Abstract
Chronic lymphocytic leukemia (CLL) is a clinically and genetically heterogenous disease. Recent next-generation sequencing (NGS) studies have uncovered numerous low-frequency mutated genes in CLL patients, with SAMHD1 emerging as a candidate driver gene. However, the biological and clinical implications of SAMHD1 mutations remain unclear. Using CRISPR/Cas9, we generated CLL models to investigate the impact of SAMHD1 deficiency on pathogenesis and explore therapeutic strategies. Moreover, we performed NGS in treatment-naïve CLL patients to characterize SAMHD1 mutations and employed RNA-sequencing to evaluate their clinical significance. Our study shows that SAMHD1 inactivation impairs the DNA damage response by reducing homologous recombination efficiency through BRCA1 and RAD51 dysregulation. Importantly, SAMHD1 colocalizes with BRCA1 at DNA damage sites in CLL cells. This research also identifies that SAMHD1-mutated cells are more sensitive to PARP inhibition. Clinically, SAMHD1 dysfunction negatively impacts clinical outcome of CLL cases: SAMHD1 mutations reduce failure-free survival (median 46 vs 57 months, p = 0.033), while low SAMHD1 expression associates with shorter time to first treatment (median 47 vs 77 months; p = 0.00073). Overall, this study elucidates that SAMHD1 dysfunction compromises DNA damage response mechanisms, potentially contributing to unfavorable clinical outcomes in CLL, and proposes PARP-inhibitors as a potential therapeutic approach for SAMHD1-mutated CLL cells.
Similar content being viewed by others
Introduction
Chronic lymphocytic leukemia (CLL) exhibits both a heterogenous clinical and biological course, reflecting the complex genetic landscape of the disease1,2. Recent large-scale next generation sequencing (NGS) studies of CLL patients have identified over 200 candidate drivers genes, predominantly exhibiting low mutational incidences (< 5%)3,4,5. Among these, SAMHD1 has been uncovered as a potential CLL driver. However, the prognostic and biological implications of its alterations are poorly understood.
SAMHD1 is a nuclear triphosphohydrolase which converts all four deoxynucleotide triphosphates (dNTPs) to deoxynucleosides and inorganic triphosphate. By regulating cellular dNTPs levels, SAMHD1 plays a key role in the maintenance of homeostasis of dNTPs pools and is essential for the preservation of genomic integrity6,7,8. An imbalance in the dNTPs levels associates with DNA replication stress, reduced genomic stability and impaired DNA breaks repair mechanisms9,10. Apart from its dNTPase function, SAMHD1 also plays a direct role in DNA double-strand breaks (DSB) repair, promoting DNA end resection to facilitate DNA lesions repair by homologous recombination (HR) through the recruitment of DSB repair machinery11.
Germline SAMHD1 mutations are reported in Aicardi-Goutières syndrome (AGS)12,13. Importantly, clinical observations have involved SAMHD1 mutations with CLL pathogenesis, as evidenced by an AGS patient with SAMHD1 mutated but lacking other CLL-related mutations who developed CLL14. In addition, somatic SAMHD1 mutations occur in ~ 2% of newly diagnosed CLL patients5 and up to 11% of relapsed or chemotherapy-refractory cases, often as early clonal events5,15. Previous studies have suggested that SAMHD1 mutations typically lead to significantly reduced mRNA and protein expression14.
Despite the huge advances in improving CLL genetic characterization, the impact of some recurrently mutated genes such as SAMHD1 remains insufficiently understood. This study aims to delineate the implications of SAMHD1 variants in CLL. To elucidate their biological impact, novel isogenic CLL cellular models with loss-of-function mutations in SAMHD1 were generated using CRISPR/Cas9. Given the known involvement of SAMHD1 in the DNA damage response (DDR), our results show that SAMHD1 dysfunction reduces HR efficiency through impaired recruitment of BRCA1 to DNA damage sites, showing a functional interaction between SAMHD1 and BRCA1. Moreover, we correlated defective DDR with increased sensitivity to olaparib, suggesting potential therapeutic benefits of PARP inhibition in SAMHD1-mutated CLL patients. Furthermore, to assess their prognostic significance, we evaluated SAMHD1 mutational status in a treatment-naïve cohort and analyzed the clinical effects of its dysfunction using genomic and transcriptomic data, revealing an unfavorable clinical outcome. These findings position SAMHD1 as a critical mediator of DNA repair and genomic stability in CLL, with important implications for both prognosis and targeted therapy.
Results
SAMHD1 deficiency disrupts the DNA damage response by impairing the recruitment of HR marker BRCA1
To investigate the biological implications of SAMHD1 mutations, we conducted an in-depth analysis using transcriptomic datasets from CLL patients collected in the CLL-map portal3). Through this approach, we identified a comprehensive list of genes whose expression is significantly correlated with SAMHD1 expression in CLL patients (Supplementary Table 5). Functional annotation of these genes highlighted the DNA damage response as one of the most significantly enriched pathways (Fig. 1A). To gain deeper insights, we generated SAMHD1KO cells in PGA1 and MEC1 CLL-derived cell lines following the experimental design shown in Supplementary Fig. 1A. The introduction of truncating mutations in SAMHD1 gene (Supplementary Fig. 1B) resulted in the absence of protein (Supplementary Fig. 1C).
