Abstract
Laboratory and pot experiments were conducted to evaluate the influence of leachates of fresh/dry parts of marigold on photosynthetic pigments and nitrogen metabolism of wheat. Considerable decline in chlorophylls and carotenoids and the activities of enzymes of nitrogen metabolism (nitrate reductase, alanine and aspartate aminotransferases, glutamate dehydrogenase and glutamate synthase) was noticed in plants treated with higher concentration of fresh (30% w/v) or dry (10% w/v) leaf and flower leachates of marigold. However, treatment of lower concentrations i.e., 5% (w/v) of leachates of fresh parts and 1% (w/v) leachates of dry parts imparted stimulatory effects. Sodium and potassium contents in different parts of wheat plants showed a significant increase with the increase in the concentration of dry leachates both at pre-flowering and flowering stages. On the other hand, nitrogen and calcium content exhibited a decline with the increase in concentration of leachates. The study indicates that identification of allelochemicals in these leachates may probably help evaluation of marigold as a natural herbicide for sustainable agriculture paving way for further study.
Similar content being viewed by others
Introduction
Interference between plants is referred either to competition for the utilization of necessary resources required for the growth and development or to allelopathy1. Exudation of soluble chemicals or release of volatile organic compounds from plants can affect the soil microbes in the surrounding environment by altering the chemical and physical properties thereby inhibiting the growth of certain plant species2. The allelopathic interactions among different biotic components in agro-ecosystems encompassing crops, weeds, trees and microbes may contribute in improvement of crop production, genetic diversity, nutrient conservation, management of noxious weeds and pests and in the maintenance of ecosystem stability3,4,5. Allelochemicals play a variety of functions/roles such as in plant defence, nutrient chelation, symbiosis and soil macro and microbiota affecting decomposition and soil fertility3,6.
As an environmental stress, allelopathy plays a significant role in stress ecotoxicity caused by secondary metabolites. It is one of the powerful biochemical weapons, that can easily cause plant physiological and biochemical imbalances7,8 by decreasing leaf stomatal conductance, transpiration9, photosynthesis10,11, expression and activity of enzymes necessary for efficient seed germination such as, acid phosphatase, peroxidase, catalase and amylase12. In addition, allelopathy decreases the activities of nitrogen metabolizing enzymes13,14,15 and also affects accumulation and transport of ions across membranes16, which ultimately inhibits plant growth and metabolism16,17.
Allelopathic compounds include secondary plant products released through volatilization, root exudation and leaching of dead and decomposed plant parts into the environment/soil18,19,20 which directly or indirectly influence the surrounding plants, a wide number of such allelochemicals have been identified affecting the germination and growth of different plants20,21,22,23. The allelochemicals released by the plant residues left in the fields after the harvest of crops may pose problems which could alternatively be used for weed and pest management. Use of cover crops, transfer of allelopathic traits to modern day cultivars, direct use of allelochemicals as natural pesticides etc. may be the strategies exploited for weed management. The purified allelochemicals and their derivatives can serve as novel agrochemicals for eco-friendly management22,23,24,25,26,27.
Wheat belonging to family Poaceae is a major cereal crop throughout the world. It is the main energy source consumed globally and is rich in proteins, minerals, vitamins and dietary fiber. In India particularly northern plains wheat is the most staple food crop and India is the fourth major wheat-producing country in the world. It plays a significant role in fulfilling food and energy requirements and in the livelihood of farmers. On the other hand, Tagetes erecta L. commonly known as marigold is an important traditional flower crop grown in India and the world over due to its adaptability to diverse environmental conditions28,29. Apart from the diverse medicinal importance of marigold29 in India marigold is profusely used in different social and religious practices and after use may be disposed off in an agricultural land. It is with this background the present study was conducted to assess the impact of marigold leachates on wheat.
Material and methods
Certified seeds of marigold (Tagetes erecta L.) cultivar Pusa Basanti Gainda (PBG) were procured from Indian Agriculture Research Institute (IARI) Pusa, New Delhi, India and plants were raised in Botanical Garden of School of Studies in Botany, Jiwaji University, Gwalior, India. The plants were uprooted and the different parts were separated. Leachates of different fresh/dry parts of marigold were prepared to evaluate the allelopathic potential of marigold. Wheat (cultivar LOK-1; seeds were obtained from Krishi Vigyan Kendra, Rajmata Vijayaraje Scindia Krishi Vishwa Vidhyalaya, Gwalior, MP) has been selected as test crop in order to evaluate the effects of marigold leachates.
