Abstract
The transcription factor MYB proto-oncogene like 2 (MYBL2) has been reported to be involved in the occurrence and development of various tumors, however, its role in gastric cancer (GC) remains to be elucidated. In this study, the Kaplan-Meier plotter was used to evaluate the prognostic value of different MYBL2 expression levels in GC patients. The UALCAN database were applied to analyze the relationships between MYBL2 and clinicopathological characteristics of GC. GC cell proliferation, cell cycle and apoptosis were determined by CCK-8 and flow cytometry assays, and proteins were examined by Western blot analysis. Next, signaling pathway enrichment analysis of MYBL2-related genes and protein expression were analyzed by Gene Set Enrichment Analysis (GSEA) and Western blot assays. The results found that MYBL2 expression was significantly upregulated in GC compared with adjacent non-malignant tissues and associated with poor patient survival, tumor, stages and lymph node metastasis. Forced expression of MYBL2 could promote cell proliferation, resulting in an accelerated S phase progression and inhibiting cell apoptosis in GC cells. Conversely, MYBL2 silencing inhibited cell proliferation, induced G2/M phase arrest and promoted cell apoptosis in GC cells. Mechanistically, Western blot analysis showed that MYBL2 silencing decreased the expression of BCL2 and upregulated the expression of Cleaved-caspase-3 and BAX in HGC-27 cells. Conversely, MYBL2 overexpression in AGS cells resulted in the opposite effects. Furthermore, enforced expression of MYBL2 activated the PI3K/AKT signaling pathway, especially AKT phosphorylation. Additionally, the AKT inhibitor MK2206 significantly reversed the proliferation capacity of GC cells induced by MYBL2 overexpression. Therefore, these results suggest that upregulated expression of MYBL2 contributes to GC cell growth and inhibits cell apoptosis by regulating the PI3K/AKT and BCL2/BAX/Cleaved-caspase-3 signaling pathways in GC cells indicating that MYBL2 may be a new therapeutic target and prognostic marker for GC.
Similar content being viewed by others
Introduction
Cancer remains a major cause of mortality worldwide, especially in China1,2,3, and GC ranks as the fifth most common cancer and ranks fourth among causes of cancer-related death globally4. Although the past decade has witnessed significant inroads in surgery, chemotherapy, radiotherapy, and biological therapy, the prognosis of GC patients remains unsatisfactory5,6. Therefore, it is particularly urgent to search for the driving genes involved in the occurrence and development of GC.
Uncontrolled cell proliferation is one of the hallmarks of cancer cells. It has been established that cell proliferation and malignant transformation represent complex biological processes involving multiple genes and signaling pathways7. Transcription factors (TFs) are essential for regulating gene expression by interacting with transcription corepressors/enhancers and play fundamental roles in the multistep process of tumorigenesis and development8. An increasing body of evidence suggests that TFs such as AP-1, Sp1, Snail1 and c-Myc are involved in the malignant proliferation, invasion and metastasis of tumor cells9,10,11,12,13.
MYBL2 (MYB proto-oncogene Like 2) is a highly conserved member of the MYB transcription factor family, which plays a key role in regulating the cell cycle, cell proliferation, apoptosis, and metastasis14,15,16 There is a growing consensus that overexpression of MYBL2 can promote the proliferation of gallbladder17 and liver cancer cells18. Consistently, increased or overexpression of MYBL2 in several invasive cancers can promote tumorigenesis in many tissue types, including human glioma19, myeloid leukemia20, breast cancer21, and non-small cell lung cancer22. However, the biological function of MYBL2 expression and GC has not been thoroughly studied. Accordingly, further studies are warranted to explore whether MYBL2 is involved in the malignant proliferation of GC cells.
In this study, we demonstrated that elevated expression of MYBL2 was significantly correlated with the different tumor grades and prognosis of GC patients, and MYBL2 contributes to GC cell growth and inhibits cell apoptosis by regulating the PI3K/AKT and BCL2/BAX/Cleaved-caspase-3 signaling pathways in GC cells, and providing a potential novel molecular target for GC patients.
Methods
Bioinformatic data mining
The survival curves of GC patients with high and low expression of MYBL2 were plotted using the Kaplan-Meier database (https://kmplot.com/analysis/). MYBL2 expression was analyzed in the gastric patient in The Cancer Genome Atlas (TCGA) data portal (https://portal.gdc.cancer.gov/) using The R Project for Statistical Computing (version 4.0.5, https://www.r-project.org/) and ActivePerl (version 5.26.3001, https://www.perl.org/) software. Moreover, the relationship between MYBL2 expression and clinicopathological characteristics was analyzed using the online tool UALCAN (http://ualcan.path.uab.edu/).
