Abstract
Imaging examinations exhibit a certain rate of missed detection for distant metastases of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). This study aims to develop and validate a risk prediction model for the distant metastases and prognosis of GEP-NENs. This study included patients diagnosed with gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) from the Surveillance, Epidemiology, and End Results (SEER) database between 2010 and 2015. External validation was performed with patients from the China-Japan Union Hospital of Jilin University. Univariate and multivariate logistic regression analyses were conducted on the selected data to identify independent risk factors for distant metastasis in GEP-NENs. A nomogram was subsequently developed using these variables to estimate the probability of distant metastasis in patients with GEP-NENs. Subsequently, patients with distant metastasis from GEP-NENs were selected for univariate and multivariate Cox regression analyses to identify prognostic risk factors. A nomogram was subsequently developed to predict overall survival (OS) in patients with GEP-NENs. Finally, the developed nomogram was validated using Receiver Operating Characteristic (ROC) curves, calibration curves, and Decision Curve Analysis (DCA). Kaplan–Meier analysis was employed to evaluate survival differences between high-risk and low-risk groups. A total of 11,207 patients with GEP-NENs were selected from the SEER database, and 152 patients from the China-Japan Union Hospital of Jilin University were utilized as an independent external validation cohort. Univariate and multivariate logistic regression analyses revealed that the primary tumor site, tumor grade, pathological type, tumor size, T stage, and N stage are independent predictors of distant metastasis in GEP-NENs. Additionally, among the 1732 patients with distant metastasis of GEP-NENs, univariate and multivariate Cox regression analyses identified N stage, tumor size, pathological type, primary site surgery, and tumor grade as independent prognostic factors. Based on the results of the regression analyses, a nomogram model was developed. Both internal and external validation results demonstrated that the nomogram models exhibited high predictive accuracy and significant clinical utility. In summary, we developed an effective predictive model to assess distant metastasis and prognosis in GEP-NENs. This model assists clinicians in evaluating the risk of distant metastasis and in assessing patient prognosis.
Similar content being viewed by others
Introduction
Neuroendocrine neoplasms (NENs) originate from neuroendocrine cells, exhibiting high heterogeneity1,2. Its incidence has shown a yearly increasing trend. According to data from the SEER database, the incidence rate increased from 5.25 per 100,000 in 2004 to 6.98 per 100,000 in 20123. The European NENs Society reported that the incidence of NENs in the United States reached 8.4 per 100,000 in 20164. In China, based on data from cancer surveillance points, the incidence rate of NENs reached 4.1 per 100,000, with a 5-year survival rate remaining stable at approximately 50%. Among these, gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) represent the most common subtype of NENs, accounting for approximately 55–70% of all NENs5,6,7. In 2022, the World Health Organization (WHO) classified NENs into well-differentiated neuroendocrine tumors (NETs), poorly differentiated neuroendocrine carcinomas (NECs), and mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs), based on morphological characteristics and proliferation indices8,9. NENs are classified into functional and non-functional types based on their ability to secrete hormones and to produce hormone-related symptoms. Additionally, based on biological behaviors, such as mitotic count and Ki-67 proliferation index, as well as prognostic indicators, NENs can be stratified into grades 1, 2, and 310.
Early-stage NENs are more prevalent and are generally amenable to surgical treatment, with a favorable prognosis11,12. The AJCC guidelines classify locally advanced and metastatic NENs into stages II through IV. Although the incidence is low,
their high invasiveness and malignancy result in an increased risk of mortality, thus warranting attention. Studies have indicated that the median survival for localized NENs exceeds 30 years, while the median survival for peritoneal metastatic NENs is only 1 year3,13.
Over the past three decades, the incidence of GEP-NENs has increased significantly, making it the second most common type of digestive system cancer4,14. GEP-NENs exhibit significant heterogeneity, with considerable variation in malignancy and invasiveness across tumors of different grades and locations4,8. Studies have shown that approximately one-third of patients with GEP-NENs present with distant metastasis at the time of diagnosis3. Imaging examinations are currently the primary methods for the initial diagnosis and staging of GEP-NENs, with computed tomography (CT) as the principal imaging technique. However, CT has a low detection rate for small lymph node metastases (< 1 cm), with detection rates of 61% for bone metastases and 79% for liver metastases. Its sensitivity for detecting extraperitoneal soft tissue metastases is 70%, and it has limited capability in detecting small peritoneal metastases15,16. Magnetic resonance imaging (MRI) is advantageous for examining the pancreas, liver, and bones; however, it may overlook small pulmonary metastases16, potentially leading to delayed diagnosis and suboptimal treatment for GEP-NENs patients with distant metastases. Functional imaging plays a pivotal role in the detection of primary and metastatic lesions in NENs, as well as in guiding therapeutic interventions. Various modalities, including 111In-DTPA-Octreotide (OctreoScan) and 68 Ga-labeled somatostatin analogue positron emission tomography (68 Ga-PET), are extensively utilized6. Moreover, the RECIST 1.1 criteria are frequently employed alongside CT to evaluate the efficacy of systemic therapies in patients with NETs and liver metastases (LMs). The study conducted by Mestier et al. demonstrated that a ≥ 10% reduction in the size of three LMs could represent a more clinically relevant criterion than the current 30% threshold outlined by RECIST 1.1 for assessing treatment efficacy in patients with advanced NETs17.