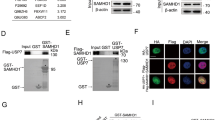
Biological effects of SAMHD1 dysfunction in DNA damage response in CLL. (A) Plots depicting GO analysis results of the top significantly enriched pathways correlated with SAMHD1 expression in CLL patients. Arrows emphasize the biological processes related with DNA damage response. (B) Left panel: HR efficiency graph for DSB repair in PGA1 cell line using a HR reporter plasmid. HR repair efficiency was calculated as the ratio of GFP + cells to the total number of DsRed + (transfected) cells. Three clones per condition in 3 independent experiments were analyzed. Results are shown as mean ± SD. Right panel: Representative plots of HR repair in SAMHD1WT and SAMHD1KO cells. (C) Correlation analysis between BRCA1 and SAMHD1 mRNA expression normalized with the vst score in CLL patients from the CLL map project. (D) Left panel: Quantification of BRCA1 foci/cell in SAMHD1WT and SAMHD1KO cells 1-h post-IR. At least fifty cells were counted per experiment in two independent clones per condition. Right panel: Representative images of BRCA1-positive cells 1 h after irradiation (2 Gy). (E) Representative immunofluorescence images showing the colocalization of SAMHD1 with BRCA1 at DNA damage sites following 2 Gy irradiation. Images include untreated control (no irradiation) and irradiated (DNA-damage induced) conditions. (F) Left panel: Measurement of RAD51 foci/cell in γH2AX positive cells 6 h post-IR. Cells were considered as γH2AX + when 5 or more foci were formed. At least fifty cells were counted per experiment. Two independent experiments were performed in three biological replicates. Right panel: Representative images of γH2AX + cells and RAD51 localization. (G) Western blot analyses depicting the expression of the proteins implicated in DNA damage signaling ATM, ATR, CHK1 and CHK2; and their phosphorylated forms. Cells were not treated (0), or treated with 2 or 4 µM of etoposide for 8 h. Two single-cell clones were analyzed for each condition.
The role of SAMHD1 in maintaining genome stability by promoting HR11 prompted us to evaluate the efficiency of the two main mechanisms of DSBs repair: HR and non-homologous end joining (NHEJ). Functional assays in CLL models demonstrated a significantly reduced HR efficiency in SAMHD1KO cells compared to SAMHD1WT counterparts, (p < 0.05) (Fig. 1B). In contrast, SAMHD1 truncation did not cause significant changes in NHEJ efficiency (Supplementary Fig. 2).
Strikingly, analysis of patients samples from the CLL-map portal revealed a significant positive correlation between SAMHD1 and BRCA1 expression (R = 0.36, p < 0.0001)(Fig. 1C). These findings led us to investigate whether SAMHD1 directly impacts BRCA1 recruitment. In response to ionizing radiation (IR), SAMHD1KO cells exhibited a statistically significant reduction of BRCA1 foci) (p < 0.0001) (Fig. 1D), indicating defective recruitment of BRCA1 to DSBs. Importantly, SAMHD1 colocalized with BRCA1 at DSBs sites following IR (Fig. 1E), suggesting a cooperation of SAMHD1 and BRCA1 in DDR. Furthermore, SAMHD1 disruption significantly impaired the localization of RAD51 to DSB sites in γH2AX-positive cells (p < 0.05) (Fig. 1F), indicating that SAMHD1 is essential for the efficient localization of these HR machinery components.
We tested in vitro how SAMHD1KO cells responded to DSBs. SAMHD1KO cells were more sensitive to DNA damage inducers such as IR (Supplementary Fig. 3A) or etoposide, validating this hypersensitivity in MEC1 cells (Supplementary Fig. 3B). Subsequently, we examined the expression levels of key protein kinases involved in the signaling pathways associated with DSB repair. Upon etoposide treatment, SAMHD1KO cells exhibited reduced phosphorylation levels of ATM, ATR and CHK1, concomitant with higher CHK2 phosphorylation (Fig. 1G). By contrast, we did not observe changes in expression levels of ATM, ATR, CHK1 and CHK2. Then, we determined cell cycle profile of PGA1 cells 48 h post-IR. As expected, SAMHD1WT cells exhibited cell cycle arrest in G2/M phase, indicative of an active repair process in response to DNA damage, whereas SAMHD1KO cells overcame this point and showed the same profile that those cells without IR exposure (Supplementary Fig. 3C). A similar cell cycle distribution was observed in MEC1 cells (Supplementary Fig. 3D). In addition, an analysis comparing SAMHD1KO to SAMHD1WT cells revealed a statistically significant reduction in G2/M arrest in CLL cells with SAMHD1 dysfunction (p < 0.001) (Supplementary Fig. 3E). These findings underscore the impact of SAMHD1 deficiency on cellular response to DSBs.