Preparation of leachates
Fresh leachate preparation
30 g fresh leaf and flower tissue of marigold plants were cut into small pieces and were soaked in 100 ml distilled water for 48 h at room temperature. Thereafter, leachates were squeezed through double layered cheese cloth and centrifuged at 3000 g for 15 min. Final volume was made up to 100 ml using distilled water and the supernatant obtained was considered as 30% leachates of fresh leaf (FLL) and fresh flower (FFL). Different concentrations (5%, 10% and 20%) were prepared by diluting these solutions and were stored at 4 ℃ until used for bioassay.
Dry leachate preparation
Oven dried samples were ground with the help of a mechanical grinder. The powder was sieved through 2 mm sieve and 10 g dry powder was soaked in 100 ml distilled water for 48 h at room temperature. Subsequently, leachates were squeezed through cheese cloth and filtered through Whatman no. 1 filter paper. The filtrate was centrifuged at 3000 g for 15 min and the final volume made up to 100 ml using distilled water (supernatant was considered as 10% leachate). Further dilutions using distilled water were done for obtaining 1 and 5% leachates of dry leaves (DLL) and dry flowers (DFL) and were stored at 4 ℃ until further use.
Experimental setup
Following three types of experiments were conducted to assess the impact of fresh and dry leachates on growth of wheat:
Laboratory experiments
Uniform and healthy seeds of selected wheat (cultivar LOK-1) were surface sterilized using 0.01% mercuric chloride solution followed by thorough washing with distilled water. Ten sterilized seeds were placed in each petri plate, lined with Whatman no. 1 filter paper and petri plates were wetted with leachates of dry plant parts (leaves/flowers) and control was maintained with distilled water. Seedlings were analysed on the 7th day of seed wetting.
Pot experiments using sand substrate
Seeds of wheat (Triticum aestivum L. cultivar LOK-1) were sown in pots (18 cm diameter) filled with acid washed sand30. Pots were wetted with 200 ml full strength Hoagland solution as given in detail by Mir et al.31. Ten days after germination, pots were divided into different groups and were treated with: (a) 5, 10, 20 and 30% leachates of fresh leaves and flowers of Tagetes erecta L in one set, and (b) 1, 5 and 10% leachates of dry leaves and flowers of Tagetes erecta L in another set. Leachates were prepared in full strength Hoagland solution and control was supplied with normal Hoagland solution. Leachate treatment (200 ml per pot) was applied on every alternate day for another 15 days. Twenty five day old seedlings were put to analysis.
Based on the screening experiments performed on 25 days old seedlings, it was obvious that dry leachates have greater impact in comparison to fresh leachates. Therefore, dry leachates were used for further experimentation i.e., laboratory and field experiments.
Pot experiments using soil substrate
Bottom perforated polythene bags (with area of 0.0962 m2) were filled with well ploughed garden soil. Fifteen days after germination, pots were divided into different groups and were treated with 1, 5 and 10% dry leaf and flower leachates (200 mL). Irrigation was avoided for five days before and after leachate treatment. Pots maintained as ‘Control’ were supplied with normal water. Pots were arranged in randomized block design with four replicates for each treatment. Plants raised were put to analysis for the different components of nitrogen metabolism, chlorophyll pigments and elements at pre-flowering and flowering stage.
Estimation of photosynthetic pigments
Chlorophylls and carotenoids were extracted by homogenising 100 mg fresh leaf tissue in 80% acetone. After centrifugation of homogenate at 3000 g for 10 min the supernatant was read spectrophotometrically at 645, 663 and 480 nm and expressed as mg g-1 FW32.
Estimation of nitrate reductase, aminotransferases, glutamate synthase and glutamate dehydrogenase activity
For determination of nitrate reductase (EC 1.6.6.1) activity method of Srivastava33 as adopted by Ahanger and Agarwal34 was followed. A 300 mg fresh plant sample was incubated in a 100 mM phosphate buffer (pH 7.5) containing 200 mM KNO3 and 0.5% n-propanol in dark for 3 h at 30 ℃. Aliquot was mixed with 1% sulphanilamide and 0.2% 1-naphthylethylenediamine dihydrochloride and optical density was recorded at 540 nm. Activity was expressed as µ mole nitrite produced h-1 g-1 FW.
Aminotransferases [aspartate aminotransferase (EC 2.6.1.1) and alanine aminotransferase (EC 2.6.1.2)] were assayed following Reitman and Frankel35. Fresh tissue was homogenized in 1.5 ml chilled sodium–potassium buffer (0.05 M, pH 7.0) and extract was centrifuged at 5000 g for 15 min. Supernatant (50 µl) was added to 100 µl substrate buffer (pH 7.0) and incubated at 37 ℃ (60 min for aspartate aminotransferase and 30 min for alanine aminotransferase) followed by addition of 0.1 ml DNPH. After 20 min, 0.4 M NaOH was added. Thereafter optical density was recorded at 540 nm.
