Abstract
Many observational studies have found an association between osteoporosis and postural instability. However, it is unclear whether there is a genetic causal relationship between osteoporosis and postural instability. In this study, we conducted a two-sample Mendelian randomization (MR) analysis to investigate the causal relationship between osteoporosis and postural instability, with osteoporosis represented by bone mineral density (BMD). We used random effects Inverse Variance Weighted (IVW), weighted median, and MR-Egger methods after Steiger filtering, followed by FDR correction, to assess the causal relationship. We also used the Cochran Q statistic and MR-PRESSO to detect and exclude heterogeneity, the MR-Egger intercept to detect horizontal pleiotropy, and the leave-one-out method for sensitivity analyses. After excluding the heterogeneity in causal estimates across different SNPs and after Steiger filtering, the inverse variance weighted analysis showed a significant negative correlation between femoral neck BMD (FN-BMD) and the occurrence of postural instability, with an OR of 0.9171 (95% CI: 0.8745–0.9617; FDR P.value = 0.0009). Similar results were obtained in the weighted median analysis, with an OR of 0.923 (95% CI: 0.8717–0.9733; FDR P = 0.0180), and in the analysis of lumbar spine BMD (LS-BMD) in IVW, with an OR of 0.9491 (95% CI: 0.9156–0.9838; FDR P.value = 0.0129). However, there was no significant correlation between forearm BMD (FA-BMD) and postural instability. Further analysis showed no horizontal pleiotropy or heterogeneity in FN-BMD and LS-BMD after excluding heterogeneous SNPs. This study demonstrates a causal association between BMD and postural instability, suggesting that individuals with osteoporosis may be at higher risk of experiencing postural instability.
Similar content being viewed by others
Introduction
The worldwide prevalence of postural instability and falls among older individuals has captured significant public health attention1. Postural instability, in itself, can contribute to falls, often resulting in severe outcomes, such as fractures and head injuries2,3. Therefore, effective prevention of postural instability is of paramount importance to mitigate the risks associated with these consequential outcomes4.
To address postural instability comprehensively, it is imperative to focus on potentially modifiable risk factors, with osteoporosis being a notable concern. Osteoporosis is a systemic condition characterized by bone loss, leading to reduced bone density and compromised bone microarchitecture5,6. This ailment frequently results in fragility fractures, accounting for approximately 9 million cases annually globally, further diminishing patients’ quality of life and increasing the risk of premature mortality7. As per the World Health Organization’s definition, the clinical diagnosis and evaluation of osteoporosis heavily relys on measuring bone mineral density (BMD)8.
Osteoporosis is identified as a potential risk factor for various conditions, and individuals with osteoporosis seem to exhibit an increased susceptibility to postural instability issues, often with poorer prognoses9,10. Given the ubiquity and severity of osteoporosis, it is paramount to elucidate the potential association between osteoporosis and postural instability, significantly impacting public health11.
Mendelian randomization serves as a valuable method for assessing causal relationships between exposure factors and outcomes12. It employs genetic variants as instrumental variables, randomly and evenly distributed during genetic inheritance and impervious to the influences of age, gender, lifestyle, or environmental factors. One key advantage of MR, in contrast to traditional clinical randomized controlled trials, was its ability to effectively mitigate potential confounding variables13.
In this study, we adopted a two-sample MR design to investigate whether a causal relationship exists between osteoporosis and postural instability. This approach provided a comprehensive understanding of the connection between these two factors and offered valuable insights into related health concerns.
Method
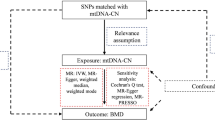
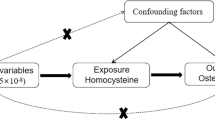
In this two-sample Mendelian randomization study, we utilized single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to evaluate the causal relationship between osteoporosis and postural instability, leveraging Genome-Wide Association Study (GWAS) data. It provides an overview of the study design and the MR study’s hypotheses. We selected relevant SNPs associated with osteoporosis and postural instability from published GWAS. The MR study is grounded in three fundamental hypotheses: The selected genetic variants, functioning as instrumental variables, exhibit a robust association with the risk factors of interest. These chosen genetic variants are not linked to potential confounders. The selected genetic variants solely impact the risk of an outcome by influencing the risk factor associated with that outcome, excluding other pathways14.