SAMHD1 KO cells are more sensitive to DNA damage inducers
Considering the potential of defects in DNA damage repair to increase sensitivity to various DNA damage-inducing agents, we tested the impact of SAMHD1 truncation on the cellular response to the alkylating agent bendamustine and the nucleoside analogue fludarabine. Notably, SAMHD1KO cells exhibited higher sensitivity to both drugs approved for CLL therapy (Fig. 2A,B). Remarkably, SAMHD1KO cells showed a significant lower fludarabine IC50 compared with SAMHD1WT (mean IC50 of SAMHD1WT: 9.8 µM vs SAMHD1KO: 2.5 µM, p < 0.0001). This higher sensitivity was also validated in SAMHD1KO MEC1 cells, which harbor TP53 dysfunction (Supplementary Fig. 4A,B). Interestingly, HG3 cells, harboring a truncating mutation in SAMHD1, showed the lowest IC50 value for fludarabine (Fig. 2C, Supplementary Table 1). This result supports that SAMHD1 inactivating mutations enhance sensitivity to fludarabine, which is a SAMHD1 substrate.
Impact of SAMHD1 disruption on response to DNA damage inducers. (A, B) Dose–response curves of bendamustine (A) and fludarabine (B) treatment in PGA1 cell line. Cells were treated with escalating doses of bendamustine (0.625–80 µM) or fludarabine (0.625–40 µM) for 72 h. Cell viability was assessed with MTT, and surviving fraction is expressed relative to DMSO control. Dashed line represents the reduction of IC50 value in SAMHD1KO cells. Data are represented as mean ± SD of three independent experiments. (C) IC50 value (µM) for fludarabine treatment in 3 CLL cell lines (HG3, PGA-1 and MEC1). Information of SAMHD1 and TP53 status were summarized below the graph. (D) Representative annexin V/PI dot-plots (left panel) to determine apoptotic (annexin V + /PI +) cells (right panel) after 7.5 µM fludarabine for 48 h. (E) PI staining for determination of subG0 population (left panel) for PGA1 cells, reflecting the increase of apoptotic SAMHD1KO cells after fludarabine treatment (right panel). (F) Cell cycle profile determined by PI staining after 48 h fludarabine treatment (7.5 µM) in PGA1 cell line. Data are represented as mean ± SD of three independent experiments.
We next demonstrated that fludarabine treatment increases apoptosis and modifies cell cycle profile. Annexin V/PI staining revealed a higher percentage of apoptotic (annexin V +) and late apoptotic SAMHD1KO cells (annexin V + PI +) (Fig. 2D) compared to SAMHD1WT cells. Apoptosis enhancement was also confirmed by the higher subG0 percentage in SAMHD1KO cells, a characteristic marker of apoptosis (Fig. 2E). In parallel, we identified a higher G1 percentage and a concomitant reduction of cells in G2/M after fludarabine treatment in SAMHD1KO cells compared to the WT counterparts (Fig. 2F, Supplementary Fig. 4C).
SAMHD1 KO cells are more sensitive to PARP inhibition by olaparib
Considering the reduced HR efficiency in SAMHD1KO cells and the well-established association between HR defects and increased sensitivity to poly-ADP-ribose polymerase (PARP) inhibitors35, we hypothesized that PARP inhibition could be an attractive treatment for SAMHD1KO cells. First, we observed that SAMHD1KO potentiated sensitivity to PARP inhibition with olaparib in PGA1 cells, which show a significant decrease of IC50 value (4.014 vs 8.383 µM), (Fig. 3A). This hypersensitivity to olaparib was corroborated in MEC1 SAMHD1KO cells (Supplementary Fig. 5A).
Response of SAMHD1KO cells to olaparib in monotherapy and combined with bendamustine/ ibrutinib. (A) Cell viability studies by MTT assay in response to increasing doses of olaparib (0.625–20 µM) for 72 h. Percentage of live cells is expressed relative to DMSO control. Differences between PGA-1 SAMHD1WT and SAMHD1KO clones are summarized by asterisks. Data are summarized as the mean ± SD of three independent experiments. (B) Drug response to olaparib (20 µM), bendamustine (20 µM) and combination of both drugs (ratio 1:1) in PGA-1 SAMHD1WT and SAMHD1KO by measuring cell viability with MTT assay after 72 h Data are summarized as the mean ± SD of three independent experiments. (C) Dose–response curve of viable cells (%, relativized to untreated cells) after treatment with olaparib, ibrutinib and their combination at a 1:1 ratio. Data are presented as the mean ± SD of three independent experiments. Combination index (CI) was calculated using Calcusyn. (D) Determination of cytotoxicity after 48-h treatment with olaparib (7.5 µM), ibrutinib (7.5 µM) and the combination of these drugs (1:1) in PGA-1 cells by measuring the percentage of apoptotic cells (annexin V + / PI +) with annexin V/PI staining. Data are presented as the mean ± SD of three independent experiments. (E) Western blot analyses reflecting PARP cleavage as an apoptosis marker after treatment with olaparib, ibrutinib and their combination (ratio 1:1).