For extraction of glutamate dehydrogenase and glutamate synthase, 100 mg fresh plant tissue was homogenised in cold 0.1 M Tris–HCl buffer (pH 8.0) containing 1 mM magnesium chloride, 1 mM cysteine, 1% PVP and 1 mM EDTA using prechilled pestle and mortar. After centrifugation at 13,000 g for 20 min at 4 ℃, supernatant was used as an enzyme source. For glutamate dehydrogenase (NADH-GDH; EC 1.4.1.2) activity Robinson et al.’s method36 was employed and consumption of NADH was recorded at 340 nm.
Glutamate synthase (NADH-GOGAT; EC 1.4.1.14) was estimated as per the method of Lea et al.37 in an assay mixture containing 0.1 mM NADH, 10 mM glutamine, 10 mM 2-oxoglutarate, 100 mM potassium phosphate buffer (pH 7.5) and 200 µL enzyme. Change in absorbance was recorded at 340 nm by monitoring the disappearance of NADH spectrophotometrically.
Estimation of sodium, potassium, calcium and nitrogen
Sodium, potassium and calcium were estimated using a flame photometer (Systronic flame photometer-128) employing different filters according to a method adopted by Ahanger et al.38. Nitrogen was estimated following the micro-Kjeldahl method as suggested by Jackson39 and modified by Iswaran and Marwaha40. 1.0 gm dry plant sample acid digested using H2SO4 supplemented with catalyst mixture containing K2SO4, FeSO4.5H2O and CuSO4.5H2O. Digested samples were subjected to distillation and N was determined by titrating the sample against NaOH.
Statistical analysis
Data presented is the mean of four replicates with standard error (± SE) calculated. ANOVA was worked out using Statistix 8.1 software and a significant difference was calculated at 0.05% level of significance.
Results
Effect of marigold leachates on photosynthetic pigments
Treatments of lower concentrations (1% w/v) of DLL and DFL of Tagetes erecta showed stimulatory effects on chlorophyll and carotenoid contents of Triticum aestivum L. whereas, at higher concentration i.e., at 10% w/v, reduction in chlorophylls and carotenoids was observed (Tables 1, 3, 4). Percent reduction in chlorophyll a, chlorophyll b, total chlorophylls and carotenoids was 30.00, 28.40, 29.19 and 50.41% due to 10% DLL and 36.15, 36.36, 35.83 and 74.38% due to 10% DFL (Table 1). On the other hand, chlorophyll a, chlorophyll b, total chlorophylls and carotenoids showed 16.92, 6.81, 15.02 and 6.61% increase in 1% DLL treated wheat seedlings and 22.69, 4.54, 18.78 and 5.78% increase in 1% DFL treated wheat seedlings (Table 1).
Wheat plants exposed to treatments of fresh leachates (both leaves and flowers) of Marigold exhibited significant increase with 5% and decrease with 30% concentrations. An increase of 10.03% and 5.45% in chlorophyll a, 4.76% and 3.61% in chlorophyll b, 7.50% and 4.57% in total chlorophylls and 14.53% and 14.96% in carotenoids was seen upon application of 5% FLL and 5% FFL leachates respectively (Table 2). Wheat plants treated with 10% fresh leachates (leaves and flowers) of Marigold exhibited hardly any impact (stimulatory or inhibitory) on chlorophyll pigments, however, reduction in pigments was gradually obvious at 20% and maximum reduction was observed with 30% leachate treatments. The reduction was 31.51% (chlorophyll a), 45.14% (chlorophyll b), 38.06% (total chlorophylls) and 28.19% (carotenoids) in 30% FLL treated plants and 29.75% (chlorophyll a), 41.52% (chlorophyll b), 35.40% (total chlorophylls) and 33.18% (carotenoids) in 30% FFL treated wheat plants (Table 2). Leachates of dry parts were more effective (stimulatory/inhibitory) at lower concentrations as compared to the leachates of fresh parts and the similar trend was noticed in both treated seedlings and older plants (Tables 1, 2, 3, 4). Reduction in pigment contents of wheat plants in response to leachates of different plant parts of marigold is an indicator of its phytotoxicity against wheat plants. Nevertheless, treatments of lower concentrations (5% w/v of fresh leachates) and (1% w/v of dry leachates) from both plant parts (flowers and leaves) of Marigold showed stimulatory effects.
