Abstract
To investigate the association between blood pressure variability (BPV) and mortality (in-hospital and 30-day) among heart failure (HF) patients, and to examine these associations across patient subgroups. This multicenter retrospective cohort study analyzed 25,591 heart failure patients from two intensive care databases (eICU Collaborative Research Database [eICU-CRD] and the Medical Information Mart for Intensive Care IV [MIMIC-IV]). BPV was assessed using coefficient of variation of systolic (SBPV), diastolic (DBPV), and mean (MBPV) blood pressure measurements. Multivariable logistic regression and Cox proportional hazards models evaluated mortality associations, adjusting for clinical parameters. The observed mortality rates were 14.7% (in-hospital) and 17.3% (30-day). Higher BPV demonstrated significant associations with increased mortality risk, with SBPV showing the strongest relationship. For in-hospital mortality, each standard deviation increase in SBPV, DBPV, and MBPV corresponded to adjusted odds ratios of 1.56 (95% CI 1.51–1.62), 1.21 (95% CI 1.16–1.25), and 1.42 (95% CI 1.37–1.48), respectively. For 30-day mortality, adjusted hazard ratios were 1.37 (95% CI 1.33–1.41) for SBPV, 1.15 (95% CI 1.12–1.19) for DBPV, and 1.30 (95% CI 1.27–1.34) for MBPV. These associations remained robust across all patient subgroups. Increased blood pressure variability during hospitalization independently predicts higher in-hospital (14.7%) and 30-day mortality (17.3%) in HF patients, with SBPV showing the strongest association (OR: 1.56, 95% CI 1.51–1.62). BPV may serve as a valuable prognostic marker for risk stratification in hospitalized heart failure patients.
Similar content being viewed by others
Introduction
Heart failure (HF) represents a significant global health burden, affecting approximately 64.3 million people worldwide and accounting for substantial mortality and healthcare expenditure. Despite advances in treatment strategies, hospital mortality rates for HF patients remain high, ranging from 4 to 11%, with 30-day mortality rates reaching up to 15%1. Recent studies have highlighted the importance of hemodynamic parameters beyond traditional risk factors in predicting outcomes for HF patients. Blood pressure variability (BPV), defined as fluctuations in blood pressure measurements over time, has emerged as a potential prognostic marker in various cardiovascular conditions2,3,4.
The significance of BPV in cardiovascular outcomes has been increasingly recognized in recent years. Visit-to-visit BPV has been associated with increased risks of all-cause mortality, coronary heart disease, left ventricular diastolic dysfunction, and stroke in various populations5,6,7,8,9,10. However, most existing studies have focused on long-term BPV in outpatient settings or specific patient populations such as those with hypertension or coronary artery disease. Short-term BPV, particularly during hospital stays, remains understudied in HF patients. Furthermore, the relationship between BPV and early mortality outcomes (in-hospital and 30-day) in this high-risk population has not been well characterized11,12.
The pathophysiology of HF, incltivation, autonomic dysfunction, and altered baroreceptor sensitivity, may render these patients particularly vulnerable to the adverse effects of BPV. While previous studies have shown conflicting resultsthe prognostic value of BPV in different clinical settings, there is a notable gap in understanding how BPV influences early mortality outcomes in HF patients, particularly in the hospital setting. Furthermore, there is limited evidence on how BPV interacts with specific subgroups, such as elderly patients or women, in predicting mortality.
Given these gaps, the primary aims of this comprehensive multirt study were to: (1) evaluate the associations between in-hospital BPV and both in-hospital and 30-day mortality among patients with HF; (2) assess the heterogeneity of these associations across diverse patient subgroups; and (3) develop and externally validate a novel risk stratification model incorporating BPV parameters. These objectives are designed to fill the gap in our understanding of the role of BPV in predicting mortality in HF patients, particularly in the early phase of their hospitalization.
