolic syndrome, diabetes and hypertension, compared to the Q1 reference group, the Q2 group (OR: 1.49; 95% CI 1.25, 1.77), Q3 group (OR: 1.72; 95% CI 1.36, 2.19), and Q4 group (OR: 2.36; 95% CI 1.73, 3.23) all exhibited a significantly increased risk, showing a clear upward trend (p for trend < 0.0001). This trend was consistent across all models, indicating that the positive association between METS-IR and the risk of OSA remains stable regardless of adjustments for confounding factors.
Subgroup analysis
Table 3 Subgroup analyses were conducted across various factors, including age (≤ 40 years, 40–60 years, > 60 years), sex (male, female), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, Other Hispanic, and other races), education level (< High School, Completed High School, > High School), smoking status (former smoker, never smoked, current smoker), alcohol consumption (former drinker, heavy drinker, mild drinker, moderate drinker, never drank), marital status (Married/Living with Partner, Widowed/Divorced/Separated/Never married), physical activity (inactive, moderate activity, both moderate and vigorous, vigorous), and the presence of metabolic syndrome (yes or no) to evaluate the stability of the association between METS-IR and OSA. Interaction tests were performed to assess whether these factors influenced the strength of the association between METS-IR and OSA.
The results revealed significant interactions for age (p-interaction = 0.026), smoking status (p-interaction = 0.01), alcohol consumption (p-interaction = 0.029), education level (p-interaction = 0.037), diabetes (p-interaction < 0.0001), hypertension (p-interaction = 0.001) and metabolic syndrome (p-interaction < 0.001), indicating that these factors significantly modified the relationship between METS-IR and OSA. However, despite the significant interactions (p < 0.05), METS-IR remained positively correlated with OSA within these subgroups. No significant interactions were observed for Race/ethnicity (p-interaction = 0.304), sex (p-interaction = 0.507), marital status (p-interaction = 0.983), or physical activity (p-interaction = 0.29), suggesting that the association between METS-IR and OSA remained stable within these subgroups.
Mediation analysis
In this study, four key metabolic markers—PLF, HSI, SII and OBS—were used as mediating variables to explore the relationship between METS-IR and obstructive sleep apnea (OSA). Figures 2, 3, 4 and 5 showed the mediation analysis results.
The mediating effect of PLF was 0.00216 (p < 0.001), accounting for 6.95% of the total effect, while the mediating effect of HSI was 0.00556 (p < 0.001), accounting for 17.87%. In contrast, SII (− 0.0127198, p = 0.95) and OBS (− 0.000175, p = 0.21) did not show significant mediating effects, so no meaningful mediating ratios were calculated. These findings suggest that PLF and HSI play a key mediating role in the relationship between METS-IR and OSA, emphasizing the critical role of liver fat accumulation in this pathway. Conversely, the mediating effects of SII and OBS were not significant, indicating that systemic inflammation and oxidative stress may have a minimal impact on the association between insulin resistance and OSA.
Furthermore, the direct effect of METS-IR on OSA remains significant, highlighting the need to consider insulin resistance when assessing OSA risk. Addressing liver health through PLF and HSI may be an effective strategy to mitigate the risk of OSA associated with insulin resistance.
Discussion
This study is the first comprehensive analysis of how liver fat accumulation, systemic inflammation and oxidative stress mediate the relationship between METS-IR and OSA. We found that in a nationally representative sample, higher METS-IR scores were significantly associated with increased OSA risk, and liver fat accumulation estimated by PLF and HSI played a key mediating role. This suggests that insulin resistance captured by METS-IR promotes OSA mainly through liver metabolic dysfunction. Interestingly, SII and OBS did not show a significant mediating effect in this pathway, suggesting that although inflammation and oxidative stress are associated with OSA14,15, they may not be the core mechanisms linking METS-IR and OSA. This highlights the importance of addressing liver health issues in managing OSA risk in people with high insulin resistance.
METS-IR combines fasting blood glucose, triglycerides and BMI to form a comprehensive index of insulin resistance, which plays a key role in the pathophysiology of OSA. Insulin resistance exacerbates OSA through a combination of metabolic disorders driven primarily by intermittent hypoxia (a hallmark of OSA)25. This intermittent hypoxia activates the sympathetic nervous system, leading to oxidative stress and systemic inflammation, which in turn deteriorates insulin sensitivity26. The resulting increase in visceral fat deposition and lipid metabolism dysfunction further aggravate the severity of OSA27.
Visceral fat, especially liver and abdominal fat, due to its dual role, is the main trigger factor for OSA: mechanical compression of the upper airway, airway collapse deterioration, and secretion of inflammatory cytokines (such as TNF-α and IL-6)28. These pro-inflammatory signals interfere with insulin signaling, enhance insulin resistance and promote further fat accumulation29. This forms a vicious cycle, where metabolic dysfunction not only aggravates OSA but also accelerates other cardiovascular risks30.
In addition, OSA-induced hypoxia leads to oxidative stress, which produces reactive oxygen species (ROS) that destroy the insulin receptor signaling pathway and impairs endothelial function31. This vascular health dysfunction is common in OSA patients, which can aggravate their overall metabolic status and promote cardiovascular complications32. Therefore, METS-IR can effectively capture these metabolic disorders and is of great significance in assessing OSA risk.