The MR design plays a pivotal role in mitigating residual confounding and reverse causation, thus bolstering the inference of causality in exposure-outcome relationships. This is attributed to the random assignment of the selected genetic variants as instrumental variables at conception, rendering them less susceptible to confounding by environmental factors and reverse causation15.
Study design
In this study, a GWAS was applied to the pooled dataset (GWAS) pooled dataset for two-sample Mendelian randomization analysis to assess the causal relationship between osteoporosis and postural instability as well as between postural instability and osteoporosis, and sensitivity analysis to test the reliability of the results (Fig. 1).
Mendelian randomization studies need to satisfy three core assumptions: (1) the instrumental variable must be strongly correlated with the exposure factor; (2) the instrumental variable must not be correlated with any confounding factors associated with the “exposure-outcome” relationship; (3) the instrumental variable can only affect the outcome variable through the exposure factor.
Data sources
We used pooled GWAS data on femoral neck BMD (FN-BMD), lumbar spine BMD (LS-BMD), and forearm BMD (FA-BMD), to evaluate the impact of these factors on postural instability. These three common skeletal sites, the femoral neck, lumbar spine, and forearm, are commonly used to measure BMD, particularly for postmenopausal women and men aged 50 years or older, which is determined using dual-energy X-ray absorptiometry. The above GWAS summary statistics for BMD (g/cm2) and postural instabilityare available from the Genetic Osteoporosis Society (GEFOS: http://www.gefos.org/)16. In addition, data on the postural instability study were obtained from the GWAS catalog website( https://www.ebi.ac.uk/gwas/studies/GCST90012857)17. It is worth noting that there is overlap between the BMD data and the postural instability data, with the detailed data provided in Supplementary Table 1.
Instrumental variable settings
We systematically evaluated the potential overfitting bias arising from sample overlap between exposure and outcome datasets18. To address this potential bias, we performed sensitivity analyses using both MR-Lap and MR-CAUSE methods19,20. Specifically, MR-Lap models the covariance structure of sample overlap through a joint likelihood framework, while MR-CAUSE employs a hierarchical model to disentangle direct causal effects of instrumental variables from confounding pathways. Importantly, both methods demonstrated high concordance between core causal estimates and traditional IVW results (MR-Lap: β = 0.32, 95% CI 0.25–0.39 vs. IVW β = 0.29, 95% CI 0.22–0.36; MR-CAUSE causal effect probability P = 0.93). To further validate these findings, simulation calibration via MR-Lap indicated a relative bias < 5% (simulated SE = 0.04) under the observed sample overlap proportion (~ 10%). Collectively, these findings suggest that partial sample overlap between exposure and outcome data exerts statistically negligible impacts on primary causal inferences.
Regarding the robustness of genetic instruments, all genetic variants selected as instrumental variables (IVs) in this study underwent rigorous filtering21,22. Notably, the minor allele frequency (MAF) threshold was set to MAF > 0.01, a criterion designed to exclude potential confounding effects from low-frequency variants while simultaneously ensuring the population representativeness of the selected IVs.Building on these methodological safeguards, we addressed the Mendelian randomization (MR) assumptions as follows. First, to satisfy the core MR requirement that SNPs must be robustly associated with the exposure (osteoporosis), we implemented stringent selection criteria: genome-wide significance (P< 5 × 10⁻⁸), linkage disequilibrium clumping (r² < 0.0001), and a genetic distance threshold of 10,000 kb. Second, to ensure that the second MR hypothesis, that genetic variation is not associated with potential confounders, we have queried the Phenoscanner database to determine that the included SNPs were not associated with known confounders23. The potential confounders we considered include Vitamin C, Vitamin D, sex, body mass index (BMI), physical activity. The hypothesis of association was further confirmed by assessing the presence of weak instrumental variable bias in the selected instrumental variables by calculating the F-statistic24,25. An F value greater than 10 indicates the absence of weak instrumental variable bias. The F-statistic is calculated as F = [(N-K-1)/K] × [R2/(1-R2)], where N is the sample size of the exposure factor, K is the number of instrumental variables, and R2 is the instrumental variable proportion of variance explained by the exposure factor. After excluding continuously unbalanced IVs, we extracted a series of SNPs from the GWAS study as IVs for investigating the effects of different BMD measurements on postural instability. Specifically, we selected 20, 22, and 3 SNPs as IVs for studying FN-BMD, LS-BMD, and FA-BMD, respectively. In addition, when studying the effect of postural instability on FN-BMD, LS-BMD, and FA-BMD at different sites, we used the same methodology and extracted 47, 47, and 50 SNPs, except for the setting of P < 5 × 10−⁶.