Methods
Study population
We conducted a retrospective cohort study using data from two large critical care databases: the Medical Information Mart for Intensive Care IV (MIMIC-IV) and eICU Collaborative Research Database (eICU-CRD). We selected these databases because of their comprehensive and diverse patient populations, which augment the generalizability of our findings to heart failure patients in intensive care settings across various regions. The MIMIC-IV database contains comprehensive clinical data from patients admitted to intensive care units at Beth Israel Deaconess Medical Center between 2008 and 201913. The eICU-CRD contains data from over 200 hospitals throughout the United States from 2014 to 201514. Access to these de-identified databases was granted to researchers who successfully completed the Collaborative Institutional Training Initiative (CITI) Program (certification numbers: 60071489 [Zhang] and 52219361 [Tang]). Given the de-identified nature of the data, informed consent and ethical approval requirements were waived. Data extraction was performed using Structured Query Language (SQL) with PostgreSQL (version 13.0) and Navicat software (version 16.0). For patients with multiple measurements of clinical parameters during hospitalization, only the initial values were included in the analysis. To ensure data accuracy and reliability, all variable extractions underwent independent verification by two researchers. This retrospective study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The studies involving human participants were reviewed and approved by MIMIC-IV and eICU-CRD databases were approved by the institutional review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Informed consent was obtained from all subjects and/or their legal guardian(s). Due to the retrospective nature of the study, institutional review board waived the need of obtaining informed consent’ in the manuscript.
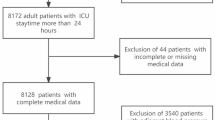
Data were extracted using a SQL server for patients fitting the study’s inclusion criteria: adults diagnosed with HF according to ICD-9 or ICD-10 codes (Supplementary Table 1). The initial dataset comprised a total of 31,855 heart failure patients, with 15,208 patients from the eICU-CRD database and 16,647 patients from the MIMIC-IV ICU database. The exclusion criteria applied were patients aged under 18 years, those with ICU stays of less than 24 h, and individuals with fewer than three blood pressure measurements. From the eICU-CRD database, 2 patients were excluded due to age, 3,006 due to ICU stay duration, and 356 due to insufficient BP measurements, resulting in 11,844 eligible patients. Similarly, from the MIMIC-IV ICU database, 2,803 patients were excluded due to ICU stay duration and 97 due to insufficient BP measurements, leaving 13,747 eligible patients. In total, 25,591 patients were included in the final analysis after combining the eligible patients from both databases. This selection process ensured that the analysis focused on a specific group of individuals relevant to the research objectives (Fig. 1). Missing data were handled using multiple imputations, and one randomly selected imputed dataset was used for analysis, with detailed missing data patterns provided in Supplementary Table 2.
Assessment of blood pressure variability
Blood pressure measurements were extracted from each database using structured query language. For each patient, we calculated 24-h BPV from the first ICU admission using the standard deviation (SD) of all recorded values. The coefficient of variation (CV) was calculated as SD divided by mean blood pressure5. These two metrics were chosen due to their clinical relevance and ability to measure both absolute and relative variability across patients with differing baseline pressures. We assessed systolic blood pressure variability (SBPV), diastolic blood pressure variability (DBPV), and blood pressure variability (MBPV) using invasive arterial line measurements recorded during the first 24 h of ICU admission. These accurate and real-time measurements allowed us to evaluate BPV’s impact on patient outcomes.
Additionally, a detailed breakdown of the frequency of blood pressure measurements is presented in Supplementary Table 3, which includes the first values, mean values, SD, variability, and frequency across the eICU-CRD and MIMIC-IV databases.
Covariates
We collected comprehensive baseline characteristics including: (1) demographic data: age, sex, and ethnicity (White or Other); (2) vital signs: body mass index (BMI), heart rate, SBP, DBP, and MAP; (3) comorbidities: hypertension, diabetes, myocardial infarction, atrial fibrillation, stroke, chronic obstructive pulmonary disease (COPD), renal failure, and cancer; (4) laboratory tests within the first 24 h of ICU admission: hemoglobin, white blood cell count (WBC), platelet count, creatinine, blood urea nitrogen (BUN), potassium, sodium, and chloride; (5) treatments: angiotensin converting enzyme inhibitor/angiotensin receptor blockers (ACEI/ARB), beta-blockers, calcium channel blockers (CCB), diuretics, vasoactive agents, hemodialysis, and mechanical ventilation.
Outcomes
The primary outcomes were in-hospital mortality and 30-day all-cause mortality. In-hospital mortality was defined as death occurring during the index hospitalization. Thirty-day mortality was defined as death occurring within 30 days after ICU admission. Both outcomes were assessed separately in the eICU-CRD and MIMIC-IV cohorts. The mortality data were extracted directly from the databases, which maintain comprehensive patient outcome records13,14. For patients with multiple ICU admissions, only the outcomes from the first admission were considered to avoid potential confounding from repeated measurements.