Cell lines and cell culture
The GC cell lines AGS and HGC-27 were obtained from the Chinese Academy of Medical Sciences & Peking Union Medical College (CAMS & PUMC, Beijing, China). All cells were cultured in DMEM (GIBCO, USA) medium containing 10% fetal bovine serum (Wisent, St. Bruno, QC, Canada), 1% streptomycin and 1% penicillin (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere at 37 °C with 5% CO2.
Cell transfection
The pcDNA3.1 vector and pcDNA3.1-MYBL2 plasmids were purchased from Youbio (Changsha, China). The plasmids were transfected into AGS cells using lipofectamine 6000 (Beyotime Biotechnology, Shanghai, China) in accordance with the manufacturer’s protocol. The interfering RNA (siRNA) for the specific inhibition of MYBL2 expression and a negative control siRNA were synthesized by GenePharma Co., Ltd. (Shanghai, China). The target sequence of MYBL2 was as follows23: MYBL2#1: 5′- GCA GAG GAC AGU AUC AAC ATT-3′; MYBL2#2: 5′- GAU CUG GAU GAG CUG CAC UTT-3′. The sequence of siRNA-NC was as follows: 5′-UUC UCC GAA CGU GUC ACG UTT − 3′. The HGC27 cells were transfected with siRNAs using lipofectamine 6000 according to the manufacturer’s information. After treatment, the cells were harvested and processed for further analysis.
Cell proliferation assays
The cells (4.5 × 103 cells per well) were seeded in 96-well culture plates for 24 h and transfected with siRNAs or plasmids DNA for 4 ~ 6 h and switched to complete medium or MK2206 (Beyotime Biotechnology, Shanghai, China) for culture for 24, 48, 72 and 96 h, respectively. Next, the CCK-8 reagent (Beyotime Biotechnology, Shanghai, China) was used to detect cell proliferation following the kit instructions.
Cell cycle and apoptosis analysis
The cells were inoculated into 6-well plates for 24 h and transfected with siRNAs or plasmids DNA for 4 ~ 6 h. After cells were cultured in complete medium for 48 h, cells were collected and washed with ice-cold PBS. Then the cells were fixed in 70% ethanol at -20 °C overnight. The next day, cells were resuspended in PBS, and stained with PI solution (Beyotime Biotechnology, Shanghai, China) for 30 min protected from light, and then cells were analyzed by flow cytometry (Beckman Coulter, Shanghai, China). At 48 h post-transfection, cells were harvested and stained with Annexin V-FITC/PI Staining Kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. GFP-expressing cells were analyzed by labeling with Annexin V-PE/7-AAD (Beyotime Biotechnology, Shanghai, China). The flow cytometry data were obtained using CytoFlex (Beckman Coulter, Shanghai, China), and analyzed by FlowJo (version 10.9.0, https://www.flowjo.com/) software.
Function enrichment analysis
GSEA enrichment analysis of MYBL2-related genes based on the TCGA database was performed using the c2.cp.kegg.v7.5.1symbols.gmt subset. Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathway enrichment analysis of MYBL2 was performed by GSEA software (version 4.3.3, https://www.gsea-msigdb.org/), and the results were plotted using R software. False Discovery Rate (FDR) < 0.05 was considered statistically significant.
Western blot analysis
Total protein was extracted using RIPA Lysis Buffer (Beyotime Biotechnology, Shanghai, China) and the protein concentrations were quantified with the BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China). Subsequently, proteins were subjected to 10% or 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Massachusetts, USA). Then, the membranes were sequentially incubated with primary antibodies at 4 °C overnight and horseradish peroxidase (HRP)-conjugated secondary antibodies for 2 h at room temperature. Antibodies for BCL2 (1:1000), p-PI3K (1:1000) and β-actin (1:4000) antibodies were purchased from ABclonal (ABclonal Science Co., Ltd., Wuhan, China). BAX (1:2000), Cleaved-caspase-3(1:1000), AKT (1:2000) and p-AKT (1:1000) antibodies were purchased from Proteintech (Proteintech Scientific Co., Ltd, Wuhan, China). Goat anti-Mouse IgG HRP (1:4000) and goat anti-Rabbit IgG HRP (1:4000) purchased from Abways (Abways Technology, Shanghai, China) were used as the secondary antibody. The immunoreactive proteins were detected using an ECL Western blotting detection system (Millipore, MA, USA).
