Abstract
Bilastine is a non-sedating, highly selective H1-antihistamine with proven efficacy and safety in treating allergic rhinoconjunctivitis and urticaria in adults and children. Allergic conjunctivitis, a common ocular condition, negatively impacts quality of life. Topical eye drops are the standard treatment, though ocular bioavailability is often low. Incorporating biopolymers such as hyaluronic acid (HA) into topical formulations enhances adhesive properties, prolongs retention on the ocular surface, and ultimately improves drug bioavailability. This study evaluated the new multidose preservative-free bilastine 0.6% solution with sodium HA against eight commercially available antiallergic eye drops. Using an ex vivo bovine cornea model, bilastine 0.6% demonstrated the highest bioadhesion strength (0.025 mJ), indicating superior retention on the ocular surface. It also showed strong protective effects against in vitro dehydration, mainly due to the presence of HA, and did not exhibit cytotoxicity in human primary conjunctival cells. In wound healing assays, preservative-free ketotifen 0.025%, bilastine 0.6%, and azelastine 0.05% promoted corneal wound repair at 72 h, outperforming preserved formulations. Overall, preservative-free bilastine 0.6% with HA enhances corneal hydration, retention, and re-epithelialization in vitro, suggesting potential benefits for the management of allergic conjunctivitis and offering promising advancements in treating this widespread condition.
Similar content being viewed by others
Introduction
Allergic conjunctivitis is a highly prevalent ocular condition that is estimated to affect 6–30% of the general population, and can lead to visual impairment and a significant reduction in the quality of life of patients1. Managing this complex and multifactorial condition begins with the difficult task of avoiding environmental irritants and allergens. However, allergen avoidance, along with non-pharmacological methods such as cold compresses and artificial tears, often prove insufficient in controlling the signs and symptoms of this ocular surface disorder2,3. The treatment options for allergic conjunctivitis typically include antihistamines, mast cell stabilizers, dual-activity agents, non-steroidal anti-inflammatory drugs, steroids and some off-label treatments4,5.
Topical ocular administration is the most effective treatment for allergic conjunctivitis, offering a faster onset of action compared to systemic agents, reducing symptoms and improving the hydration of the ocular surface6. However, despite its convenience, ophthalmic drug delivery faces significant challenges to various anatomic and physiologic barriers, resulting in more than 90% of drug loss at the application site7,8. To address this issue, drug delivery formulations are designed to enhance drug bioavailability and prolong therapeutic contact time on the eye9. Most ophthalmic preparations in the market are available as conventional eye drops, which represent a non-invasive and convenient route of topical drug administration, and the preferred option for many ocular diseases, including allergic conjunctivitis10,11. Still, one of the main challenges associated with conventional eye drops is the poor ocular bioavailability, primarily due to nasolacrimal drainage and limited corneal epithelium permeability.
One strategy to enhance topical ocular bioavailability is the incorporation of biopolymers or naturally derived macromolecules. These substances increase the viscosity of the tear film and exhibit mucoadhesive properties, which help prolong the retention of the formulation at the application site, thereby improving the therapeutic effect12,13. Macromolecules like xyloglucan, hyaluronic acid (HA) and chitosan not only increase the viscosity of the formulation but also interact closely with the mucin layer covering the corneal and conjunctival epithelia14,15,16. This bioadhesion capacity enhances the contact time of the formulation with the ocular surface, improving the bioavailability of the active agent17,18. In particular, the water-soluble polymer HA, also known as hyaluronan or hyaluronate, is frequently incorporated in eye drops because it increases precorneal retention time due to its viscous and mucoadhesive properties, leading to an increase of drug bioavailability19. In addition to its viscoelasticity, HA’s strong water-binding properties provide lubricating and moisturizing effects, helping to maintain proper hydration levels and reduce surface friction20,21,22.
A key aspect of treating allergic conjunctivitis is controlling ocular surface inflammation, which can result from epithelial damage and environmental stressors. The condition often alters tear film composition and causes corneal epithelial damage due to itching-induced rubbing, worsening inflammation and leading to pain13. Therefore, treatment should not only be aimed at preventing the release of mediators of allergy mediators and control the allergic inflammatory response, but also protect the ocular surface from further damage and promote healing. In this context, the hydrophilic biopolymer HA, known to stimulate corneal epithelial cell migration and play an aid wound healing23,24,25,26, may help improve ocular tissue health when included in antiallergic formulations.
The treatment of allergic conjunctivitis with eye drops should avoid inducing side effects that disturb tear film homeostasis or trigger excessive inflammation. The use of antiallergic eye drops with low ocular bioavailability, requiring high doses or frequent administration to achieve the desired therapeutic effect, can result in large cumulative doses that may increase the risk of adverse ocular reactions and reduce patient compliance27,28.
Preservatives, commonly included in multidose presentations, are often responsible for ocular surface complications. Benzalkonium chloride (BAC), a quaternary ammonium compound, is the most widely used preservative in ophthalmic medications due to its effectiveness against most ocular pathogens29. However, it exhibits toxic effects on human corneal epithelial, inhibiting mitochondrial function30,31. The extent of BAC’s negative impact depends on both the frequency of use and the concentration of the preservative.
Hence, there is a clear need for effective and safe antiallergic topic therapies that preserve ocular surface integrity while delivering therapeutic effects without exacerbating pre-existing ocular surface conditions. To address this, new preservative-free formulations are being developed to avoid the adverse effects typically associated with preservatives in eye drops29,32,33. Currently, some antiallergic preservative-free eye drops are available in single-dose presentations (ketotifen fumarate 0.025% and azelastine hydrochloride 0.05%), while a limited number are available in multidose formats (ketotifen fumarate 0.025% and olopatadine 0.1%). Recently, a novel once-daily multidose preservative-free eye drop formulation of bilastine 0.6% (w/v) containing sodium HA has been developed for the symptomatic treatment of allergic conjunctivitis34. Bilastine is a non-sedating and highly selective H1-antihistamine with proven efficacy and safety in the treatment of the overall symptoms of allergic rhinoconjunctivitis and urticaria in adults and children35,36,37,38,39. This novel formulation offers several advantages over existing treatments, including once-daily administration which can enhance compliance and lead to a better control of symptoms. Additionally, bilastine ophthalmic formulation contains HA and is available as a preservative-free, phosphates-free sterile solution, which can reduce the risk of adverse events and ocular surface changes24,40.
While the effects of BAC on the ocular surface have been studied in antiglaucoma formulations, there is a lack of in vitro studies on its impact in antiallergic formulations. In this study, we investigated the bioadhesion properties of the new sodium hyaluronate multidose preservative-free bilastine 0.6% and the main commercially available BAC-preserved and preservative-free eye drops indicated for the treatment of allergic conjunctivitis. We also aimed to compare the in vitro protective effects of these formulations against dehydration and their wound healing properties in human ocular epithelial cells.
Materials and methods
Eye drop formulations
In this study, we selected the most commonly used antiallergic ophthalmic formulations available on the market at the time, all with the same indication and application as the once-daily multidose preservative-free bilastine 0.6% formulation. A total of eight commercially available products, along with bilastine 0.6%, were evaluated, in both single-dose or multidose presentations, with or without preservatives, as listed in Table 1. A code consisting of three letters (EDF, representing ‘Eye Drop Formulation’), followed by consecutive numbers from one to nine, was assigned to each formulation.
AH: H1-receptor antagonistic action; BAC: Benzalkonium chloride; DUAL: histamine H1 receptor antagonistic action and mast cell stabilization action; EDF: eye drop formulation; PF: preservative-free.
