Abstract
Rheumatoid arthritis (RA) is a global epidemic. We conducted a cross-sectional study using the Global Burden of Disease (GBD) 2021 dataset to examine RA trends in patients aged 20–54 years worldwide. Key outcomes included incidence, mortality, and disability-adjusted life years (DALYs), as well as trends over time, stratifying by region, country, age, sex, and Sociodemographic Index (SDI). We also assessed the contribution of smoking to RA-related mortality and DALYs. Over the past 32 years, the global RA-related incidence rate increased from 11.66 (95% UI 9.60–13.94) to 13.48 (95% UI 11.08–16.06) per 100,000 population. RA-related DALYs rate increased from 26.37 (95% UI 18.43–36.99) to 30.71 (95% UI 20.82–44.08) per 100,000 population, with females bearing a higher burden. And the RA-related mortality rate decreased from 0.09 (95% UI 0.08–0.1) to 0.06 (95% UI 0.05–0.07) per 100,000 population. Regional disparities were evident, with lower SDI regions experiencing the larger change. Smoking remained a significant risk factor, accounting for 9.01% of RA-related mortality in 2021. Overall, we highlighted the rising global burden of RA, particularly among females and in lower SDI regions, emphasizing disparities in healthcare resources, prevention, and early diagnosis.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease, with a prevalence of 0.5–1.0% according to most epidemiological studies1. It predominantly affects females, with a male-to-female ratio of 1:2 to 1:3, and can manifest at any age, peaking around 60 years2,3. The incidence of RA displays both temporal and geographical variability, likely influenced by genetic and environmental factors4. Among these, smoking is a well-established environmental risk factor5, with secondhand smoke exposure in young individuals recognized as an independent risk factor for RA onset6,7. This disease imposes a heavy burden on patients and their families and has become a global public health issue.
According to the Global Burden of Disease (GBD) study, the number of people with rheumatoid arthritis (RA) has been increasing year by year from 1990 to 20218. Recently, the population of RA patients has been rising annually, yet epidemiological studies on RA in young and middle-aged groups have not been reported. In this study, we analyzed the trends in the incidence, mortality, and disability-adjusted life years (DALY) associated with RA among individuals aged 20–54 from 1990 to 2021 using the GBD database, as well as the impact of smoking on the survival of RA patients. We hope this interpretation of the 2021 GBD estimates will promote the development of new prevention and treatment strategies to mitigate the health risks of RA.
Methods
Overview and data collection
We employed the Global Health Data Exchange query tool, developed by GBD collaborators, to collect data on RA among individuals aged 20 to 54, including standardized disease definitions and prevalence information9. The 2021 GBD study evaluated the incidence, mortality, and DALYs for 371 diseases and injuries across 204 countries and regions from 1990 to 2021, providing corresponding incidence rates and uncertainty intervals9,10. To summarize the age distribution of the RA burden, we categorized patients into seven groups: 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, and 50–54 years. This study gathered data on RA case numbers, incidence rates, RA-related mortality, and RA-related DALYs, along with their rates at global, regional, and national levels. The GBD database does not include race and ethnicity data, as these categories were not collected. We calculated the average estimated annual percentage change (EAPC) using linear regression and collected data on risk factors associated with smoking-related mortality and DALYs in RA patients11. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Socio demographic index
The Socio-Demographic Index (SDI) quantifies the development level of a country or region by integrating fertility rates, educational attainment, and per capita income. The SDI ranges from 0 to 1, with higher values indicating a higher degree of socio-economic development. Previous research has established a correlation between SDI and both disease incidence and mortality rates. In this study, we categorized countries and geographical regions into five SDI categories (low, low middle, middle, high middle, and high) to investigate the relationship between the burden of RA and socio-economic development9.
Statistical analysis
Numbers of incidence, mortality, DALYs, and their corresponding rates were the main indicators used to describe the burden of RA. Each rate is reported per 100,000 population, along with 95% uncertainty interval (UI) according to the GBD algorithm12. The dynamics of RA were analyzed by calculating estimated annual percentage changes (EAPCs) and average annual percent change (AAPC) to identify temporal trends in disease burden13; the 95% confidence intervals (CIs) of EAPCs were estimated using linear modeling14. If the upper limit of both the EAPC and its 95% CI is negative, the corresponding rate indicates a decreasing trend; conversely, if the lower limit of both the EAPC and its 95% CI is positive, the corresponding rate indicates an increasing trend. Joinpoint regression analysis indicated that the annual percentage change (APC). Additionally, the RA-related mortality and DALYs rates caused by smoking were assessed. The population attributable fraction (PAF) quantifies the proportion of RA-related disease in the population that can be attributed to smoking. All calculations were performed using R Studio, version 4.1.2 (R Project for Statistical Computing). All P values were 2-sided, and P < 0.05 was considered statistically significant.