Over the years, treatment options for GEP-NENs have expanded18; however, no therapies targeting specific molecular markers of GEP-NENs have been approved to date. 177Lu-DOTATATE radioligand therapy is the only treatment that requires preselection based on SSTR2-positive molecular imaging. Further research is ongoing to develop more precision therapies for GEP-NENs19.
For GEP-NENs, the American Joint Committee on Cancer (AJCC and WHO TNM staging systems serve as the primary basis for assessing prognosis20,21. However, other factors, including tumor grade, size, and location, also significantly impact patient outcomes22,23. Currently, effective evaluation tools for assessing distant metastasis and prognosis of GEP-NENs are lacking in clinical practice. Therefore, the research and development of relevant predictive models are crucial.
This study utilizes the SEER database, from which basic clinical information on patients diagnosed with GEP-NENs was extracted. Combined with 152 samples from a single center for external validation, statistical analysis was conducted to identify risk factors associated with distant metastasis in GEP-NENs, as well as prognostic factors for patients with distant metastasis. Additionally, diagnostic and prognostic predictive models were developed to generate numerical probabilities for individual patients concerning the occurrence of distant metastasis and survival time. These models aim to address the clinical need for effective management and personalized treatment of patients with GEP-NENs.
Materials and methods
Patient selection
In this study, clinical information from patients was obtained from the SEER database. As our study is limited to the 7th edition of the AJCC, we extracted GEP-NENs-related data from the SEER database covering the years 2010 to 2015 to investigate survival outcomes and prognostic factors. Patients diagnosed with GEP-NENs from January 2010 to December 2015, as recorded in the “Incidence—SEER Research Data, 17 Registries, Nov 2022 Sub (2000–2020)” database, were selected as the cohort for developing the distant metastasis model (model group). Furthermore, to adhere to the AJCC 7th edition and ensure the availability of sufficiently long follow-up data, we performed an independent external validation by analyzing data from 152 GEP-NENs patients diagnosed at the China-Japan Union Hospital of Jilin University between January 2010 and January 2018.
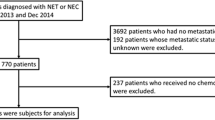
Inclusion criteria were as follows: (1) Patients with primary site classifications according to the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), including C16.0-C16.6; C16.8-C16.9; C17.0-C17.3; C17.8-C17.9; C18.0-C18.9; C19.9; C20.9; C21.0-C21.8; C25.0-C25.4; C25.7-C25.9; C26.0-C26.9. (2) Pathology types classified according to ICD-O-3, including 8150–8156; 8240–8244; 8246; 8249. Histological classification followed the WHO 2010 criteria (aligned with SEER coding during 2010–2015). (3) Complete follow-up information to ensure accurate assessment of patient status. Exclusion criteria included: (1) Patients with missing information on sex, age, year of diagnosis, race, histological type, primary site, tumor size, TNM staging, surgery, radiotherapy, chemotherapy, survival status, time of death, and cause of death. (2) Patients with GEP-NENs not diagnosed histologically or cytologically. (3) Patients with a survival time of less than one month or a follow-up time of less than one month after initial diagnosis (Fig. 1).
As part of this study, 152 patients were retrospectively recruited from China-Japan Union Hospital of Jilin University between January 2010 and January 2018 for external validation. Both the SEER database and patient information from our hospital met the aforementioned inclusion and exclusion criteria. The SEER database is a publicly accessible repository of anonymized data. All patients provided written informed consent upon inclusion, and the study was approved by the local institutional review board. Additionally, the study was approved by the Ethics Committee of the China-Japan Union Hospital of Jilin University in accordance with the Declaration of Helsinki (2024091103), and all experiments were performed in accordance with relevant guidelines and regulations. All participants provided written informed consent to be involved in this study.
Study variables and outcomes
Variables extracted from the SEER database included sex, age, year of diagnosis, race, histological type, primary site, tumor size (< 2 cm, 2–5 cm, ≥ 5 cm), TNM staging, surgery, radiotherapy, chemotherapy, survival status, time of death, and cause of death. This study uses overall survival (OS) as the primary outcome measure, defined as the duration from the diagnosis of GEP-NENs to death from any cause.