Cell lines and culture conditions
Human Primary Conjunctival Epithelial Cells (HConEpiC, P10870, Innoprot, Bizkaia, ESP) were cultured on collagen coated flasks in Corneal Epithelial Cell Medium (CEpiCM, P60131; Innoprot, Bizkaia, ESP), containing 500 mL of Corneal Epithelial Cell basal medium, 25 mL of Fetal Bovine Serum (FBS, A5256701 Gibco/Thermo Fisher Scientific, Waltham, Massachusetts USA), 5 mL of Epithelial Cell Growth Supplement (CEpiCGS, P60106-GS; Innoprot, Bizkaia, ESP) and 5 mL of Penicillin/Streptomycin solution (Corning, 25-051-CI).
Human Primary Corneal Epithelial Cells (HCEpC) (H-6048, Cell Biologics, Campbell Park Drive, Chicago, USA) were cultured on collagen coated flasks in Epithelial Cell Medium (EpiCM, P60106, Innoprot, Bizkaia, ESP), containing 500 mL of Epithelial basal medium, 10 mL of FBS, 5 mL of CEpiCGS, and 5 mL of Penicillin/Streptomycin solution; all of these components were included in kit P60106 of EpiCM.
The SV40 Immortalized Human Corneal Epithelial Cells HCE-2 (50.B1) were purchased from the American Type Culture Collection (CRL-11135, ATCC, Manassas, VA, USA). HCE-2 cells were cultured in Dulbecco Modified Eagle’s Medium with HAM F12 mixture (DMEM/F12) (BE12-719 F, Lonza, Verviers, BEL), supplemented with 10% FBS (DE14-801 F, Lonza Verviers, BEL), 10 ng/mL EGF (85570 C, Sigma–Aldrich, St. Louis, Missouri, USA), 5 µg/mL insulin (I9278, Sigma–Aldrich, St. Louis, Missouri, USA), 0.1 µg/mL cholerae toxin (C8180, Sigma–Aldrich, St. Louis, Missouri, USA), 0.5% v/v dimethyl sulfoxide (DMSO) (D2650, Sigma–Aldrich, St. Louis, Missouri, USA) and 50 U/mL Penicillin/Streptomycin solution (30-002-CI, Corning, New York, USA).
All cell cultures were maintained at 37ºC in a humidified atmosphere with 5% CO2. The medium was changed every 2–3 days, and cells were subcultured when they reached 80–90% confluence. Cells were detached with trypsin/EDTA (T4174, Sigma–Aldrich, St. Louis, Missouri, USA), and the enzyme mixture inactivated with 10% FBS.
Corneal Bioadhesion Assay
Corneal bioadhesion was studied in an ex vivo bovine cornea model for the selected antiallergic eye drop formulations. Similarly to a previously described protocol41, corneas were obtained from bovine eye samples collected at the slaughterhouse Compostela de Tambre S.L. in Santiago de Compostela (Spain) and transported to the laboratory in phosphate-buffered saline (PBS) at 4˚C. Only eyes with intact corneas were used, discarding those with opaque or hemorrhagic corneas. The corneas were excised by sectioning the eye and leaving 1–2 mm of surrounding scleral tissue, taking special care to avoid damage. Afterwards, the corneas were washed with artificial tears and maintained in the same solution until the system was assembled. Bovine corneas were selected for the test for two primary reasons: their ready availability and their structural similarity to human corneas, which makes them a suitable model for the assay. Additionally, the larger size of bovine corneas facilitates easier handling during the test. Bovine corneas are also widely used in drug evaluation studies, including standardized protocols such as the Bovine Corneal Opacity and Permeability (BCOP) assay42.
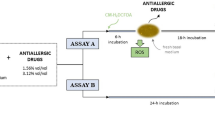
Corneas were evaluated in quintuplicate by using a TA.XT Plus Texture analyzer (StableMicro Systems Products, Godalming, GBR), as previously described43. Figure 1 shows a scheme of the cornea model. Plaster supports were made with the curved shape of the anterior part of the eyeball, which was obtained through a mold made by bovine eyeball contact printing on alginate paste. A scalpel blade was used to carefully excise the cornea of the eye from the eyeball, with a 1–2 mm margin of the surrounding sclerotic tissue. Bovine corneas were fixed to plaster supports with cyanoacrylate adhesive, which were attached to the upper probe of the texture analyzer with the same adhesive. Formulations were deposited in weighing bottles (40 mm of diameter and 20 mm of height) and placed in the lower part of the analyzer. Then, corneas were lowered 2 mm into the formulation at a speed of 1 mm/s, and the contact was kept for 30 s. Then, the corneas were pushed back at a speed of 1 mm/s, until its complete separation from the formulation. Force-displacement curve was recorded, and the work of adhesion (mJ) was calculated from the area under the curve (AUC) obtained during the traction phase.

(Adapted from Díaz-Tomé V et al., 2021. International Journal of Pharmaceutics 597, p. 6). Corneas were immersed 2 mm deep into the formulation at a 1 mm/s speed, and were kept in touch for 30 s. Then, corneas returned to the initial position at a 1 mm/s speed, and work (mJ) was measured. Bioadhesion work was calculated from the area under the curve (AUC) obtained during the traction phase.
Ex vivo bovine cornea model for the analysis of the bioadhesion properties of the selected antiallergic eye drop formulations.
Evaluation of hydration activity
The protective effect of the selected antiallergic eye drop formulations against dehydration was evaluated using an in vitro dryness model in primary epithelial cells based on previously reported protocols28,44.
Specifically, HConEpiC cells were grown until 70–80% confluence on 96-well tissue-culture flat bottom plates. The medium was then removed, and cells were treated with medium lacking FBS and growth factors, containing 100 µL of the ophthalmic formulations at different serial dilutions (6.25%, 3.13%, 1.56%, 0.78%, 0.39%, 0.195% v/v). The cells were incubated for 17 h at 37 °C with 5% CO2 in a humidified environment. After incubation, the drug-containing medium was removed and cells were incubated in dehydrated conditions for 40 min at 37 °C with 5% CO2. For the negative control, the medium was removed, and cells were incubated in dehydrated conditions for 40 min at 37 °C with 5% CO2 (untreated and dehydrated control cells). For the positive control, cells remained in the medium throughout the 40-minute period. Cell viability was then evaluated by MTT [3-(4,5-dimethyl-2thiazolyl)-2,5-diphenyl-2 H-tetrazolium bromide] assay. Briefly, cells were washed with PBS and stained with MTT reagent (475989, Sigma–Aldrich, St. Louis, Missouri, USA) for 2 h at 37 °C and 5% CO2 in a humidified environment. After removing the MTT solution, precipitates were solubilized with 100 µL of dimethyl sulfoxide. After 15 min at room temperature, the absorbances were measured at 540 nm using a spectrophotometer (Multiskan spectrum 1500, Thermo Fisher Scientific, Thermo Fisher Scientific, Waltham, Massachusetts, USA). The experiments were performed in sextuplicate, and the results were expressed as the percentage of cell viability (%) relative to the untreated and dehydrated control cells (negative control).
Evaluation of wound healing activity
A scratch wound healing assay with HCE-2 and HCEpC cultures was performed to evaluate the in vitro wound healing capacity of the selected antiallergic eye drops, as previously described45,46. Immortalized HCE-2 cells were initially used for their ease of culture, maintenance, and expansion, enabling the evaluation of all formulations at different concentrations. While primary HCEpC cells offer greater physiological relevance, they are more difficult to culture and maintain. Therefore, HCEpC cells were used to confirm the key results observed in HCE-2 cells.