Result
RA: Global trends
Incidence
In 2021, the global incidence of RA among individuals aged 20 to 54 years was 508,185 (95% UI 417,807–605,687). Between 1990 and 2021, the incidence in this age group increased by 81.21% (95% UI 74.44–88.97%). The incidence rate rose from 11.66 (95% UI 9.60–13.94) to 13.48 (95% UI 11.08–16.06) per 100,000 individuals, with an EAPC of 0.62 (95% CI 0.57–0.67).
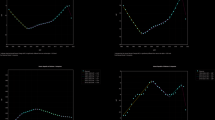
In 2021, the incidence of RA among this age group increased with age (AAPC = 0.469; 95% CI 0.451–0.486; p < 0.001). The age group with the highest incidence for both males and females was 50–54 years, with male and female incidence rates of 13.15 (95% UI 8.80–18.54) and 28.98 (95% UI 19.50–40.45), respectively. The rate of increase was more pronounced in males (AAPC = 0.57; 95% CI 0.55–0.59; p < 0.001) than in females (AAPC = 0.43; 95% CI 0.41–0.44; p < 0.001). Joinpoint regression analysis indicated that the APC from 2018 to 2021 was 0.14 (95% CI 0.07–0.22; p = 0.001), with female incidence rates increasing more than those of males (0.22; 95% CI 0.15–0.28; p < 0.001) compared to males (0.08; 95% CI 0.01–0.15; p < 0.001) (Table 1 and Fig. 1A, Fig. 2A, B, C).
Trends in annual percent change (APC) in Rheumatoid Arthritis Incidence, Deaths, and Disability-Adjusted Life-Years (DALYs) Among People Aged 20–54 years from 1990 to 2021, Abbreviations: APC, Annual Percent Change. (A) APC in incidence rate of both females and males. (B) APC in incidence rate of females. (C) APC in incidence rate of males. (D) APC in death rate of both females and males. (E) APC in death rate of females. (F) APC in death rate of males. (G) APC in DALYs rate of both females and males. (H) APC in DALYs rate of females. (I) APC in DALYs rate of males.
Mortality
Over the past 32 years, the global number of RA-associated deaths among individuals aged 20 to 54 years increased by 2.97%. In contrast, the RA-associated death rate decreased from 0.09 (95% UI 0.08–0.11) per 100,000 in 1990 to 0.06 (95% UI 0.05–0.07) per 100,000 in 2021, with an EAPC of − 1.13 (95% CI − 1.24 to − 1.11) and an AAPC of − 1.36 (95% CI − 1.62 to − 1.10; p < 0.001) (eTable 1 in Supplement 1). The joinpoint regression model indicated an APC of − 1.55 (95% CI − 1.74 to − 1.36; p < 0.001) for the period 2011–2021.
In 2021, the number of RA-associated deaths among females aged 20–54 years was higher than that among males, with 1542 deaths (95% UI 1357–1811) compared to 766 deaths (95% UI 496–984). The mortality rate for females was 0.08 (95% UI 0.07–0.10; APCC = − 1.66 [95% CI − 1.88 to − 1.44; p < 0.001]), significantly higher than the rate for males, which was 0.04 (95% UI 0.03–0.05; APCC = − 0.75 [95% CI − 1.04 to − 0.46; p < 0.001]). The joinpoint regression analysis revealed that the APC for females during 2016–2021 was − 2.21 (95% CI − 2.63 to − 1.78; p < 0.001), while the APC for males from 2012 to 2021 was − 0.95 (95% CI − 1.20 to − 0.70; p < 0.001). Mortality rates gradually increased among individuals aged 20 to 54 years, with the most significant increase (10.45%) observed in the 50–54 age group. In 2021, the RA-associated mortality rate among individuals aged 20 to 54 years was higher in females (0.30; 95% UI 0.26–0.34) compared to males (0.14; 95% UI 0.09–0.19). (Fig. 1B, Fig. 2D, E, F).
DALYs
The global number of DALYs associated with RA among individuals aged 20 to 54 years increased by 82.63% (95% UI 0.74 to 0.89) from 1990 to 2021. The EAPC was 0.62 (95% CI 0.57 to 0.67), while the AAPC was 0.49 (95% CI 0.45–0.53; p < 0.001) (Table 1 in Supplement 1). The joinpoint regression model indicated that the APC for the period 2012–2018 was 0.87 (95% CI 0.71–1.01, p < 0.001).