Statistical analysis
Data were analyzed using IBM SPSS Statistics (version 25.0, https://www.ibm.com/spss) software (IBM Corporation, Armonk, NY, USA), and graphs were generated using GraphPad Prism (version 8.0.1, https://www.graphpad.com/)(GraphPad Software, La Jolla, CA, USA). Results were presented as mean ± SD. For all experiments, one-way ANOVA or Student’s t-test was used to analyze the differences between groups. A log-rank test was used to compare survival differences across groups for Kaplan-Meier survival analysis. The Wilcoxon rank sum test, Wilcoxon signed-rank test or Kruskal-Wallis test was used to analyze the relationship between MYBL2 expression and clinicopathological characteristics. A two-sided P-value < 0.05 was statistically significant.
Results
Elevated expression of MYBL2 was significantly correlated with the clinicopathological features of GC patients
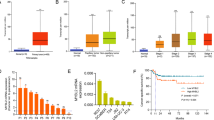
It has been established that the upregulation of MYBL2 expression is associated with the progression and poor prognosis of prostate cancer24. To explore the correlation between MYBL2 expression in GC and the clinicopathological features and prognostic significance, we first used Kaplan-Meier to analyze the effect of MYBL2 on the survival of GC patients. The results showed a significant relationship between MYBL2 and overall survival (P < 0.01, Fig. 1A), progression-free survival (P < 0.001, Fig. 1B), and post-progression survival (P < 0.05, Fig. 1C) in GC patients, suggesting that high MYBL2 expression correlated with a poor prognosis in GC patients. Next, we analyzed the expression of MYBL2 in GC tissues through the TCGA database. The results showed that MYBL2 expression was significantly increased in GC tissues (n = 375) compared with gastric mucosal tissues (n = 32) (P < 0.001, Fig. 1D). In addition, we analyzed 27 paired GC and adjacent tissues and found that MYBL2 was highly expressed in GC tissues (P < 0.001, Fig. 1E). Moreover, MYBL2 expression in GC tissues with different tumor grades (Fig. 1F), cancer stages (Fig. 1G) and lymph node metastatic grades (Fig. 1H) was significantly higher than that in peri-tumor tissues. Taken together, MYBL2 may be involved in the malignant phenotype of GC cells and represents is a prognostic marker and potential therapeutic target in GC patients.
The expression level of MYBL2 was significantly correlated with the clinicopathological features of GC patients. (A–C) Kaplan-Meier analysis of the effect of MYBL2 expression on overall survival (A), progression-free survival (B), and post-progression survival (C) in GC patients. (D–E) MYBL2 expression in gastric mucosal tissues and GC tissues (D), MYBL2 expression in 27 paired of GC tissues and adjacent tissues (E). (F–H) MYBL2 expression level in normal gastric tissue and different grades (F), stages (G) and lymph node metastasis (H) of GC patients. **P < 0.01; ***P < 0.001; *: compared with normal gastric tissue.
MYBL2 affects cell proliferation by modulating G2/S cell phase transition in GC cells
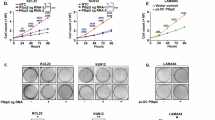
Abnormal cell proliferation is a typical feature observed after the malignant transformation of cells. Previous studies have shown that MYBL2 regulates the proliferation of lung and bladder cancer cells17,22. Accordingly, we first transfected HGC27 cells with a siRNA (target to MYBL2) and MYBL2 overexpression vector, respectively. Next, the proliferation ability of the GC cells was detected by the CCK-8 assay at 24, 48, 72, and 96 h, respectively. The results showed that MYBL2 silencing inhibited cell proliferation, whereas overexpression of MYBL2 promoted GC cell proliferation (Fig. 2A, P < 0.05). Flow cytometry analysis showed that the G2/M phase cells were significantly increased after MYBL2 silencing in HGC27 cells (Fig. 2B, P < 0.05), while the S phase cells of AGS cells increased significantly after MYBL2 overexpression (Fig. 2C, P < 0.05). The above results suggest that MYBL2 affects the proliferation of GC cells by regulating the cell cycle of GC cells.
Effects of MYBL2 on the proliferation and cell cycle progression of GC cells. (A) HGC27 cells were treated with siRNA, AGS cells were treated with overexpression vector and empty vector, and then the cell proliferation was detected by CCK-8 at 24, 48, 72, and 96 h, respectively. (B) Distribution of cells in HGC27 cell cycle after 48 h of MYBL2 silencing by flow cytometry, the percentage of cells in G0/G1, S and G2/M phases as shown in the bar graph. (C) The distribution of MYBL2 overexpression in AGS cells after 48 h was detected by flow cytometry, and the percentage of cells in the G0/G1 phase, S phase and G2/M phase is shown in the bar graph. *P < 0.05; **P < 0.01.