HCE-2 and HCEpC cells were seeded into 96-well flat bottom culture plates at a density of 3.5 × 104 and 3 × 104 cells/well (100 µl/well), respectively. HCE-2 were maintained in serum-free DMEM/F12 supplemented with 1% bovine serum albumin (BSA) for 12 h at 37 °C with 5% CO2, in a humidified environment, whereas HCEpC cells, because of their sensitivity to stress, were maintained in serum-free CEpiCM medium supplemented with 50% CEpiCGS and 1% BSA for 12 h at 37 °C with 5% CO2, in a humidified environment. After incubation, circular wounds were created in confluent cultures. Then, the wells and the de-epithelialized areas were imaged using an Olympus IX71 microscope (Olympus, Hamburg, DEU), and the images were analyzed with Cell^B software (Olympus). The initial wound at time 0 served as the reference point. To ensure consistency, wells were evenly distributed across conditions, maintaining equivalent average wound areas at time 0.
Subsequently, wounded HCE-2 cells were incubated for 72 h with antiallergic eye drops, at dilutions 3.13% and 0.78% v/v. However, EDF 7 could not be tested in these experiments because the ophthalmic suspension formed a precipitate that interfered with the assay. Wounded HCE-2 cells were incubated with DMEM/F12 containing 1% BSA as the negative control to maintain cell viability without inducing proliferation, while HCE-2 medium with 10% FBS, rich in growth factors, served as the positive control to benchmark the tested formulations. In the case of HCEpC cells, wounded cells were incubated for 72 h with EDF 1, EDF 2, EDF 3, and EDF 6 antiallergic eye drops, at dilutions 3.13% and 0.78% v/v. Serum-free CEpiCM medium supplemented with 50% CEpiCGS and 1% BSA was used as the negative control, while serum-free CEpiCM medium supplemented with 100% CEpiCGS and 2% FBS served as the positive control.
Serial bright-field images of wounds were captured every 12 h (i.e., 0, 12, 24, 36, 48, 60 and 72 h post-scratch) and the evolution of wound healing area at these time points for each treatment were measured using FIJI software (Wayne Rasband, National Institute of Mental Health, Bethesda, Maryland, USA)47 and a plugin specialized in wound surface quantification48, and compared to the wound area at 0 h. Epithelial wound closure was examined by calculating the percentage of the remaining wound area at a given time point compared with the initially wound area at 0 h.
Experiments in HCE-2 cells were performed in quintuplicate with three biological replicates per experiment, while in HCEpC cells, a single biological replicate was performed in sextuplicate due to challenges in cultivation and maintenance. The easier maintenance of HCE-2 cells allowed for a greater number of biological replicates.
Statistical analysis
Bioadhesion data were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post-hoc tests for multiple comparisons. Differences were considered to be significant at p < 0.05. Statistical analyses were performed with GraphPad Prism 9.0 (GraphPad Software, Inc., San Diego, California, USA).
The protective effect of the selected antiallergic eye drop formulations against dehydration was evaluated by cell viability, and analyses were performed using GraphPad Prism version 9, GraphPad Software. Statistical comparisons were conducted using variance analysis (ANOVA) models and Dunnett´s multiple comparison test. Differences between groups were considered significant when p < 0.05.
For the wound healing assay, data were analyzed with the open-source statistical software Jamovi v.1.6.3 (https://www.jamovi.org, Amsterdam, NLD). Changes were examined using the Shapiro-Wilk test (normality of distribution) and Levene’s test (equality of variances). One-way analysis of variance (ANOVA) followed by Tukey’s HSD, Games–Howell or Dwass-Steel-Critchlow-Fligner post hoc tests, when appropriate, to compare groups. Differences were considered significant when p < 0.05.
Results
Bioadhesion study
Bioadhesion studies were conducted to evaluate the adhesive strength of the antiallergic formulations using an ex vivo bovine cornea model. Corneal bioadhesion was quantified as the work required to completely detach the formulation from the corneal surface. The results, presented in Fig. 2, show that EDF 3 exhibited the highest bioadhesion value (0.025 ± 0.001 mJ), while EDF 5 had the lowest (0.00 ± 0.00 mJ). Statistically significant differences (p < 0.05) were observed for EDF 2, EDF 4, EDF 5, EDF 7, and EDF 8, compared to EDF 3, whereas EDF 1 and EDF 9 demonstrated comparable bioadhesion values to EDF 3.
Bioadhesion work (mJ) obtained for each antiallergic ophthalmic formulation using fresh bovine cornea model as a substrate. Results are presented as means ± standard deviations. Asterisks indicate significant differences (p < 0.05) in comparison with EDF 3. EDF 1 (0.025% ketotifen fumarate); EDF 2 (0.05% azelastine hydrochloride); EDF 3 (0.6% bilastine); EDF 4 (0.222% olopatadine hydrochloride); EDF 5 (0.05% azelastine hydrochloride); EDF 6 (0.776% olopatadine hydrochloride); EDF 7 (0.05% levocabastine hydrochloride); EDF 8 (0.1% olopatadine hydrochloride); EDF 9 (0.025% ketotifen fumarate).
Evaluation of hydration activity using an in vitro induced dryness cell model
The results of the quantitative assessment of the protective effect of the selected antiallergic eye drop formulations against dehydration in HConEpiC cells are presented in Fig. 3. The formulations were tested at various dilutions, ranging from 6.25 to 0.195% (v/v), depending on the eye drop. Cell viability in the positive control group remained consistently high at nearly 100% for all formulations, serving as a reference for optimal conditions.
Figure 3a illustrates cell viability after treatment with EDF 1, EDF 2, EDF 3, and EDF 6 formulas, while Fig. 3B presents the results for EDF 3, EDF 4, EDF 5, EDF 7, EDF 8, and EDF 9. Formulations have been divided into these two groups to improve the readability of the results. EDF 1, EDF 2, and EDF 6 have been grouped together with EDF 3 due to their shared characteristics. Specifically, EDF 1 and EDF 2, like EDF 3, are preservative-free formulations, while EDF 6 and EDF 3 share a once-daily administration schedule. EDF 3 has been included in both figures to facilitate direct comparisons with the other formulations.
Figure 3a shows that at the highest dilution (6.25% v/v), EDF 3 exhibited the highest cell viability values among all formulations, significantly exceeding those of EDF 1, EDF 2, and EDF 6. Notably, the cell viability for EDF 2 and EDF 6 dropped significantly below 10% at this dilution. At the 3.13% dilution, EDF 3 also demonstrated significantly greater recovery in cell viability compared to the other formulations. At this concentration, EDF 2 showed evidence of recovery, whereas EDF 6 did not, with its viability remaining below 20%. A similar trend was observed at the 1.56% dilution. At lower concentrations (0.78%, 0.39%, and 0.195%), all formulations displayed cell viability comparable to that of the negative control. Interestingly, at the 0.195% dilution, EDF 6 exhibited a significantly higher viability compared to both the negative control and EDF 3.
Figure 3b illustrates that at the 1.56% dilution, EDF 3 exhibited the highest cell viability, significantly greater (p < 0.05) than that of the other formulations (EDF 4, EDF 5, EDF 7, EDF 8, and EDF 9), all of which fell significantly below the negative control values (p < 0.05). At the 0.78% dilution, most formulations demonstrated comparable cell viability with no significant differences relative to the negative control. However, EDF 3 exhibited the highest viability, significantly surpassing that of EDF 5, EDF 7, EDF 8, and EDF 9 (p < 0.05). At the 0.39% and 0.195% dilutions, no significant differences were observed either relative to the negative control or among the groups, except for EDF 5 at 0.195%, which showed significantly higher cell viability compared to EDF 3.
Evaluation of wound healing activity using an in vitro scratch assay
The in vitro scratch assay was performed on HCE-2 and HCEpC cells to assess the re-epithelialization effects of the selected antiallergic eye drop formulations after inducing epithelial defects. As stated in the Materials and Methods section, immortalized HCE-2 cells were used for their ease of culture and expansion, allowing evaluation of all formulations at different concentrations. Primary HCEpC cells, though more physiological relevant, are more challenging to maintain, so they were used to confirm the key results from HCE-2 cells.
