Abstract
To investigate the impact of varying altitudes on the functional components of the leaves of Zanthoxylum planispinum var. Dintanensis, this research collected leaf samples from three different elevations: 610 m, 833 m, and 1083 m. Utilizing water and ethanol as extraction solvents, the study optimized extraction parameters via an ultrasonic-assisted technique to maximize the yield of total flavonoids. Following extraction, five types of macroporous adsorption resins were employed for purification. Significant flavonoid constituents within the purified extracts were qualitatively analyzed using liquid chromatography-mass spectrometry (LC-MS). The antioxidant activity of the extracts was also assessed pre- and post-purification. Findings indicated that water was a more effective solvent than ethanol for flavonoid extraction, yielding optimal results at 70 °C, with a solid-to-liquid ratio of 1:70, 30 min, and 480 W. Conversely, ethanol extraction yielded optimal results at a concentration of 65%, a liquid-to-solid ratio of 1:30, 60 °C, 30 min, and 360 W. Among the tested resins, AB-8 demonstrated the highest efficacy for purifying flavonoid extracts, with adsorption data conforming best to the Freundlich isotherm model. Optimal conditions for AB-8 purification included a crude extract concentration of 2.50 mg/mL, pH 5, and temperature 25 °C, eluted with 10 mL of 60% (v/v) ethanol. A notable increase in total flavonoid content was observed, rising from an average of 3.43–16.00%, with a recovery yield of 82.12%. Leaves collected at 830 m contained the highest total flavonoid content, with rutin predominating over naringenin chalcone and naringenin. At 1083 m, naringenin chalcone was most abundant, while the highest concentration of naringenin was recorded at 610 m. This study provides optimized protocols for the extraction and purification of total flavonoids from Z. planispinum var. Dintanensis leaves, contributing to the development of potential applications for these bioactive compounds in various fields.
Similar content being viewed by others
Introduction
The efficacy and quality of medicinal plants are intrinsically linked to the environmental factors that influence their growth and development. It is well-established that the production of secondary metabolites, the pharmacologically active constituents in these plants, represents an adaptive mechanism enabling them to cope with environmental stressors. Numerous studies have demonstrated the significant impact of environmental factors, particularly altitude, on the growth and secondary metabolism of medicinal plants, ultimately influencing the content of active ingredients. Liu et al.1., reported that ecological factors, including annual average precipitation, July mean temperature, frost-free period, sunshine duration, soil pH, soil organic matter, and soil potassium availability, significantly affected the content, though not the type, of active ingredients in the anti-cancer plant Sinopodophyllum hexandrum (Royle) T.S. Ying, with annual average precipitation emerging as the most influential determinant factor. Similarly, Sun et al.2., found that both chemical analyses and delayed luminescence (DL) measurements revealed a correlation between altitude and the quality and composition of rhubarb, suggesting the potential of DL parameters as a novel tool for assessing rhubarb quality. Saffariha et al.3., observed that the optimal harvest time for extracting the highest oil content in Salvia limbata was during the vegetative stage at 1500 m, whereas the highest content of monoterpenes, including α-pinene and β-pinene, could be obtained at 2000 m during the same phenological stage. Conversely, sesquiterpene content reached its peak in the ripening stage at both 1500 and 2500 m. Qiao et al.4., reported that the flavonoid content in the leaves and roots of Lamiophlomis rotata increased with altitude. Elkady et al.5., demonstrated that altitude influences the essential oil compositions of Pinus halepensis L., which consequently affects its anthelmintic and antimicrobial activity. These findings underscore the crucial role of environmental factors in determining the medicinal properties of plants. Therefore, controlling environmental conditions becomes paramount in ensuring the consistent production and preservation of desired therapeutic efficacy in medicinal plant cultivation.
Zanthoxylum planispinum var. Dintanensis, belonging to the Rutaceae family, is a semi-deciduous shrub or small tree, typically reaching a height of 2–4.5 m6. The stems and branches are characterized by sharp, reddish-brown thorns, distinguished by their wide and flat bases. Since 1992, large-scale plantings of Z. planispinum var. Dintanensis have been established in a region characterized by a typical karst plateau canyon landscape. This environment is dominated by carbonate rocks, which contribute to the rapid loss of nutrients through water and soil, resulting in infertile and ecologically fragile soil conditions. Z. planispinum’s high adaptability, ease of cultivation, calcium preference, drought resistance, and notable soil and water conservation properties make it a suitable species for this challenging environment7. Z. planispinum is rich in volatile oils, which impart its distinctive aroma and flavor. Research has demonstrated the presence of various medicinal properties in Z. planispinum, including antioxidant, antitumor, analgesic, anti-inflammatory, and antimicrobial activities8. These beneficial effects are attributed to the presence of bioactive compounds such as amides, alkaloids, flavonoids, lignans, and coumarins9.
Flavonoids, a diverse class of polyphenolic compounds, are universally present in plants, ranging from roots to fruits. To date, over 8000 distinct flavonoids have been identified, including anthocyanins, quercetin, catechin, apigenin, and luteolin10. As secondary metabolites, they play pivotal roles in various plant processes, such as photosynthesis, respiration, growth, development, and defense against environmental stresses11. Flavonoids possess a wide range of medicinal properties, including enhancing human nutrition, offering potential benefits in treating excitotoxicity, oxidative stress, inflammation, and thrombin’s cellular toxicity, as well as protecting the blood-brain barrier and contributing to a reduced risk of cardiovascular diseases, immune-mediated diseases, cancer, and Alzheimer’s disease12,13. Notably, flavonoids exhibit strong free radical scavenging and antioxidant capacity, particularly evident in Chinese prickly ash14. To optimize flavonoid extraction, advanced techniques such as ultrasound-assisted, microwave-assisted, and enzyme-assisted extraction methods have gained widespread acceptance15,16.
