Abstract
We investigated plasma phosphorylated tau217 (p-tau217) and p-tau181 efficacy in predicting positive amyloid positron emission tomography (PET) results and cognitive stage transitions. Plasma p-tau217 and p-tau181 were measured in participants who were cognitively unimpaired (CU, n = 121), had mild cognitive impairment (n = 102), or dementia (n = 75) from two independent cohorts (Cohort 1: KBASE-V and Cohort 2: Asan) who underwent amyloid PET. In Cohort 1, plasma p-tau217 (area under the curve [AUC] = 0.938, P < 0.001) outperformed p-tau181 (AUC = 0.857, P < 0.001) in predicting amyloid PET positivity (Pdifference < 0.001). In Cohort 2, p-tau217 (AUC = 0.893, P < 0.001) and p-tau181 (AUC = 0.856, P < 0.001) showed comparably good discrimination for predicting amyloid PET positivity (P = 0.377). P-tau217 (AUC = 0.852, P < 0.001) and p-tau181 (AUC = 0.828, P < 0.001) demonstrated similarly good discriminations for predicting cognitive stage transition (Pdifference = 0.093). Plasma p-tau217 and p-tau181 predicted amyloid PET positivity and cognitive stage transitions well. Plasma p-tau217 might perform better, especially in the early stages.
Similar content being viewed by others
Introduction
With the emergence of antiamyloid agents and the rising burden of Alzheimer’s disease (AD)1,2, promptly detecting individuals with amyloid pathology and identifying those at a high risk of progression is essential. Amyloid positron emission tomography (PET) imaging and cerebrospinal fluid (CSF) analyses accurately detect amyloid positivity; however, these approaches are expensive or invasive3. The implementation of blood-based biomarkers overcomes these limitations.
Plasma phospho-tau (p-tau) biomarkers, including p-tau217, p-tau181, and p-tau231, are useful blood-based biomarkers of AD4. These biomarkers strongly correlate with the extent of amyloid pathology detected by amyloid PET imaging and CSF analysis5,6,7,8,9,10. Plasma p-tau217 demonstrated equivalent diagnostic performance to CSF p-tau217 in predicting amyloid PET positivity; p-tau181 and p-tau231 did not11. Different assays for measuring plasma p-tau may affect the performance of p-tau; therefore, a head-to-head comparison of diverse assays in patients with mild cognitive impairment (MCI) has been performed12 This report showed that the mass spectrometry–based measurement of p-tau217 exhibited the best performance in predicting amyloid positivity or disease progression12. Although plasma p-tau217 might be a promising diagnostic tool for AD, its extensive assessment has been impeded due to the limited accessibility of commercial tests13. In addition, information on the ethnic variation of plasma p-tau is lacking, and differences in accuracy might depend on the laboratory environment.
We investigated the efficacy of plasma p-tau217 and p-tau181 measured using a commercially available Simoa immunoassay for predicting amyloid PET positivity and cognitive stage transition. We analyzed two independent Korean cohorts as follows: the Korea Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE-V, Cohort 1) and the Asan Medical Center (Asan, Cohort 2).