In 2021, the number of DALYs among females aged 20–54 years with RA was significantly higher (834,310; 95% UI 567,949–1,190,281) compared to males (323,562; 95% UI, 218,169 to 465,243). The DALYs rate for females was also greater (44.66; 95% UI, 30.40 to 63.71) than that for males (17.02; 95% UI 11.47–24.47). Furthermore, the APCC for females (0.45; 95% CI 0.41–0.49; p < 0.001) was lower than that for males (0.59; 95% CI 0.51–0.65; p < 0.001). From 1990 to 2021, RA-associated DALYs increased across all age groups, with the most significant rise (13.80%) observed in individuals aged 50 to 54 years. In 2021, the rate of RA-associated DALYs among individuals aged 20 to 54 years was higher in females than in males (Fig. 1C, Fig. 2G, H, I).
RA: SDI regional trends
Incidence
In 2021, the middle SDI region reported the highest number of RA cases, totaling 180,000 (95% UI 147,792–215,051). Conversely, the high SDI region exhibited the highest incidence rate at 19.73 per 100,000 individuals (95% UI 16.38–23.51). The low SDI region experienced a dramatic increase in incident cases, rising by 186.13% (95% UI, 180.13–193.19%). Notably, the most significant increase in RA incidence was observed in the low-middle SDI region, with an EAPC of 1.06 (95% CI 0.95–1.17) (Table 1 and Fig. 3A).
Mortality
Among the five SDI regions, the high SDI (18.30%) and high-middle SDI (36.64%) regions showed a decrease in RA mortality. Conversely, among the remaining three SDI regions, the low SDI region experienced the most significant increase in RA-associated mortality (79.86%). In 2021, the middle SDI region reported the highest number of RA-related deaths (1,101; 95% UI 903–1291), while the low SDI region recorded the fewest (71; 95% UI 36–170). Additionally, the RA-associated mortality rate was highest in the middle SDI region (0.09; 95% UI 0.07–0.11) and lowest in the low SDI region (0.02; 95% UI 0.01–0.04). Notably, the high SDI region exhibited the lowest EAPC in RA-associated mortality rate (− 1.92; 95% UI − 2.07 to − 1.78) (eTable 1 in Supplement 1 and Fig. 3B).
DALYs
In 2021, the middle SDI region reported the highest number of DALYs associated with RA, totaling 439,138 (95% UI 301,260–623,170). Conversely, the high SDI region exhibited the highest rate of RA-associated DALYs at 39.96 per 100,000 population (95% UI 27.38–57.01). Notably, the low SDI region experienced the most significant increase in DALYs, rising by 178.64% (95% UI 159.19–193.87%) from 1990 to 2021. Additionally, the greatest annual percentage change in RA-associated DALYs was observed in the low-middle SDI region, with an EAPC of 0.99 (95% CI 0.90–1.08) (eTable 1 in Supplement 1 and Fig. 3C).
RA: Geographic regional trends
Incidence
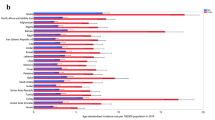
In 2021, East Asia reported the highest number of RA cases among 21 geographic regions, with a total of 132,280 cases (95% UI 106,413–161,093). Conversely, Oceania recorded the fewest cases, totaling 220 (95% UI 173–275). The incidence rate of RA was highest in Andean Latin America, at 30.02 per 100,000 population (95% UI 25.05–35.14), while Oceania exhibited the lowest incidence rate at 3.50 per 100,000 (95% UI 2.75–4.36).
From 1990 to 2021, all regions except Southern Sub-Saharan Africa experienced a decline in RA incidence (EAPC, − 0.33; 95% CI − 0.41 to − 0.24). Andean Latin America saw the most significant increase in incidence (EAPC, 1.75; 95% CI 1.67–1.84), whereas the high-income Asia Pacific region had the smallest increase (EAPC, 0.27; 95% CI 0.17–0.38) (eTable 1 in Supplement). In 2021, the global SDI was 0.67. Twelve regions had RA incidence rates above the global mean, while nine regions were below it. Additionally, there was a significant positive correlation between geographic regional RA incidence rates and SDI levels (ρ = 0.70, p < 0.01) (Fig. 4A).
Mortality
In 2021, East Asia recorded the highest number of deaths associated with RA, totaling 794 (95% UI 576–1023). Central Latin America had the highest mortality rate from RA, at 0.27 (95% UI 0.23–0.32).
The high-income Asia–Pacific region experienced the most significant decline in RA-associated mortality rates, with an EAPC of − 4.34 (95% CI − 4.89 to − 3.78). In contrast, Central Asia saw the largest increase in mortality rates, with an EAPC of 6.02 (95% CI 4.29–7.78). In total, nine regions reported RA-associated mortality rates above the global mean, while twelve regions reported lower rates. Furthermore, a bimodal distribution was observed between the death rate and the SDI level across geographic regions (ρ = 0.50, p < 0.01) (etable 1 in Supplement 1 and Fig. 4B).