Previous research has established the potential of Z. planispinum var. Dingtanensis in various industries, emphasizing its sensitivity to altitude and identifying key flavonoid metabolites, such as naringenin chalcone and naringenin, which are involved in environmental stress response Wa17. This study aimed to further elucidate the influence of varying altitudes (610 m, 833 m, and 1083 m) on the functional components of Z. planispinum var. Dingtanensis leaves. Ultrasonic-assisted extraction methods were optimized for both water and ethanol to maximize total flavonoid (TF) yield. Subsequently, crude TF extracts were purified using macroporous resin. Key flavonoid compounds, including rutin, naringenin, and chalcone, were qualitatively analyzed using liquid chromatography-mass spectrometry (LC-MS). Additionally, the antioxidant activity of TF extracts was measured both pre- and post-purification. This study introduces a novel method for the extraction and purification of flavonoids from the leaves of Z. planispinum var. Dintanensis, systematically examining how altitude affects flavonoid composition. Additionally, it identifies specific flavonoid constituents within the plant. This research contributes to a deeper understanding of phytochemical extraction processes and highlights their potential applications.
Materials and methods
Sample collection
Leaf samples were collected from 7-year-old Z. planispinum var. Dintanensis trees grown at three distinct altitudes, specifically 610 m.a.s.l. (A1), 833 m.a.s.l. (A2), and 1083 m.a.s.l. (A3). The collected leaves were dried in a hot air oven at 70 °C for 48 h, then ground and sieved through 100 mesh screens to obtain the powdered leaf material for further experiment.
Determination of total flavonoids
The total flavonoid content was determined using the methodology described by Masci et al.18. The calculation was performed according to the following formula:
Where C is the concentration of the rutin standard solution (mg/mL), V is the volume of the sample solution (mL), N is the dilution factor, M is the weight of the leaf (g).
Extraction of total flavonoids using water as the solvent
The extraction of TF was conducted using distilled water as the solvent via ultrasonic-assisted extraction (UAE). A single-factor experimental design was employed to initially assess the impact of material-to-liquid ratio (1:30, 1:50, 1:70, 1:90, 1:110), extraction temperature (20 °C, 30 °C, 40 °C, 60 °C, 70 °C, 80 °C), extraction time (10 min, 20 min, 30 min, 40 min, 50 min, 60 min, 80 min, 100 min), and ultrasonic power (320 W, 400 W, 480 W, 560 W, 640 W) on extraction efficiency. Based on these results, an orthogonal design was implemented to optimize the extraction process considering material-to-liquid ratio, temperature, ultrasonic power, and time .
Extraction of total flavonoids using ethanol as the solvent
The optimization of total flavonoid extraction using an UAE method with ethanol as the solvent was investigated. A series of single-factor experiments were also conducted to explore the effects of various parameters, including ultrasonic power (320 W, 400 W, 480 W, 560 W, 640 W), temperature (20 °C, 30 °C, 40 °C, 50 °C, 60 °C, 70 °C, 80 °C), and ethanol concentration (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%). According to the results of the single-factor experiment, design-Expert v.8.0 software was used to investigate the effects of four independent variables on the yield of TFs, including ethanol concentration (A), material-liquid ratios (B), extraction power (C), and temperature (D). The four-factor three-level response is shown in .
Purification of total flavonoids with macroporous resin
To enhance TF in Z. planispinum leaf extract, five different macroporous resins (HPD600, HPD100, XAD-2, AB-8, and D101) were employed to separate the flavonoids from crude TF extracts, following the methodology described by Wang et al.19. Static adsorption and desorption tests of TF on macroporous resins were conducted according to the protocol outlined by Xie et al.20. The optimal resin was selected based on a comprehensive evaluation of adsorption capacities, adsorption ratios, and desorption rates. The following equations were used to quantify the equilibrium adsorption capacity and adsorption ratio and desorption ratio:
where Q is the equilibrium adsorption capacity at adsorption equilibrium; R is the adsorption rate; E is the desorption rate (%); C0 and C1 are the initial and equilibrium concentrations of total flavonoids in the solution, respectively (mg/mL); C2 is the concentration of total flavonoids in the desorption solution (mg/mL); V is the volume of the initial sample solution (mL); W is the weight of resin (g).
The adsorption isotherms of total flavonoids on selected resin were investigated by mixing 1 g of resin with 100 mL of sample solutions containing varying concentrations of total flavonoids (0.07–0.40 mg/mL). The mixtures were continuously agitated at 150 rpm for 5 h at temperatures of 25 °C, 30 °C, and 35 °C. The equilibrium adsorption data were analyzed using the Langmuir and Freundlich models.
In these models, Qe (mg/g) denotes the theoretical maximum adsorption capacity, Ce (mg/mL) represents the concentration of total flavonoids, Kad (L/mg) is the Langmuir adsorption constant, while n and K [mg/g (L/mg)^(1/n)] refer to the Freundlich constants.