Methods
Study participants
We analyzed participants from two independent cohorts. The KBASE-V contains a nationwide cohort, including cognitively unimpaired (CU), MCI, and clinically probable AD dementia (ADD) participants, from nine hospitals across South Korea from April 2015 to August 2016. All participants were aged from 55 to 90 years with a reliable informant who could provide investigators with the requested information. From the KBASE-V study, we analyzed 198 individuals who underwent amyloid PET (Cohort 1), including 113 CU, 49 MCI, and 36 clinically probable ADD. We analyzed plasma p-tau181 in 170 and p-tau217 in 153 individuals (Fig. 1A). Detailed methods, including the inclusion and exclusion criteria of the KBASE-V, have been described14. The KBASE-V study was approved by the Institutional Review Board (IRB) of each participating center (INHAUH 2015-03-021). All participants with CU and MCI provided written informed consent to participate in the study, while written informed consent for ADD patients was obtained from both the patients and their legal representatives prior to participation. All CU participants had an average education-adjusted performance on memory tests and a clinical dementia rating (CDR) scale score of 0 points. The participants with MCI met the criteria proposed by Petersen et al.15: (1) a CDR score of 0.5 points, (2) memory complaint, preferably corroborated by an informant, (3) impaired memory function for age and education, (4) preserved general cognitive function, (5) intact activities of daily living, and (6) was not demented. Participants with ADD met (1) the diagnostic criteria for dementia, as per the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV-TR) and (2) the criteria for probable ADD as per the NINCDS-ADRDA criteria16. All participants in the KBASE-V underwent physical and neurological examinations, including the Mini-Mental State Examination (MMSE)17, CDR scale18, and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) yearly19. CERAD is an assessment battery containing tests for verbal fluency, Boston naming test, MMSE, constructional praxis, word list immediate recall, word list delayed recall, word list recognition, and constructional praxis recall. It was used as a reference for assessing the cognitive stage of participants. All participants underwent 3.0T brain magnetic resonance imaging (MRI). Based on our objective, 134 participants (CU = 93, MCI = 41) were analyzed for future cognitive stage transitions.
The participants of Cohort 2 were enrolled prospectively from the memory clinic of the Asan Medical Center, South Korea, from June 2018 to July 2020, as described previously20. The inclusion criteria were (1) age > 40 and < 90 years, and (2) no evidence of parenchymal lesions that could influence cognitive function based on brain MRI. This study was approved by the IRB of the Asan Medical Center (Approval #2018–0614). All participants or their proxies provided written informed consent. We analyzed 100 participants (CU = 8, MCI = 53, and ADD = 39, Fig. 1B). The plasma p-tau181 was analyzed in all participants in Cohort 2 and p-tau217 in 97 (97%) participants. The criteria for CU, MCI, and ADD in both cohorts were the same, except for the limitations of the CDR score in Cohort 1 (a score of 0 points for CU and 0.5 for MCI). Plasma p-tau181 and p-tau217 were analyzed using the same method in both cohorts. Differences in participant characteristics between cohort 1 and cohort 2 are described in Table S1.
Positron emission tomography images
All participants were assigned to the Aβ+ and Aβ− groups according to the amyloid status on PET, irrespective of their cognitive status. In Cohort 1, positive amyloid pathology by Aβ-PET was defined as a cutoff point of 10 Centiloid units21,22. All participants underwent Aβ-PET (11C-PiB PET or 18F-flutemetamol PET) at baseline. The standard uptake value ratio was obtained using the pons as the reference region for 18F-flutemetamol PET and the cerebellar gray matter for 11C-PiB PET. Centiloid replication analysis was performed as described14,23.
All participants in Cohort 2 underwent 18F-florbetaben amyloid PET. All PET images were obtained using Discovery 690, 710, and 690 Elite PET/CT scanners (GE Healthcare; Chicago, IL, USA). Amyloid PET images were collected for 20 min, beginning 90 min after injecting 300 ± 30 MBq 18F-florbetaben. Two neurologists and two nuclear medicine physicians reviewed the PET scans according to predefined regional cortical tracer uptake (RCTU) and brain amyloid plaque load (BAPL) scoring systems. In general, four regions of interest, the frontal, temporal, and parietal cortices and the posterior cingulate/precuneus, were interpreted in the visual assessment of 18F-florbetaben PET scans. The RCTU scores were condensed into a single three-grade scoring system for each PET scan (BAPL score) as follows: 1 = no Aβ load, 2 = a minor Aβ load, and 3 = a significant Aβ load. The final score was reached by consensus, with BAPL scores of 1 regarded as amyloid beta–negative (Aβ−) and scores of 2 and 3 as amyloid beta–positive (Aβ+)24.