DALYs
In 2021, East Asia reported the highest number of deaths associated with RA, totaling 794 (95% UI 576–1023). Central Latin America exhibited the highest RA-associated mortality rate, recorded at 0.27 (95% UI 0.23–0.32).
The Asia Pacific region classified as high-income experienced the most significant decline in RA-associated mortality rate, with an EAPC of − 4.34 (95% CI–4.89 to − 3.78). Conversely, Central Asia saw the largest increase in mortality rate, with an EAPC of 6.02 (95% CI 4.29–7.78). In 2021, nine regions reported RA-associated mortality rates above the global mean, while twelve regions reported rates below the mean. The relationship between death rates and the SDI across geographic regions demonstrated a bimodal distribution (ρ = 0.50, p < 0.01) (eTable 1 in Supplement and Fig. 4C).
RA: National trends
Incidence
In 2021, China reported the highest number of RA cases among 204 countries, with a total of 128,221 cases (95% UI 102,852–156,715). Ireland exhibited the highest incidence rate at 37.74 per 100,000 population (95% UI 28.10–47.51) (eTable 2 and eFigure 1A in Supplement).
Chile experienced the most significant increase in RA incidence, with an EAPC of 2.09 (95% CI 1.95–2.23), while South Africa showed the largest decrease, with an EAPC of − 0.42 (95% CI − 0.52 to − 0.32) (eTable 2 and eFigure 2A in Supplement). Additionally, Ireland had the highest SDI for RA incidence at 0.87, whereas Papua New Guinea had the lowest at 0.42. The global incidence of RA in 2021 was 13.48 per 100,000 population (95% UI, 11.08–16.07), with 70 countries reporting incidences above the global mean and 134 countries reporting below it (ρ = 0.61, p < 0.01).
Mortality
In 2021, China reported the highest number of RA-associated deaths, totaling 757 (95% UI, 545–986) (eTable 2 and eFigure 1B in Supplement). Lithuania exhibited the highest RA-associated mortality rate, recorded at 0.43 (95% CI 0.37–0.50) (eTable 2 and eFigure 1B in Supplement).
Turkmenistan experienced the most significant increase in mortality rate, with an EAPC of 11.14 (95% CI 9.47–12.83), while Norway showed the largest decrease, with an EAPC of − 5.26 (95% CI − 5.72 to − 4.8) (eTable 2 and eFigure 2B in Supplement). In terms of SDI, Lithuania (SDI, 0.85) had the highest RA-associated mortality rate, in contrast to Cabo Verde (SDI, 0.53), which had the lowest. The global RA-associated mortality rate in 2021 was estimated at 0.06 (95% UI 0.05–0.07), with rates exceeding the global mean in 57 countries and falling below the mean in 147 countries (ρ = 0.32, p < 0.01).
DALYs
In 2021, China reported the highest number of RA-associated deaths, totaling 757 (95% UI, 545–986) (eTable 2 and eFigure 1C in Supplement). Lithuania exhibited the highest RA-associated mortality rate, recorded at 0.43 (95% CI 0.37–0.50) (eTable 2 and eFig. 1C in Supplement).
Turkmenistan experienced the most significant increase in mortality rate, with an EAPC of 11.14 (95% CI 9.47–12.83), while Norway showed the largest decrease, with an EAPC of − 5.26 (95% CI − 5.72 to − 4.8) (eTable 2 and eFigure 2C in Supplement). In terms of SDI, Lithuania (SDI, 0.85) had the highest RA-associated mortality rate, in contrast to Cabo Verde (SDI, 0.53), which had the lowest. The global RA-associated mortality rate in 2021 was 0.06 (95% UI 0.05–0.07); the rates were above the global mean in 57 countries and below the global mean in 147 countries (ρ = 0.32, p < 0.01).
Risk factor for RA
The GBD database highlights the impact of smoking on RA. In 2021, smoking was responsible for 9.01% of RA-related deaths globally, with a PAF of 19.50% in males and 3.80% in females (eFigure 3A in Supplement). Furthermore, smoking accounted for 7.31% of RA-related DALYs, with percentages of 16.38% in males and 3.81% in females (eFig. 3B in Supplement).
Between 1990 and 2021, the global proportion of RA-related deaths attributable to smoking exhibited a declining trend (AAPC, − 1.74; 95% CI − 2.10 to − 1.39, P < 0.001), with a more pronounced decrease in females (AAPC, − 2.74; 95% CI − 2.97 to − 2.51, P < 0.001) compared to males (AAPC, − 1.25; 95% CI − 1.59 to − 0.90, P < 0.001). Joinpoint regression analysis further confirmed this decline in recent years (APC, − 2.16; 95% CI − 2.46 to 1.85, P < 0.001), again noting a greater reduction in females (APC, − 3.72; 95% CI − 3.85 to − 3.57, P < 0.001) compared to males (APC, − 1.51; 95% CI − 1.80 to − 1.21, P < 0.001) (eFig. 4A, B, C in Supplement).