The adsorption kinetics of the total flavonoids on the selected resin were investigated. A sample solution with a total flavonoids concentration of 3.00 mg/mL was blended with 1 g of the AB-8 resin at a shaking speed of 150 rpm and a temperature of 25 ℃ for 12 h. The adsorption capacity of the AB-8 resin was measured at various time intervals, ranging from 0 to 12 h, with a 0.5-h increment. Additionally, The effects of pH on the adsorption capacity of the selected resin were investigated. The influence of ethanol concentration and elution volume on the desorption ratio was examined to determine the optimal purification conditions. Following flavonoid adsorption saturation in resin columns loaded using the wet loading method, a sequential elution protocol is employed. Initially, the column is rinsed with water at a flow rate of 2 bed volumes per hour until the effluent clarifies. Subsequently, the column is eluted with 60% ethanol at the same flow rate, and the ethanol-eluted fractions are collected. An elution curve is then plotted accordingly, illustrating the optimal elution conditions for flavonoid recovery.
Analysis of LC-MS
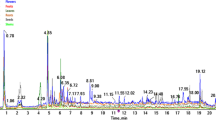
The quantitative analysis of three flavonoids, namely rutin, naringin chalcones, and naringin, present in Z. zanthoxylum extracts, was carried out using LC-MS. The analysis was executed on an Agilent 1100 liquid chromatography mass spectrometry system equipped with an Agilent Poroshell 120 EC-C18 2.7 μm column (3 × 50 mm) maintained at a column temperature of 35 ℃. The mobile phases comprised of 0.5% formic acid in water (solvent A) and acetonitrile (solvent C), employing a gradient elution method. The elution program comprised the following steps: (1) 0–1 min, 0-95% A; (2) 1–8 min, 95–75% A; (3) 8–12 min, 75–40% A; (4) 13–16 min, 40–0% A; (5) 16–16.1 min, 0–95% A; (6) 16.1–20 min, 95–95% A.The flow rate was set at 0.6 mL/min, and the injected sample volume was 10 µL.
HOSC assay and FRAP assay
The antioxidant properties of crude TF extracts and purified final products were comprehensively evaluated using the hydroxyl radical scavenging capacity (HOSC) assay and the ferric reducing antioxidant power (FRAP) assay, following the methodologies described by Dienaitė et al.21 and Zhao et al.22.
Statistical analysis
The results were statistically analyzed using one-way analysis of variance (ANOVA) and Tukey’s post-hoc test at a 95% confidence level, employing SPSS statistical software version 26.0 (https://www.ibm.com/support/pages/downloading). Additionally, regression analysis and response surface optimization were performed using Design-Expert software version 8.0.6 (www.statease.com/software/design-expert/). All measurements were carried out in triplicate and expressed as mean ± standard deviation. Graphical representations were generated using Origin software version 2022 (https://www.originlab.com/index.aspx?go=Products/Origin/2022&pid=4418).
Results
Effect extraction of total flavonoids with water as solvent
Regarding the extraction of total flavonoids using ultrasound with water as the solvent (Fig. 1), the yield initially increased but subsequently decreased as the material-to-liquid ratio, temperature, time, and ultrasonic power were increased. The single-factor experiments revealed that the optimal conditions for maximum adsorption were a material-to-liquid ratio of 1:70, a temperature of 70 ℃, an extraction time of 30 min, and an ultrasound power of 480 W.
Effect extraction of total flavonoids with ethanol as solvent
Similarly, the extraction of total flavonoids using ultrasound with ethanol as the solvent (Fig. 2) showed an initial increase in the adsorption ratio followed by a decrease as the ethanol concentration, material-to-liquid ratio, ultrasound power, temperature, and extraction time were increased. The single-factor experiments indicated that the maximum adsorption ratio was obtained with an ethanol concentration of 60%, a material-to-liquid ratio of 1:70, an ultrasound power of 320 W, and a temperature of 60 °C.
Optimization of extraction process by orthogonal experiment
The results of the orthogonal experiment, which employed a four-factor, three-level (L9 (34)) design, are presented in Table 1. Range analysis indicated that the material-to-liquid ratio (B) has the most significant influence on total flavonoid extraction rate, followed by extraction power (C), then extraction time (A), and finally temperature (D). The optimal extraction conditions were determined to be A3B2C1D3, corresponding to ultrasound-assisted extraction at 70 °C, a material-to-liquid ratio of 1:70, an extraction time of 30 min, and a ultrasound power of 480 W.
Optimization of extraction process by response surface experiment
The Response surface design scheme and results are shown in Table 2. a multiple quadratic response surface regression model was established: Y = 2.19 + 0.016 A-0.11B-0.014 C -8.333*10-3- 0.090AB-0.030AC + 0.013AD + 0.060BC + 0.015BD + 0.053CD-0.69A2-0.32B2-0.37C2-0.34D2.
As illustrated in Table 3, the model exhibited statistical significance with an F-value of 53.22 (p < 0.0001), indicating a strong fit. The lack-of-fit test produced a non-significant p-value of 0.403, suggesting that the lack of fit was not statistically significant when compared to pure error (p = 0.017). The high adjusted R2 value of 0.963 demonstrated a substantial correlation between the observed and predicted values. These findings indicate the model’s accurate representation of the relationship between the independent variables and the outcomes. Additionally, the factors influencing the yield of total flavonoids, in order of significance, were material-to-liquid ratio (B), ethanol concentration (A), extraction power (C), and temperature (D).