The formulations were initially tested at 12.5%, 6.25%, 3.13%, and 0.78% v/v dilutions in HCE-2 cells. Cell toxicity at 12.5% and 6.25% negatively impacted re-epithelialization in most formulations (data not shown). Therefore, 3.13% and 0.78% v/v dilutions were selected for evaluating monolayer re-epithelialization. Accordingly, in the second assay, the same dilutions were tested in HCEpC cells.
At 3.13% dilution (Fig. 4), EDF 1, EDF 2 and EDF 3 showed significant reductions in the damaged area of HCE-2 cells, with nearly 100%, 75%, and 60% decreases at 72 h, respectively, compared to 0 h. In contrast, treatment with EDF 4, EDF 5, EDF 6, EDF 8, and EDF 9 showed minimal wound re-epithelialization (10 to 40% improvement, approximately), which was lower than the negative control. Statistically significant differences compared to EDF 3 were observed for EDF 6 at 36, 48 and 60 h (p < 0.05), and for EDF 1 at 36 h (p < 0.05) (statistical results not shown in the figure).
At the highest dilution (0.78% v/v) (Fig. 5), EDF 4 exhibited the lowest wound closure capacity (20% reduction at 72 h), even lower than the negative control. EDF 1, EDF 2 and EDF 3 presented a significant increase in wound closure evolution, reaching nearly 100% at 72 h, similar to the positive control for re-epithelialization. However, the wound closure evolution for EDF 5, EDF 6, EDF 8, and EDF 9 reached a plateau at around 60–70% reduction after 24 h of treatment. They showed complete closure of the wound area after 72 h at both dilutions. EDF 7 could not be tested in these experiments due to the formation of a precipitate in the ophthalmic suspension, which interfered with the assay.
In both Figs. 4a and 5a, error bars have been omitted to maintain visual clarity, as the graphs contain multiple closely spaced lines. Including error bars for each data point would result in significant overlap, especially in regions where the lines converge, obscuring individual trends and making the graphs difficult to interpret. Instead, the mean values and standard deviations (SDs) for these datasets are provided in Supplementary Table S1. This supplementary data allows a detailed examination of the variability in the measurements while ensuring the figures remain clear and straightforward to interpret. In addition, in Figs. 4b and 5b, intermediate micrographs have been omitted to avoid redundancy.
Quantitative determination of the protective effect of selected antiallergic aye drop formulations against dehydration in Human Primary Conjunctival Epithelial Cells (HConEpiC). (a) Cell viability after 17-hour treatment with EDF 1, EDF 2, EDF 3, and EDF 6. (b) Cell viability after 17-hour treatment with EDF 3, EDF 4, EDF 5, EDF 7, EDF 8, and EDF 9. Cell viability is expressed as percentage (%) relative to the positive control. Results are presented as means ± standard deviations. Statistically significant differences compared to the negative control (untreated and dehydrated control cells): *p < 0.05. Statistically significant differences compared to EDF 3 (bilastine 0.6%): #p < 0.05. EDF 1 (0.025% ketotifen fumarate); EDF 2 (0.05% azelastine hydrochloride); EDF 3 (0.6% bilastine); EDF 4 (0.222% olopatadine hydrochloride); EDF 5 (0.05% azelastine hydrochloride); EDF 6 (0.776% olopatadine hydrochloride); EDF 7 (0.05% levocabastine hydrochloride); EDF 8 (0.1% olopatadine hydrochloride); EDF 9 (0.025% ketotifen fumarate).
In Vitro Wound Healing Assay of Immortalized Human Corneal Epithelial Cells (HCE-2). After the scratch, HCE-2 cells were incubated in fresh medium with or without EDF 1, EDF 2, EDF 3, EDF 4, EDF 5, EDF 6, EDF 8, and EDF 9 formulations 3.13% v/v dilution for 72 h. The wound area was imaged and quantified at 0, 12, 24, 36, 48, 60 and 72 h post-scratching. (a) Quantification of Wound Healing Activity. Wound healing rate was expressed as the percentage of the remaining wound area compared to 0 h. (b) Representative transmitted light optical microscopy images (4x objective) of wounds at 0 h, 24 h, 48 h, and 72 h (scale bar: 200 μm). Intermediate images have been omitted to avoid redundancy. EDF 1 (0.025% ketotifen fumarate); EDF 2 (0.05% azelastine hydrochloride); EDF 3 (0.6% bilastine); EDF 4 (0.222% olopatadine hydrochloride); EDF 5 (0.05% azelastine hydrochloride); EDF 6 (0.776% olopatadine hydrochloride); EDF 8 (0.1% olopatadine hydrochloride); EDF 9 (0.025% ketotifen fumarate); C+: positive control; C-: negative control.
In Vitro Wound Healing Assay of Immortalized Human Corneal Epithelial Cells (HCE-2). After the scratch, HCE-2 cells were incubated in fresh medium with or without EDF 1, EDF 2, EDF 3, EDF 4, EDF 5, EDF 6, EDF 8, and EDF 9 formulations at 0.78% v/v dilution for 72 h. The wound area was imaged and quantified at 0, 12, 24, 36, 48, 60 and 72 hours post-scratching. (a) Quantification of Wound Healing Activity. Wound healing rate was expressed as the percentage of the remaining wound area compared to 0 h. (b) Representative transmitted light optical microscopy images (4x objective) of wounds at 0 h, 24 h, 48 h, and 72 h (scale bar: 200 µm). Intermediate images have been omitted to avoid redundancy. EDF 1 (0.025% ketotifen fumarate); EDF 2 (0.05% azelastine hydrochloride); EDF 3 (0.6% bilastine); EDF 4 (0.222% olopatadine hydrochloride); EDF 5 (0.05% azelastine hydrochloride); EDF 6 (0.776% olopatadine hydrochloride); EDF 8 (0.1% olopatadine hydrochloride); EDF 9 (0.025% ketotifen fumarate); C+: positive control; C-: negative control.
In Vitro Wound Healing Assay of Human Primary Corneal Epithelial Cells (HCEpC). After the scratch, HCEpC cells were incubated in fresh medium with or without EDF 1, EDF 2, EDF 3, EDF 6 formulations at 3.13% v/v dilution for 72 h. The wound area was imaged and quantified at 0, 12, 24, 36, 48, 60 and 72 h post-scratching. (a) Quantification of Wound Healing Activity. Wound healing rate was expressed as the percentage of the remaining wound area compared to 0 h. (b) Representative transmitted light optical microscopy images (4x objective) of wounds at 0 h, 12 h, 24 h, 48 h, and 72 h (scale bar: 200 μm). Intermediate images have been omitted to avoid redundancy. EDF 1 (0.025% ketotifen fumarate); EDF 2 (0.05% azelastine hydrochloride); EDF 3 (0.6% bilastine); EDF 6 (0.776% olopatadine hydrochloride); C+: positive control; C-: negative control.
To confirm the results obtained with HCE-2 cells, we also performed the scratch wound assay in primary HCEpC cells using the formulations that showed the highest protective effect against dehydration in conjunctival HConEpiC cells, and the highest re-epithelialization capacity in HCE-2 cells (EDF 1, EDF 2, and EDF 3). Additionally, EDF 6, which showed the lowest re-epithelialization activity at 3.13% v/v in HCE-2 cells, was included for comparison. EDF 1 and EDF 2 showed similar results in HCEpC cells, although their re-epithelialization activity slightly decreased at both dilutions (3.13% and 0.78% v/v) compared to the activity observed in HCE-2 cells (Figs. 6 and 7). EDF 3 also demonstrated high wound closure capacity, reducing the wound area by 85–90% at both dilutions. In contrast, EDF 6 exhibited the lowest re-epithelialization activity, reducing the damaged area by 20% and 70% at 3.13% and 0.78% v/v, respectively. Significant differences were found when comparing EDF 6 to the positive control after 12-h treatment and throughout the assay (p < 0.05 at both dilutions), and between EDF 6 and EDF 3 at all time points (statistical comparison results not shown in the figure).