The global proportion of RA-associated DALYs attributable to smoking has shown a decreasing trend (AAPC, − 0.45; 95% CI − 0.53 to − 0.37, P < 0.001). This decline is more pronounced in females than in males (female AAPC, − 0.96; 95% CI − 1.00 to − 0.91, P < 0.001; male AAPC, − 0.14; 95% CI − 0.24 to − 0.04, P < 0.01). Joinpoint regression analysis further confirmed this downward trend in recent years (APC, − 0.62; 95% CI − 0.71 to − 0.54, P < 0.001), with females experiencing a greater decline than males (female APC, − 1.41; 95% CI − 1.49 to − 1.34, P < 0.001; male APC, − 0.76; 95% CI 1.23 to − 0.28, P < 0.001) (eFigure 4D, E, F in Supplement).
Factors influencing EAPCs
EAPCs significantly differed from incidence, mortality rate, and number of DALYs in 1990; they significantly differed from SDI in 2021. The incidence in 1990 represents the disease pool at baseline, whereas SDI can be considered an index of the level of medical care. The EAPC in incidence was positively correlated with SDI (Spearman r = 0.698; P < 0.001), and the EAPC in mortality rate was positively correlated with SDI (Spearman r = 0.498; P < 0.001). EAPCs were positive correlated with rate of DALYs (Spearman r = 0.675; P < 0.001).
Discussion
Over the past 32 years, the global prevalence of RA has steadily increased, leading to significant medical and social costs and marking it as a critical public health issue worldwide. This study examines the incidence, mortality, and DALYs associated with RA in relation to smoking as a risk factor among individuals aged 20–54 across all GBD regions and countries from 1990 to 2021. Our findings shed light on the burden of RA over these three decades in regions with varying SDI levels. The results align with previous studies indicating an escalating RA burden in certain regions and countries. A comprehensive evaluation of RA epidemiological patterns can aid policymakers and clinicians in formulating effective prevention and management strategies.
Previous analyses had demonstrated that the incidence, mortality, and DALYs associated with RA are higher in females than in males globally15,16,17,18. Our study corroborated these findings within the 20–54 age group. The increased prevalence of RA in females is attributed to hormonal influences, particularly the effects of estrogen16,17. Females exhibited greater susceptibility to the disease during middle and older age, with those experiencing early menopause showing heightened disease activity and a poorer quality of life compared to their counterparts with late menopause19. Additionally, our study revealed that the incidence and DALYs related to RA in females increased at a slower rate than in males, while female mortality rates related to RA decreased more rapidly. Joinpoint regression analysis indicates a recent slight acceleration in the incidence rate among women compared to men. Females typically bear a greater burden from RA, facing more severe disease progression, higher disability risk, and significant impairments in quality of life. Biologically, the pathogenesis of RA is primarily characterized by immune system dysregulation20. Estrogen may exacerbate the inflammatory response in females by increasing immune cell activity. Additionally, immune-related genes located on the X chromosome may contribute to stronger immune responses in females, owing to the dual role of the X chromosome21. Social and environmental factors further amplify this burden, especially in regions with low SDI and low-middle SDI, where females encounter greater challenges in accessing healthcare resources. Moreover, females often juggle multiple roles within the family and society, which may increase psychological and emotional stress in managing RA22. A busy lifestyle can also lead to irregular dietary habits and insufficient physical activity. Future research should examine these gender-specific differences from multiple angles, including biological mechanisms, social support, mental health, and healthcare accessibility. Targeted interventions are needed to alleviate the burden on female RA patients and enhance their quality of life.
Age is a significant risk factor for RA23. Previous studies indicated that the increasing proportion of the aging population correlates with a rise in both the incidence and DALYs associated with RA-related diseases24,25. Our analysis revealed that from 1990 to 2021, the incidence and DALYs of RA-related diseases among the global population aged 20 to 54 increased, while mortality rates decreased slightly, despite a general rise in global deaths. This trend reflected a shift in the disease spectrum. The etiological factors contributing to RA were varied, and with the advancement of industrialization and modernization, more individuals were exposed to these factors through occupational and other conditions26. Given that RA remained incurable, patients experienced substantial challenges regarding their quality of life and functional capacity, which contributed to the persistent rise in DALYs. However, the introduction of biological agents, such as tumor necrosis factor (TNF) inhibitors and other immunosuppressive drugs, significantly slowed disease progression and improved average life expectancy27,28.