Then, we tested whether olaparib could be used in combination with standard agents of CLL treatment such as the alkylating agent bendamustine or the BCR inhibitor ibrutinib, which could enhance olaparib cytotoxicity by synthetic lethality30. In monotherapy, SAMHD1KO cells were more sensitive to bendamustine (Fig. 3B), but not to ibrutinib (Supplementary Fig. 5B). Strikingly, combining bendamustine with olaparib resulted in synergistic effects in reducing cell survival of PGA1 cells (Combination Index–CI: SAMHD1WT = 0.712; SAMHD1KO = 0.751), being dual treatment more effective in SAMHD1KO cells (Fig. 3B, Supplementary Fig. 5C). Furthermore, BCR inhibition with ibrutinib synergistically potentiated the effect of PARP inhibition (CI-SAMHD1WT = 0.862; CI-SAMHD1KO = 0.681), especially in SAMHD1KO cells (Fig. 3C). Similarly, combining olaparib with ibrutinib increased induction of apoptosis particularly in SAMHD1KO cells, demonstrated by a higher percentage of apoptotic cells (Fig. 3D, Supplementary Fig. 5D) and an enhanced PARP cleavage (Fig. 3E). Overall, these data indicate that olaparib, either in monotherapy or combined with other CLL treatments, effectively sensitizes SAMHD1KO. Additionally, the reduced RAD51 localization in DSB lesions was consistent with an increase in sensitivity to the RAD51 inhibitor B02, as well as to the ATR inhibitor AZD6738 in SAMHD1KO cells (Supplementary Fig. 5E).
SAMHD1 mutations are infrequent but its dysfunction associates with dismal prognosis in CLL
Finally, we evaluated the clinical impact of SAMHD1 dysfunction through NGS analysis in 487 CLL patients. We identified 12 SAMHD1 mutations in 8 cases (1.64%). These mutations included 3 nonsense and 9 missense substitutions that were predicted in silico as damaging for SAMHD1 protein (Fig. 4A, Supplementary Table 6). All variants were present at high variant allele frequencies (VAF) (30–55%), suggesting its clonal nature. Moreover, SAMHD1 mutated (SAMHD1MUT) patients harbored other high-risk genetic alterations such as TP53 or ATM mutations or losses (Supplementary Table 6).
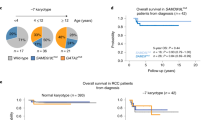
Clinical impact of SAMHD1 dysfunction in CLL patients. (A) Diagrammatic representation illustrating the distribution of SAMHD1 mutations identified in CLL patients from the local cohort (up) and those previously reported in three CLL cohorts (down)3,14,15. These variants are represented according to the amino acid change at the protein level (Transcript: ENST00000262878). Each circle represents a SAMHD1 mutation, and its color means the type of mutation (missense, nonsense or frameshift). (B) Kaplan–Meier curve of failure free survival analysis of SAMHD1 mutated (SAMHD1MUT, red line) vs SAMHD1 non-mutated (SAMHD1WT, purple line) CLL patients from the CLL-map portal3. (C) Kaplan–Meier plots showing the association between SAMHD1 expression and the time to first treatment (left) and overall survival (right) in CLL from the CLL-map portal3. CLL patient were grouped according to SAMHD1 expression levels: low (dark red line) and high (light purple line).
In additional CLL cohorts, the frequency of SAMHD1 mutations varied depending on the patient population. In the largest cohort of pre-treatment patients analyzed (n = 1,009), the frequency was 1.5%3, while a separate study of treatment-naïve patients (n = 200) reported a slightly higher frequency of 3%14. Interestingly, SAMHD1 variants were more prevalent among relapsed/refractory patients who had received immunochemotherapy, reaching 11% in a smaller cohort (n = 63)14. This elevated frequency was consistent with findings from another relapsed/refractory cohort, where the mutation rate was 9.6% (n = 114)15. Across these studies, 43 SAMHD1 mutations were reported in 40 CLL patients3,14,15 (Supplementary Table 7). 75% (36/48) of these mutations were missense, being most of them (~ 80%, 29/36) annotated by bioinformatic predictors as likely pathogenic. In addition, 7 nonsense variations and 5 frameshift mutations were detected. The mutations were broadly distributed throughout the protein structure, affecting functional domains, allosteric and catalytic sites (Fig. 4A). The median VAF was 59.3% (range 8.15%- 99.65%), with over 90% having a VAF > 25%, suggesting that these variants were mainly clonal, as previously reported14 (Supplementary Table 7).