Construction and validation of diagnostic and prognostic models
A total of 11,207 GEP-NEN patients were extracted from the SEER database to establish the model cohort. The patients were randomly allocated into a training set (n = 7,844) and a validation set (n = 3,363) in a 7:3 ratio. Clinical information between the training and validation sets was compared for differences. Variables with P-values < 0.05 in the univariate logistic regression analysis were included in the multivariate regression analysis. Risk factors for distant metastasis in GEP-NENs were identified based on the results of the multivariate logistic regression analysis. A clinical prediction model for distant metastasis in GEP-NENs was developed using the training set to estimate the likelihood of distant metastasis. The model was further evaluated using ROC curves, calibration curves, and DCA. Both internal and external validations of the prediction model were conducted using the validation set and an independent external validation cohort.
Subsequently, 1,732 patients with distant metastasis of GEP-NENs were identified. These patients were divided into a training set (n = 1,212) and a validation set (n = 520) in a 7:3 ratio and subjected to univariate and multivariate Cox regression analyses. Independent risk factors affecting the prognosis of patients with distant metastasis of GEP-NENs were identified and used to construct a prediction model to estimate the probabilities of survival for less than 1-year, 2-year, 3-year, 4-year, and 5-year. Risk prediction scores for each patient were calculated by summing the scores associated with each variable to estimate the 1-year, 2-year, 3-year, 4-year, and 5-year survival rates. The model was validated both internally and externally using the validation set and an independent external validation cohort. Furthermore, based on the median of the model prediction scores, patients with distant metastasis of GEP-NENs were categorized into high-risk and low-risk groups, and OS was compared between the two groups.
Statistical analysis
In this study, all statistical analyses were performed using R software (version 4.3.2) and the Zstats statistical platform. Chi-square tests were employed to compare the distribution of clinical variables between the training and validation sets. Logistic regression and Cox regression analyses were employed to assess the risk factors for distant metastasis and prognosis of GEP-NENs, respectively. Patients were categorized into high-risk and low-risk groups according to the median score of the prognostic model, and survival differences between the two groups were analyzed using the Log-Rank test.
Results
Baseline characteristics of GEP-NEN patients
The SEER database contained data on 11,207 patients diagnosed with GEP-NENs between January 2010 and December 2015, including 7,844 patients in the training set and 3,363 patients in the validation set. In both groups, 45.76% of patients were aged 60 years or older, 48.95% were male, and 51.05% were female. Among the patients, 76.97% were White, 14.82% were Black, and 8.21% were of other races, including American Indian/Alaskan Native, Asian, or Pacific Islander. Lesions were located in the intestinal tract in 72.04% of patients, while 20.85% had lesions in the pancreas, and 7.10% had lesions in the stomach. Tumors in the SEER database are classified by differentiation into Grade I, Grade II, Grade III, and Grade IV, corresponding to well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated tumors, respectively. Of these tumors, 91.59% were Grade I/II, while 8.41% were Grade III/IV. Additionally, neuroendocrine tumors were the most prevalent histological type, comprising 59.91% of cases. Tumors smaller than 2 cm were the most frequent, representing 57.96%. In the TNM staging of the tumors, stages T1 and N0 were the most common, accounting for 43.45% and 64.98%, respectively. Among all patients diagnosed with GEP-NENs, 15.45% had experienced distant metastasis at the time of diagnosis. Additionally, 152 patients diagnosed with GEP-NENs at our institution between 2010 and 2018 were independently validated and assessed (Table 1).
Analysis of risk factors for distant metastasis in GEP-NENs
To develop a nomogram for predicting distant metastasis in GEP-NENs, we compared the baseline characteristics of the training and validation sets. No statistically significant differences were observed between the two sets, indicating data consistency. Nine clinical variables were subsequently included in the regression analysis. The univariate analysis identified age, race, tumor location, tumor grade, histological type, tumor size, T stage, and N stage as risk factors for distant metastasis in GEP-NENs (P < 0.05). The multivariate logistic regression analysis determined the following as independent risk factors for distant metastasis in GEP-NENs: pancreatic tumor location, Grade III/IV tumor grade, histological type, tumor size, advanced T stage, and advanced N stage (P < 0.05) (Table 2).
Development and validation of the nomogram diagnostic model for distant metastasis in GEP-NENs
Based on the results of univariate and multivariate logistic regression analyses, tumor location, tumor grade, histological type, tumor size, T stage, and N stage were identified as predictors for the nomogram of distant metastasis in GEP-NENs, leading to the construction of a prediction model (Fig. 2). In the nomogram developed from the training set, the area under the ROC curve (AUC) was 0.85 (Fig. 3A), indicating that the model demonstrates satisfactory diagnostic performance. The calibration curve demonstrated good fit (Fig. 3B), and the DCA curve exhibited excellent clinical utility (Fig. 3C). The ROC curve for the validation set (AUC = 0.85), along with the calibration curve and DCA curve (Fig. 3E–G), demonstrated satisfactory accuracy and discrimination. Furthermore, ROC curves for each of the six independent risk factors incorporated into the prediction model were plotted separately for both the training and validation sets (Fig. 3D,H). The results indicated that the nomogram exhibited significantly superior predictive ability compared to each individual risk factor and demonstrated high discrimination capability.