As with Figs. 4a and 5a, error bars have been omitted in Figs. 6a and 7a to maintain visual clarity due to the overlap that would occur with closely spaced lines. The corresponding mean values and SDs are available in Supplementary Table S2, allowing for a detailed analysis of variability while keeping the figures easy to interpret. In addition, in Figs. 6b and 7b, intermediate micrographs have been omitted to avoid redundancy.
In Vitro Wound Healing Assay of Human Primary Corneal Epithelial Cells (HCEpC). After the scratch, HCEpC cells were incubated in fresh medium with or without EDF 1, EDF 2, EDF 3, EDF 6 formulations at 0.78% v/v dilution for 72 h. The wound area was imaged and quantified at 0, 12, 24, 36, 48, 60 and 72 h post-scratching. (a) Quantification of Wound Healing Activity. Wound healing rate was expressed as the percentage of the remaining wound area compared to 0 h. (b) Representative transmitted light optical microscopy images (4x objective) of wounds at 0 h, 24 h, 48 h, and 72 h (scale bar: 200 μm). Intermediate images have been omitted to avoid redundancy. EDF 1 (0.025% ketotifen fumarate); EDF 2 (0.05% azelastine hydrochloride); EDF 3 (0.6% bilastine); EDF 6 (0.776% olopatadine hydrochloride); C+: positive control; C-: negative control.
Discussion
One of the major challenges in ophthalmic delivery of topical formulations is achieving and maintaining an optimal drug concentration on the ocular surface. Given that the adhesion properties of these formulations provide an estimate of their retention on the eye, we conducted bioadhesion measurements with the topical antiallergic formulations to assess their permanence on the ocular surface.
Bioadhesion results showed that EDF 3 presented the highest bioadhesion value (0.025 mJ) followed by EDF 1 (0.021 mJ), EDF 9 (0.021 mJ), EDF 6 (0.019 mJ) and EDF 7 (0.019 mJ), although not all differences reached statistical significance. These results suggest a trend toward superior viscosity in the EDF3 formulation, indicating a potential increase in retention time on the ocular surface compared to the other formulations. Considering that the formulations contain similar excipient ingredients, the higher bioadhesion values of bilastine 0.6% could be attributed to the presence of HA, which is only incorporated in this formulation and has been shown to enhance the permanence and bioavailability of ophthalmic formulations19,49,50. It is worth mentioning that although EDF 3 showed a trend toward higher bioadhesion capacity compared to EDF 1 and EDF 9, the difference was not statistically significant. For EDF 1 and EDF 9, both formulations containing ketotifen 0.025% exhibited the same bioadhesion capacity. Since they share the same qualitative composition, except for the presence of BAC in EDF 9, we can infer that their bioadhesion capacity is primarily attributed to glycerol and is not significantly affected by the preservative.
Our study also evaluated and compared the in vitro protective effects of all formulations, which were tested at various dilutions to simulate the changes in the concentration of the antiallergic agent in the tear film following in vivo instillation. Among all the tested products, only EDF 3 demonstrated a strong protective effect against dehydration at the highest concentrations (6.25%, 3.13%, and 1.56% v/v), significantly increasing the survival rates of conjunctival cells compared to the dehydrated control cells (negative control). The results also indicate a potential cytotoxic effect of EDF 2 at 6.25% and 3.13% dilutions, as well as EDF 4, EDF 5, EDF 6, EDF 7, EDF 8, and EDF 9 at 1.56%. Interestingly, EDF 6 exhibited a significant increase in survival rates at the lowest concentration tested (0.195% v/v), raising questions about the underlying mechanisms that might promote cell growth at this low drug concentration.
The observed dose-dependent increase in the protective effect of EDF 3 against dehydration suggests that its active pharmaceutical ingredient does not induce toxic effects. Markedly, among all tested products, EDF 3 is the only eye drop containing sodium HA, a compound traditionally used in topical ophthalmic formulations for its viscoelastic properties, safety profile and physiological benefits19,51,52. HA is a hydrophilic biopolymer naturally present in various ocular tissues, including the cornea, aqueous humor, vitreous humor, and retina53,54. It is well known for its ability to increase viscosity, enhance retention time, and optimize hydration and lubrication of the ocular surface19,55,56. Our results evidence the superior hydration capacity of the sodium HA-containing antiallergic formulation EDF 3, which exceed the protective hydration effects of all other tested formulations. Additionally, bilastine 0.6% contains other excipients with humectant, viscous and bioadhesion properties such as glycerol and methylcellulose57,58,59, that not only prolong the contact time of the drug with the ocular surface, but also favor hydration of the cornea, consequently improving tear film stability.
Although the formulation EDF 6 contains polyethylene glycol and hydroxypropyl methylcellulose—agents known to enhance tear film viscosity and exhibit mucoadhesive properties59— it induced cytotoxic effects at higher concentrations, leading to a significant reduction of cell viability. The substantial differences in cell viability observed between EDF 3 and EDF 6 can likely be attributed to two key factors. First, HA has been shown outperform polyethylene glycol and hydroxypropyl methylcellulose in increasing tear film viscosity and stability60. Second, and more critically, the cytotoxic effects observed with EDF 6 at higher concentrations are likely due to the presence of the preservative.
Indeed, all BAC-preserved antiallergic eye drops (EDF 4 to EDF 9) demonstrated cytotoxicity on conjunctival cells, suggesting that BAC plays a pivotal role in these effects. Previous studies have consistently shown that exposure to BAC has a direct detrimental impact on the ocular surface and can potentially enhance the toxicity of other drugs and excipients within the same formulation61.
The cytotoxicity results of the three olopatadine formulations—EDF 4, EDF 6, and EDF 8—containing 0.01%, 0.015%, and 0.01% BAC, respectively, suggest that the preservative BAC, along with the concentration of the active ingredient, may contribute to the observed effects. At the 1.56% v/v dilution, slight differences were observed between EDF 4 and EDF 8, which are likely attributable to variations in the active ingredient concentration. In contrast, EDF 6, which contains both the highest concentration of BAC and the active ingredient, exhibited markedly greater cytotoxicity.
Comparatively, both EDF 2 and EDF 5 contain azelastine 0.05% with identical compositions, except for the presence of BAC (0.0125%) in EDF 5. At 1.56% v/v dilution, EDF 2 showed no protection, with similar viability to the dehydration control (~ 60%), while EDF 5 reduced viability to ~ 20%, highlighting BAC’s role in cytotoxicity.
A similar pattern was observed for EDF 1 (preservative-free) and EDF 9 (BAC-preserved, 0.01%), both containing ketotifen 0.025%. At 1.56% v/v, EDF 9 reduced viability more than EDF 1, further implicating BAC. These findings align with previous studies showing cytotoxic effects of these formulations on HConEpiC cells after 24-hour incubation, corroborating our results62.
The preservative-free formulations EDF 1 and EDF 2 did not protect conjunctival cells against dehydration but also did not exhibit significant cytotoxic effects, unlike the BAC-preserved formulations. An exception was EDF 2 at the highest concentration (6.25% v/v dilution), which significantly reduced cell viability beyond the negative dehydration control. In this case, any of the excipients present in EDF2 and/or the active ingredient might have contributed to this toxicity.
This assay was also conducted with primary corneal HCEpiC cells, although none of the formulations showed a protective effect against dehydration (data not shown).
The in vitro scratch wound healing assay in corneal cells was used to evaluate the effects of different concentrations of antiallergic eye drop formulations on re-epithelialization. It is important to note that the supplementary data reveals considerable variability in some measurements of the wounds in the in vitro scratch assay, as indicated by the SD. This variability can be attributed to factors such as the manual creation of the wounds, which made standardization challenging, as well as excessive desquamation and debris formation in some wells. Although automated measurements were employed, manual adjustments were frequently necessary to address these issues. Despite this, we do not believe the error bars undermine the validity of the assay. Rather, they reflect the natural complexity of the assay.