MYBL2 inhibits cell apoptosis through Mitochondria-mediated pathway in GC cells
The ability of cancer cells to evade apoptosis is established as one of the hallmarks of cancer7. To elucidate the role of MYBL2 in GC cell apoptosis, the effects of MYBL2 silencing/overexpression on GC cell apoptosis were detected by Annexin V-FITC/PI or Annexin V-PE/7-AAD fluorescent staining assay. As shown in Fig. 3, MYBL2 depletion resulted in increased early apoptosis levels in HGC27 cells (Fig. 3A), whereas ectopic expression of MYBL2 significantly reduced early apoptosis of AGS cells (Fig. 3B). To further explore the mechanism of MYBL2 inhibiting apoptosis of HGC27 cells, we detected the expression of pro-apoptotic protein BAX, anti-apoptotic protein BCL2 and Cleaved-caspase-3 protein by Western blotting assay. The results indicated that the knockdown MYBL2 significantly enhanced the BAX and Cleaved-caspase-3 expression and reduced BCL-2 levels in HGC27 cells (Fig. 3C). As expected, overexpression of MYBL2 yielded the opposite result (Fig. 3D). The above results suggest that MYBL2 inhibits apoptosis in GC cells via BCL2/BAX/Cleaved-caspase-3 signaling pathway in GC cells.
Effects of MYBL2 on apoptosis of GC cells. (A) MYBL2 silencing significantly promoted the apoptosis of HGC27 cells by flow cytometry (Annexin V/PI staining) analysis. (B) MYBL2 overexpression significantly inhibited the apoptosis of AGS cells by flow cytometry (Annexin V-PE/7-AAD staining) analysis. (C) Western blot analysis of the expression levels of apoptosis-related proteins MYBL2, BAX, BCL2, Cleaved-caspase-3 p17 and Cleaved-caspase-3 p19 after treating HGC27 cells with siRNA for 48 h, respectively. (D) Changes in the expression levels of apoptosis-related proteins MYBL2, BAX, BCL2 and Cleaved-caspase-3 p17 and Cleaved-caspase-3 p19 were analyzed by Western blotting after treating AGS cells with the overexpression vector for 48 h. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NC.
GSEA enrichment analysis of MYBL2-related genes based on the TCGA database
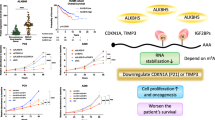
Our results above confirmed that the high expression of MYBL2 can inhibit the apoptosis and promote the proliferation of GC cells, in order to further explore the mechanism underlying the effect of MYBL2 on the occurrence and development of GC, gene set enrichment analysis of MYBL2-related genes were performed by GSEA. The results revealed that MYBL2 were significantly enriched in cell cycle, pyrimidine metabolism, DNA replication, calcium signaling pathway, ECM receptor interaction, etc25,26 (Fig. 4). The results are shown in Additional file 1: Table S1. It has been reported that altered cell cycle progression27, DNA replication stress28, and metabolic stress contribute to the hyper-proliferation in cancer cells. In addition, the PI3K/AKT signaling pathway, which is widely activated in tumor tissues and cells29, plays a crucial role in cell proliferation, differentiation, apoptosis, metabolism, and angiogenesis30, and it is widely acknowledged that the activation of the PI3K/AKT signal pathway can promote the proliferation of GC cells29. So, we speculate that MYBL2 may promote the proliferation of GC cells by activating PI3K/AKT signal pathway.
MYBL2 promotes GC cell proliferation through the PI3K/AKT signaling pathway in GC cells
To further confirm our hypothesis that MYBL2 induced GC cell proliferation is related to PI3K/AKT signal pathway, Western blot was used to detect the key proteins in PI3K/AKT signal transduction pathway in MYBL2 silenced and overexpressed GC cells. As shown in Fig. 5, the protein expression levels of p-PI3K and p-AKT were significantly downregulated after transfection of the MYBL2-targeted siRNA in HGC27 cells (Fig. 5A). Conversely, transfection of MYBL2 plasmid could significantly upregulate the protein expression of p-PI3K and p-AKT compared with the control group (Fig. 5B). The above results suggest that MYBL2 can promote the proliferation of GC cells by activating the PI3K/AKT signaling pathway. In addition, the AKT inhibitor (MK2206) significantly reversed the proliferation of GC cells induced by MYBL2 overexpression (Fig. 5C). Taken together, our findings suggest that MYBL2 overexpression can promote proliferation and inhibit apoptosis of GC cells by regulating the PI3K/AKT and BCL-2/BAX/Cleaved-caspase-3 signaling pathways in GC cells (Fig. 5D).