Statistical analysis
This study employs a two-sample Mendelian randomization analysis, incorporating Steiger filtering and False Discovery Rate(FDR) correction. The IVW, weighted median, and MR-Egger methods are utilized to assess the potential causal relationship between osteoporosis and postural instability, with odds ratios as the primary measure. The relevant formulas for calculating OR using IVW, MR Egger, and weighted median can be found in Supplementary Table 226,27,28.Traditional inverse variance weighting analysis methods are susceptible to instrumental bias or pleiotropy; hence, sensitivity analyses were performed to validate the reliability and robustness of IVW results. To mitigate the impact of horizontal pleiotropy on results, the association between osteoporosis and postural instability was additionally scrutinized using two other established MR techniques: the weighted median and MR-Egger regression methods. Furthermore, the robustness of the findings was assessed through various heterogeneity tests, including the MR-Egger intercept test, Cochran’s Q statistic, and leave-one-out analysis. Notably, SNPs exhibiting significant heterogeneity were identified and excluded using the MR-PRESSO method, followed by a reanalysis to ensure result reliability.
All data analyses were executed using the ‘two samples’ package in R (version 4.3.1). A significance threshold of FDR P.value < 0.05was employed. The data utilized in this study were publicly available, thus obviating the need for ethical approval.
Results
The causal relationship between BMD and postural instability at different sites
The 20, 22, and 3 SNPs from GWAS were found to be associated with BMD at different sites, including FN-BMD, LS-BMD, and FA-BMD. After conducting the Steiger analysis, 19, 20, and 3 SNPs remained. Following IVW analysis and FDR correction, we found strong evidence suggesting a causal relationship between FN-BMD and LS-BMD for postural instability (FDR P < 0.05), underscoring the potential association between osteoporosis and postural instability (Fig. 2).
To ascertain result stability, Cochran’s Q statistical test was performed, revealing heterogeneity in FN-BMD (P = 0.012) and FA-BMD (P = 0.013) (Table 1). Subsequently, the MR-PRESSO assay was employed to exclude 1 SNP from FN-BMD, followed by another IVW analysis. This iteration yielded consistent evidence of a causal link between femoral neck BMD and postural instability (P < 0.05), further substantiating the strong connection between osteoporosis and postural instability. The weighted median method provided similar results (P < 0.05) (Fig. 3).
All datasets underwent MR-Egger testing, which did not reveal any potential horizontal pleiotropy (P > 0.05) (Table 2). Moreover, leave-one-out sensitivity analyses were conducted to assess the influence of each SNP locus on overall causality. These analyses consistently indicated that systematically removing individual SNPs and rerunning MR analysis did not lead to significant differences in the established causality. This further emphasizes that the estimated effects could not be attributed to any single genetic instrument except for FA-BMD on postural instability (Fig. 4).
Mendelian randomization analysis for investigating the causal association between FN-BMD, LS-BMD, FA-BMD and postural instability. (A) Scatter plot of FN-BMD on postural instability. (B) Scatter plot of LS-BMD on postural instability. (C) Scatter plot of FN-BMD on postural instability after removing significant heterogeneity SNP. (D) Scatter plot of FA-BMD on postural instability. (E) Leave-one-out sensitivity analysis of FN-BMD on postural instability. (F) Leave-one-out sensitivity analysis of LS-BMD on postural instability. (G) Leave-one-out sensitivity analysis of FN-BMD on postural instability after removing significant heterogeneity SNP. (H) Scatter plot of FA-BMD on postural instability of FA-BMD on postural instability.
The causal relationship between postural instability and BMD at different sites
In this study, we employed the same statistical methods to investigate the relationship between postural instability and BMD at different sites, including IVW, the weighted median method, and the MR-Egger method. The consistent results obtained from these approaches strongly indicate the absence of a causal relationship between postural instability and BMD (Fig. 5). Furthermore, in order to account for data heterogeneity, we conducted Cochran’s Q test, the results of which demonstrated the absence of significant heterogeneity within the dataset under investigation (Table 3).