According to the data obtained, formulations EDF 1, EDF 2, and EDF 3 showed the highest re-epithelialization activity in human-established corneal epithelial cells, with EDF 1 exhibiting the greatest effect. In human primary corneal epithelial cells, the order of activity was EDF 1/EDF 3 (depending on the concentration) followed by EDF 2. Specifically, EDF 1 significantly accelerated wound closure, achieving complete or near-complete closure after 72 h, with no significant difference compared to the positive control. EDF 2 also accelerated wound closure, reaching 75% at 72 h with a 3.13% v/v dilution, and nearly 90% at 0.78% v/v dilution. For EDF 3 (bilastine 0.6%), the formulation stimulated corneal re-epithelialization at 3.13% v/v dilution, reducing the wound area by approximately 60%. At the highest dilution (0.78% v/v), EDF 3 exhibited even more significant improvement, with wound closure percentages of 70% at 24 h and nearly 100% at 72 h.
The main differences in the chemical composition of these three antiallergic eye drops, aside from the active ingredient, are the excipients: glycerol (in EDF 1 and EDF 3); polyvinyl alcohol, hydroxypropyl methylcellulose and sorbitol (in EDF 2); and methylcellulose and HA (in EDF 3). Several studies indicate that sodium HA plays an important role in suppressing inflammation, promoting corneal epithelial cell migration and proliferation, thus enhancing wound healing24,63,64,65,66,67. Glycerol and methylcellulose also aid in corneal epithelial wound healing59,68, although sodium HA has been shown to accelerate healing more effectively than hydroxypropyl methylcellulose69. Additionally, exposure to polyvinyl alcohol has been associated with corneal epithelial damage in rabbit eyes70, which could not explain the wound healing capacity observed in EDF 2. Therefore, the differences among EDF 1, EDF 2 and EDF 3 may primarily stem from the active pharmaceutical ingredient.
The results of this assay also evidenced that BAC-preserved agents greatly delayed corneal wound healing compared to preservative-free formulations. Notably, EDF 9 and EDF 1, which contain the same active ingredient (ketotifen 0.025%), exhibited substantial differences in wound closure capacity. EDF 1 promoted healing at all dilution, while the BAC-preserved EDF 9 showed minimal activity, only at the most diluted concentration. Similar results were observed for EDF2 and EDF5, which contain the same active ingredient (azelastine 0.05%), but EDF5 is BAC-preserved. These findings support the notion that BAC toxicity hinders corneal epithelial wound healing71, consistent with studies showing that preservative-free formulations, or those with soft preservatives, improve ex vivo corneal healing72. In line with our results, an in vitro study using a scraping model in human corneal and conjunctival epithelial cell lines showed that antiglaucoma formulations containing BAC delay the wound-healing process. Additionally, BAC concentrations above 0.01% did not promote wound closure in the short term, but worsened the epithelial defect73.
Overall, the new multidose preservative-free bilastine 0.6% formulation containing sodium hyaluronate demonstrated superior bioadhesion properties compared to the other antiallergic eye drops evaluated. Although not all differences reached statistical significance, this trend in performance can likely be attributed to the presence of HA in the formulation. In addition, EDF 3 offered highly protective effects against dehydration and stimulated re-epithelialization of corneal cells after scratch, exhibiting remarkable wound healing properties. Although extrapolating in vitro results to in vivo contexts can be challenging, the findings of the present study are in line with previously reported evidence, particularly regarding the hydration and re-epithelialization effects of HA. Specifically, in an animal model of dry eye disease, high molecular weight hyaluronic acid (HMWHA) demonstrated superior protection over low molecular weight hyaluronic acid (LMWHA) and diquafosol sodium (DQ) eye drops74. This highlights the greater efficacy of HMWHA in promoting tear film stability and epithelial healing, which aligns with the results observed with EDF3 in our study. These findings strongly support the use of the preservative-free bilastine 0.6% eye drops containing sodium HA in the treatment of patients with allergic conjunctivitis. Besides its antihistamine effect, the prolonged use of bilastine 0.6% is unlikely to cause ocular surface damage and may actually help improve the ocular surface conditions often compromised by the disease itself.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Kausar, A., Akhtar, N. & Akbar, N. Epidemiological aspects of allergic conjunctivitis. J. Ayub Med. Coll. Abbottabad. 34 (1), 135–140. https://doi.org/10.55519/JAMC-01-9432 (2022).
Dupuis, P., Prokopich, C. L., Hynes, A. & Kim, H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 16, 5. https://doi.org/10.1186/s13223-020-0403-9 (2020).
Kimchi, N. & Bielory, L. The allergic eye: recommendations about pharmacotherapy and recent therapeutic agents. Curr. Opin. Allergy Clin. Immunol. 20 (4), 414–420. https://doi.org/10.1097/ACI.0000000000000669 (2020).
Kari, O. & Saari, K. M. Updates in the treatment of ocular allergies. J. Asthma Allergy. 3, 149–158. https://doi.org/10.2147/JAA.S13705 (2010).
Leonardi, A. et al. Management of ocular allergy. Allergy 74 (9), 1611–1630. https://doi.org/10.1111/all.13786 (2019).
del Cuvillo, A. et al. Allergic conjunctivitis and H1 antihistamines. J. Investig Allergol. Clin. Immunol. 19 (Suppl 1), 11–18 (2009).
Lin, S. et al. Overcoming the anatomical and physiological barriers in topical eye surface medication using a Peptide-Decorated polymeric micelle. ACS Appl. Mater. Interfaces. 11 (43), 39603–39612. https://doi.org/10.1021/acsami.9b13851 (2019).
Gote, V., Sikder, S., Sicotte, J. & Pal, D. O. Drug delivery: present innovations and future challenges. J. Pharmacol. Exp. Ther. 370 (3), 602–624. https://doi.org/10.1124/jpet.119.256933 (2019).
Yang, Y. & Lockwood, A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp Eye Res. ;218:109006. (2022). https://doi.org/10.1016/j.exer.2022.109006. Epub 2022 Mar 4. PMID: 35248559.
Wang, Y. & Wang, C. Novel eye drop delivery systems: advance on formulation design strategies targeting anterior and posterior segments of the eye. Pharmaceutics 14 (6), 1150. https://doi.org/10.3390/pharmaceutics14061150 (2022).
Davies, N. M. Biopharmaceutical considerations in topical ocular drug delivery. Clin. Exp. Pharmacol. Physiol. 27 (7), 558–562. https://doi.org/10.1046/j.1440-1681.2000.03288.x (2000).
Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv Rev. 57 (11), 1595–1639. https://doi.org/10.1016/j.addr.2005.07.005 (2005).
Račić, A. & Krajišnik, D. Biopolymers in mucoadhesive eye drops for treatment of dry eye and allergic conditions: application and perspectives. Pharmaceutics 15 (2), 470. https://doi.org/10.3390/pharmaceutics15020470 (2023).
Silva, B., São, B. B., Delgado, E. & Gonçalves, L. Colloidal nanosystems with mucoadhesive properties designed for ocular topical delivery. Int. J. Pharm. 606, 120873. https://doi.org/10.1016/j.ijpharm.2021.120873 (2021).
Krishnaswami, V., Kandasamy, R., Alagarsamy, S., Palanisamy, R. & Natesan, S. Biological macromolecules for ophthalmic drug delivery to treat ocular diseases. Int. J. Biol. Macromol. 110, 7–16. https://doi.org/10.1016/j.ijbiomac.2018.01.120 (2018).
Ways, M., Lau, T. M. & Khutoryanskiy, W. M. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polym. (Basel). 10 (3), 267. https://doi.org/10.3390/polym10030267 (2018).