Plasma sampling and analysis
Plasma samples were collected according to the procedure manual of Alzheimer’s Disease Neuroimaging Initiative 225. Blood samples were collected after overnight fasting of at least 6 h, centrifuged within an hour, and stored at − 80 °C. Plasma levels of p-tau181 were quantified using the Simoa Human p-Tau181 Advantage V2 assay (Quanterix PN/103,714; Boston, MA, USA); levels of p-tau217 in plasma were assessed using the Simoa Human ALZpath p-tau217 V2 assay (Quanterix, PN/104371)13 The standard protocol allocated plasma samples and controls into 96-well Quanterix plates for duplicate assessments. The instrument facilitated onboard dilution with a 4 × dilution for p-tau181 and a 3 × dilution for p-tau217. The assay was performed using a Simoa HD-X Analyzer (Quanterix) through a two-step digital immunoassay following the manufacturer’s protocol. The plasma samples underwent a single cycle of thawing and refreezing before being used for p-tau217 analysis.
The APOE genotype was identified by extracting genomic deoxyribonucleic acid from the venous blood. APOE-risk allele status was modeled as one variable coded for the presence of the ε4 allele (1 for ε4 carriers and 0 for noncarriers).
Cognitive stage transition
The cognitive stage transition (from CU to MCI or dementia or from MCI to dementia) during the 3-year study period was analyzed in Cohort 1. The diagnosis of MCI was based on the clinical criteria for MCI established by the National Institute on Aging and Alzheimer’s Association (NIA-AA) workgroups26 and the modified criteria of Petersen et al.15, as described earlier14,27. The diagnosis of dementia was based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for dementia28; the diagnosis of probable ADD was based on the NIA-AA core clinical criteria29.
Statistical analysis
Pearson’s chi-squared and Mann–Whitney U tests were used to compare baseline demographics, clinical data, and biomarker levels. The discrimination accuracies of plasma biomarkers were determined using receiver operating characteristic (ROC) curve analysis, including p-tau181 and p-tau217, for correctly identifying amyloid status on PET or individuals with cognitive stage transition. All models were adjusted for age and sex. The AUCs of different ROC curves were compared using DeLong’s method. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp.; Armonk, NY) and R version 4.2.0 (R Foundation for Statistical Computing). Statistical significance was defined as a two-sided P < 0.05.
Results
Participant characteristics
The baseline characteristics and blood biomarker levels of the participants in both cohorts are presented in Table 1. In Cohort 1, 71 (35.9%) participants were Aβ+. Participants who were Aβ+ were older, had less education, greater rates of dementia and carrying APOE ε4, lower MMSE and higher CDR scores, and greater p-tau181 and p-tau217 levels compared to participants who were Aβ−. In Cohort 2, participants who were Aβ+ were younger, had lower MMSE scores, a lower rate of diabetes mellitus, a greater rate of carrying APOE ε4, and a greater level of plasma p-tau181 and p-tau217. Other demographics and the risk factor proportions did not differ between the Aβ− and Aβ+ groups.
Predicting amyloid positivity confirmed by PET
In the total participants of Cohort 1 (Table 1 and Fig. 2A), both plasma p-tau181 (mean ± standard deviation: 4.14 ± 2.42 vs. 2.14 ± 1.83 pg/mL; P < 0.001) and p-tau217 (1.98 ± 0.76 vs. 0.34 ± 0.29 pg/mL; P < 0.001) were greater in the Aβ+ group than the Aβ− group. Plasma p-tau217 (AUC = 0.938, 95% confidence interval [CI]: 0.899–0.976; P < 0.001) outperformed p-tau181 (AUC = 0.857, 95% CI: 0.797–0.917; P < 0.001) for predicting amyloid PET positivity (Pdifference < 0.001, Fig. 2A). The total participants of Cohort 2 also had greater plasma p-tau181 (4.43 ± 1.82 vs. 2.30 ± 1.16 pg/mL; P < 0.001) and p-tau217 (1.29 ± 0.77 vs. 0.41 ± 1.29 pg/mL; P < 0.001) levels in the Aβ+ group. P-tau217 (AUC = 0.893, 95% CI: 0.830–0.957; P < 0.001) and p-tau181 (AUC = 0.856, 95% CI: 0.780–0.932; P < 0.001) showed comparably high discriminative accuracy for predicting amyloid PET positivity (Pdifference = 0.377, Fig. 2B).