Statistical analysis
All continuous and categorical variables at baseline were presented as means (SD) and percentages. A Chi-square test or independent sample t-test was used to examine the baseline differences between included and excluded participants. Descriptive statistics summarized the baseline characteristics of participants. BPV measures (SBPV, DBPV, and MBPV) were standardized using z-score transformation to facilitate comparison and interpretation.
We constructed four sequential models to analyze the association between blood pressure variability and mortality. Model 1 was unadjusted. Model 2 adjusted for demographic characteristics (age, gender, ethnicity). Model 3 adjusted for Model 2 covariates plus vital signs (BMI, heart rate) and comorbidities (hypertension, diabetes, myocardial infarction, atrial fibrillation, stroke, COPD, renal failure, cancer). Model 4 was fully adjusted by adding laboratory parameters (hemoglobin, WBC, platelet, creatinine, BUN, potassium, sodium, chloride) and treatments (ACEI/ARB, beta-blockers, CCB, diuretics, vasoactive agents, hemodialysis, mechanical ventilation) to Model 3.
Logistic regression was used to examine the association between BPV and in-hospital mortality, while Cox proportional hazards regression was employed to analyze the relationship with 30-day mortality. These regression methods were selected as they account for potential confounders and allow for precise estimation of associations between BPV and mortality. In the Cox regression analysis, patients who were lost to follow-up were censored at their last known status. To visualize survival differences, we constructed Kaplan–Meier survival curves stratified by quartiles of BPV measures, with the log-rank test used to assess statistical differences between these curves.
Stratified analyses were performed to evaluate potential effect modifications by key characteristics, including database source (eICU-CRD vs MIMIC-IV), age (≤ 65 vs > 65 years), gender (female vs male), race (White vs Other), BMI (< 25 vs ≥ 25 kg/m2), comorbidities (hypertension, myocardial infarction, stroke, renal failure), and treatments (ACEI/ARB, CCB, vasoactive agents, hemodialysis). Within each stratum, we calculated odds ratios (ORs) with 95% confidence intervals (CIs) using the fully adjusted Model 4. Interaction tests were performed to assess the presence of effect modification, with a P-value < 0.05 indicating significant interaction. Results were presented using forest plots to visualize consistency of associations across strata.
To assess the robustness of our findings, we conducted sensitivity analyses by excluding patients with missing data. The primary analysis utilized all available data, while sensitivity analysis was restricted to patients with complete data for all variables. We repeated the primary analyses, including fully adjusted models (Model 4) and stratified analyses, in this complete-case cohort.
All analyses were performed using R Statistical Software (Version 4.2.2, http://www.R-project.org, The R Foundation) and Free Statistics analysis platform (Version 2.0, Beijing, China, http://www.clinicalscientists.cn/freestatistics). Statistical significance was set at p < 0.05 for all tests.
Results
Baseline demographic and clinical characteristics
The study included 25,591 heart failure patients, with significant differences between the eICU-CRD (11,844) and MIMIC-IV (13,747) cohorts (Table 1). The MIMIC-IV cohort was older (median age 74 vs. 72 years, P < 0.001) and had a higher percentage of males (56.2% vs. 52.9%, P < 0.001). Racial composition varied, with more White patients in the eICU-CRD cohort (74.3% vs. 68.4%, P < 0.001). The MIMIC-IV cohort exhibited higher rates of hypertension (85.7% vs. 63.9%, P < 0.001), diabetes (41.2% vs. 20.2%, P < 0.001), and other comorbidities. Vital signs and lab results also differed, with the MIMIC-IV group showing lower blood pressure variability and worse renal function markers. Treatment differences were marked, with more frequent use of ACEI/ARB, beta-blockers, and mechanical ventilation in the MIMIC-IV cohort. Mortality rates were higher in the MIMIC-IV group for both in-hospital (16.4% vs. 12.8%, P < 0.001) and 30-day mortality (21.6% vs. 12.3%, P < 0.001).