Validation of nomogram model for GEP-NENs distant metastasis. (A–D) ROC curves, calibration curves, and DCA curves for the training set, as well as comparisons of the ROC curves for various independent risk factors and the nomogram. (E–H) ROC curves, calibration curves, and DCA curves for the validation set, as well as comparisons of the ROC curves for various independent risk factors and the nomogram. (I–L) ROC curves, calibration curves, and DCA curves for the external validation set, as well as comparisons of the ROC curves for various independent risk factors and the nomogram.
To further assess the model’s performance, we utilized an external validation set comprising GEP-NENs patients diagnosed at our institution from January 2010 to January 2018 (n = 152). The results were satisfactory (Fig. 3I–L), with the ROC curve area under the curve (AUC) being 0.84, demonstrating that the prediction model also exhibits strong predictive performance in the external validation set. Overall, the GEP-NENs distant metastasis prediction model we developed demonstrates outstanding performance.
Analysis of prognostic risk factors for patients with distant metastasis of GEP-NENs
Among the 11,207 patients with GEP-NENs identified from the SEER database, 1,732 had distant metastasis. Patients with distant metastasis were allocated to a training set (n = 1212) and a validation set (n = 520) in a 7:3 ratio. Twelve clinical variables were analyzed using univariate and multivariate Cox regression. Univariate Cox regression analysis identified tumor location, tumor grade, histological type, tumor size, primary site surgery, radiotherapy, chemotherapy, T stage, and N stage as risk factors for the prognosis of patients with distant metastasis from GEP-NENs (P < 0.05). Multivariate Cox regression analysis determined tumor grade III/IV, tumor size ≥ 5 cm, and high N stage as independent risk factors for the prognosis of patients with distant metastasis from GEP-NENs (HR > 1; P < 0.05). For patients with distant metastasis of GEP-NENs, a pathological diagnosis of Neuroendocrine tumor and surgical treatment of the primary site are independent protective factors for prognosis (HR < 1; P < 0.05) (Table 3).
Development and validation of the prognostic model for patients with distant metastasis of GEP-NENs
Based on the results of the multivariate Cox regression analysis, we developed a nomogram for the prognosis of GEP-NENs patients with distant metastasis (Fig. 4) to predict the 1-year, 2-year, 3-year, 4-year, and 5-year survival probabilities. For the nomogram developed using the training set, the ROC curve areas under the curve (AUC) for 1-year, 2-year, 3-year, 4-year, and 5-year survival were 0.87, 0.88, 0.85,0.83 and 0.82, respectively (Fig. 5A), indicating good predictive ability. The calibration curves also demonstrated high predictive accuracy (Fig. 6A–E). Additionally, the predictive performance of our nomogram significantly surpassed that of individual independent risk factors (Fig. 5B). Furthermore, we stratified patients into high-risk and low-risk groups based on the median survival prediction scores from the model. Results indicated that the survival probability for the high-risk group was significantly lower than that for the low-risk group (Fig. 5C). The results were similarly satisfactory in the validation set (Figs. 5D–F, 6F–J), demonstrating the model’s robust performance.
ROC curves were used to assess the 1-year, 2-year, 3-year, 4-year, and 5-year survival predictions for GEP-NENs patients with distant metastasis in the training set (A), validation set (D), and external validation set (G). ROC curves were used to evaluate the predictive performance of the nomogram model against various independent prognostic factors in the training set (B), validation set (E), and external validation set (H). Patients were categorized into high-risk and low-risk groups based on the median survival prediction scores from the nomogram model. Survival time differences between these groups were compared in the training set (C), validation set (F), and external validation set (I).
We then screened 152 GEP-NENs patients diagnosed at our hospital and identified 37 cases with distant metastasis, which were used as an independent external validation set for the prognostic model. The results showed that the ROC curves, calibration curves, and other evaluations demonstrated high predictive accuracy and discriminative ability of the model (Figs. 5G,H, 6K–O). Risk stratification based on the median prediction scores of the model indicated that the low-risk group had a significantly better prognosis compared to the high-risk group (Fig. 5I). In summary, the developed nomogram demonstrates excellent diagnostic and prognostic evaluation capabilities, with satisfactory accuracy and discriminative ability.