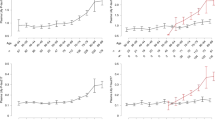
We analyzed the performance of plasma p-tau217 and p-tau181 (combined with age and sex) to predict amyloid PET positivity according to the cognitive stage. In the participants with CU in Cohort 1 (n = 113), plasma p-tau217 (AUC = 0.881, 95% CI: 0.793–0.969; P < 0.001) outperformed p-tau181 (AUC = 0.719, 95% CI: 0.568–0.870; P = 0.006) in predicting amyloid PET positivity (Pdifference = 0.004, Fig. 3A). Both p-tau217 and p-tau181 showed high discriminative values. In patients with MCI in Cohort 1 (n = 49), both p-tau217 (AUC = 0.969, 95% CI: 0.920–1.000; P < 0.001) and p-tau181 (AUC = 0.964, 95% CI: 0.912–1.000; P < 0.001) showed comparably high discriminative values (Pdifference = 0.692, Fig. 3B). In patients with dementia in Cohort 1 (n = 36), plasma p-tau217 (AUC = 0.952, 95% CI: 0.827–1.000; P = 0.011) outperformed p-tau181 (AUC = 0.706, 95% CI: 0.472–0.940; P = 0.186; Pdifference = 0.034) (Fig. 3C). Unadjusted suggested cutoff values of p-tau217 and p-tau181 for Aβ+ in Cohort 1 were 0.50 pg/mL and 2.36 pg/mL, respectively (Table S2).
ROC curve using p-tau217 or p-tau181 for predicting Aβ status using PET. ROC curve analysis of p-tau217 and p-tau181 for predicting Aβ status in the Cohort 1 (A–C) and Cohort 2 (D–F) subgroups. ROC curves for p-tau217 and p-tau181 are compared in the CU (A and D), MCI (B and E), and dementia (C and F) groups. *AUCs of different ROC curves compared using the DeLong method. CU, cognitive unimpaired; MCI, mild cognitive impairment; P-tau, phosphorylated tau; AUC, area under the curve; ROC, receiver operating characteristic; PET, positron emission tomography; Aβ, amyloid beta.
In the participants with CU in Cohort 2 (n = 8), the AUCs for plasma p-tau217 and p-tau181 for predicting amyloid PET positivity were both 1.0 (Fig. 3D). In patients with MCI in Cohort 2 (n = 53), both p-tau217 (AUC = 0.867, 95% CI: 0.761–0.973; P < 0.001) and p-tau181 (AUC = 0.809, 95% CI: 0.687–0.932; P < 0.001) showed comparably high discriminative values for predicting amyloid PET positivity (Pdifference = 0.450, Fig. 3E). In patients with dementia in Cohort 2 (n = 39), both plasma p-tau217 (AUC = 0.940, 95% CI: 0.862–1.000; P < 0.001) and p-tau181 (AUC = 0.913, 95% CI: 0.813–1.000; P < 0.001) showed comparably high discriminative values (Pdifference = 0.328, Fig. 3F). Unadjusted suggested cutoff values of p-tau217 and p-tau181 for Aβ+ in Cohort 2 were 0.62 pg/mL and 2.93 pg/mL, respectively (Table S2).