The SDI in various regions and countries was positively correlated with the incidence and DALYs rates of RA, highlighting the significance of socioeconomic factors in RA management and prevention. In line with previous research29, the low SDI regions reported the most significant increases in RA incidence. RA exerts significant clinical and policy-level impacts in regions with low and low-middle SDI scores. These impacts encompass key areas such as medical resource allocation, early diagnosis and treatment, chronic disease management, and socioeconomic support30. These regions often face challenges, including resource shortages and unequal access to healthcare. These should focus on improving access to diagnosis and treatment, reducing healthcare costs, strengthening basic healthcare infrastructure, and fostering international cooperation. Through collaborative efforts, the quality of life for RA patients in low SDI and low-middle SDI regions can be enhanced, the social burden reduced, and public health development promoted. In addition, areas with high SDI also have high incidence rates. Populations in high-SDI regions were more susceptible to mental health challenges such as stress, anxiety, and depression, which influenced both the incidence and progression of RA. In addition, improvements in medical care and RA diagnostics-particularly the widespread implementation of early detection methods-led to an increased identification of RA cases, particularly in economically developed regions. This phenomenon explained the higher incidence of RA-related diseases in areas with elevated SDI31.
The rising incidence of RA in high-SDI regions reflects enhanced disease recognition and the adoption of advanced diagnostic technologies, such as rheumatology diagnostic equipment, anti-cyclic citrullinated peptide (CCP) antibody testing, and imaging techniques32. These advancements have facilitated earlier diagnosis and improved case reporting, meaning that the observed increase in incidence is not indicative of a higher disease burden, but rather a result of improved diagnostic and reporting capabilities33. Thus, access to advanced diagnostic tools is crucial for ensuring the accuracy of disease burden data. Additionally, the observed decline in RA mortality in high-SDI regions underscores the significant role of advanced medical care in extending patient life expectancy. High-SDI regions provide timely pharmacological treatments, personalized interventions, and biologic therapies, all of which enhance patients’ prognosis and quality of life34,35. Through early diagnosis, personalized treatment, and precise disease monitoring, doctors can better manage patients’ conditions and reduce the burden and functional loss caused by the disease. At the same time, standardized tools also provide a solid foundation for the formulation of public health policies and scientific research34,35,36.However, mortality rates in economically developed regions and countries were also higher compared to those in less developed areas. Even in economically advanced countries, income inequality could impact the incidence and mortality rates of the disease. Low-income groups in developed nations may face higher health risks due to an inability to afford medical expenses or a lack of health education37.
The incidence, mortality and DALYs burden of RA in different regions are affected by multiple factors, including access to healthcare, environmental exposure and socioeconomic conditions38. Regions with high healthcare accessibility, such as the high-income Asia–Pacific, typically exhibit lower mortality rates and DALYs due to timely diagnosis and access to advanced medical treatments39. Conversely, regions with limited healthcare access, including Central Asia and certain low-SDI areas, often face resource shortages, delayed diagnoses, and insufficient treatment options, resulting in a substantially higher disease burden. Regarding environmental factors, pollutants such as PM2.5 and nitrogen oxides have been linked to the onset and progression of RA40. For regions with occupational and environmental exposure, such as Central Asia and Andean Latin America, in-depth environmental and occupational health research is needed to develop effective prevention strategies41. Higher environmental regions typically benefit from stronger environmental regulations, better air quality monitoring, and cleaner water sources, leading to improved living conditions. Socioeconomically, individuals in economically underdeveloped regions often face challenges such as limited education, poor health awareness, and inadequate health infrastructure42. To address these disparities, economically underdeveloped regions require greater international cooperation to provide affordable and effective treatments, increase disease awareness, and reduce the impact of environmental exposures on RA. Furthermore, enhancing global health advocacy is essential to securing international attention and support for chronic disease management and promoting healthier development in low-income countries.
Smoking is a significant risk factor for RA-associated DALYs, particularly in males, although its impact has decreased over time43,44. Since 2013, The analysis of risk factors revealed the global RA-related mortality and DALYs attributable to smoking had shown a yearly decline, and the decline rate for females was greater than that for males. This reduction was largely due to the implementation of stricter smoking regulations in many countries and regions, leading to a global decrease in smoking prevalence45. The decline in smoking-related PAF and DALYs serves as a key indicator of the effectiveness of tobacco control policies and public health interventions. Looking ahead, it is essential to develop more personalized and regionally tailored public health strategies. These strategies should focus on targeted tobacco control education for high-risk groups, such as low-income populations and adolescents, while also implementing smoking cessation policies and providing adequate support for cessation. Multinational cooperation between both low-income and high-income countries will be crucial in advancing tobacco control efforts and alleviating the global health burden caused by smoking46. By further strengthening tobacco control policies, enhancing cessation support systems, and optimizing resource allocation, we can reduce smoking-related health losses and promote global health equity.