Discussion
GEP-NENs were historically considered rare and slow-growing tumors with unclear pathogenesis. However, increasing research has indicated that the biological behavior of GEP-NENs is more aggressive than previously thought18. Furthermore, over the past two decades, the incidence of GEP-NENs has risen5,14,24,25. As the second most common cancer in the digestive system, this warrants increased attention. Therefore, in-depth research on the diagnosis and prognosis of GEP-NENs is crucial for advancing our understanding and improving patient outcomes.
The clinical presentation of GEP-NENs varies widely, primarily based on the tumor’s pathological type and its hormone-secreting capability26. Non-functional GEP-NENs typically lack distinct clinical features. Studies have indicated that approximately 27% of GEP-NENs are metastatic at the time of initial diagnosis3. Furthermore, the invasiveness of GEP-NENs is highly variable based on the primary site. NENs located in the small intestine tend to have higher malignancy but show relatively slow progression after metastasis. In contrast, NENs in the stomach and rectum have a lower metastatic potential; however, once distant metastasis occurs, progression is rapid4,27.
For early-stage GEP-NENs, surgery remains the primary treatment, significantly improving patient survival28,29, However, in advanced GEP-NENs with distant metastasis, effective surgical resection is often not feasible. In recent years, the pharmacological treatment of NENs has progressed significantly. Octreotide and lanreotide are two somatostatin analogs (SSAs) approved for the treatment of tumor growth in advanced low- and intermediate-grade NETs. However, as an antiproliferative therapy, certain clinical indications remain controversial30,31,32,33. Peptide Receptor-Radionuclide Therapy (PRRT) and various chemotherapy regimens have provided varying degrees of relief in the prognosis of GEP-NENs34,35,36,37,38. Targeted drugs, such as sunitinib and everolimus, have shown effective antiproliferative effects in progressive pancreatic NENs39. These have been approved for the treatment of progressive, locally advanced, or metastatic pancreatic NENs40, but their efficacy in tumors at other sites requires further investigation. Immunotherapy, as a novel treatment option, is currently being evaluated in clinical trials for its efficacy in GEP-NENs41,42,43.
Currently, imaging techniques such as CT and MRI still have a certain rate of missed detection for small metastatic foci of GEP-NENs15,16. Tumor markers associated with GEP-NENs, such as chromogranin A (CgA), neuron-specific enolase (NSE), and serotonin, hold diagnostic and prognostic value for GEP-NENs6,44,45,46,47. However, they also present certain limitations. For example, although serum CgA levels correlate with tumor burden and recurrence in neuroendocrine tumors (NETs), their clinical utility is restricted by low specificity and sensitivity6. Consequently, the 9th edition of the AJCC does not recommend their routine use48. At present, there is a deficiency of effective predictive indicators or references for distant metastasis in GEP-NENs within clinical practice. Therefore, conducting in-depth research on the risk factors for distant metastasis in GEP-NENs and developing relevant preventive strategies are crucial for improving the prognosis of GEP-NENs patients.
Nomograms are extensively utilized in cancer diagnosis and prognosis, converting complex statistical prediction models into a single numerical estimate of event probability49. Specifically, nomograms integrate patient clinical information and disease characteristics to serve as a reference for disease diagnosis and prognosis assessment, characterized by their simplicity, accuracy, and practicality. Studies have demonstrated that nomograms exhibit superior performance in survival prediction and metastasis risk assessment for various cancers, including gastric cancer, breast cancer, small-cell lung cancer, and liver cancer50,51,52,53. They aid clinicians in formulating individualized treatment strategies and in the prevention of distant metastasis.
This study extracted clinical information on GEP-NEN patients from the SEER database, and coupled with independent external validation from our institution, identified independent risk factors through statistical analysis, ultimately developing a nomogram for distant metastasis in GEP-NENs. In the nomogram used for diagnosing GEP-NENs, a scoring system was developed by assigning points to each independent variable based on their respective regression coefficients derived from multivariate logistic regression analysis. The absolute value of each coefficient was used to proportionally allocate points along the scale, with the variable exhibiting the highest regression coefficient being assigned the maximum score. Subsequently, the scores for all risk factors present in an individual patient were summed to generate a total risk score. This total score was then mapped onto a probability scale to estimate the likelihood of distant metastasis. Additionally, we further selected cases of distant metastasis among GEP-NENs patients and developed a prognostic nomogram for distant metastasis through relevant statistical analyses. In the nomogram used to evaluate the prognosis of patients with distant metastatic GEP-NENs, a similar point assignment method was employed, where each independent variable was weighted according to its hazard ratio (HR) derived from the Cox regression model. The variable with the most significant effect on survival was assigned the highest score, and all other variables were proportionally weighted. The total risk score was used to estimate individualized survival probabilities at 1-year, 2-year, 3-year, 4-year, and 5-year intervals. The results indicate that, in addition to T and N staging, tumor grade, pathology type, and tumor size each exert varying degrees of influence on distant metastasis in GEP-NENs. Among GEP-NEN patients with established distant metastasis, tumor grade significantly impacts prognosis. Surgical treatment of the primary site significantly reduces the prognostic risk score and is associated with a longer postoperative survival period. Contrary to common clinical perspectives, statistical analysis indicates that radiotherapy and chemotherapy do not significantly affect the prognosis of patients with distant metastases of GEP-NENs. The development of these nomograms facilitates a rapid assessment of the probability of distant metastasis based on clinical information and tumor characteristics during the initial diagnosis of GEP-NENs. Moreover, the prognostic models can assess patient outcomes, offering greater accuracy and discrimination compared to traditional TNM staging. This aids clinicians in formulating more tailored treatment plans and helps minimize distant metastasis, thereby enabling personalized and precise medical care.