Extending our investigations to the CLL-map project3, SAMHD1MUT CLL cases (n = 15) had significantly shorter failure free-survival (FFS) compared to SAMHD1WT patients (n = 994) (median 46 vs 57 months; p = 0.033 (Fig. 4B). Moreover, SAMHD1MUT patients exhibited a trend towards reduction of time to first treatment (TFT) (median 32 vs 67 months; p = 0.069) (Supplementary Fig. 6A). Stratifying CLL patients from two independent cohorts based on SAMHD1 expression levels, CLL cases with low SAMHD1 expression associated with shorter TFT (median 47 vs 77 months; p = 0.00073) and overall survival (OS) (median 154 months vs not reached; p = 0.0046) compared to those with high SAMHD1 expression in the CLL-map portal (Fig. 4C). These findings were independently validated in the GSE22762 dataset, in which low SAMHD1 expression was similarly correlated with reduced TFT and OS (Supplementary Fig. 6B and C). Thus, both loss-of-function mutations and reduced expression of SAMHD1 are implicated in adverse prognosis of CLL patients.
Discussion
Despite significant advances from large-scale sequencing studies over the last years3,5,18,19, little is known about the biological and prognostic impact of most putative drivers in CLL such as SAMHD1. Our work provides a comprehensive analysis of the biological impact of SAMHD1 dysfunction in CLL disease. Using novel CRISPR/Cas9-edited CLL cellular models, we show that loss of SAMHD1 negatively affects the DDR and makes CLL cells hypersensitive to therapeutic approaches based on PARP inhibitors. Moreover, our results reinforce that not only mutations, but also altered gene expression of SAMHD1, are clinically relevant in CLL. Thus, our data underscore SAMHD1 alterations as a possible biomarker of worse prognosis in CLL14, as it has been previously proposed in other solid tumors such as colon cancer22,37.
Our findings uncover that SAMHD1 dysfunction reduced HR efficiency in the context of CLL disease. Similarly, Daddacha et al. observed that SAMHD1 localizes at DSBs in response to DNA damage to facilitate HR by promoting RAD51 recruitment11. In our study, we demonstrate that SAMHD1 depletion not only impairs RAD51 recruitment but also disrupts BRCA1 recruitment to DSBs. Furthermore, we confirm the colocalization of SAMHD1 with the HR marker BRCA1 at DNA damage foci. This observation aligns with a previous study that identified the colocalization of SAMHD1 with 53BP1, another DDR-related protein14. Additionally, through transcriptomic analysis of CLL patient data, we reveal a significant linear correlation between SAMHD1 and BRCA1 expression in CLL patients, providing further evidence of their functional relationship. Overall, our results substantiate a novel mechanistic role for SAMHD1 in enabling efficient BRCA1-mediated repair, as initially described in solid tumor models11. Beyond this impact on HR, SAMHD1 deficiency compromises DDR signaling as SAMHD1 loss impairs cell cycle arrest in G2/M phase in response to DNA damage.We hypothesize that the moderate overexpression of p-CHK2 in SAMHD1KO cells relative to SAMHD1WT could enhance cell cycle arrest in G1 phase22,23,24. This hypothesis aligns with previous studies reporting that defects in dNTPase function caused by SAMHD1 deficiency may lead to a G1 arrest8,17,25 and genomic instability26. Taken together, our findings propose SAMHD1 as a critical mediator of HR and DDR integrity, offering new insights into its role in genomic stability.
Our work provides evidence of hypersensitivity to olaparib in SAMHD1KO cells. PARP inhibition is an approved treatment for HR-deficient solid tumors34 and a potential therapeutic approach for hematological malignancies16,28, especially in CLL cells with DDR-related gene mutations20,30. Furthermore, bendamustine or ibrutinib synergistically enhance olaparib sensitivity in CLL cells, mainly in the context of SAMHD1KO, similar to previous observations in ATM-deficient CLL cells30,38. These findings suggest that PARP inhibition could be a therapeutic strategy to use in CLL cells with defective DDR. In addition, previous research have demonstrated that ibrutinib produced a HR-impairment through RAD51 dysregulation which could explain the synergy effects of this drug with olaparib in SAMHD1KO cells30.
Moreover, we showed that SAMHD1KO increases efficacy of fludarabine in CLL cells. Previous studies have identified that SAMHD1 influences the response to nucleoside analogues21. Moreover, in various hematological malignancies, depletion of SAMHD1 increases the cytarabine cytotoxicity, showing better chemotherapy responses in those patients with low SAMHD1 expression39,40,41,42. However, this observation is contradictory with the higher SAMHD1 mutational frequency observed in relapsed/refractory to chemotherapy CLL cohorts. The presence of high-risk genetic alterations in relapsed CLL cases, even when SAMHD1 is mutated, may contribute to poorer chemotherapy responses14,15. In fact, SAMHD1MUT CLL patients of the sequenced cohort harbored other high-risk alterations. In this way, SAMHD1KO cells could be selected after fludarabine-based treatment, generating a non-death cellular pool with higher genomic instability that could increase relapse risk. In addition, DNA damaging agents such as fludarabine, bendamustine or etoposide further potentiate DNA repair defects and increase apoptosis in SAMHD1KO cells, reinforcing the vulnerability of cells with SAMHD1 mutations to DNA damage inducers11.