Association between BP variability and in-hospital, 30-day mortality
In the fully adjusted logistic and Cox regression models (Model 4) (Table 2), SBPV, DPBV, and MBPV were all significantly associated with increased in-hospital and 30-day mortality. In Model 4, the odds ratio (OR) for in-hospital mortality was 1.56 (95% CI 1.51–1.62) for SBPV, 1.21 (95% CI 1.16–1.25) for DBPV, and 1.42 (95% CI 1.37–1.48) for MBPV. The hazard ratio (HR) for 30-day mortality in Model 4 was 1.37 (95% CI 1.33–1.41) for SBPV, 1.15 (95% CI 1.12–1.19) for DBPV, and 1.3 (95% CI 1.27–1.34) for MBPV. In all BPV quartiles, higher BPV was associated with increased mortality risk compared to the reference quartile.
For SBPV, DBPV, and MBPV (Fig. 2), survival analysis reveals that patients in higher quartiles (Q3 and Q4) experience a more rapid decline in survival probability compared to those in lower quartiles (Q1 and Q2), with significant differences observed (P < 0.001). The survival probability consistently decreases more quickly in the higher BPV quartiles across all BPV measures, with Q4 showing the lowest survival probability by the end of the 30-day follow-up period.
Stratified analysis
Across all subgroups, no significant differences were observed between groups, indicating consistent associations between BPV and mortality risk. The HR and OR for each subgroup remained statistically significant (Supplementary Figs. 1–6).
Sensitivity analysis
The sensitivity analysis (Table 3) illustrates the associations between BPV and in-hospital and 30-day mortality using logistic and Cox regression models after excluding missing data. The results consistently demonstrate that higher SBPV, DBPV, and MBPV are significantly associated with increased risks of both in-hospital and 30-day mortality across all models. The fully adjusted Model 4 shows that these associations remain robust, with higher BPV quartiles indicating progressively greater mortality risks compared to the lowest quartile.
Discussion
In this large multicenter cohort study of 25,591 heart failure patients from two critical care databases, we found that increased BPV was independently associated with higher risks of both in-hospital and 30-day mortality. After full adjustment for potential confounders, each standard deviation increase in SBPV, DPBV, and MBPV was associated with 56% (OR 1.56, 95% CI 1.51–1.62), 21% (OR 1.21, 95% CI 1.16–1.25), and 42% (OR 1.42, 95% CI 1.37–1.48) higher odds of in-hospital mortality, respectively. Similar associations were observed for 30-day mortality, with hazard ratios of 1.37 (95% CI 1.33–1.41) for SBPV, 1.15 (95% CI 1.12–1.19) for DBPV, and 1.30 (95% CI 1.27–1.34) for MBPV. These associations remained consistent across various subgroups including age, gender, ethnicity, and comorbidities. Sensitivity analyses excluding patients with missing data confirmed the robustness of these findings.
Our findings extend existing literature on BPV in cardiovascular diseases, focusing on HF patients in the ICU. Muntner et al. (2015) found visit-to-visit SBPV was associated with all-cause mortality in the general population, with HR of 1.1 to 1.4, lower than the stronger associations observed in our study. These differences may be due to the unique pathophysiology of HF, where autonomic dysfunction and altered baroreceptor sensitivity amplify BPV’s adverse effects. Additionally, our study aligns with Stevens et al. (2021), who demonstrated BPV’s prognostic value in hypertensive patients. However, our findings differ by focusing on critically ill HF patients, where the acute nature of BPV—exacerbated by medical interventions and clinical instability—may increase its prognostic significance. This underscores BPV’s potential as a predictive marker in more vulnerable populations.
The biological mechanisms underlying the association between BPV and mortality in heart failure patients are likely multifaceted. Previous research has revealed that increased BPV may reflect impaired autonomic function and baroreceptor sensitivity. Stevens et al. demonstrated that blood pressure fluctuations trigger inflammatory responses and oxidative stress, potentially contributing to organ dysfunction in critical illness. Additionally, increased BPV is associated with microcirculatory dysfunction and tissue hypoperfusion. These mechanisms may be particularly relevant in heart failure patients, where compromised cardiovascular reserve amplifies the adverse effects of BP fluctuations.
The pathophysiological impact of BPV in HF appears to be distinct from other cardiovascular conditions. HF patients exhibit unique patterns of autonomic dysfunction that may make them particularly susceptible to the adverse effects of BP fluctuations15. Parati et al. demonstrated through hemodynamic monitoring that BPV in HF patients is closely linked to variations in cardiac output and systemic vascular resistance16. Furthermore, increased BPV is associated with higher levels of neurohormonal activation and inflammatory markers in HF patients compared to other cardiovascular conditions17,18.