Predicting cognitive stage transition within three years (Cohort 1)
The efficacy of plasma p-tau181 and p-tau217 in predicting cognitive stage transition within 3 years was analyzed in participants with CU and MCI in Cohort 1 (n = 162). The baseline characteristics of the participants according to cognitive stage transition are shown in Table S3. Participants with cognitive stage transition were 3 years older, had lower MMSE score, had higher CDR and CDR-SOB scores, and showed greater Centiloid scores, p-tau217, and p-tau181 levels. In participants with CU or MCI (Fig. 4A), p-tau217 (AUC = 0.852, 95% CI: 0.757–0.934; P < 0.001) and p-tau181 (AUC = 0.828, 95% CI: 0.742–0.913; P < 0.001) showed comparably high discriminative values in predicting cognitive stage transition (P = 0.093). These results were similar when separately considering participants with CU (p-tau217: AUC = 0.777, 95% CI: 0.660–0.895; p-tau181: AUC = 0.813, 95% CI: 0.718–0.907; Pdifference = 0.714) and MCI (p-tau217: AUC = 0.905, 95% CI: 0.794–1.000; p-tau181: AUC = 0.902, 95% CI: 0.802–1.000; Pdifference = 0.195) (Fig. 4B and C). Performance of p-tau in predicting cognitive stage transition were not significantly different with that of Centiloid score (Figure S1).
ROC curve using p-tau217 or p-tau181 for predicting cognitive stage transition. ROC curve analysis of p-tau217 and p-tau181 for discriminating a cognitive stage transition in total (A), CU (B), and MCI (C) patients over 3 years in cohort 1. *AUC of different ROC curves compared using the DeLong method. CU, cognitive unimpaired; MCI, mild cognitive impaired; P-tau, phosphorylated tau; ROC, receiver operating characteristic; ROC, receiver operating characteristic.
Discussion
This study demonstrated the utility of measuring plasma p-tau217 and p-tau181 with a commercially available Simoa immunoassay to discriminate amyloid PET positivity and predict cognitive stage transition in two independent cohorts. Both p-tau217 and p-tau181 showed good efficacy in predicting amyloid PET positivity. Plasma p-tau217 significantly outperformed p-tau181 in predicting amyloid PET positivity in Cohort 1 but not in Cohort 2; p-tau217 tended to perform slightly better discriminative values. For predicting the cognitive stage transition within 3 years, p-tau217 and p-tau181 showed comparably high predictive values in participants with CU or MCI in Cohort 1.
The finding that p-tau217 outperformed p-tau181 in discriminating amyloid positivity is consistent with previous reports11,12,30. A previous study compared the ability of ten plasma p-tau assays to predict Aβ positivity; p-tau217 measured by mass spectrometry had the highest AUC (0.947), followed by p-tau217 measured by immunoassays developed by Lilly Research Laboratories (AUC = 0.886) and Janssen (AUC = 0.858)12. Another study showed high diagnostic performance for plasma p-tau217 (AUC = 0.91) measured by the Simoa immunoassay developed by Janssen to identify amyloid PET positivity11. In the current study, plasma p-tau217 measured by the commercial ALZpath Simoa immunoassay showed comparable performance with previous studies in identifying amyloid PET positivity in both Cohort 1 (AUC = 0.938) and Cohort 2 (AUC = 0.893).
Several characteristics of our study should be considered. First, we used a commercially available assay to measure plasma p-tau levels. Second, we analyzed various groups of patients, ranging from CU to dementia. Despite using samples that were thawed and refrozen once before analyzing plasma p-tau217, we found that they maintained high performance. In addition, the analysis of p-tau in two cohorts consisting solely of Asians showed performance similar to previous studies.
Plasma p-tau217 outperformed p-tau181 in predicting amyloid PET positivity (Fig. 3A), especially in the participants with CU. A previous study analyzing Aβ positivity–related changes in plasma p-tau31. showed that plasma p-tau217 increased significantly more in CU individuals who were amyloid-positive than in those who were amyloid-negative. This difference was not observed in plasma p-tau181 levels. CSF p-tau217 might increase earlier than p-tau18132. These findings suggest that p-tau217 changes earlier than p-tau181 in patients who were amyloid-positive and shows efficacy in detecting amyloid positivity earlier. In Cohort 2, only 8 participants with CU were analyzed. This limitation might be why no significant difference was found between plasma p-tau217 and p-tau181 for predicting amyloid PET positivity in Cohort 2.