The TF regression model demonstrated substantial linear effects of ethanol concentration, material-to-liquid ratio, extraction power, and temperature, as well as significant interactions between the four independent variables (p < 0.05). Specifically, the interaction between ethanol concentration and material-to-liquid ratio (AB) exhibited a significant effect. The impacts of the independent variables on the TF are illustrated in Fig. 3. An increase in ethanol concentration, coupled with an increasing material-to-liquid ratio, resulted in a higher TF yield. Regression analysis predicted that the optimal extraction parameters were an ethanol concentration of 63.70%, a material-to-liquid ratio of 28.09:1 (mL/g), a temperature of 62.62 °C, an extraction time of 30 min, and an ultrasonic power of 360 W.
The effects of independent variables on the TF. (a) interaction between material-liquid ratios and temperature, (b) interaction between extraction power and temperature, (c) interaction between ethanol concentration and material-liquid ratios, (d) interaction between ethanol concentration and extraction power, (e) interaction between ethanol concentration and temperature, (f) interaction between material-liquid ratios and extraction power.
TF content of Z. planispinum leaves at different elevation
The yield of total flavonoids extracted using water (ranging from 2.13 to 3.85%) was significantly higher than that obtained through alcohol solvent extraction (which ranged from 1.11 to 2.26%), as presented in Tables 1 and 2. As a result, water was selected as the solvent for extracting total flavonoids from the leaves of Z. zanthoxylum at varying elevations. The flavonoid content was highest in sample A2, with a concentration of 3.74%, followed by sample A3 at 3.58% and sample A1 at 3.15%.
Screening of macroporous resin
The adsorption and desorption properties of five macroporous resins (XAD-2, AB-8, HPD-100, D101, and HPD-600) were rigorously investigated for the purification of total flavonoids. As shown in Fig. 4, AB-8 exhibited the most favorable performance among these resins, demonstrating significantly enhanced adsorption capacity (Q), adsorption rate (R), and desorption rate (E) across various experimental samples. Notably, the adsorption rate of AB-8 on sample A2 was lower than those of the other resins. Nevertheless, based on a comprehensive comparative analysis, AB-8 was ultimately chosen as the optimal resin for purifying crude TF extracts from the leaves of Z. planispinum.
Adsorption isotherms and adsorption kinetics of AB-8 resin
The adsorption capacity of the AB-8 resin exhibits a gradual decline as the adsorption temperature increases. Notably, the highest adsorption capacity is attained at 25 ℃, significantly surpassing the capacities observed at other temperatures, as depicted in Fig. 5a–c. These findings suggested that 25℃represents the optimal conditions for the adsorption process. A comparative analysis of the Freundlich and Langmuir isotherm models revealed that the former provides a superior fit to the experimental data for parameters of Langmuir and Freundlich equations for the adsorption of AB-8 resin, evident from its higher correlation coefficient R2 (0.98519–0.99541) within the tested temperature range. This indicated that the adsorption of total flavonoids from Z. planispinum leaves onto the AB-8 resin follows a monolayer adsorption behavior. Furthermore, the adsorption kinetics curves for total flavonoids on AB-8 resins (Fig. 5d) demonstrated a rapid increase in adsorption capacity with time, reaching equilibrium at approximately 8 h and achieving 48–50 mg/g.
Effect of pH value on adsorption capacity of AB-8 resin and maximum adsorption capacity
The influence of pH value on the adsorption capacity of AB-8 resin is illustrated in Fig. 6a. The results showed that pH significantly impact the adsorption capacity of the resin. Specifically, the adsorption capacity of AB-8 resin at pH 5 is substantially higher than at other pH levels, a trend consistently observed across all three samples.
The adsorption performance of AB-8 resin for total flavonoids was thoroughly investigated through an equilibrium adsorption study at varying concentrations of the crude flavonoid extracts. As depicted in Fig. 6b, the adsorption capacity of total flavonoids initially increased with the concentration of the crude extracts, but subsequently decreased. The maximum adsorption capacity was observed when the concentration of the crude flavonoid extracts was 2.50 mg/mL, indicating that the optimal concentration for the adsorption of flavonoids onto the AB-8 resin is 2.5 mg/mL.
Dynamic desorption
The breakthrough point was defined as the instant at which the effluent’s total flavonoid concentration reached 10% of the initial value, as reported by Liu et al.23.The breakthrough curves obtained from the AB-8 resin-packed column are illustrated in Fig. 7a. To optimize operational efficiency, the breakthrough volume was set at 10 ml, indicating the complete penetration of total flavonoids through the resin. The influence of ethanol concentration on the desorption rate was investigated, as depicted in Fig. 7b. An initial increase in ethanol concentration led to a rise in the desorption rate, which subsequently decreased upon further increases. Notably, at an ethanol concentration of 60%, the desorption rate peaked, surpassing 60%, with this trend consistently observed across all three samples. Additionally, gradient elution was examined within the context of dynamic desorption. It was determined that when the eluent volume was maintained at 10 mL, the concentration of total flavonoids reached its maximum value of 16 mg/mL (Fig. 7c). Consequently, the optimal conditions for desorption were identified as an ethanol concentration of 60% and an eluent volume of 10 mL.