MYBL2 activates the PI3K/AKT signaling pathway in GC cells. (A) The p-PI3K, p-AKT and AKT protein expression were examined by Western blot after transfecting siRNA targeting MYBL2 for 48 h in HGC27 cells. (B) Western blot analysis of the expression of p-PI3K, p-AKT and AKT proteins after transfecting AGS cells for 48 h with MYBL2 overexpression vector. (C) Effect of the AKT inhibitor MK2206 on MYBL2-induced cell. (D) MYBL2 contributes to GC cell growth and inhibits cell apoptosis by regulating PI3K/AKT and BCL2/BAX/Cleaved-caspase-3 signaling pathways in GC cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NC.
Discussion
GC remains a major public health issue affecting the life and health of the Chinese population, and incidence rates of early-onset GC have been rising in recent years31. Although unprecedented medical progress has been achieved, the prognosis of patients with GC remains dismal32. Thus, further research is warranted to explore the potential causes of GC development and progression and develop the corresponding targeted interventions.
There is a rich literature available substantiating that transcription factors and their regulatory networks play an important role in the tumorigenesis and development of various tumors, including GC33,34,35,36. Previous research has shown that GC cells are regulated by transcription factors in terms of proliferation, migration and invasion36. MYBL2 is a member of the MYB transcription factor family, and upregulated MYBL2 expression has been correlated with a poor prognosis in prostate and colorectal cancer24,37. In the present study, we consistently observed that the expression of MYBL2 was significantly higher in GC tissues than in normal paracancerous tissues and correlated with poor patient outcomes (Fig. 1). Subsequent cell experiments demonstrated that MYBL2 silencing significantly inhibited the growth of HGC27 cells, and the overexpression of MYBL2 could enhance the proliferation of AGS cells, which indicated that the MYBL2 gene could enhance cell proliferation (Fig. 2A). This finding is consistent with studies on hepatocellular carcinoma, bladder cancer and esophageal squamous cell carcinoma16,18,38.
The regulation of cell proliferation is a complex regulatory network, and the abnormal cell cycle can change the behavior of cell differentiation and proliferation. Emerging evidence suggests that MYBL2 can contribute to cell cycle dysfunction, promote cell carcinogenesis and malignant proliferation, and then damage the organisms, which plays an important role in cell proliferation, metastasis and recurrence of tumor cells39,40. Further study found that MYBL2 silencing can inhibit cell proliferation and induce G2/M arrest in U251 cells19. In this study, MYBL2 silencing inhibited the proliferation of GC cells by inducing G2/M cell cycle arrest. On the contrary, overexpression of MYBL2 could increase the number of cells in S phase, promote cell division and promote cell proliferation. This is consistent with the results of previous studies that the percentage of S phase cells increased significantly after overexpression of MYBL2 in colorectal cancer41. Therefore, we speculate that MYBL2 may promote cell proliferation by regulating the process of cell cycle, but the specific mechanism is still unclear.
Apart from cell cycle arrest, the imbalance of apoptosis can also lead to changes in cell proliferation behavior. Apoptosis is an evolutionarily conservative form of programmed cell death, which is a critical limiting factor that limits the expansion of cell populations, and plays an indispensable regulatory role in the maintenance and survival of cell homeostasis42. Dysregulation of apoptotic cell death is a hallmark of cancer43. Apoptosis can be triggered intrinsically through the mitochondrial pathway44. It is well-established that the BCL2 families of proteins are key regulators of the mitochondrial apoptotic pathway. BCL2 and BCL-XL are major anti-apoptotic proteins, while BAD, BID, BIK, BAX and BAK are major pro-apoptotic proteins45,46,47. Studies have suggested that the MYBL2 gene promotes T-cell survival by enhancing the expression of the proto-oncogene BCL248. In this study, we first assessed the expression of BCL2 and BAX in HGC27 and AGS cells treated with MYBL2 silencing and overexpression. The results showed that MYBL2 silencing upregulated the expression of BAX but decreased BCL2 levels, while MYBL2 over-expression could inhibit the apoptosis of AGS cells by suppressing the expression of BAX and upregulating BCL2 (Fig. 3). Apoptosis is mainly executed by a family of Caspases49, and Caspase-3 is one of the most important members of the caspase protein family and a major executioner caspase in apoptosis50. In this study, MYBL2 silencing showed increased Cleaved-caspase-3 p17 and Cleaved-caspase-3 p19 protein expression by western analysis, compared to MYBL2 overexpressing cells. Taken together, our results substantiate that MYBL2 can inhibit apoptosis of human GC cells through the mitochondrial pathway.