Both p-tau217 and p-tau181 showed high discriminative values in predicting cognitive stage transitions. In participants with CU or MCI, p-tau217 (AUC = 0.852) showed slightly higher discriminative value in predicting cognitive stage transition than p-tau181 (AUC = 0.828), although the difference was not statistically significant (P = 0.093). In patients with MCI, p-tau217 and p-tau181 predicted future dementia within 3 years with high accuracy (AUC = 0.905 and 0.902, respectively; Fig. 4). These values for predicting future progression to dementia are comparable to previous p-tau217 values measured by mass spectrometry (AUC = 0.932, mean follow-up period: 4.9 years)12. In participants with CU in our study, p-tau181 showed a greater but not significantly different AUC than p-tau217 for predicting cognitive stage transition. Further studies are needed to confirm and compare the prognostic value of p-tau with a larger number of patients over a longer follow-up period.
This study had some limitations. First, it included participants of only a single ethnic group. In previous studies, including a study that analyzed the effect of p-tau181 in a multi-ethnic population, information on Asians was lacking33. Although several studies have compared the efficacy of p-tau217 and p-tau181, how they differ between ethnicities is unclear; validating their discriminative value within a single ethnicity is important. Second, our study analyzed few participants from the memory clinics of large hospitals. Therefore, replicating our results with a larger population, including primary care centers, is necessary. Third, the criteria for determining amyloid PET positivity differed between the two cohorts. The Centiloid scale was used in Cohort 1; the BAPL score was used in Cohort 2. However, it is also valuable to demonstrate the difference in the discriminative value of p-tau217 and p-tau181, even when different criteria for amyloid PET positivity were used as outcomes. To identify the early accumulation of Aβ, we used a cutoff value of 10 for the Centiloid scale21 This difference in criteria for amyloid PET positivity might have contributed to why only Cohort 1 showed a significant difference between the predictive values of p-tau217 and p-tau181. Nevertheless, that the blood biomarkers p-tau217 and p-tau181 exhibited high discriminative value is important, even when considering different standards of amyloid PET positivity as outcomes.
Despite these limitations, our results provide evidence for the discriminative value of plasma p-tau217 and p-tau181 in detecting Aβ pathology and cognitive stage transition. Regardless of disease severity, plasma biomarkers are reliable, cost-saving, and noninvasive methods for identifying individuals.
In summary, we demonstrated the robust discriminative value of plasma p-tau217 and p-tau181 for detecting Aβ-PET positivity in two independent Korean cohorts. Plasma p-tau217 showed better performance, especially in the early stage. P-tau217 and p-tau181 showed comparably high performance predicting cognitive stage transition within 3 years. Establishing the cutoff value of plasma p-tau based on the detection method and ethnicity and verifying the suitability for implementing these methods in primary care centers will be important in future research.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Aβ:
-
Amyloid beta
- AD:
-
Alzheimer’s disease
- ADD:
-
Alzheimer’s disease dementia
- APOE:
-
Apolipoprotein E
- AUC:
-
Area under the curve
- BAPL:
-
Brain amyloid plaque load
- CDR-SOB:
-
Clinical dementia rating-sum of box
- CU:
-
Cognitively unimpaired
- KBASE-V:
-
Korea Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease
- MCI:
-
Mild cognitive impairment
- PET:
-
Positron emission tomography
- p-tau:
-
Plasma phosphorylated tau
- RCTU:
-
Regional cortical tracer uptake
- ROC:
-
Receiver operating characteristic
- Simoa:
-
Single-molecule array
References
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21. https://doi.org/10.1056/NEJMoa2212948 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527. https://doi.org/10.1001/jama.2023.13239 (2023).