Based on the aforementioned experimental findings, an investigation into the purification process of total flavonoids from Z. planispinum crude flavonoid extracts was conducted on AB-8 resin under optimized parameters. A crude flavonoid extract solution, with a concentration of 2.50 mg/mL, a pH of 5, and a temperature of 25 °C, was loaded onto a pre-treated AB-8 resin column. After adsorptive saturation, the column was eluted with 10 mL of 60% (v/v) ethanol. This process resulted in a final product containing 16.00% total flavonoids, corresponding to an 82.13% recovery rate. These results indicate the feasibility and reliability of the AB-8 resin for the preparative purification of flavonoids.
Content of Rutin, naringin Chalcones, and naringin in TF extracts
The LC-MS chromatogram revealed three distinct peaks with retention times of 7.11–7.33, 11.1, and 11.4 min, corresponding to rutin, naringin chalcones, and naringin, respectively. The content of these flavonoids exhibited significant variation among the three samples analyzed (Table 4). Specifically, the rutin content followed the order of A2 > A3 > A1, while the naringin content was ordered as A1 > A2 > A3. The naringin chalcone content was significantly higher in A3, followed by A2 and then A1. Across all samples, rutin had the highest concentration, whereas naringin chalcone had the lowest concentration.
Antioxidant activity of total flavonoids
Herein, we present a comprehensive analysis of the antioxidant activity of crude TF extracts and the final products purified using AB-8 resin. As demonstrated in Fig. 8, within the concentration range of 0.007–0.035 mg/mL, both before and after purification, the the clearance ratio of TF on ·OH increased with the rising TF concentration. Notably, HOSC value was significantly higher than that of Vitamin C (Vc). Additionally, as depicted in Fig. 9, within the concentration range of 2–10 µg/mL, FRAP capacity of the TF extracts increased proportionally with the TF concentration. The FRAP strength hierarchy for the crude TF extract was observed as follows: Vc > A2 = A3 > A1. Conversely, for the TF extracts purified with AB-8 resin, the order was A2 > A3 > Vc > A1. In addition, the IC50 values of HOSC assay for the crude TF extracts from sample A1 and A3 were significantly lower than those of the purified products (Table 5). Similarly, FRAP assay performed on the crude TF extract from sample A1 indicated a significantly reduced IC50 value (Table 6). These results suggest that the purification process enhances the antioxidant activity of TF.
Discussion
The total flavonoid yield was found to be highest when using the ultrasound extraction method with water as the solvent, compared to using ethanol as the solvent. This finding is consistent with the study by González-Centeno et al.24, who utilized water for the extraction of phenolic compounds, flavonoids, and antioxidants from grape pomace. Similarly, Egüés et al.25 reported the successful extraction of bioactive compounds from apple pomace using an ultrasound-assisted technique with water as the solvent. Water, being a highly polar solvent, is more effective in extracting highly polar flavonoid compounds, such as flavonoid glycosides with multiple hydroxyl groups26. In contrast, ethanol, with its lower polarity, results in a relatively lower extraction rate for flavonoid compounds. The solubility of flavonoid compounds in different solvents significantly influences the extraction efficiency. For certain flavonoid compounds, their solubility in water may be higher than in ethanol, leading to a greater extraction yield when water is used as the solvent27,28. Flavonoid compounds in plants often exist as flavonoid glycosides, which contain one or more sugar molecules, increasing their polarity and water solubility29. Therefore, water is more suitable for extracting these water-soluble flavonoid glycosides, suggesting that the leaves of Z. planispinum var. Dintanensis contain a higher proportion of water-soluble substances, such as polysaccharides and polyphenols.
The yield of TF extracted from Z. planispinum leaves at an altitude of 830 m (A2) is significantly higher than that at 1083 m (A3) and 610 m (A1). Various environmental factors associated with different altitudes, including light, temperature, moisture, and soil conditions, have a substantial impact on the accumulation of secondary metabolites in Z. planispinum. Rocky desertification areas, especially at higher altitudes, typically receive more direct sunlight and ultraviolet (UV) radiation, which prompts the plants to produce more protective secondary metabolites, such as flavonoids, to mitigate UV-induced damage and reduce photooxidative stress30. Moreover, extreme temperature fluctuations in rocky desertification areas can adversely affect the normal growth of Z. planispinum, leading them to adjust their secondary metabolite production as a means of adaptation31. Severe water shortages in these regions further stimulate Z. planispinum to synthesize additional secondary metabolites, such as acrylic acid derivatives and specific alkaloids, to enhance drought resistance and maintain cellular water balance32. Furthermore, the reduced atmospheric pressure and nutrient availability at higher altitudes may stimulate metabolic pathways involved in flavonoid biosynthesis33. Consequently, the increased total flavonoid content at higher altitudes results from a synergy of environmental stress factors and physiological adaptations, significantly impacting secondary metabolite production. Therefore, the significant environmental differences at various altitudes, which influence secondary metabolite production, result in varying total flavonoid yields in Z. planispinum leaves when subjected to ultrasound-assisted extraction.
This study compared the adsorption performance of five different macroporous resins (AB-8, D101, HPD-100, HPD-600, and XAD-2) and found that AB-8 resin exhibited the best adsorption data for total flavonoid extracts. This result aligns with the findings of Hou et al.34, who established the AB-8 macroporous resin column chromatography (MRCC) method for the purification of total flavonoids from S. tonkinensis. The unique macroporous structure and pore size of AB-8 resin effectively adsorb and release large molecules such as flavonoids, reducing molecular diffusion resistance and improving adsorption efficiency. Its chemical properties enhance flavonoid affinity and selectivity, thereby increasing adsorption capacity, reducing non-target adsorption, and improving purity. Compared to other resins, AB-8 also has the highest desorption rate, enabling the rapid adsorption and release of target compounds, thus improving production efficiency and reducing energy consumption35.