Our findings on the biological impact of SAMHD1 mutations highlights the importance of SAMHD1 in the cellular response to DNA damage to ensure genomic stability. We could hypothesize that the defective DNA damage repair likely influences clinical outcomes, with SAMHD1 dysfunction in CLL patients associating with unfavorable prognosis. Considering the molecular features of CLL, the identification of SAMDH1 as a valuable prognostic marker contributes to our understanding of the molecular landscape of this disease and set the basis to the development of successful individual therapies. Moreover, our results emphasize the important role of DNA damage repair in CLL pathogenesis, as mutations in TP53 and ATM occur frequently in CLL patients, underscoring this pathway as a promising therapeutic target.
In conclusion, our results provide evidence of the significance of SAMHD1 mutations in the cellular response to DNA damage and uncover SAMHD1 as a potential prognostic marker with clinical relevance. We propose that PARP inhibition in monotherapy or combined with DNA damage inducers or BTK inhibitors could be promising options for treatment of CLL patients with SAMHD1 alterations. Thus, this study contributes to gain insight into the heterogeneous landscape of CLL mutations and propose promising therapeutic approaches for a subgroup of high-risk CLL patients.
Methods
Generation of SAMHD1KO CLL cell lines
The human CLL-derived cell lines PGA1, HG3 and MEC1 were used. SAMHD1 mutational status was analyzed with a custom NGS panel. SAMHD1 wild-type PGA1 and MEC1 cell lines were selected for CRISPR/Cas9 experiments (Supplementary Table 1).
PGA1 and MEC1 Cas9-expressing cell lines were previously established36 and tested for Cas9 activity43. To generate CLL cells knockout for SAMHD1 (SAMHD1KO), single-guide RNAs (sgRNAs) targeting SAMHD1 (exon 4) were designed using CRISPick44, prioritizing those with high on-target efficiency and low off-target effects (Supplementary Table 2). A non-targeting sgRNA served as a negative control (5’-ACGGAGGCTAAGCGTCGCAA-3’). SgRNAs were cloned into the pLKO5.sgRNA.EFS.tRFP plasmid (Addgene_#57823)27 and nucleofected into PGA1-Cas9 and MEC1-Cas9 using Amaxa Nucleofector II (Lonza, program X-005). 72 h post-transfection, mCherry + cells were single-cell sorted using a FACSAria II cytometer (BD Biosciences) and clones were expanded and screened for the presence of SAMHD1 truncating mutations (Supplementary Fig. 1A and 1B). Three SAMHD1 loss-of-function clones were selected for functional studies.
Patients
The study involved 487 CLL patients diagnosed according to the International Workshop on CLL (iwCLL) criteria45,46. Samples and clinical data from 16 Spanish institutions were centrally analyzed at the Molecular Cytogenetics Unit of the Cancer Research Center in Salamanca, Spain (Supplementary Table 3). This study was approved by the ethics committee of Hospital Universitario de Salamanca (Comité Ético de Investigación Clínica, Hospital Universitario de Salamanca). Written informed consent was obtained from all participants before they entered the study in accordance to the Declaration of Helsinki. CLL samples were analyzed before first-line treatment. Additional clinical information was obtained from three previously documented CLL cohorts3,14,15 and the CLL-map portal3.
Next generation sequencing (NGS)
NGS analyses were performed in 487 CLL patients. Genomic DNA of CD19 + B-lymphocytes was isolated from peripheral blood or bone marrow by magnetically activated cell sorting (B-cell purity > 98%)29,30,31,32. Libraries of exonic regions from 54 CLL-associated genes were prepared using the Agilent SureSelectQXT Target Enrichment system for Illumina Multiplexed Sequencing (Agilent Technologies) (Supplementary Table 4). Paired-end sequencing (151-bp reads) was conducted on an Illumina NextSeq instrument (see Supplementary methods).
Bioinformatic analyses
The clinical implications of SAMHD1 mutations in CLL were assessed in 1,009 patients from the CLLmap portal3. The impact of low SAMHD1 expression was evaluated in two transcriptomic data sets, one with 5663 and another with 107 CLL cases33. Time to first treatment (TFT), overall survival (OS) and failure free survival (FFS) were estimated by the Kaplan–Meier method, with group comparisons performed using the log-rank test. Overrepresented biological processes associated with SAMHD1 dysfunction were identified using a Pearson correlation-based approach, focusing on genes with a Pearson Correlation coefficient |R-squared|> 0.3 and adjusted p-value < 0.05. Gene enrichment and functional annotation analyses were conducted using DAVID, focusing on gene ontology (GO) at biological process (BP) level (see supplementary methods).
Statistical approach
Statistical analyses were performed with GraphPad Prism v6. Data are represented as mean ± standard deviation (SD). Student´s t test, Mann–Whitney and ANOVA were used to determine statistical significance. P-values < 0.05 were considered statistically significant. Experiments were performed in 3 independent SAMHD1WT and SAMHD1KO clones.
Data availability
All data and information concerning this study will be made available from the corresponding authors (jmhr@usal.es and mariahs@ucm.es) upon reasonable request.
References
Gaidano, G., Foà, R. & Dalla-Favera, R. Molecular pathogenesis of chronic lymphocytic leukemia. J. Clin. Investig. 122(10), 3432–3438 (2012).