The clinical implications of our findings are substantial. Meta-analyses suggest that incorporating BPV assessment into risk stratification models could improve prognostic accuracy in cardiovascular patients19. Recent studies have shown that continuous BP monitoring and variability assessment might help identify patients at risk of acute decompensation earlier than traditional monitoring approaches20. This is particularly relevant in the critical care setting, where early identification of high-risk patients could enable more timely interventions. Additionally, emerging evidence suggests that BPV-guided therapy might represent a novel therapeutic target in HF management21,22.
The differential associations we observed across BP parameters have important implications for monitoring strategies. The stronger association of systolic BPV with outcomes aligns with previous studies demonstrating SBPV to be a more sensitive marker of cardiovascular dysfunction23,24. The ADVANCE trial showed that SBPV was independently associated with cardiovascular events and mortality in high-risk patients25. Similarly, the ALLHAT study found that visit-to-visit variability in SBPV was a stronger predictor of outcomes than diastolic variability26.
Our findings regarding the timing of BPV assessment are particularly noteworthy. The strong associations observed during the first 24 h of ICU admission suggest that early BPV assessment may be crucial for risk stratification. This aligns with previous studies showing that early hemodynamic patterns predict outcomes in critical illness27,28. The persistence of these associations after adjustment for MBP and other risk factors supports the independent prognostic value of BPV.
The impact of comorbidities on the relationship between BPV and outcomes deserves special consideration. Our stratified analyses revealed consistent associations across different patient subgroups, supporting the robustness of BPV as a prognostic marker. This finding extends previous work showing that BPV predicts outcomes in various cardiovascular conditions2,29. The stronger associations observed in certain subgroups may reflect underlying pathophysiological differences or varying degrees of cardiovascular reserve.
Advantages and limitations
The present investigation offers several methodological innovations that advance the understanding of BPV in critical care settings. First, the concurrent analysis of two large-scale critical care databases (MIMIC-IV and eICU-CRD) yielded both a demographically diverse patient population and enhanced external validity for our findings. Second, the study extended beyond conventional single-parameter approaches through comprehensive assessment of variability across multiple BP components (systolic, diastolic, and mean arterial pressure), facilitating a more nuanced understanding of hemodynamic instability. Third, the implementation of standardized BPV measures through z-score transformation addressed a methodological gap in the current literature regarding the comparability of BP parameters21. Additionally, the dual-model analytical approach, incorporating both logistic regression and Cox proportional hazards models, enabled comprehensive temporal assessment of BPV’s relationship with patient outcomes.
Several limitations should be acknowledged. First, despite comprehensive adjustment for confounders, the retrospective nature of our study prevents causal inference30. Second, electronic health record data may contain systematic biases affecting BP measurements31. Third, we could not assess the impact of newer heart failure therapies on BPV due to the study period. Fourth, our study population was limited to ICU patients, potentially affecting generalizability to less severe cases. Fifth, we lacked detailed data on medication timing and adherence, which could influence BPV32.
Conclusions
In this large multicenter cohort study, we demonstrated that elevated BPV exhibited a robust independent association with increased risks of both in-hospital and 30-day mortality among patients with heart failure. Furthermore, SBPV demonstrated superior prognostic value compared with other BP parameters. The integration of BPV assessment into routine monitoring protocols could potentially enhance risk stratification and facilitate the optimization of management strategies for HF patients in the intensive care setting. Further investigation is warranted to establish standardized BPV measurement protocols and evaluate targeted interventions for BPV reduction in HF patients, with the ultimate goal of improving clinical outcomes in this vulnerable population.
Data availability
The data were available on the MIMIC-IV website at https://mimic.physionet.org/, https://doi.org/10.13026/C2HM2Q and eICU-CRD at. The data in thisarticle can be reasonably applied to the corresponding author.