Several studies have reported on models related to GEP-NENs. For instance, Salvia et al. analyzed 34 articles related to prognostic models of pancreatic NENs and found that gender played a critical role in the prognosis of pancreatic NENs in a small number of models; however, no definitive conclusions could be drawn54. Li et al. developed a nomogram for predicting distant metastasis and prognosis of GEP-NENs using 9,145 clinically documented GEP-NEN patients from the SEER database8. Zhao et al. constructed a prognostic model for stage II-IV CRNENs patients by screening 668 cases from the SEER database and combining them with 62 cases from a single-center external validation55. In comparison to these models, the model we developed is more comprehensive. It incorporates more comprehensive clinical information and benefits from a larger and more detailed external validation cohort, thus enhancing its robustness and credibility.
Nevertheless, this study has several limitations. As a retrospective analysis, it is subject to inherent selection biases. The SEER database lacks specific details on surgical methods and comprehensive radiotherapy and chemotherapy regimens, which prevents the incorporation of more precise variables into the final nomogram. Moreover, several biomarkers closely linked to tumor progression and prognosis, including the Ki-67 index, CgA, pancreastatin, and SPP1, are not documented in the SEER database56,57,58. Additionally, the SEER database does not include functional imaging data for GEP-NENs58. Owing to the evolving classification criteria for NENs, the categorization of GEP-NENs in the SEER database may not align with the most recent NENs classification standards. Furthermore, our model employs the SEER-derived grading system (Grades I-IV), which is inconsistent with the WHO 2022 three-tier system (NET G1/G2/G3, NEC). Thus, this discrepancy must be taken into account when applying the model in clinical practice. Clinicians should integrate relevant laboratory tests and imaging studies to optimize diagnostic accuracy and therapeutic outcomes. Furthermore, given the heterogeneity of GEP-NENs, the external validation cohort (n = 152) is relatively limited in size, necessitating larger multicenter cohorts in future studies to validate the clinical significance of this model. Future studies should prioritize the inclusion of more comprehensive clinical variables, particularly patients’ specific treatment regimens, as these factors may substantially impact prognosis. Notably, the incorporation of relevant blood biomarkers could further enhance the predictive performance of the model.
Conclusion
In summary, we have developed a highly effective nomogram for predicting distant metastasis and prognosis in GEP-NENs, which assists clinicians in assessing metastasis risk and evaluating prognosis during the initial diagnosis of GEP-NEN patients. This model meets the clinical need for individualized precision treatment. Future research should include larger multicenter cohorts to further evaluate and validate its performance and to enhance its clinical applicability.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
References
Fang, J. M., Li, J. & Shi, J. An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. World J. Gastroenterol. 28(10), 1009–1023 (2022).
Sedlack, A. J. H. et al. Update in the management of gastroenteropancreatic neuroendocrine tumors. Cancer 13, 3090 (2024).
Dasari, A. et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3(10), 1335–1342 (2017).
Cives, M. & Strosberg, J. R. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J. Clin. 68(6), 471–487 (2018).
Xu, Z. et al. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA Netw. Open 4(9), e2124750 (2021).
Modica, R. et al. Recent advances and future challenges in the diagnosis of neuroendocrine neoplasms. Minerva Endocrinol. (Torino) 49(2), 158–174 (2024).
Lu, L. et al. Epidemiologic trends and prognostic risk factors of patients with pancreatic neuroendocrine neoplasms in the US: An updated population-based study. Future Oncol. 17(5), 549–563 (2021).
Li, X. et al. Risk factors, prognostic factors, and nomograms for distant metastases in patients with gastroenteropancreatic neuroendocrine tumors: A population-based study. Front. Endocrinol. (Lausanne) 15, 1264952 (2024).
Rindi, G. et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr. Pathol. 33(1), 115–154 (2022).
Uccella, S. Molecular classification of gastrointestinal and pancreatic neuroendocrine neoplasms: Are we ready for that?. Endocr. Pathol. 35(2), 91–106 (2024).
Effraimidis, G. et al. Multiple endocrine neoplasia type 1 (MEN-1) and neuroendocrine neoplasms (NENs). Semin. Cancer Biol. 79, 141–162 (2022).