The GBD dataset has made significant contributions to global health research. However, it has several limitations, including missing race/ethnicity data, biases introduced by imputation methods, regional discrepancies in data collection, and delays in disease classification. These limitations compromise the representativeness and accuracy of the data. In particular, data quality is poor in low-SDI countries, where health information is often inconsistent, and imputation assumptions may not accurately reflect local conditions, especially when health data vary widely across regions. To address these issues, future GBD datasets should prioritize racial and ethnic health disparities, with a focus on in-depth analyses of specific ethnic groups to identify potential health risks. To mitigate the bias introduced by imputation, multiple imputation schemes could be incorporated, and sensitivity analyses should be conducted to evaluate the impact of different assumptions. Additionally, field surveys and data collection efforts in regions with limited data should be expanded. As the disease landscape shifts and new public health challenges emerge, the GBD system will need to update its disease classification system regularly to capture evolving global health trends more accurately.
Conclusions
The trends in RA incidence, mortality, and DALYs present a multifaceted public health challenge that necessitates a comprehensive approach. While advanceable treatment may be contributing to decreased mortality rates, the significant rise in incidence and DALYs signal that RA is becoming increasingly prevalent, particularly in low and middle SDI regions. To address this issue effectively, healthcare policies must prioritize equitable access to care, targeted prevention strategies, and education on risk factors such as smoking. Additionally, further research is warranted to understand the underlying causes of the observed gender disparities and to develop tailored interventions that can effectively mitigate the burden of RA globally.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Aletaha, D. & Smolen, J. S. Diagnosis and management of rheumatoid arthritis. Jama 320(13), 1360 (2018).
Okada, Y. et al. Genetics of rheumatoid arthritis: 2018 status. Ann Rheum Dis 78(4), 446–453 (2019).
Serhal, L. et al. Rheumatoid arthritis in the elderly: Characteristics and treatment considerations. Autoimmun. Rev. 19(6), 102528 (2020).
Myasoedova, E. et al. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985–2014. Ann Rheum Dis 79(4), 440–444 (2020).
Ishikawa, Y. & Terao, C. The impact of cigarette smoking on risk of rheumatoid arthritis: A narrative review. Cells 9(2), 2020 (2020).
Kronzer, V. L. et al. Investigating asthma, allergic disease, passive smoke exposure, and risk of rheumatoid arthritis. Arthritis Rheumatol 71(8), 1217–1224 (2019).
Burke, H. et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 129(4), 735–744 (2012).
Collaborators, G.B.D.R.A., Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol, 2023. 5(10): e594–e610.
The Lancet, P. Global Burden of disease 2021: Mental health messages. Lancet Psychiatry 11(8), 573 (2024).
Diseases, G. B. D. & Injuries, C. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 403(10440), 2133–2161 (2024).
Collaborators, G.B.D.R.F., Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet, 403(10440), 2162–2203 (2024).
Mortality, G.B.D. and C. Causes of Death, Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet, 385(9963), 117–71 (2015).
Ding, Q. et al. Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology 98(3), e279–e290 (2022).
Yang, Y., Fu, W. J. & Land, K. C. A methodological comparison of age-period-cohort models: The intrinsic estimator and conventional generalized linear models. Sociol. Methodol. 34(1), 75–110 (2004).
Aleixo, P. et al. Ankle kinematics and kinetics during gait in healthy and rheumatoid arthritis post-menopausal women. Somatosens. Motor Res. 36(2), 171–178 (2019).
Islander, U. et al. Estrogens in rheumatoid arthritis; the immune system and bone. Mol. Cellul. Endocrinol. 335(1), 14–29 (2011).
Favalli, E. G. et al. Sex and management of rheumatoid arthritis. Clin Rev Allergy Immunol 56(3), 333–345 (2019).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388(10055), 2023–2038 (2016).
Park, E. H. et al. Impact of early age at menopause on disease outcomes in postmenopausal women with rheumatoid arthritis: A large observational cohort study of Korean patients with rheumatoid arthritis. RMD Open 9(1), e002722 (2023).
Firestein, G. S. & McInnes, I. B. Immunopathogenesis of rheumatoid arthritis. Immunity 46(2), 183–196 (2017).
Khan, D. & Ansar Ahmed, S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 6, 6353 (2015).
Dey, M. et al. Association between social deprivation and disease activity in rheumatoid arthritis: A systematic literature review. RMD Open 8(1), e002058 (2022).
Chalan, P. et al. Rheumatoid arthritis, immunosenescence and the hallmarks of aging. Curr. Aging Sci. 8(2), 131–146 (2015).