Abbreviations
- ACEI/ARB:
-
Angiotensin converting enzyme inhibitor/angiotensin receptor blockers
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BPV:
-
Blood pressure variability
- BUN:
-
Blood urea nitrogen
- CCB:
-
Calcium channel blockers
- CI:
-
Confidence interval
- CITI:
-
Collaborative institutional training initiative
- COPD:
-
Chronic obstructive pulmonary disease
- CV:
-
Coefficient of variation
- DBP:
-
Diastolic blood pressure
- DBPV:
-
Diastolic blood pressure variability
- eICU-CRD:
-
EICU collaborative research database
- GUI:
-
Graphical user interface
- HF:
-
Heart failure
- HR:
-
Hazard ratio
- ICD:
-
International classification of diseases
- ICU:
-
Intensive care unit
- MAP:
-
Mean arterial pressure
- MBPV:
-
Mean blood pressure variability
- MIMIC-IV:
-
Medical information mart for intensive care IV
- OR:
-
Odds ratio
- SBP:
-
Systolic blood pressure
- SBPV:
-
Systolic blood pressure variability
- SD:
-
Standard deviation
- SQL:
-
Structured query language
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- WBC:
-
White blood cell count
References
Ziaeian, B. & Fonarow, G. C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 13(6), 368–378. https://doi.org/10.1038/nrcardio.2016.25 (2016).
Gosmanova, E. O. et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J. Am. Coll. Cardiol. 68(13), 1375–1386. https://doi.org/10.1016/j.jacc.2016.06.054 (2016).
Stevens, S. L. et al. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. Bmj 354, i4098. https://doi.org/10.1136/bmj.i4098 (2016).
Narita, K., Shimbo, D. & Kario, K. Assessment of blood pressure variability: Characteristics and comparison of blood pressure measurement methods. Hypertens. Res. 47(12), 3345–3355. https://doi.org/10.1038/s41440-024-01844-y (2024).
Muntner, P. et al. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from NHANES III, 1988 to 1994. Hypertension 57(2), 160–166. https://doi.org/10.1161/hypertensionaha.110.162255 (2011).
Rothwell, P. M. et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375(9718), 895–905. https://doi.org/10.1016/s0140-6736(10)60308-x (2010).
Park, J. H. et al. Increased blood pressure variability over a 16-year period is associated with left ventricular diastolic dysfunction in a population-based cohort. Am. J. Hypertens. 37(3), 168–178. https://doi.org/10.1093/ajh/hpad106 (2024).
Masugata, H. et al. Visit-to-visit variability in blood pressure over a 1-year period is a marker of left ventricular diastolic dysfunction in treated hypertensive patients. Hypertens. Res. 34(7), 846–850. https://doi.org/10.1038/hr.2011.54 (2011).
Alves, M. A. M. et al. Relationship between blood pressure variability and blood pressure phenotypes: A home blood pressure monitoring study. J. Hypertens. 43(3), 456–463. https://doi.org/10.1097/hjh.0000000000003925 (2025).
Saren, J. et al. Elevated blood pressure variability is associated with an increased risk of negative health outcomes in adults aged 65 and above—A systematic review and meta-analysis. Age Ageing https://doi.org/10.1093/ageing/afae262 (2024).
Dasa, O. et al. Association of 1-year blood pressure variability with long-term mortality among adults with coronary artery disease: A post hoc analysis of a randomized clinical trial. JAMA Netw. Open 4(4), e218418. https://doi.org/10.1001/jamanetworkopen.2021.8418 (2021).
Wu, C. et al. Visit-to-visit blood pressure variability and mortality and cardiovascular outcomes among older adults: The health, aging, and body composition study. Am. J. Hypertens. 30(2), 151–158. https://doi.org/10.1093/ajh/hpw106 (2017).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10(1), 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Pollard, T. J. et al. The eICU collaborative research database, a freely available multi-center database for critical care research. Sci. Data 5, 180178. https://doi.org/10.1038/sdata.2018.178 (2018).
Floras, J. S. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. J. Am. Coll. Cardiol. 54(5), 375–385. https://doi.org/10.1016/j.jacc.2009.03.061 (2009).
Parati, G., Faini, A. & Valentini, M. Blood pressure variability: Its measurement and significance in hypertension. Curr. Hypertens. Rep. 8(3), 199–204. https://doi.org/10.1007/s11906-006-0051-6 (2006).
Mehlum, M. H. et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur. Heart J. 39(24), 2243–2251. https://doi.org/10.1093/eurheartj/ehx760 (2018).
Webb, A. J. et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet 375(9718), 906–915. https://doi.org/10.1016/s0140-6736(10)60235-8 (2010).