Zenz, T., Mertens, D., Küppers, R., Döhner, H. & Stilgenbauer, S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat. Rev. Cancer 10(1), 37–50 (2010).
Knisbacher, B. A. et al. Molecular map of chronic lymphocytic leukemia and its impact on outcome. Nat. Genet. 54(11), 1664–1674 (2022).
Puente, X. S. et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 526(7574), 519–524 (2015).
Landau, D. A. et al. Mutations driving CLL and their evolution in progression and relapse. Nature 526(7574), 525–530 (2015).
Goldstone, D. C. et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480(7377), 379–382 (2011).
Powell, R. D., Holland, P. J., Hollis, T. & Perrino, F. W. Aicardi-Goutières syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J. Biol. Chem. 286(51), 43596–43600 (2011).
Franzolin, E. et al. The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 110(35), 14272–14277 (2013).
Mathews, C. K. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat. Rev. Cancer 15(9), 528–539 (2015).
Rentoft, M. et al. Heterozygous colon cancer-associated mutations of SAMHD1 have functional significance. Proc. Natl. Acad. Sci. U. S. A. 113(17), 4723–4728 (2016).
Daddacha, W. et al. SAMHD1 promotes DNA end resection to facilitate DNA repair by homologous recombination. Cell Rep. 20(8), 1921–1935 (2017).
Rice, G. I. et al. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41(7), 829–832 (2009).
Crow, Y. J. & Rehwinkel, J. Aicardi-Goutie’res syndrome and related phenotypes: Linking nucleic acid metabolism with autoimmunity. Hum. Mol. Genet. 18(R2), 130–136 (2009).
Clifford, R. et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood 123(7), 1021–1031 (2014).
Guièze, R. et al. Presence of multiple recurrent mutations confers poor trial outcome of relapsed/refractory CLL. Blood 126(18), 2110–2117 (2015).
Fong, P. C. et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. N. Engl. J. Med. 361(2), 123–134 (2009).
Coggins, S. A., Mahboubi, B., Schinazi, R. F. & Kim, B. SAMHD1 functions and human diseases. Viruses 12(4), 1–28 (2020).
Quesada, V. et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat. Genet. 44(1), 47–52 (2012).
Puente, X. S. et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 475(7354), 101–105 (2011).
Knecht, K. M. et al. The structural basis for cancer drug interactions with the catalytic and allosteric sites of SAMHD1. Proc. Natl. Acad. Sci. U. S. A. 115(43), E10022–E10031 (2018).
Schneider, C. et al. SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia. Nat. Med. 23(2), 250–255 (2017).
Zhang, Z. et al. Involvement of SAMHD1 in dNTP homeostasis and the maintenance of genomic integrity and oncotherapy (Review). Int. J. Oncol. 56(4), 879–888 (2020).
Zannini, L., Delia, D. & Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 6(6), 442–457 (2014).
Maréchal, A. & Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 5(9), 1–18 (2013).
Bonifati, S. et al. SAMHD1 controls cell cycle status, apoptosis and HIV-1 infection in monocytic THP-1 cells. Virology 495, 92–100 (2016).
Kretschmer, S. et al. SAMHD1 prevents autoimmunity by maintaining genome stability. Ann. Rheum. Dis. 74(3), 1–8 (2015).
García-Tuñón, I. et al. The CRISPR/Cas9 system efficiently reverts the tumorigenic ability of BCR/ABL in vitro and in a xenograft model of chronic myeloid leukemia. Oncotarget 8(16), 26027–26040 (2017).
Skelding, K. A. & Lincz, L. F. PARP inhibitors and haematological malignancies—Friend or foe?. Cancers 13, 5328 (2021).
Quijada-Álamo, M. et al. Dissecting the role of TP53 alterations in del(11q) chronic lymphocytic leukemia. Clin. Transl. Med. https://doi.org/10.1002/ctm2.304 (2021).
Quijada-Álamo, M. et al. Biological significance of monoallelic and biallelic BIRC3 loss in del(11q) chronic lymphocytic leukemia progression. Blood Cancer J. 11(7), 1–11 (2021).
Pérez-Carretero, C. et al. Chronic lymphocytic leukemia patients with IGH translocations are characterized by a distinct genetic landscape with prognostic implications. Int. J. Cancer 147(10), 2780–2792 (2020).
Pérez-Carretero, C. et al. TRAF3 alterations are frequent in del-3’IGH chronic lymphocytic leukemia patients and define a specific subgroup with adverse clinical features. Am. J. Hematol. 97(7), 903–914 (2022).
Herold, T. et al. An eight-gene expression signature for the prediction of survival and time to treatment in chronic lymphocytic leukemia. Leukemia 25(10), 1639–1645 (2011).
Paik, E. S., Chang, H. K. & Lee, S. Prevalence of homologous recombination deficiency in first-line PARP inhibitor maintenance clinical trials and further implication of personalized treatment in ovarian cancer. Cancers (Basel) 15(12), 3095 (2023).
Helleday, T., Bryant, H. E. & Schultz, N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle 4(9), 1176–1178 (2005).