Holmager, P. et al. Surgery in patients with gastro-entero-pancreatic neuroendocrine carcinomas, neuroendocrine tumors G3 and high grade mixed neuroendocrine-non-neuroendocrine neoplasms. Curr. Treat. Options Oncol. 23(6), 806–817 (2022).
Madani, A. et al. Peritoneal metastases from gastroenteropancreatic neuroendocrine tumors: Incidence, risk factors and prognosis. Ann. Surg. Oncol. 24(8), 2199–2205 (2017).
Yao, J. C. et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 26(18), 3063–3072 (2008).
Kim, J. H. et al. Pancreatic neuroendocrine tumour (PNET): Staging accuracy of MDCT and its diagnostic performance for the differentiation of PNET with uncommon CT findings from pancreatic adenocarcinoma. Eur. Radiol. 26(5), 1338–1347 (2016).
Pavel, M. et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 31(7), 844–860 (2020).
de Mestier, L. et al. Proposal of early CT morphological criteria for response of liver metastases to systemic treatments in gastroenteropancreatic neuroendocrine tumors: Alternatives to RECIST. J. Neuroendocrinol. 35(6), e13311 (2023).
Zappi, A. et al. Chemotherapy in well differentiated neuroendocrine tumors (NET) G1, G2, and G3: A narrative review. J. Clin. Med. 12(2), 717 (2023).
Fazio, N. & La Salvia, A. Precision medicine in gastroenteropancreatic neuroendocrine neoplasms: Where are we in 2023?. Best Pract. Res. Clin. Endocrinol. Metab. 37(5), 101794 (2023).
Klöppel, G., Perren, A. & Heitz, P. U. The gastroenteropancreatic neuroendocrine cell system and its tumors: The WHO classification. Ann. N. Y. Acad. Sci. 1014, 13–27 (2004).
Kulke, M. H. et al. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Cancer Netw. 13(1), 78–108 (2015).
Broadbent, R. et al. Prognostic factors for relapse in resected gastroenteropancreatic neuroendocrine neoplasms: A systematic review and meta-analysis. Cancer Treat. Rev. 101, 102299 (2021).
Li, Y. et al. Pathological characteristics and survival analysis of 355 patients with gastroenteropancreatic neuroendocrine neoplasms. Zhonghua Zhong Liu Za Zhi 42(5), 426–431 (2020).
Ito, T. et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: Diagnosis, treatment, and follow-up: A synopsis. J. Gastroenterol. 56(11), 1033–1044 (2021).
Modlin, I. M. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 9(1), 61–72 (2008).
Zhang, X. et al. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: A retrospective study. BMC Endocr. Disord. 14, 54 (2014).
Gonzalez, R. S. Diagnosis and management of gastrointestinal neuroendocrine neoplasms. Surg. Pathol. Clin. 13(3), 377–397 (2020).
Díez, M., Teulé, A. & Salazar, R. Gastroenteropancreatic neuroendocrine tumors: Diagnosis and treatment. Ann. Gastroenterol. 26(1), 29–36 (2013).
Howe, J. R. et al. The North American neuroendocrine tumor society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas 49(1), 1–33 (2020).
Zhang, X. B. et al. Gastroenteropancreatic neuroendocrine neoplasms: Current development, challenges, and clinical perspectives. Mil. Med. Res. 11(1), 35 (2024).
Pavel, M. et al. Efficacy and safety of high-dose lanreotide autogel in patients with progressive pancreatic or midgut neuroendocrine tumours: CLARINET FORTE phase 2 study results. Eur. J. Cancer 157, 403–414 (2021).
La Salvia, A. et al. Targeting neuroendocrine tumors with octreotide and lanreotide: Key points for clinical practice from NET specialists. Cancer Treat. Rev. 117, 102560 (2023).
Kulke, M. H. et al. Real-world treatment patterns and clinical outcomes in advanced gastrointestinal neuroendocrine tumors (GI NET): A multicenter retrospective chart review study. Oncologist 24(8), 1056–1065 (2019).
Kunz, P. L. et al. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 41(7), 1359–1369 (2023).
Puliani, G. et al. New Insights in PRRT: Lessons from 2021. Front. Endocrinol. (Lausanne) 13, 861434 (2022).
Rosery, V. et al. Identification of a new prognostic score for patients with high-grade metastatic GEP-NEN treated with palliative chemotherapy. J. Cancer Res. Clin. Oncol. 149(8), 4315–4325 (2023).
Sorbye, H. et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 24(1), 152–160 (2013).
Strosberg, J. R. et al. (177)Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 22(12), 1752–1763 (2021).
Pavel, M. et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 103(2), 172–185 (2016).
Raymond, E. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364(6), 501–513 (2011).