Lee, E. H. et al. Serum neurofilament light chain level as a predictor of cognitive stage transition. Alzheimers Res. Ther. 14, 6. https://doi.org/10.1186/s13195-021-00953-x (2022).
Park, S. A., Jang, Y. J., Kim, M. K., Lee, S. M. & Moon, S. Y. Promising blood biomarkers for clinical use in Alzheimer’s disease: a focused update. J. Clin. Neurol. (Seoul, Korea) 18, 401–409. https://doi.org/10.3988/jcn.2022.18.4.401 (2022).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433. https://doi.org/10.1016/S1474-4422(20)30071-5 (2020).
Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 26, 387–397. https://doi.org/10.1038/s41591-020-0762-2 (2020).
Janelidze, S. et al. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 78, 149–156. https://doi.org/10.1001/jamaneurol.2020.4201 (2021).
Ashton, N. J. et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathologica 141, 709–724. https://doi.org/10.1007/s00401-021-02275-6 (2021).
Thijssen, E. H. et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: A retrospective diagnostic performance study. Lancet Neurol 20, 739–752. https://doi.org/10.1016/s1474-4422(21)00214-3 (2021).
Kwon, H. S. et al. Predicting amyloid PET positivity using plasma p-tau181 and other blood-based biomarkers. Alzheimer’s Dementia (Amsterdam, Netherlands) 15, e12502. https://doi.org/10.1002/dad2.12502 (2023).
Therriault, J. et al. Equivalence of plasma p-tau217 with cerebrospinal fluid in the diagnosis of Alzheimer’s disease. Alzheimers Dement 19, 4967–4977. https://doi.org/10.1002/alz.13026 (2023).
Janelidze, S. et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain J. Neurol. 146, 1592–1601. https://doi.org/10.1093/brain/awac333 (2023).
Ashton, N. J. et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 81, 255–263. https://doi.org/10.1001/jamaneurol.2023.5319 (2024).
Hwang, J. et al. Clinical and biomarker characteristics according to clinical spectrum of Alzheimer’s disease (AD) in the validation cohort of korean brain aging study for the early diagnosis and prediction of AD. J. Clin. Med. 8, 341. https://doi.org/10.3390/jcm8030341 (2019).
Petersen, R. C. et al. Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. https://doi.org/10.1001/archneur.58.12.1985 (2001).
Dubois, B. et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746. https://doi.org/10.1016/s1474-4422(07)70178-3 (2007).
Jin, J. H. et al. Association between sleep parameters and longitudinal shortening of telomere length. Aging (Albany NY) 14, 2930–2944. https://doi.org/10.18632/aging.203993 (2022).
Choi, S. H. et al. Interchanging scores between clinical dementia rating scale and global deterioration scale. Alzheimer Dis. Assoc. Disord. 17, 98–105. https://doi.org/10.1097/00002093-200304000-00008 (2003).
Lee, D. Y. et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J. Int. Neuropsychol. Soc. 10, 72–81. https://doi.org/10.1017/S1355617704101094 (2004).
Park, S. H. et al. The relationship of soluble TREM2 to other biomarkers of sporadic Alzheimer’s disease. Sci. Rep. 11, 13050. https://doi.org/10.1038/s41598-021-92101-6 (2021).
de Souza, G. S. et al. Amyloid-β PET classification on cognitive aging stages using the Centiloid scale. Mol. Imaging Biol. 24, 394–403. https://doi.org/10.1007/s11307-021-01660-7 (2022).
Kwon, H. S. et al. Predicting cognitive stage transition using p-tau181, Centiloid, and other measures. Alzheimers Dement 19, 4641–4650. https://doi.org/10.1002/alz.13054 (2023).
Klunk, W. E. et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 11(1–15), e11-14. https://doi.org/10.1016/j.jalz.2014.07.003 (2015).