Quijada-Álamo, M. et al. CRISPR/Cas9-generated models uncover therapeutic vulnerabilities of del(11q) CLL cells to dual BCR and PARP inhibition. Leukemia 34(6), 1599–1612 (2020).
Park, K. et al. Aicardi-Goutières syndrome-associated gene SAMHD1 preserves genome integrity by preventing R-loop formation at transcription–replication conflict regions. PLoS Genet. 17, 1–24 (2021).
Stankovic, T. & Skowronska, A. The role of ATM mutations and 11q deletions in disease progression in chronic lymphocytic leukemia. Leuk. Lymphoma. 55(6), 1227–1239 (2014).
Rassidakis, G. Z. et al. Low-level expression of SAMHD1 in acute myeloid leukemia (AML) blasts correlates with improved outcome upon consolidation chemotherapy with high-dose cytarabine-based regimens. Blood Cancer J. https://doi.org/10.1038/s41408-018-0134-z (2018).
Wang, T. et al. SAMHD1 mutations and expression in mantle cell lymphoma patients. Front. Oncol. 11(December), 1–10 (2021).
Roider, T. et al. The impact of SAMHD1 expression and mutation status in mantle cell lymphoma: An analysis of the MCL Younger and Elderly trial. Int. J. Cancer 148(1), 150–160 (2021).
Xagoraris, I. et al. Expression of the novel tumour suppressor sterile alpha motif and HD domain-containing protein 1 is an independent adverse prognostic factor in classical Hodgkin lymphoma. Br. J. Haematol. 193(3), 488–496 (2021).
Doench, J. G. et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32(12), 1262–1267 (2014).
CRISPick [Internet]. https://portals.broadinstitute.org/gppx/crispick/public (2023).
Swerdlow, S. H. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127(20), 2375–2390 (2016).
Hallek. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute Working Group 1996 guidelines (Blood (2008) (111)). Blood.112(13), 5259 (2008).
SwimmerPlot GitHub public site [Internet]. https://cllmap.org/viz/swimmer-plot/ (2023).
Acknowledgements
We thank Almudena Martín-Martín, Sara González, Irene Rodríguez, Teresa Prieto, Mª Ángeles Ramos, Filomena Corral, Mª Almudena Martín, Ana Díaz, Ana Simón, María del Pozo, Isabel M Isidro, Vanesa Gutiérrez, Sandra Pujante, Mª Ángeles Hernández, Sandra Santos and Cristina Miguel from the Cancer Research Center of Salamanca, Spain, for their technical support. We are grateful to Ángel Prieto and Ana I García from the Microscopy Unit and María Luz Sánchez from Cytometry Unit, from the Cancer Research Center of Salamanca for their technical assistance. We thank Javier Borrajo from the Service of NUCLEUS, University of Salamanca for his help with the irradiation experiments. We are deeply grateful to Teresa González, Julio Dávila-Valls, María Jesús Vidal-Manceñido, Josefina Galende, José Antonio Queizán, Carlos Aguilar and José Ángel Hernández-Rivas for providing patient samples and clinical information. This study has been funded by Instituto de Salud Carlos III (ISCIII) through the project "PI21/00983" and co-funded by the European Union, by grants (RD12/0036/0069) from Red Temática de Investigación Cooperativa en Cáncer (RTICC), Centro de Investigación Biomédica en Red de Cáncer (CIBERONC CB16/12/00233), Residual disease assessment in hematologic malignancies to improve patient-relevant outcomes across Europe (RESOLVE, GA number 101136502), HORIZON-MISS-2023-CANCER-01-03 and grant PID2023-149241OA-I00 funded by MICIU/AEI/10.13039/501100011033 and by ERDF/EU. ARS is fully supported by predoctoral research fellowship 2022 from Junta de Castilla y León and cofinancied by the European Social Fund Plus (FSE +) (JCYL-EDU/1868/2022 PhD scholarship). CPC is supported by a research grant from FEHH (“Fundación Española de Hematología y Hemoterapia”; Beca de Investigación FEHH 2022).
Author information
Authors and Affiliations
Contributions
ARS and MHS designed and performed the experiments, conducted data analysis and wrote the manuscript. MQA and CPC contributed to the design of the functional experiments, interpreted the results and critically reviewed the manuscript. ABH designed DNA damage repair experiments and interpreted the results. AAB contributed to bioinformatic data analysis. JDV, AR, AGC provided patient´s clinical data. RBS and AERV designed sequencing studies and contributed to data analysis. JMHR conceived the study, supervised the research and critically reviewed and approved the final version of the manuscript. All authors discussed the results and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study was approved by the ethics committee of Hospital Universitario de Salamanca and written informed consent was obtained from all participants before they entered the study in accordance to the declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rodríguez-Sánchez, A., Quijada-Álamo, M., Pérez-Carretero, C. et al. SAMHD1 dysfunction impairs DNA damage response and increases sensitivity to PARP inhibition in chronic lymphocytic leukemia. Sci Rep 15, 10446 (2025). https://doi.org/10.1038/s41598-025-93629-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93629-7