The LC-MS analysis of the three flavonoid compounds revealed that the content of rutin in sample A2 was significantly higher than in samples A3 and A1, suggesting that the increased total flavonoid yield in A2 may be attributed to its higher rutin content. However, sample A3 exhibited a significantly higher concentration of naringenin chalcone compared to A2 and A1, while sample A1 demonstrated a significantly higher concentration of naringenin relative to A2 and A3. The biosynthesis of naringenin chalcone, a critical intermediate in the flavonoid pathway, plays an essential role in the production of diverse flavonoid classes. Naringenin chalcone is synthesized through the condensation of p-coumaroyl-CoA with three malonyl-CoA molecules, a reaction catalyzed by the enzyme chalcone synthase (CHS). The resultant naringenin chalcone is subsequently converted to naringenin by the action of chalcone isomerase (CHI), which serves as the branching point for the further diversification of flavonoids, including isoflavones, flavanones, flavones, flavanols, flavan-3-ols, and anthocyanins36. The differential accumulation of naringenin chalcone in the leaves of Z. planispinum var. Dintanensis across varying elevational environments suggests its adaptive significance in response to environmental conditions, underscoring the importance of this metabolic intermediate in the regulation of flavonoid biosynthesis. This result is consistent with the study by Giupponi et al.37, which demonstrated that environmental conditions influenced by elevation are significant factors in inducing variations in the secondary metabolite composition of hemp inflorescences, including terpenes, cannabinoids, and flavonoids. All plants grown at higher altitudes exhibited a higher total amount of terpenes compared to those grown at lower elevations, with β-Myrcene, trans-Caryophyllene, and α-Humulene being the main contributors. The unique environmental conditions of karst rocky desertification mountainous areas, characterized by prolonged ultraviolet radiation exposure and harsh environmental stresses, exert a significant influence on the flavonoid content of plants in these regions. This influence is likely a consequence of the plants’ high adaptability to fluctuating climatic and geographical conditions, suggesting a strong correlation between flavonoid levels and resilience to these environmental pressures.
Based on HOSC and FRAP assays, the antioxidant activity of the TF final product significantly improved through AB-8 resin purification compared to the crude extract. Furthermore, the antioxidant capacity of TF increased in a dose-dependent manner. Similarly, in the investigation of blackened jujube (Ziziphus jujuba Mill.), the purified triterpenic acids exhibited stronger antioxidative capacity than the crude extract and demonstrated excellent protective effects against H2O2-induced damage in HUVEC cells38. The difference in the types of compounds between the final product and the crude extract may also contribute to variations in their antioxidant capacity19. Purification resulted in the enrichment of more active components, which significantly enhanced the antioxidant activity of the TF extract.
Conclusion
This study investigated the extraction and purification of flavonoid compounds from Z.planispinum var. dintanensis leaves using ultrasonic-assisted extraction. Two solvents, water and ethanol, were employed to evaluate their efficacy in extracting flavonoids. The optimal conditions for water extraction were determined to be: 70℃ temperature, 1:70 solid-to-liquid ratio, 30 min extraction duration, and 480 W power. For ethanol extraction, optimal conditions were: 65% ethanol concentration, 1:30 liquid-to-solid ratio, 60℃ temperature, 30 min duration, and 360 W power. The results indicated that water extraction yielded a higher total flavonoid content compared to ethanol extraction. Purification of the crude flavonoid extracts was conducted using five macroporous adsorption resins (AB-8, D101, HPD-100, HPD-600, and XAD-2). Among these, AB-8 resin demonstrated the most effective adsorption, with the adsorption data best fitting the Freundlich isotherm model. The optimal adsorption conditions for AB-8 were: 2.50 mg/mL crude flavonoid extract solution concentration, pH 5, and 25℃ temperature. Elution with 10 mL of 60% (v/v) ethanol resulted in a significant increase in total flavonoid content, reaching an average of 16.00% with a 4.67-fold increase from the initial 3.43%. The recovery yield of the purification process was 82.12%. Furthermore, it was found that the highest total flavonoid content was present in the leaves of Z. planispinum var. dintanensis at an altitude of 830 m (A2). The rutin content significantly exceeded that of naringenin chalcone and naringenin. At an altitude of 1083 m (A3), the naringenin chalcone content in leaves was the highest, while at 610 m (A1), the naringenin content was the highest.The findings of this study will contribute to a deeper understanding of the impact of altitude on the functional components of Z. planispinum var. dingtanensis, a species with significant potential in various industries. The optimized extraction and purification methods developed in this research can also be applied to the efficient and effective isolation of valuable bioactive compounds from this plant.
Data availability
All data analyzed during this study are included in this published article and supplementary information file.
References
Liu, W., Liu, J., Yin, D. & Zhao, X. Influence of ecological factors on the production of active substances in the anti-cancer plant Sinopodophyllum hexandrum (Royle) T.S. Ying. PLoS ONE. 10 (4), e0122981. https://doi.org/10.1371/journal.pone.0122981 (2015).
Sun, M. et al. Effects of growth altitude on chemical constituents and delayed luminescence properties in medicinal rhubarb. J. Photochem. Photobiol. B. 162, 24–33. https://doi.org/10.1016/j.jphotobiol.2016.06.018 (2016).