Zou, W. et al. Increasing global burden of rheumatoid arthritis: An epidemiological analysis from 1990 to 2019. Arch. Med. Sci. 19(4), 1037–1048 (2023).
Yang, W. et al. Global, regional and national burden of rheumatoid arthritis, and attributable risk factors from 1990 to 2019: Update from the Global Burden of Disease 2019 study. Clin. Exp. Rheumatol. 41(7), 429–442 (2022).
Romao, V. C. & Fonseca, J. E. Etiology and risk factors for rheumatoid arthritis: A state-of-the-art review. Front Med (Lausanne) 8, 689698 (2021).
Nam, J. L. et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: A systematic literature review informing the EULAR recommendations for the management of RA. Ann. Rheumat. Dis. 69(6), 976–986 (2010).
Jang, D. I. et al. The role of tumor necrosis factor alpha (TNF-alpha) in autoimmune disease and current TNF-alpha inhibitors in therapeutics. Int J Mol Sci 22(5), 2719 (2021).
Rudan, I. et al. Prevalence of rheumatoid arthritis in low- and middle-income countries: A systematic review and analysis. J Glob Health 5(1), 010409 (2015).
Yang, G. et al. Does socioeconomic status affect outcomes in early inflammatory arthritis? Data from a Canadian multisite suspected rheumatoid arthritis inception cohort. J. Rheumatol. 42(1), 46–54 (2015).
de Thurah, A. et al. Use of primary health care and radiological imaging preceding a diagnosis of rheumatoid arthritis: A nationwide cohort study in Denmark. Rheumatology (Oxford) 62(2), 555–564 (2023).
Radner, H. et al. Performance of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis: A systematic literature review. Ann. Rheum. Dis. 73(1), 114–123 (2014).
Willame, C. et al. Incidence rates of autoimmune diseases in european healthcare databases: A contribution of the ADVANCE project. Drug Saf. 44(3), 383–395 (2021).
Calabretta, M. M. et al. Precision medicine, bioanalytics and nanomaterials: Toward a new generation of personalized portable diagnostics. Analyst 145(8), 2841–2853 (2020).
Parimbelli, E. et al. Patient similarity for precision medicine: A systematic review. J. Biomed. Inform. 83, 87–96 (2018).
Ho, D. et al. Enabling technologies for personalized and precision medicine. Trends Biotechnol. 38(5), 497–518 (2020).
Kondo, N. et al. Income inequality, mortality, and self rated health: Meta-analysis of multilevel studies. BMJ 339, b4471 (2009).
Safiri, S. et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis 78(11), 1463–1471 (2019).
Beck, K. C. et al. Educational inequalities in adult mortality: A systematic review and meta-analysis of the Asia Pacific region. BMJ Open 12(8), e059042 (2022).
Adami, G. et al. Association between environmental air pollution and rheumatoid arthritis flares. Rheumatology (Oxford) 60(10), 4591–4597 (2021).
Coman, A. et al. An assessment of the occupational and environmental health needs in seven Southeastern European and West-Central Asian countries. J Epidemiol Glob Health 5(4), 375–384 (2015).
Brereton, C. F. & Jagals, P. Applications of systems science to understand and manage multiple influences within children’s environmental health in least developed countries: A causal loop diagram approach. Int. J. Environ. Res. Public Health 18(6), 3010 (2021).
Scherer, H. U., Häupl, T. & Burmester, G. R. The etiology of rheumatoid arthritis. J. Autoimmun. 110, 1024005 (2020).
Maisha, J. A., El-Gabalawy, H. S. & O’Neil, L. J. Modifiable risk factors linked to the development of rheumatoid arthritis: Evidence, immunological mechanisms and prevention. Front Immunol https://doi.org/10.3389/fimmu.2023.1221125 (2023).
Collaborators, G. B. D. T. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 389(10082), 1885–1906 (2017).
Reddy, K. S. et al. Integrating tobacco control into health and development agendas. Tob Control 21(2), 281–286 (2012).
Acknowledgments
We thank the Global Burden of Disease Study 2021 (GBD 2021) and GBD 2021 collaborators for providing the data used in this study. Beyond the usual salary, no one received financial compensation for their contribution.
Funding
This work was financially supported by the Shandong Natural Science Foundation General Project (Grant No. ZR2020MH094), China University Industry-University-Research Innovation Fund (Grant No. 2024GR050).
Author information
Authors and Affiliations
Contributions
Z Z and G X are considered co-first authors. Drs Z Y and W J had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: L S, W Q and W Y. Critical revision of the manuscript for important intellectual content: H S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Gao, X., Liu, S. et al. Global, regional, and national epidemiology of rheumatoid arthritis among people aged 20–54 years from 1990 to 2021. Sci Rep 15, 10736 (2025). https://doi.org/10.1038/s41598-025-92150-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92150-1