Through mediation analysis, PLF and HSI play a key role in assessing the relationship between liver fat content, METS-IR and OSA. PLF directly estimates liver fat content, reflecting the actual fat accumulation in liver tissue and is closely related to metabolic dysfunction such as insulin resistance and nonalcoholic fatty liver disease. Increased liver fat directly affects insulin signal transduction, aggravates insulin resistance, and promotes common metabolic disorders in OSA patients33. This link underscores the central role of liver fat in OSA progression and the need for clinical interventions aimed at reducing liver fat to reduce OSA risk, especially in individuals with high insulin resistance. On the other hand, HSI can be used as an indirect measurement of liver fat, calculated by metabolic factors such as liver enzyme levels (AST/ALT), BMI and the presence of diabetes. An increased HSI value indicates hepatic steatosis, which aggravates insulin resistance and is common in OSA patients. Since HSI is also associated with liver function, strategies to improve liver health such as optimizing liver enzyme levels and addressing NAFLD are crucial19. While reducing liver fat, improving liver function may further enhance insulin sensitivity and metabolic regulation34. This shows that by changing lifestyle, diet adjustment and drug intervention, not only focusing on lipid reduction but also on clinical strategies to improve liver function can more effectively reduce the impact of insulin resistance on OSA and improve the clinical outcomes of patients with metabolic disorders35,36.
Although systemic inflammation and oxidative stress are known to play a role in the pathology of OSA, the results of this study show that SII and OBS do not mediate the METS-IR-OSA relationship15. This suggests that although chronic low-grade inflammation and oxidative stress are elevated in OSA patients, their effects may not be significant in the case of insulin resistance. Nevertheless, future research should further explore whether SII and OBS play a greater role in specific subpopulations, where the interaction between insulin resistance, inflammation, and oxidative stress may be more pronounced.
Moreover, even after considering the mediating effect of liver fat through PLF and HSI, METS-IR still showed a significant direct association with OSA. This suggests that insulin resistance itself is an independent driver of OSA. Clinicians should give priority to insulin resistance when assessing the risk of OSA.Improving insulin sensitivity by changing lifestyle (such as weight control, physical activity and diet adjustment) can be used as an effective strategy to reduce the risk of OSA.
This study has several advantages. First, the use of NHANES data provides a large, nationally representative sample that enhances the general applicability of the research findings. Additionally, by controlling for various confounding factors and conducting mediation analysis between METS-IR, PLF, HSI, SII, OBS and OSA, we gain a deeper understanding of the metabolic pathways involved in OSA.
However, one limitation is that the assessment of OSA relied on self-report questionnaires, which may introduce recall and detection bias. Participants might misinterpret or fail to accurately remember their symptoms. Although this approach allows for a comprehensive sample, it introduces uncertainties regarding assessment accuracy. Future research should utilize objective measures, such as polysomnography, to validate these findings.
Despite this limitation, our results are significant as they reveal important pathways linking components of metabolic syndrome with OSA risk, highlighting the need to address metabolic health in this population. This can provide valuable insights into how targeted interventions aimed at improving metabolic health can help reduce the risk of OSA.
Looking ahead, future experiments could involve longitudinal studies to investigate how changes in metabolic syndrome components over time impact the development and progression of OSA. Additionally, randomized controlled trials of interventions that focus on improving metabolic health, such as dietary modifications or exercise programs, could provide further evidence of their effectiveness in reducing OSA risk. By expanding our research to include these experimental approaches, we can deepen our understanding of the connections between metabolic health and OSA and develop more effective preventative strategies for at-risk populations.
Conclusion
This study shows that the METS-IR is significantly associated with the risk of OSA, in which PLF and HSI play a key mediating role. As scoring systems, PLF and HSI capture liver metabolic abnormalities and play a crucial role in connecting insulin resistance and OSA. The results suggest that improving liver health and managing insulin resistance may be effective strategies to reduce OSA risk in people with high METS-IR scores. Future research should focus on improving these scoring systems in order to better predict and manage OSA risks and apply them to clinical practice.
Data availability
The data used in this study are available on the National Health and Nutrition Examination Survey website: https://www.cdc.gov/nchs/nhanes/index.htm
Abbreviations
- NHANES:
-
National Health and Nutrition Examination Survey
- CDC:
-
Centers for Disease Control and Prevention
- METS-IR:
-
Metabolic Score for Insulin Resistance
- PLF:
-
Percentage of liver fat
- HSI:
-
Hepatic Steatosis Index
- SII:
-
Systemic Immunity-inflammation Index
- OBS:
-
Oxidative Balance Score
- NAFLD:
-
Non-alcoholic fatty liver disease
- BMI:
-
Body mass index
- PIR:
-
A ratio of family income to the poverty threshold
References
Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 13(3), 479–504 (2017).
Somers, V. K. et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J. Am. Coll. Cardiol. 52(8), 686–717 (2008).
Punjabi, N. M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 5(2), 136–143 (2008).