Ali, Y. & Lehmussaari, K. Industrial perspective in ocular drug delivery. Adv. Drug Deliv Rev. 58 (11), 1258–1268. https://doi.org/10.1016/j.addr.2006.07.022 (2006).
Zhang, X. et al. Dry eye management: targeting the ocular surface microenvironment. Int. J. Mol. Sci. 18 (7), 1398. https://doi.org/10.3390/ijms18071398 (2017).
Salzillo, R. et al. Optimization of hyaluronan-based eye drop formulations [published correction appears in carbohydr polym. 181:1235–1236 (2018)]. Carbohydr. Polym. 153, 275–283. https://doi.org/10.1016/j.carbpol.2016.07.106 (2016).
Rah, M. J. A review of hyaluronan and its ophthalmic applications. Optometry 82 (1), 38–43. https://doi.org/10.1016/j.optm.2010.08.003 (2011).
Nakamura, M. et al. Characterization of water retentive properties of hyaluronan. Cornea 12 (5), 433–436. https://doi.org/10.1097/00003226-199309000-00010 (1993).
Ali, S. et al. Crosslinked hyaluronic acid with liposomes and Crocin confers cytoprotection in an experimental model of dry eye. Molecules 26 (4), 849. https://doi.org/10.3390/molecules26040849 (2021).
Kawano, Y. et al. Wound healing promotion by hyaluronic acid: effect of molecular weight on gene expression and In vivo wound closure. Pharmaceuticals (Basel). 14 (4), 301. https://doi.org/10.3390/ph14040301 (2021).
Gomes, J. A., Amankwah, R., Powell-Richards, A. & Dua, H. S. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br. J. Ophthalmol. 88 (6), 821–825. https://doi.org/10.1136/bjo.2003.027573 (2004).
Fallacara, A. et al. Novel artificial tears containing Cross-Linked hyaluronic acid: an In vitro Re-Epithelialization study. Molecules 22 (12), 2104. https://doi.org/10.3390/molecules22122104 (2017).
Nishida, T., Nakamura, M., Mishima, H. & Otori, T. Hyaluronan stimulates corneal epithelial migration. Exp. Eye Res. 53 (6), 753–758. https://doi.org/10.1016/0014-4835(91)90110-z (1991).
Ilochonwu, B. C., Urtti, A., Hennink, W. E. & Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control Release. 10;326, 419–441. https://doi.org/10.1016/j.jconrel.2020.07.031 (2020).
Campos, P. M., Petrilli, R. & Lopez, R. F. V. The prominence of the dosage form design to treat ocular diseases. Int. J. Pharm. 586, 119577. https://doi.org/10.1016/j.ijpharm.2020.119577 (2020).
Goldstein, M. H., Silva, F. Q., Blender, N., Tran, T. & Vantipalli, S. Ocular Benzalkonium chloride exposure: problems and solutions. Eye (Lond). 36 (2), 361–368. https://doi.org/10.1038/s41433-021-01668-x (2022).
Datta, S., Baudouin, C., Brignole Baudouin, F., Denoyer, A. & Cortopassi, G. A. The eye drop preservative Benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Invest. Ophthalmol. Vis. Sci. 58 (4), 2406–2412. https://doi.org/10.1167/iovs.16-20903 (2017).
Rosin, L. M. & Bell, N. P. Preservative toxicity in glaucoma medication: clinical evaluation of Benzalkonium chloride-free 0.5% timolol eye drops. Clin. Ophthalmol. 7, 2131–2135. https://doi.org/10.2147/OPTH.S41358 (2013).
Kagkelaris, K., Panayiotakopoulos, G. & Georgakopoulos, C. D. Nanotechnology-based formulations to amplify intraocular bioavailability. Ther. Adv. Ophthalmol. 14, 25158414221112356. https://doi.org/10.1177/25158414221112356 (2022).
Campolo, A., Crary, M. & Shannon, P. A. Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops. Biomed J Sci Tech Res. 45(1)-2022. BJSTR. MS.ID.007130 (2022).
Gomes, P. J. et al. Bilastine 0.6% preservative-free eye drops, an effective once-daily treatment to reduce signs and symptoms of allergic conjunctivitis: A pooled analysis of two randomized clinical trials. J. Investig Allergol. Clin. Immunol. https://doi.org/10.18176/jiaci.0940 (2023). Published online September 21, 2023.
Church, M. K., Tiongco-Recto, M., Ridolo, E. & Novák, Z. Bilastine: a lifetime companion for the treatment of allergies. Curr. Med. Res. Opin. 36 (3), 445–454. https://doi.org/10.1080/03007995.2019.1681134 (2020).
Carter, N. J. Bilastine: in allergic rhinitis and urticaria. Drugs 72 (9), 1257–1269. https://doi.org/10.2165/11209310-000000000-00000 (2012).
Ridolo, E. et al. Bilastine: new insight into antihistamine treatment. Clin. Mol. Allergy. 13 (1), 1. https://doi.org/10.1186/s12948-015-0008-x (2015).
Ochoa, D. et al. Pharmacokinetics and safety of a Bilastine Once-Daily, Preservative-Free, ophthalmic formulation. Adv. Ther. 38 (7), 4070–4081. https://doi.org/10.1007/s12325-021-01801-y (2021).
Gomes, P. J., Ciolino, J. B., Arranz, P., Hernández, G. & Fernández, N. Efficacy of once-daily ophthalmic Bilastine for the treatment of allergic conjunctivitis: a dose-finding study. J. Investig Allergol. Clin. Immunol. 1, 0. https://doi.org/10.18176/jiaci.0800 (2022).
Suarez-Cortes, T. et al. The new once-daily multidose preservative-free 0.6% Bilastine eye drop formulation containing sodium hyaluronate preserves ocular surface integrity and tear film homeostasis in a comparative in vivo study. Invest. Ophthalmol. Vis. Sci. 64 (8), 209 (2023).
Doughty, M. J., Petrou, S. & Macmillan, H. Anatomy and morphology of the cornea of bovine eyes from a slaughterhouse. Can. J. Zool. 73 (11), 2159–2165. https://doi.org/10.1139/z95-253 (1995).
OECD. Test No. 437: Bovine Corneal Opacity and Permeability Test Method for Identifying Ocular Corrosives and Severe Irritants (OECD Publishing, 2009). https://doi.org/10.1787/9789264076303-en
Díaz-Tomé, V. et al. In situ forming and mucoadhesive ophthalmic Voriconazole/HPβCD hydrogels for the treatment of fungal keratitis. Int. J. Pharm. 597, 120318. https://doi.org/10.1016/j.ijpharm.2021.120318 (2021).
Rangarajan, R., Kraybill, B., Ogundele, A. & Ketelson, H. A. Effects of a hyaluronic acid/hydroxypropyl Guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J. Ocul Pharmacol. Ther. 31 (8), 491–497. https://doi.org/10.1089/jop.2014.0164 (2015).
Martinotti, S. & Ranzato, E. Scratch wound healing assay. Methods Mol. Biol. 2109, 225–229 (2020). 10.1007/7651_2019_259.
Rodriguez, L. G., Wu, X. & Guan, J. L. Wound-healing assay. Methods Mol. Biol. 294, 23–29. https://doi.org/10.1385/1-59259-860-9:023 (2005).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9 (7), 676–682. https://doi.org/10.1038/nmeth.2019 (2012). Published 2012 Jun 28.
Suarez-Arnedo, A. et al. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS One. 15 (7), e0232565. https://doi.org/10.1371/journal.pone.0232565 (2020). Published 2020 Jul 28.
Alvarez-Lorenzo, C., Hiratani, H. & Concheiro, A. Contact lenses for drug delivery achieving sustained release with novel systems. Am. J. Drug Deliv. 4 (3), 131–151 (2006).