Syed, Y. Y. & Deeks, E. [(18)F]Florbetaben: A review in beta-amyloid PET imaging in cognitive impairment. CNS Drugs 29, 605–613. https://doi.org/10.1007/s40263-015-0258-7 (2015).
Beckett, L. A. et al. The Alzheimer’s Disease Neuroimaging Initiative phase 2: Increasing the length, breadth, and depth of our understanding. Alzheimers Dement 11, 823–831. https://doi.org/10.1016/j.jalz.2015.05.004 (2015).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia J. Alzheimer’s Assoc. 7, 270–279. https://doi.org/10.1016/j.jalz.2011.03.008 (2011).
Koh, S. H. et al. Telomere shortening reflecting physical aging is associated with cognitive decline and dementia conversion in mild cognitive impairment due to Alzheimer’s disease. Aging 12, 4407–4423. https://doi.org/10.18632/aging.102893 (2020).
Association, A. P. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington DC. USA (2000).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia J. Alzheimer’s Assoc. 7, 263–269. https://doi.org/10.1016/j.jalz.2011.03.005 (2011).
Thijssen, E. H. et al. Association of plasma P-tau 217 and P-tau 181 with clinical phenotype, neuropathology, and imaging markers in Alzheimer’s disease and frontotemporal lobar degeneration: A retrospective diagnostic performance study. Lancet Neurol. 20, 739–752. https://doi.org/10.1016/s1474-4422(21)00214-3 (2021).
Ashton, N. J. et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med. 28, 2555–2562. https://doi.org/10.1038/s41591-022-02074-w (2022).
Suárez-Calvet, M. et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Aβ pathology are detected. EMBO Mol. Med. 12, e12921. https://doi.org/10.15252/emmm.202012921 (2020).
Nakamura, A. et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554, 249–254. https://doi.org/10.1038/nature25456 (2018).
Acknowledgements
We would like to thank Bredis Healthcare Inc. (Seoul, South Korea; http://www.bredis.co.kr) for assisting with the immunoassay experiments under the supervision of Dr. H. Hwang.
Seong-Ho Koh, Seong Hye Choi, and Jae-Hong Lee are senior co-authors.
Funding
This work was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute and Korea Dementia Research Center (KDRC) funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (RS-2021-KH112636, RS-2021-KH112011, and RS-2024-00344521); the National Research Council of Science & Technology (NST) Aging Convergence Research Center (CRC22013-600); the Institute of Information & communications Technology Planning & Evaluation (IITP) (No. 2022-0-00448/RS-2022-II220448); the Basic Science Research Program through the National Research Foundation of Korea (NRF) (RS-2022-00165945,RS-2022-NR070008, RS-2023-00278819, RS-2023-00278819, RS-2024-00431471, and NRF-2020M3E5D2A01084721) funded by the Ministry of Science and ICT, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Kwon HS, Hwang M, Koh SH, Choi SH, and Lee JH designed the study; Kwon HS and Hwang M drafted the manuscript; Kwon HS prepared figures; Koh SH, Choi SH, and Lee JH supervised and revised the manuscript; Kim HJ, Park SH, and Jeong JH collected the data and supervised the study; Kwon HS performed the data analysis; Hwang M and Park HH performed the experiments; Kwon HS, Hwang M, Kim JY, and Han MH interpreted the data. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Consent statement
This study was performed in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by the Institutional Review Boards of the Asan Medical Center (Approval #2018-0614), Inha University Hospital (INHAUH 2015-03-021), and each participating center. All participants or their proxies provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kwon, H.S., Hwang, M., Koh, SH. et al. Comparison of plasma p-tau217 and p-tau181 in predicting amyloid positivity and prognosis among Korean memory clinic patients. Sci Rep 15, 7791 (2025). https://doi.org/10.1038/s41598-025-90232-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90232-8
Keywords
This article is cited by
-
Biomarkers and therapeutic strategies targeting microglia in neurodegenerative diseases: current status and future directions
Molecular Neurodegeneration (2025)