Saffariha, M. et al. Changes in the essential oil content and composition of Salvia limbata C.A. Mey at different growth stages and altitudes. Biomed. Chromatogr. BMC. 35 (8), e5127. https://doi.org/10.1002/bmc.5127 (2021).
Qiao, F. et al. Flavonoid synthesis in lamiophlomis rotata from Qinghai-Tibet plateau is influenced by soil properties, microbial community, and gene expression. J. Plant Physiol. 287, 154043. https://doi.org/10.1016/j.jplph.2023.154043 (2023).
Elkady, W. M., Gonaid, M. H., Yousif, M. F., El-Sayed, M. & Omar, H. A. N. Impact of altitudinal variation on the phytochemical profile, anthelmintic and antimicrobial activity of two Pinus species. Mol. (Basel Switz). 26 (11), 3170. https://doi.org/10.3390/molecules26113170 (2021).
Appelhans, M. S., Reichelt, N., Groppo, M., Paetzold, C. & Wen, J. Phylogeny and biogeography of the Pantropical genus Zanthoxylum and its closest relatives in the proto-Rutaceae group (Rutaceae). Mol. Phylogenet. Evol. 126, 31–44. https://doi.org/10.1016/j.ympev.2018.04.013 (2018).
Song, Y., Yu, Y., Li, Y. & Du, M. Leaf litter chemistry and its effects on soil microorganisms in different ages of Zanthoxylum planispinum Var. Dintanensis. BMC Plant Biol. 23 (1), 262. https://doi.org/10.1186/s12870-023-04274-z (2023).
Kim, J. A. et al. Genome-wide transcriptome profiling of the medicinal plant Zanthoxylum planispinum using a single-molecule direct RNA sequencing approach. Genomics 111 (4), 973–979. https://doi.org/10.1016/j.ygeno.2018.06.004 (2019).
Wang, Y. et al. Insecticidal and repellent efficacy against stored-product insects of oxygenated monoterpenes and 2-dodecanone of the essential oil from Zanthoxylum planispinum Var. Dintanensis. Environ. Sci. Pollut. Res. Int. 26 (24), 24988–24997. https://doi.org/10.1007/s11356-019-05765-z (2019).
Liga, S., Paul, C. & Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants (Basel Switz). 12 (14), 2732. https://doi.org/10.3390/plants12142732 (2023).
Zhu, J. et al. Identification and characterization of chalcone isomerase genes involved in flavonoid production in Dracaena Cambodiana. Front. Plant Sci. 12, 616396. https://doi.org/10.3389/fpls.2021.616396 (2021).
Zhou, J. et al. Novel insight into the therapeutical potential of flavonoids from traditional Chinese medicine against cerebral ischemia/reperfusion injury. Front. Pharmacol. 15, 1352760. https://doi.org/10.3389/fphar.2024.1352760 (2024).
Luiz-Ferreira, A. et al. TRAIL-sensitizing effects of flavonoids in Cancer. Int. J. Mol. Sci. 24 (23), 16596. https://doi.org/10.3390/ijms242316596 (2023).
Zheng, T., Han, J., Su, K. X., Sun, B. Y. & Liu, S. M. Regulation mechanisms of flavonoids biosynthesis of Hancheng Dahongpao peels (Zanthoxylum bungeanum Maxim) at different development stages by integrated metabolomics and transcriptomics analysis. BMC Plant Biol. 22 (1), 251. https://doi.org/10.1186/s12870-022-03642-5 (2022).
Shangguan, Y. et al. Response surface methodology-optimized extraction of flavonoids from pomelo peels and isolation of naringin with antioxidant activities by Sephadex LH20 gel chromatography. Curr. Res. Food Sci. 7, 100610. https://doi.org/10.1016/j.crfs.2023.100610 (2023).
Singh, T. et al. Ultrasound assisted extraction of phytochemicals from Piper betel L. Ultrason. Sonochem. 106, 106894. https://doi.org/10.1016/j.ultsonch.2024.106894 (2024).
Wang, J. et al. Response of blade in Zanthoxylum planispinum Var. Dintanensis to differences altitudes through integrated metabolomics and transcriptomics analysis. S. Afr. J. Bot. 162, 45–51. https://doi.org/10.1016/j.sajb.2023.08.070 (2023).
Masci, A. et al. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chemistry, 202, 59–69. https://doi.org/10.1016/j.foodchem.2016.01.106 (2016).
Wang, Z., Yang, S., Gao, Y. & Huang, J. Extraction and purification of antioxidative flavonoids from Chionanthus retusa leaf. Front. Bioeng. Biotechnol. 10, 1085562. https://doi.org/10.3389/fbioe.2022.1085562 (2022).
Xie, Y., Guo, Q. S. & Wang, G. S. Preparative separation and purification of the total flavonoids in Scorzonera Austriaca with macroporous resins. Mol. (Basel Switz). 21 (6), 768. https://doi.org/10.3390/molecules21060768 (2016).
Dienaitė, L. et al. Isolation of strong antioxidants from Paeonia Officinalis roots and leaves and evaluation of their bioactivities. Antioxid. (Basel Switzerland). 8 (8), 249. https://doi.org/10.3390/antiox8080249 (2019).
Zhao, S. et al. Optimal extraction, purification and antioxidant activity of total flavonoids from endophytic fungi of Conyza blinii H. Lév PeerJ. 9, e11223. https://doi.org/10.7717/peerj.11223 (2021).