Various oncogenes and growth factor receptors can activate phosphoinositide 3-kinase (PI3K) activity, and elevated PI3K signal transduction is considered a hallmark of carcinomas51. In recent years, Ser/Thr kinases AKT has become the focus of research in different fields of biology and medicine52. The activation of PI3K requires the phosphorylation of two key regulatory residues, Thr308 and Ser473, on AKT53, whereby maximum activation of AKT is observed upon phosphorylation of Ser47354. Besides, AKT phosphorylates related target proteins directly or indirectly, promoting cell survival, growth and metabolism55. Herein, we revealed that the expression of p-PI3K and p-AKT/AKT ratio significantly decreased after MYBL2 silencing in HGC27 cells, which indicates that MYBL2 silencing could inhibit the proliferation of GC cells. However, the overexpression of MYBL2 induced the proliferation by increasing the expression of p-PI3K and p-AKT/AKT ratio in AGS cells (Fig. 5). This is consistent with previous studies on colorectal cancer cells41 and lung cancer cells56. Additionally, an AKT inhibitor (MK2206) significantly reversed the cell proliferation capacity induced by MYBL2 overexpression in GC cells. Overall, these data suggest that MYBL2 can promote the proliferation and inhibit apoptosis by activating the PI3K/AKT signaling pathway in GC cells.
Conclusion
In summary, our results suggest that MYBL2 expression was significantly upregulated in GC tissues and its high expression level is associated with poor prognosis of GC. Mechanistically, upregulated expression of MYBL2 contributes to GC cell growth and inhibits cell apoptosis by regulating the PI3K/AKT and BCL2/BAX/Cleaved-caspase-3 signaling pathways in GC cells, and MYBL2 might serve as a potential novel target for treatment of GC patients.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors. Gene Expression Data for GC Patients Downloaded from TCGA Database (https://portal.gdc.cancer.gov/).
References
Frick, C. et al. Quantitative estimates of preventable and treatable deaths from 36 cancers worldwide: A population-based study. Lancet Glob. Health 11, e1700–e1712. https://doi.org/10.1016/s2214-109x(23)00406-0 (2023).
Wang, Y. et al. Overview and countermeasures of cancer burden in China. Sci. China Life Sci. 66, 2515–2526. https://doi.org/10.1007/s11427-022-2240-6 (2023).
He, S. et al. Cancer profiles in China and comparisons with the USA: A comprehensive analysis in the incidence, mortality, survival, staging, and attribution to risk factors. Sci. China Life Sci. 67, 122–131. https://doi.org/10.1007/s11427-023-2423-1 (2024).
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Ly, Q. P. & Sasson, A. R. Modern surgical considerations for gastric cancer. J. Natl. Compr. Cancer Netw. 6, 885–894. https://doi.org/10.6004/jnccn.2008.0067 (2008).
Ham, I. H. et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 18, 68. https://doi.org/10.1186/s12943-019-0972-8 (2019).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674. https://doi.org/10.1016/j.cell.2011.02.013 (2011).
Lee, T. I. & Young, R. A. Transcriptional regulation and its misregulation in disease. Cell 152, 1237–1251. https://doi.org/10.1016/j.cell.2013.02.014 (2013).
Xia, Y. et al. Chrysin inhibits tumor promoter-induced MMP-9 expression by blocking AP-1 via suppression of ERK and JNK pathways in gastric cancer cells. PLoS One 10, e0124007. https://doi.org/10.1371/journal.pone.0124007 (2015).
Liu, H. T. et al. LncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell. Death Differ. 29, 627–641. https://doi.org/10.1038/s41418-021-00879-9 (2022).
Wu, L. et al. PLAGL2 promotes the proliferation and migration of gastric cancer cells via USP37-mediated deubiquitination of Snail1. Theranostics 11, 700–714. https://doi.org/10.7150/thno.47800 (2021).
Li, Z. Y. et al. c-Myc-activated intronic miR-210 and LncRNA MIR210HG synergistically promote the metastasis of gastric cancer. Cancer Lett. 526, 322–334. https://doi.org/10.1016/j.canlet.2021.11.006 (2022).
Liu, M. et al. PRMT5-dependent transcriptional repression of c-Myc target genes promotes gastric cancer progression. Theranostics 10, 4437–4452. https://doi.org/10.7150/thno.42047 (2020).
Liu, Q. et al. A targetable MYBL2-ATAD2 axis governs cell proliferation in ovarian cancer. Cancer Gene Ther. 30, 192–208. https://doi.org/10.1038/s41417-022-00538-2 (2023).
Musa, J., Aynaud, M. M., Mirabeau, O., Delattre, O. & Grünewald, T. G. MYBL2 (B-Myb): A central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell. Death Dis. 8, e2895. https://doi.org/10.1038/cddis.2017.244 (2017).