Additionally, the MR-Egger test revealed no signs of potential horizontal pleiotropy, thereby reinforcing the reliability of our findings (Table 4). To further confirm the robustness of our results, a sensitivity analysis was carried out, excluding individual genetic variants one by one (Fig. 6).
Mendelian randomization analysis for investigating the causal association between postural instability and FN-BMD, LS-BMD, FA-BMD. (A)Scatter plot of postural instability on FN-BMD. (B) Scatter plot of postural instability on LS-BMD. (C) Scatter plot of postural instability on FA-BMD. (D) Leave-one-out sensitivity analysis of postural instability on FN-BMD. (E) Leave-one-out sensitivity analysis of postural instability on LS-BMD. (F) Leave-one-out sensitivity analysis of FA-BMD on postural instability.
Discussion
Osteoporosis, a skeletal disorder characterized by decreased bone strength and increased risk of fracture, has become a major public health problem worldwide29,30. As the global population ages, osteoporosis raises increasing concerns31,32. Previous observational studies have mentioned a strong association between osteoporosis and Alzheimer’s disease, of which postural instability is a common symptom33. However, a recent Mendelian randomization study systematically analyzed and denied a causal relationship between osteoporosis and Alzheimer’s disease, which contradicts past observational studies34. This study, however, revealed a striking association between osteoporosis, particularly femoral neck osteoporosis, and postural instability. Meanwhile, a retrospective study suggests that BMD may be a risk factor for impaired postural stability. Alexander Simon’s team screened 1,086 patients and found a correlation between lower BMD T-scores in the femur and a longer path to Romberg’s test35. A cross-sectional study by Akira Okayama also emphasized the association between osteoporosis and postural instability in postmenopausal women with instability36. Previously, the causal relationship between the two could not be well explained due to the interference of numerous confounding factors37. In the present study, genetic variants were used as instrumental variables to minimize the interference of acquired confounders and further strengthen the evidence for a causal relationship between osteoporosis and increasing postural instability. In previous studies, incipient deformity in osteoporosis usually manifests as thoracic or thoracolumbar kyphosis. However, in older patients with osteoporosis, mobility is more severely limited especially in the lumbar region, particularly in the range of lumbar extension38. The range of extension of the lower lumbar spine, in particular, may inhibit the lumbar spine’s ability to compensate for thoracic kyphosis, leading to increased postural instability associated with lumbar kyphosis39. This is consistent with our findings.
In this study, we used a Mendelian randomization method using BMD of the femoral neck, lumbar spine, and forearm to assess the degree of osteoporosis and systematically evaluated the causal relationship between osteoporosis and postural instability. Gene prediction results show that osteoporosis of the femoral neck and lumbar spine may be associated with an increased risk of postural instability. Through sensitivity analysis, we further confirmed the positive association, which was not present for the forearm.
It is worth noting that genetic variation is stable over the individual life cycle and alleles are randomly distributed and fixed. Thus, the results of the present study represent a lifelong risk of postural instability in patients with osteoporosis, and bias caused by confounding factors and reverse causality can be avoided.
The prevalence of osteoporosis among the elderly is approximately 15.7%, and this number continues to increase with the aging of the population globally40. Previous studies have examined the factors that contribute to the increased risk of falls, which include aging, history of falls, muscle weakness, home environmental hazards, drug use (especially psychotropic drugs), and cardiac pacing accompanied by carotid sinus hypersensitivity, which have all been reported as risk factors for falls41. In addition, postural instability has also been identified as a risk factor for falls42. With demographic changes, the number of osteoporosis patients is expected to increase significantly in the coming decades, especially with the aging of the population. In the face of a large population of osteoporosis patients, we should pay more attention to their risk of postural instability to prevent the health hazards and economic burden associated with fractures due to falls43.
It is worth noting that this study has some limitations. First, the original GWAS data analyzed in this study were from a European population, so its findings may not apply to other ethnic groups. Second, the limitations of the pooled GWAS data did not allow for stratified analyses based on general factors such as age and gender. Third, it was difficult to completely exclude the influence of horizontal pleiotropy effects. Therefore, the study conducted a series of sensitivity analyses to ensure the reliability of the results.