Mateo Orobia, A. J., Saa, J., Ollero Lorenzo, A. & Herreras, J. M. Combination of hyaluronic acid, Carmellose, and osmoprotectants for the treatment of dry eye disease. Clin. Ophthalmol. 12, 453–461. https://doi.org/10.2147/OPTH.S157853 (2018).
Shimmura, S. et al. Sodium hyaluronate Eyedrops in the treatment of dry eyes. Br. J. Ophthalmol. 79 (11), 1007–1011. https://doi.org/10.1136/bjo.79.11.1007 (1995).
Johnson, M. E., Murphy, P. J. & Boulton, M. Effectiveness of sodium hyaluronate Eyedrops in the treatment of dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 244 (1), 109–112. https://doi.org/10.1007/s00417-005-0028-1 (2006).
Morra, M. Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules 6 (3), 1205–1223. https://doi.org/10.1021/bm049346i (2005).
Allyn, M. M., Luo, R. H., Hellwarth, E. B. & Swindle-Reilly, K. E. Considerations for polymers used in ocular drug delivery. Front. Med. (Lausanne). 8, 787644. https://doi.org/10.3389/fmed.2021.787644 (2022).
Dogru, M. & Tsubota, K. Pharmacotherapy of Dryeye. Expert Opin. Pharmacother. 12 (2), 325–334 (2011).
Paugh, J. R., Nguyen, A. L., Ketelson, H. A., Christen-sen, M. T. & Meadows, D. L. Precorneal residence timeof artificial tears measured in dry eye subjects. Optom. Vis. Sci. 85, 725–731 (2008).
Hornby, S., Rizer, R. L. & Appa, Y. Therapeutic glycerin-based ointment provides healing benefits. J. Am. Acad. Dermatol. 60 (3). https://doi.org/10.1016/j.jaad.2008.11.880 (2009). suppl. 1, AB203.
Sezgin, S. et al. An experimental study on the comparison of the effects of triester glycerol oxide on wound repair. Oral Maxillofac Surg. 20(3):273-9; (2017). https://doi.org/10.1007/s10006-016-0566-1. Erratum in: Oral Maxillofac Surg. ;21(1):107 (2016).
Tundisi, L. L., Mostaço, G. B., Carricondo, P. C. & Petri, D. F. S. Hydroxypropyl Methylcellulose: physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 159, 105736. https://doi.org/10.1016/j.ejps.2021.105736 (2021).
Posarelli, C. et al. Cross-Linked hyaluronic acid as tear film substitute. J. Ocul Pharmacol. Ther. 35 (7), 381–387. https://doi.org/10.1089/jop.2018.0151 (2019).
Agarwal, P., Craig, J. P. & Rupenthal, I. D. Formulation considerations for the management of dry eye disease. Pharmaceutics 13 (2), 207. https://doi.org/10.3390/pharmaceutics13020207 (2021).
Arana, E. et al. New preservative-free once-daily 0.6% Bilastine multidose eye drops containing sodium hyaluronate preserve the ocular surface epithelial integrity in a comparative in vitro study. Abstracts Allergy. 76, 583–637. https://doi.org/10.1111/all.15097 (2021).
Zhong, J. et al. Hyaluronate Acid-Dependent Protection and Enhanced Corneal Wound Healing against Oxidative Damage in Corneal Epithelial Cells. J Ophthalmol. :6538051; (2016). https://doi.org/10.1155/2016/6538051 (2016).
Inoue, M. & Katakami, C. The effect of hyaluronic acid on corneal epithelial cell proliferation. Invest. Ophthalmol. Vis. Sci. 34 (7), 2313–2315 (1993).
Weigel, P. H., Frost, S. J., McGary, C. T. & LeBoeuf, R. D. The role of hyaluronic acid in inflammation and wound healing. Int. J. Tissue React. 10 (6), 355–365 (1988).
Pauloin, T., Dutot, M., Joly, F., Warnet, J. M. & Rat, P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol. Vis. 15, 577–583 (2009).
Wu, H. et al. Genoprotective effect of hyaluronic acid against Benzalkonium chloride-induced DNA damage in human corneal epithelial cells. Mol. Vis. 17, 3364–3370 (2011).
Lin, C. P. & Boehnke, M. Influences of Methylcellulose on corneal epithelial wound healing. J. Ocul Pharmacol. Ther. 15 (1), 59–63. https://doi.org/10.1089/jop.1999.15.59 (1999).
Shahraki, K. et al. Effects of topical 1% sodium hyaluronate and hydroxypropyl Methylcellulose in treatment of corneal epithelial defects. Med. Hypothesis Discov Innov. Ophthalmol. 5 (4), 136–144 (2016).
Fukuda, M. et al. Polyvinyl alcohol-iodine induced corneal epithelial injury in vivo and its protection by topical rebamipide treatment. PLoS One. 13 (11), e0208198. https://doi.org/10.1371/journal.pone.0208198 (2018).
Seino, S. et al. Topical hyaluronan alone promotes corneal epithelial cell migration whereas combination with Benzalkonium chloride impairs epithelial wound healing. Cutan. Ocul Toxicol. 39 (1), 13–20. https://doi.org/10.1080/15569527.2019.1673402 (2020).
Schrage, N., Frentz, M. & Spoeler, F. The ex vivo eye irritation test (EVEIT) in evaluation of artificial tears: Purite-preserved versus unpreserved eye drops. Graefes Arch. Clin. Exp. Ophthalmol. 250 (9), 1333–1340. https://doi.org/10.1007/s00417-012-1999-3 (2012).
Liang, H., Baudouin, C., Daull, P., Garrigue, J. S. & Brignole-Baudouin, F. In Vitro Corneal and Conjunctival Wound-Healing Assays as a Tool for Antiglaucoma Prostaglandin Formulation Characterization. Front Biosci (Landmark Ed). 27(5):147. (2022). https://doi.org/10.31083/j.fbl2705147. PMID: 35638414.
Kojima, T. et al. The effects of high molecular weight hyaluronic acid eye drop application in environmental dry eye stress model mice. Int. J. Mol. Sci. 21 (10), 3516. https://doi.org/10.3390/ijms21103516 (2020). PMID: 32429217; PMCID: PMC727891.
Acknowledgements
Blanca Martínez-Garriga, PhD (Trialance SCCL) provided medical writing assistance with the preparation of this manuscript, supported by FAES FARMA SA (Spain), according to Good Publication Practice guidelines.
Author information
Authors and Affiliations
Contributions
EA: Experimental work, Validation, Writing - Review & EditingAG: Supervision, Writing - Review & EditingNA: Supervision, Writing - Review & EditingMPG: Validation, ReviewFO: Validation, ReviewVD: Validation, ReviewFG: Validation, ReviewPGF: Validation, ReviewGH: Supervision, ReviewTSC: Experimental work/design, Validation, Writing - Review & Editing, Project administration and funding. All authors have reviewed and approved the submitted version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: EA, GH, AG and TSC are employees of FAES FARMA SA (Spain). FO, VD, FG, and PGF received fees from FAES FARMA SA (Spain) through subcontracting analyses during the conduct of the study. NA and MPG declare no potential conflict of interest. EA was granted a partial scholarship by Basque Government – BIKAINTEK Program (48-AF-W2-2018-00008). This work was partially supported by the Basque Country Government (Economic Development & Infrastructures Depart.), HAZITEK program [grant No. ZE-2019/00004]. The funding organizations had no role in the study design, data collection, decision to publish, or preparation of manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Arana, E., Gonzalo, A., Andollo, N. et al. The new bilastine eye drop formulation protects against conjunctival dehydration and promotes corneal wound healing in a comparative in vitro study. Sci Rep 15, 7987 (2025). https://doi.org/10.1038/s41598-025-91743-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91743-0