Liu, W. et al. MYBL2 promotes proliferation and metastasis of bladder cancer through transactivation of CDCA3. Oncogene 41, 4606–4617. https://doi.org/10.1038/s41388-022-02456-x (2022).
Liang, H. B. et al. MYBL2 is a potential prognostic marker that promotes cell proliferation in gallbladder cancer. Cell. Physiol. Biochem. 41, 2117–2131. https://doi.org/10.1159/000475454 (2017).
Frau, M. et al. Mybl2 expression is under genetic control and contributes to determine a hepatocellular carcinoma susceptible phenotype. J. Hepatol. 55, 111–119. https://doi.org/10.1016/j.jhep.2010.10.031 (2011).
Zhang, X., Lv, Q. L., Huang, Y. T., Zhang, L. H. & Zhou, H. H. Akt/FoxM1 signaling pathway-mediated upregulation of MYBL2 promotes progression of human glioma. J. Exp. Clin. Cancer Res. 36, 105. https://doi.org/10.1186/s13046-017-0573-6 (2017).
Dolz, S. et al. Study of the S427G polymorphism and of MYBL2 variants in patients with acute myeloid leukemia. Leuk. Lymphoma. 57, 429–435. https://doi.org/10.3109/10428194.2015.1049167 (2016).
Chen, J. & Chen, X. MYBL2 is targeted by miR-143-3p and regulates breast cancer cell proliferation and apoptosis. Oncol. Res. 26, 913–922. https://doi.org/10.3727/096504017X15135941182107 (2018).
Xiong, Y. C., Wang, J., Cheng, Y., Zhang, X. Y. & Ye, X. Q. Overexpression of MYBL2 promotes proliferation and migration of non-small-cell lung cancer via upregulating NCAPH. Mol. Cell. Biochem. 468, 185–193, https://doi.org/10.1007/s11010-020-03721-x (2020).
Klein, D. K. et al. Cyclin F suppresses B-Myb activity to promote cell cycle checkpoint control. Nat. Commun. 6, 5800. https://doi.org/10.1038/ncomms6800 (2015).
Bi, X. et al. METTL3 promotes the initiation and metastasis of ovarian cancer by inhibiting CCNG2 expression via promoting the maturation of pri-microRNA-1246. Cell. Death Discov. 7, 237. https://doi.org/10.1038/s41420-021-00600-2 (2021).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963( (2023).
Weston, W. A. & Barr, A. R. A cell cycle centric view of tumour dormancy. Br. J. Cancer. 129, 1535–1545. https://doi.org/10.1038/s41416-023-02401-z (2023).
Zeng, W., Long, X., Liu, P. S. & Xie, X. The interplay of oncogenic signaling, oxidative stress and ferroptosis in cancer. Int. J. Cancer 153, 918–931. https://doi.org/10.1002/ijc.34486 (2023).
Li, J. et al. Mir-20a-5p induced WTX deficiency promotes gastric cancer progressions through regulating PI3K/AKT signaling pathway. J. Exp. Clin. Cancer Res. 39, 212. https://doi.org/10.1186/s13046-020-01718-4 (2020).
Pompura, S. L. & Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. https://doi.org/10.1002/JLB.2MIR0817-349R (2018).
Wang, F. H. et al. The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 41, 747–795. https://doi.org/10.1002/cac2.12193 (2021).
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. T. & Lordick, F. Gastric cancer. Lancet 396, 635–648. https://doi.org/10.1016/s0140-6736(20)31288-5 (2020).
Guo, Z. et al. The transcription factor RUNX2 fuels YAP1 signaling and gastric cancer tumorigenesis. Cancer Sci. 112, 3533–3544. https://doi.org/10.1111/cas.15045 (2021).
Grunberg, N. et al. Cancer-Associated fibroblasts promote aggressive gastric Cancer phenotypes via heat shock factor 1-Mediated secretion of extracellular vesicles. Cancer Res. 81, 1639–1653. https://doi.org/10.1158/0008-5472.CAN-20-2756 (2021).
Liu, H., Ni, S., Wang, H., Zhang, Q. & Weng, W. Charactering tumor microenvironment reveals stromal-related transcription factors promote tumor carcinogenesis in gastric cancer. Cancer Med. 9, 5247–5257. https://doi.org/10.1002/cam4.3133 (2020).
Abadi, A. J. et al. The role of SOX family transcription factors in gastric cancer. Int. J. Biol. Macromol. 180, 608–624. https://doi.org/10.1016/j.ijbiomac.2021.02.202 (2021).
Ren, F. et al. MYBL2 is an independent prognostic marker that has tumor-promoting functions in colorectal cancer. Am. J. Cancer Res. 5, 1542–1552 (2015).