Conclusion
In summary, this study found that osteoporosis of the femoral neck and lumbar spine was positively associated with the risk of postural instability through the analysis of the Mendelian randomization method, while there was no significant causal relationship between postural instability and osteoporosis at different sites. This finding may open up a whole new avenue for the prevention of postural instability and the treatment of osteoporosis. Therefore, the management of patients with osteoporosis should be strengthened to reduce the risk of postural instability and the incidence of fall injuries caused by it. In addition, due to genetic differences between different ethnic groups, countries, and regions, further studies are needed in different populations.
Data availability
All data generated or analysed during this study are included in this manuscript.
References
Stemplewski, R. et al. The effect of sleep deprivation on postural stability among physically active young adults. Sci. Rep. 13(1), 17477 (2023).
Horak, F. B. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 35 (Suppl 2), ii7–ii11 (2006).
Castro, I. P. R. et al. Predictors of falls with injuries in people with Parkinson’s disease. Mov. Disorders Clin. Pract. 10(2), 258–268 (2023).
Kannan, L., Bhatt, T. & Ajilore, O. Cerebello-cortical functional connectivity May regulate reactive balance control in older adults with mild cognitive impairment. Front. Neurol. 14, 1041434 (2023).
Rachner T D, Khosla, S. & Hofbauer, L. C. Osteoporosis: now and the future. Lancet (London England). 377 (9773), 1276–1287 (2011).
Camacho P. M. et al. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr. Practice: Official J. Am. Coll. Endocrinol. Am. Association Clin. Endocrinologists, 26(Suppl 1): 1–46. (2020).
Reid, I. R., Bolland, M. J. Calcium and/or Vitamin D Supplementation for the Prevention of Fragility Fractures: Who Needs It? Nutrients 12 (4), 1011 (2020).
Bellver, M. et al. Bone mineral density and bone mineral content among female elite athletes. Bone 127, 393–400 (2019).
Yong E. L. & Logan S. Menopausal osteoporosis: screening, prevention and treatment. Singapore Med. J. 62 (4), 159–166 (2021).
Management of osteoporosis in postmenopausal Women: the 2021 Position Statement of the North American Menopause Society. Menopause (New York, NY) 28(9), 973–997 (2021).
Miko, I., Szerb, I. & Szerb, A. Effect of a balance-training programme on postural balance, aerobic capacity and frequency of falls in women with osteoporosis: A randomized controlled trial. J. Rehabil. Med. 50(6), 542–547 (2018).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4 (4), 330–345 (2017).
Titova, O. E., Michaëlsson, K. & Larsson, S. C. Sleep duration and stroke: prospective cohort study and Mendelian randomization analysis. Stroke 51(11), 3279–3285 (2020).
Evans, D. M. & Davey Smith, G. Mendelian randomization: new applications in the coming age of Hypothesis-Free causality. Annu. Rev. Genom. Hum. Genet. 16, 327–350 (2015).
Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45(6), 1961–1974 (2016).
Zheng, H. F. et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 526(7571), 112–117 (2015).
Trajanoska, K. et al. Genetic basis of falling risk susceptibility in the UK biobank study. Commun. Biology 3(1), 543 (2020).
Burgess, S., Davies, N. M. & Thompson, S. G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40(7), 597–608 (2016).
Mounier N. & Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. Genet. Epidemiol. 47 (4), 314–331 (2023).
Morrison, J. et al. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 52(7), 740–747 (2020).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81(3): 559–575. (2007).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open. Res. 4, 186 (2019).
Kamat, M. A. et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinf. (Oxford England). 35 (22), 4851–4853 (2019).
Deng, L. et al. Approximation of bias and mean-squared error in two-sample Mendelian randomization analyses. Biometrics 76(2), 369–379 (2020).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian Randomization Analysis With Multiple Genetic Variants Using Summarized Data. Genet. Epidemiol. 37 (7), 658–665 (2013).
Burgess, S. & Thompson S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32(5), 377–389 (2017).
Bowden, J., Davey Smith G. & Burgess S. J. Mendelian randomization with invalid instruments: effect Estimation and bias detection through Egger regression. International journal of epidemiology 44(2), 512–525 (2015).