Liu, W. et al. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresour. Technol. 101 (12), 4667–4675. https://doi.org/10.1016/j.biortech.2010.01.058 (2010).
González-Centeno, M. R. et al. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.) - a response surface approach. Ultrason. Sonochem. 21 (6), 2176–2184. https://doi.org/10.1016/j.ultsonch.2014.01.021 (2014).
Egüés, I., Hernandez-Ramos, F., Rivilla, I. & Labidi, J. Optimization of. Molecules (Basel Switzerland). 26 (13), 3783. https://doi.org/10.3390/molecules26133783 (2021).
Latos-Brozio, M., Masek, A. & Piotrowska, M. Effect of enzymatic polymerization on the thermal stability of flavonoids. J. Therm. Anal. Calorim. Article. 148, 5357–5374. https://doi.org/10.1007/s10973-023-12089-1 (2023).
Kumar, K., Srivastav, S. & Sharanagat, V. S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 70, 105325. https://doi.org/10.1016/j.ultsonch.2020.105325 (2021).
Carreira-Casais, A. et al. Benefits and drawbacks of Ultrasound-Assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health. 18 (17), 9153. https://doi.org/10.3390/ijerph18179153 (2021).
Dias, M. C., Pinto, D. C. G. A. & Silva, A. M. S. Plant flavonoids: chemical characteristics and biological activity. Molecules (Basel Switzerland). 26 (17), 5377. https://doi.org/10.3390/molecules26175377 (2021).
Jiang, Z., Lian, Y. & Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration,Earth-Science Reviews,132,1–12. (2014). https://doi.org/10.1016/j.earscirev.2014.01.005
Han, X. et al. Spatiotemporal dynamic evolution and driving factors of desertification in the mu Us sandy land in 30 years. Sci. Rep. https://doi.org/10.1038/s41598-020-78665-9 (2020).
Li, C. et al. Drivers and impacts of changes in China’s drylands. Nat. Reviews Earth Environ. (2), 858–873. https://doi.org/10.1038/s43017-021-00226-z (2021).
Ma, D. et al. Accumulation characteristics of plant flavonoids and effects of cultivation measures on their biosynthesis: A review. Plant. Physiol. Biochemistry: PPB. 215, 108960. https://doi.org/10.1016/j.plaphy.2024.108960 (2024).
Hou, M. et al. Preparative purification of total flavonoids from Sophora tonkinensis Gagnep. By macroporous resin column chromatography and comparative analysis of flavonoid profiles By HPLC-PAD. Molecules (Basel Switzerland). 24 (17), 3200. https://doi.org/10.3390/molecules24173200 (2019).
Wang, Z. et al. Adsorption and desorption characteristics of polyphenols from Eucommia ulmoides Oliv. Leaves with macroporous resin and its inhibitory effect on α-amylase and α-glucosidase. Annals Translational Med. 8 (16), 1004. https://doi.org/10.21037/atm-20-5468 (2020).
Kumar, K., Debnath, P., Singh, S. & Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 3 (3), 570–585. https://doi.org/10.3390/stresses3030040 (2023).
Giupponi, L., Leoni, V., Pavlovic, R. & Giorgi, A. Influence of altitude on phytochemical composition of hemp inflorescence: A metabolomic approach. Molecules (Basel Switzerland). 25 (6), 1381. https://doi.org/10.3390/molecules25061381 (2020).
Fu, Y., Zhang, Y. & Zhang, R. Purification and antioxidant properties of triterpenic acids from blackened jujube (Ziziphus Jujuba Mill.) by macroporous resins. Food Sci. Nutr. 9 (9), 5070–5082. https://doi.org/10.1002/fsn3.2464 (2021).
Funding
This study was supported by Guiyang College PhD start-up Funding, the Program for Natural Science Research in Guizhou Education Department (QJJ-[2023]-024), the Program of Innovative Training for College Students in Guizhou Province(2024109760072), the Sixth Batch of Guizhou Province High-level Innovative Talent Training Program (GCC〔2022〕009), and the program of Excellent Innovation Talents in Guizhou Province (GCC[2023]071).
Author information
Authors and Affiliations
Contributions
Jiyue wang, Xianqi Huang wrote the main manuscript; Zhenyu Chen, Nian Chen, Mingli Yang for methodology, validation, formal analysis; Jiyue Wang, Zhenyu Chen, and Mingli Yang for investigation; Jiyue Wang, Xianqi Huang, Nian Chen, and Mingli Yang for data curation; Chenggan Lian for English essay revision; Yanghua Yu for resources; Denghong Shi for funding acquisition, All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Huang, X., Chen, Z. et al. Extraction and purification of total flavonoids from Zanthoxylum planispinum Var. Dintanensis leaves and effect of altitude on total flavonoids content. Sci Rep 15, 7080 (2025). https://doi.org/10.1038/s41598-025-91528-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91528-5
Keywords
This article is cited by
-
Application of cinnamon essential oil microcapsules in anti-fungal preservation of Spatholobi caulis
Scientific Reports (2026)
-
Extraction process and antioxidant and antimicrobial activities of total flavonoids from Broussonetia papyrifera leaves
Scientific Reports (2025)
-
Mass Spectral Data of Primary and Secondary Metabolites Changes in Medicinal Plants by Solvent Polarity
Scientific Data (2025)