Qin, H. D. et al. Genomic characterization of esophageal squamous cell carcinoma reveals critical genes underlying tumorigenesis and poor prognosis. Am. J. Hum. Genet. 98, 709–727. https://doi.org/10.1016/j.ajhg.2016.02.021 (2016).
Joaquin, M. & Watson, R. J. Cell cycle regulation by the B-Myb transcription factor. Cell. Mol. Life Sci. 60, 2389–2401. https://doi.org/10.1007/s00018-003-3037-4 (2003).
Sun, Y., Liu, Y., Ma, X. & Hu, H. The influence of cell cycle regulation on chemotherapy. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22136923 (2021).
Fan, X. et al. B-Myb accelerates colorectal cancer progression through reciprocal feed-forward transactivation of E2F2. Oncogene 40, 5613–5625. https://doi.org/10.1038/s41388-021-01961-9 (2021).
Cheng, X. & Ferrell Jr, J. E. Apoptosis propagates through the cytoplasm as trigger waves. Science 361, 607–612. https://doi.org/10.1126/science.aah4065 (2018).
Pistritto, G., Trisciuoglio, D., Ceci, C., Garufi, A. & D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 8, 603–619. https://doi.org/10.18632/aging.100934 (2016).
Wong, R. S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res.https://doi.org/10.1186/1756-9966-30-87 (2011).
Adams, J. M. & Cory, S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 26, 61–66. https://doi.org/10.1016/s0968-0004(00)01740-0 (2001).
Kroemer, G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat. Med. 3, 614–620. https://doi.org/10.1038/nm0697-614 (1997).
Wei, M. C. et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730. https://doi.org/10.1126/science.1059108 (2001).
Grassilli, E., Salomoni, P., Perrotti, D., Franceschi, C. & Calabretta, B. Resistance to apoptosis in CTLL-2 cells overexpressing B-Myb is associated with B-Myb-dependent bcl-2 induction. Cancer Res. 59, 2451–2456 (1999).
Ghorbani, N., Yaghubi, R., Davoodi, J. & Pahlavan, S. How does caspases regulation play role in cell decisions? Apoptosis and beyond. Mol. Cell. Biochem. https://doi.org/10.1007/s11010-023-04870-5 (2023).
Thornberry, N. A. & Lazebnik, Y. Caspases: Enemies within. Science 281, 1312–1316. https://doi.org/10.1126/science.281.5381.1312 (1998).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635. https://doi.org/10.1016/j.cell.2017.07.029 (2017).
Manning, B. D. & Toker, A. AKT/PKB signalig: Navigating the network. Cell 169, 381–405. https://doi.org/10.1016/j.cell.2017.04.001 (2017).
Alessi, D. R. et al. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 15, 6541–6551 (1996).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101. https://doi.org/10.1126/science.1106148 (2005).
Dibble, C. C. & Manning, B. D. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell. Biol. 15, 555–564. https://doi.org/10.1038/ncb2763 (2013).
Jin, Y. et al. B-Myb is up-regulated and promotes cell growth and motility in non-small cell lung cancer. Int. J. Mol. Sci. 18, 860. https://doi.org/10.3390/ijms18060860 (2017).
Acknowledgements
We thank all the contributors for maintaining and sharing data on Kaplan-Meier Plotter (http://kmplot.com/analysis/index.php?p=background), The Cancer Genome Atlas (https://www.cancer.gov/ccg/research/genome-sequencing/tcga), The UALCAN database https://ualcan.path.uab.edu/).
Funding
The study was supported by the Natural Science Foundation of Bengbu Medical College (2022byfy002), and the Natural Science in Higher Education of Anhui Province (KJ2021A0787), Graduate Research Innovation Program of Bengbu Medical College (Byycx24005, Byycx23014, Byycx22007), the University Synergy Innovation Program of Anhui Province(GXXT-2022-065), the Natural Science Program of Bengbu Medical University (2023byzd030).
Author information
Authors and Affiliations
Contributions
Conception and design: Y.L.Z. and H.Z.W., J.Y.C., Z.L.J. and D.W. conducted and analyzed the experiments., Writing—original draft: J.Y.C., D.W., S.Y.W., Z.L.J., G.S.P. and W.J.Z., Review & editing: J.Y.C., Y.L.Z. and H.Z.W., Funding acquisition: H.Z.W. and M.J.H. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, J., Ji, Z., Wu, D. et al. MYBL2 promotes cell proliferation and inhibits cell apoptosis via PI3K/AKT and BCL2/BAX/Cleaved-caspase-3 signaling pathway in gastric cancer cells. Sci Rep 15, 9148 (2025). https://doi.org/10.1038/s41598-025-93022-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93022-4