Feng, Z. et al. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat. Cancer 3(5), 581–594 (2022).
Pan, W. X. et al. Progress in immunotherapy for neuroendocrine neoplasm of the digestive system. World J. Gastroenterol. 29(26), 4174–4185 (2023).
Popa Ilie, I. R. & Georgescu, C. E. Immunotherapy in gastroenteropancreatic neuroendocrine neoplasia. Neuroendocrinology 113(2), 262–278 (2023).
Uhlig, R. et al. Synaptophysin and chromogranin A expression analysis in human tumors. Mol. Cell Endocrinol. 555, 111726 (2022).
Lou, X. et al. The interplay of four main pathways recomposes immune landscape in primary and metastatic gastroenteropancreatic neuroendocrine tumors. Front. Oncol. 12, 808448 (2022).
Boons, G. et al. Longitudinal copy-number alteration analysis in plasma cell-free DNA of neuroendocrine neoplasms is a novel specific biomarker for diagnosis, prognosis, and follow-up. Clin. Cancer Res. 28(2), 338–349 (2022).
Almobarak, B. et al. Exposure to nonanoic acid alters small intestinal neuroendocrine tumor phenotype. BMC Cancer 23(1), 267 (2023).
Chauhan, A. et al. Critical updates in neuroendocrine tumors: Version 9 American Joint Committee on Cancer staging system for gastroenteropancreatic neuroendocrine tumors. CA Cancer J. Clin. 74(4), 359–367 (2024).
Iasonos, A. et al. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 26(8), 1364–1370 (2008).
Dong, D. et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol. 30(3), 431–438 (2019).
Min, Y. et al. Risk factors, prognostic factors, and nomogram for distant metastasis in breast cancer patients without lymph node metastasis. Front. Endocrinol. (Lausanne) 12, 771226 (2021).
Wang, S. et al. Development and validation of a nomogram prognostic model for SCLC patients. J. Thorac. Oncol. 13(9), 1338–1348 (2018).
Wang, X. et al. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int. J. Biol. Sci. 15(1), 221–228 (2019).
La Salvia, A., et al. Gender impact on pancreatic neuroendocrine neoplasm (PanNEN) prognosis according to survival nomograms. Endocrine (2024).
Zhao, F. et al. Epidemiological trends and novel prognostic evaluation approaches of patients with stage II-IV colorectal neuroendocrine neoplasms: A population-based study with external validation. Front. Endocrinol. (Lausanne) 14, 1061187 (2023).
Kidess, E. et al. Osteopontin is a prognostic circulating biomarker in patients with neuroendocrine neoplasms. J. Cancer Res. Clin. Oncol. 149(12), 10925–10933 (2023).
Hoff, C. O. et al. A neuroendocrine biomarker revolution from monoanalyte to multianalyte biomarkers in non-functioning gastro-entero-pancreatic neuroendocrine neoplasms. Crit. Rev. Oncol. Hematol. 203, 104460 (2024).
Alexandraki, K. I., Zatelli, M. C. & Grossman, A. B. “The past is a different country, they do things differently there”: Using the SEER data-base to assess prognosis in neuroendocrine tumours. Endocrine 75(3), 725–727 (2022).
Acknowledgements
Thanks to SEER database contributors for sharing the data. We thank Professor and Chair Xin-Yuan Song of the Department of Statistics, The Chinese University of Hong Kong, for his careful guidance on the statistical methods and survival analysis applied in this study.
Funding
This study was supported by the Natural Science Foundation of Jilin Province (Grant No. YDZJ202201ZYTS118), the Specialized Health Research Talents in Jilin Province Funding (Grant No. 2023SCZ09) and Jilin Provincial Department of Science and Technology Excellence Talent Program for Young and Middle-aged Science and Technology Innovation and Entrepreneurship (Grant No.20240601013RC).
Author information
Authors and Affiliations
Contributions
Shuo-Hui Gao: Conceptualization, Funding acquisition, Project administration, Writing-original draft. Jian-Peng Xing: Conceptualization, Funding acquisition, Project administration, Writing-original draft. Xuan-Peng Zhou: Data curation, Investigation, Methodology, Writing-original draft, Writing-review & editing. Luan-Biao Sun: Data curation, Writing-original draft. Wen-Hao Liu: Data curation, Investigation, Methodology. Xin-yuan Song: Data curation, Writing-original draft. All authors contributed to the article and approved the submitted version. Yang Gao: Methodology, Data curation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
All experiments were performed in accordance with relevant guidelines and regulations. All participants provided written informed consent to be involved in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, XP., Sun, LB., Liu, WH. et al. Development and validation of predictive models for distant metastasis and prognosis of gastroenteropancreatic neuroendocrine neoplasms. Sci Rep 15, 9510 (2025). https://doi.org/10.1038/s41598-025-92974-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92974-x