Burgess, S., Dudbridge, F. & Thompson S. G. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Statistics in medicine 35(11), 1880–1906 (2016).
Genant H. K. et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporosis international 10 (4), 259–264 (1999).
Muñoz, M., Robinson, K. & Shibli-Rahhal, A. Bone health and osteoporosis prevention and treatment. Clin. Obstet. Gynecol. 63 (4), 770–787 (2020).
Chandra, A. & Rajawat, J. Skeletal aging and osteoporosis: mechanisms and therapeutics. Int. J. Mol. Sci. 22(7), 3553 (2021).
Arceo-Mendoza R. M., & Camacho, P. M. Postmenopausal osteoporosis latest guidelines. Endocrinol. Metab. Clin. North Am. 50(2), 167–178 (2021).
Fehsel, K. & Christl, J. Comorbidity of osteoporosis and Alzheimer’s disease: is `AKT `-ing on cellular glucose uptake the missing link?. Ageing Res. Rev. 76, 101592 (2022).
Hu, H. et al. No genetic causal association between Alzheimer’s disease and osteoporosis: A bidirectional two-sample Mendelian randomization study. Front. Aging Neurosci. 15, 1090223 (2023).
Simon, A. et al. Evaluation of Postural Stability in Patients Screened for Osteoporosis: A Retrospective Study of 1086 Cases. Gait & posture 88, 304–310 (2021).
Okayama, A. et al. Prevalence of sarcopenia and its association with quality of life, postural stability, and past incidence of falls in postmenopausal women with osteoporosis: A Cross-Sectional study. Healthcare (Basel, Switzerland) 10(2), 192 (2022).
Lynn, S. G., Sinaki, M. & Westerlind, K. C. Balance characteristics of persons with osteoporosis. Arch. Phys. Med. Rehabil. 78 (3), 273–277 (1997).
Duangkaew, R. et al. PROTOCOL: exercise interventions to improve back shape/posture, balance, falls and fear of falling in older adults with hyperkyphosis: A systematic review. Campbell Syst. Reviews 16 (3), e1101 (2020).
Fechtenbaum, J. et al. Sagittal balance of the spine in patients with osteoporotic vertebral fractures. Osteoporosis international 27 (2), 559–567 (2016).
Carey, J. J., Chih-Hsing Wu, P. & Bergin, D. Risk Assessment Tools for Osteoporosis and Fractures in 2022. Best practice & research Clinical rheumatology 36 (3), 101775 (2022).
Dautzenberg, L. et al. Interventions for preventing falls and fall-related fractures in community-dwelling older adults: A systematic review and network meta-analysis. J. Am. Geriatr. Soc. 69 (10), 2973–2984 (2021).
Booth, V., Hood, V. & Kearney, F. Interventions incorporating physical and cognitive elements to reduce falls risk in cognitively impaired older adults: a systematic review. JBI Database Syst. Reviews Implement. Rep. 14 (5), 110–135 (2016).
Khandelwal, S. & Lane, N. E. Osteoporosis: Review of etiology, mechanisms, and approach to management in the aging population. Endocrinol. Metab. Clin. North Am. 52(2), 259–275 (2023).
Acknowledgements
We thank Dr. Wenjia Peng for his assistance in the revision.
Funding
Medical Innovation Foundation from Spinal deformity clinical and research center of Anhui province (AHJZJX-GG2023-004). Natural Science Research Project of Anhui Educational Committee (2024AH051233). Science Research Project of Anhui Health Commission (AHWJ2023A30070). Excellent scientific research and innovation team of Anhui universities(2024AH010020).
Author information
Authors and Affiliations
Contributions
Qingqiang Yao, Rui Zhao and Haiyang Yu designed the project. Rui Zhao and Kun Zhu conducted the literature review. Kun Zhu, Zijie An and YaWei Li is responsible for the computer code design and code test running. ZiJie An, Feng Zhang and Qiaoyu Zhang was responsible for writing of the manuscript and performed the statistical analysis. Kun Zhu and ZiJie An edited and polished the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, K., An, Z., Li, Y. et al. A causal association between osteoporosis and postural instability: a Mendelian randomization study. Sci Rep 15, 10234 (2025). https://doi.org/10.1038/s41598-025-93793-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93793-w