Tai, C. et al. Prognostic significance of visit-to-visit systolic blood pressure variability: A meta-analysis of 77,299 patients. J. Clin. Hypertens. 17(2), 107–115. https://doi.org/10.1111/jch.12484 (2015).
Stergiou, G. S. et al. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: Current status and future directions—Position statement of the European Society of Hypertension Working Group on blood pressure monitoring and cardiovascular variability. J. Hypertens. 34(9), 1665–1677. https://doi.org/10.1097/hjh.0000000000000969 (2016).
Cushman, W. C. et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 362(17), 1575–1585. https://doi.org/10.1056/NEJMoa1001286 (2010).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39(33), 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Pierdomenico, S. D. et al. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am. J. Hypertens. 22(8), 842–847. https://doi.org/10.1038/ajh.2009.103 (2009).
Palatini, P. et al. Added predictive value of night-time blood pressure variability for cardiovascular events and mortality: The ambulatory blood pressure-international study. Hypertension 64(3), 487–493. https://doi.org/10.1161/hypertensionaha.114.03694 (2014).
Patel, A. et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 370(9590), 829–840. https://doi.org/10.1016/s0140-6736(07)61303-8 (2007).
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Jama 288(23), 2981–2997. https://doi.org/10.1001/jama.288.23.2981 (2002).
Vincent, J. L. et al. Clinical review: Update on hemodynamic monitoring—a consensus of 16. Crit. Care 15(4), 229. https://doi.org/10.1186/cc10291 (2011).
De Backer, D. et al. Monitoring the microcirculation in the critically ill patient: Current methods and future approaches. Intensive Care Med. 36(11), 1813–1825. https://doi.org/10.1007/s00134-010-2005-3 (2010).
Kario, K. Perfect 24-h management of hypertension: Clinical relevance and perspectives. J. Hum. Hypertens. 31(4), 231–243. https://doi.org/10.1038/jhh.2016.65 (2017).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61(4), 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Goldstein, B. A. et al. Opportunities and challenges in developing risk prediction models with electronic health records data: A systematic review. J. Am. Med. Inform. Assoc. 24(1), 198–208. https://doi.org/10.1093/jamia/ocw042 (2017).
Hamdidouche, I. et al. Drug adherence in hypertension: From methodological issues to cardiovascular outcomes. J. Hypertens. 35(6), 1133–1144. https://doi.org/10.1097/hjh.0000000000001299 (2017).
Funding
This work was supported by the Shandong Province Traditional Chinese Medicine Science & Technology Project (No. Z2023116) and Jining City Science and Technology Key Research and Development Program (No. 2021YXNS069).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. The manuscript, including figures and tables has not been previously published and that the manuscript is not under consideration elsewhere. Conceptualization and methodology–Zhiqiang Zhang, Shanshan Tang, Lei Chen and Xiqing Wei Investigation and data curation–Zhiqiang Zhang, Shanshan Tang, Lei Chen,Tenglong Hu, Yangyu Zhao, Na Sun, Qiang Sun, Wenyan Liang and Xiqing Wei Supervision–Zhiqiang Zhang, Shanshan Tang, Lei Chen,Tenglong Hu, Yangyu Zhao, Na Sun, Qiang Sun, Wenyan Liang and Xiqing Wei Validation–Zhiqiang Zhang, Shanshan Tang, Lei Chen and Xiqing Wei Formal analysis–Zhiqiang Zhang, Shanshan Tang, Lei Chen and Xiqing Wei Project Administration, Resources and Software–Zhiqiang Zhang, Shanshan Tang, Lei Chen and Xiqing Wei Visualisation and writing, original draft–Zhiqiang Zhang, Shanshan Tang, Lei Chen and Xiqing Wei Writing, preparation, review and editing–Zhiqiang Zhang, Shanshan Tang, Lei Chen,Tenglong Hu, Yangyu Zhao, Na Sun, Qiang Sun, Wenyan Liang and Xiqing Wei.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The studies involving human participants were reviewed and approved by MIMIC-IV and eICU-CRD databases were approved by the institutional review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Tang, S., Chen, L. et al. Blood pressure variability associated with in-hospital and 30-day mortality in heart failure patients: a multicenter cohort study. Sci Rep 15, 9911 (2025). https://doi.org/10.1038/s41598-025-93384-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93384-9