Shahar, E. et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 163(1), 19–25 (2001).
Peppard, P. E. et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 177(9), 1006–1014 (2013).
Shamsuzzaman, A. S., Gersh, B. J. & Somers, V. K. Obstructive sleep apnea: Implications for cardiac and vascular disease. JAMA 290(14), 1906–1914 (2003).
Ip, M. S. et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 165(5), 670–676 (2002).
Bello-Chavolla, O. Y. et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur. J. Endocrinol. 178(5), 533–544 (2018).
Kotronen, A. et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 137(3), 865–872 (2009).
Kahl, S. et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One 9(4), e94059 (2014).
Détrait, M. et al. Short-term intermittent hypoxia induces simultaneous systemic insulin resistance and higher cardiac contractility in lean mice. Physiol. Rep. 9(5), e14738 (2021).
Xu, X. et al. Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosis. Lipids Health Dis. 14, 158 (2015).
Güneş, Z. Y. & Günaydın, F. M. The relationship between the systemic immune-inflammation index and obstructive sleep apnea. Sleep Breath 28(1), 311–317 (2024).
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S. & Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 5(1), 9–19 (2012).
Gabryelska, A., Łukasik, Z. M., Makowska, J. S. & Białasiewicz, P. Obstructive sleep apnea: From intermittent hypoxia to cardiovascular complications via blood platelets. Front. Neurol. 3(9), 635 (2018).
Liu, J. et al. Oxidative balance score reflects vascular endothelial function of Chinese community dwellers. Front. Physiol. 17(14), 1076327 (2023).
Zhang, W. et al. Association between the Oxidative Balance Score and Telomere Length from the National Health and Nutrition Examination Survey 1999–2002. Oxid. Med. Cell Longev. 2022, 1345071 (2022).
Wang, H. et al. Association between oxidative balance scores and all-cause and cardiovascular disease-related mortality in patients with type 2 diabetes: Data from the national health and nutrition examination survey (2007–2018). BMC Public Health 24(1), 2642 (2024).
Lee, J. H. et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42(7), 503–508 (2010).
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20(23), 6212–6222 (2014).
Cavallino, V. et al. Antimony and sleep health outcomes: NHANES 2009–2016. Sleep Health 8, 373–379 (2022).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112, 2735–2752 (2005).
Chobanian, A. V. et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 42(6), 1206–1252 (2003).
Kilpatrick, E. S., Bloomgarden, Z. T. & Zimmet, P. Z. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: Response to the International Expert Committee. Diabetes Care 32(12), e159 (2009).
Kohler, M. & Stradling, J. R. Mechanisms of vascular damage in obstructive sleep apnea. Nat. Rev. Cardiol. 7(12), 677–685 (2010).
Harsch, I. A. et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 169(2), 156–162 (2004).
Deng, H. et al. Association of adiposity with risk of obstructive sleep apnea: A population-based study. BMC Public Health 23(1), 1835 (2023).
Carey, D. G. Abdominal obesity. Curr. Opin. Lipidol. 9(1), 35–40 (1998).
Unamuno, X. et al. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Invest. 48(9), e12997 (2018).
Javaheri, S. et al. Sleep apnea: Types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 69(7), 841–858 (2017).
Zhou, L., Chen, P., Peng, Y. & Ouyang, R. Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. Oxid. Med. Cell Longev. 2016, 9626831 (2016).
Narkiewicz, K. & Somers, V. K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 177(3), 385–390 (2003).
Mesarwi, O. A., Loomba, R. & Malhotra, A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am. J. Respir. Crit. Care Med. 199(7), 830–841 (2019).
Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 52(5), 1836–1846 (2010).
Ng, S. S. S. et al. Effect of weight loss and continuous positive airway pressure on obstructive sleep apnea and metabolic profile stratified by craniofacial phenotype: A randomized clinical trial. Am. J. Respir. Crit. Care Med. 205(6), 711–720 (2022).
Murillo, R. et al. Association of self-reported physical activity with obstructive sleep apnea: Results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prev. Med. 93, 183–188 (2016).
Acknowledgements
Special thanks to all of the NHANES participants who freely gave their time to make this and other studies possible.
Funding
This work was supported by Key Project of Shandong Province Traditional Chinese Medicine Science and Technology Program (No. Z-2023019) and Shandong Medical system staff science and Technology Innovation Program project (SDYWZGKCJH2023020).
Author information
Authors and Affiliations
Contributions
S.Y.S. participated in the literature search, study design, data collection, data analysis, data interpretation and wrote the manuscript. X.H.L., Y.C.L., X.X.W. and W.H.Z. conceived the study and participated in its design, coordination, data collection and analysis. J.G.Y. and Y.X.L. participated in the study design and provided critical revision. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The National Center for Health Statistics Research Ethics Review Board authorized the NHANES study protocols in compliance with the revised Declaration of Helsinki. All participants provided informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, S., Li, X., Liu, Y. et al. Liver steatosis mediates the association between metabolic score for insulin resistance and obstructive sleep apnea. Sci Rep 15, 32364 (2025). https://doi.org/10.1038/s41598-025-89850-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89850-z