Effect of marigold leachates on activity of nitrogen metabolizing enzymes
Activity of nitrate reductase (NR)
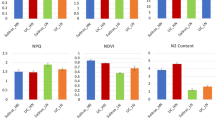
Nitrate reductase activity increased in wheat seedlings/plants receiving the treatments of low concentration (1% w/v) of dry leachates (leaves and flowers) of Tagetes erecta L. whereas, at higher concentrations i.e. 5% w/v of leachates of dry leaves and flowers slight reduction was noticed in NR activity and the maximum reduction in the activity of NR was found at 10% w/v concentration (Fig. 1A, D; Fig. 3A; Fig. 4A). Wheat plants raised using sand substrates showed 26.48 and 24.08% increase in NR activity upon application of 1% DLL and 1% DFL. On the other hand, NR activity was reduced by 17.09 and 21.25% upon application of 10% DLL and DFL treatments (Fig. 3A). Treatments of fresh parts (leaves and flowers) were effective at higher concentration as compared to those of dry parts i.e. stimulatory at 5% w/v and inhibitory at 10% w/v and above. Conspicuous reduction was observed at 30% w/v treatments of fresh leachates (leaves and flowers) of Marigold (Fig. 2A). An increase of 17.94 and 21.32% was recorded in wheat plants treated with 5% FLL and 5% FFL (Fig. 2A). Treatments with 10% concentrations of fresh leachates showed hardly any alteration (stimulatory/inhibitory) in the activity of NR, however, reduction in the activity of NR was observed at 20% leachate treatments and the maximum reduction i.e., up to 47.41 and 43.44% was recorded upon application of 30% FLL and 30% FFL concentrations (Fig. 2A).
Nitrate reductase, alanine and aspartate aminotransferase, glutamate synthase and glutamate dehydrogenase activity in leaves (A-E) of wheat (Triticum aestivum L.) plants raised using sand substrate (25 DAS) treated with leachates of fresh leaves and flowers of Tagetes erecta L. Data followed by same letters are not significantly different at p < 0.05.
Nitrate reductase, alanine and aspartate aminotransferase, glutamate synthase and glutamate dehydrogenase activity in leaves (A-E) of wheat (Triticum aestivum L.) plants raised using sand substrate (25 DAS) treated with leachates of dry leaves and flowers of Tagetes erecta L. Data followed by same letters are not significantly different at p < 0.05.
Nitrate reductase, alanine and aspartate aminotransferase, glutamate synthase and glutamate dehydrogenase activity in flag leaves of wheat (Triticum aestivum L.) plants raised using soil substrate at preflowering (A–E) and flowering (F–J) treated with leachates of dry leaves and flowers of Tagetes erecta L. Data followed by same letters are not significantly different at p < 0.05.
The flag leaves of older wheat plants i.e., 85 DAS plants showed greater NR activity as compared to younger plants i.e., 55 DAS plants raised in the field using soil substrate. On application of treatments of different concentrations of leachates of dry marigold parts, wheat plants showed alteration in the activity of nitrate reductase. The 10% DLL and 10% DFL treated plants showed greater reduction in NR activity i.e. by 58.57 and 54.06% at vegetative phase (Fig. 4A) and by 36.01 and 41.67% at flowering phase (Fig. 4F). However, wheat plants applied with 1% DLL and 1% DFL treatments showed enhancement in NR activity by 14.24 and 31.25% at vegetative stage (Fig. 4A) and by 17.61 and 22.25% at flowering phase respectively (Fig. 4F).
Activity of alanine and aspartate aminotransferases (AlaAT and AsAT)
Under laboratory conditions the activity of aminotransferases (alanine and aspartate aminotransferase) in wheat seedlings showed reduction upon exposure to treatments of higher concentration (10% w/v) leachates of dry parts of marigold. On the other hand treatments of lower concentration (1% w/v) leachates of dry parts enhanced the activity of aminotransferases (Fig. 1B, C, E, F).
Wheat plants raised using sand substrates receiving treatments of 30% FLL and 30% FFL showed reduction in the activity of AlaAT by 26.22 and 31.47% and AsAT by 33.04 and 21.15% (Fig. 2B, C), decrease in activity of AlaAT by 4.87 and 7.64% and AsAT by 10.52 and 24.67% was also recorded upon application of 10% DLL and 10% DFL treatments (Fig. 3B, C).
Nevertheless, an increase in the activity of AlaAT by 32.61 and 12.54% and AsAT by 11.59 and 11.30% was noticed in leaves of wheat plants treated with 5% FLL and 5% FFL leachates (Fig. 2B-C) and these stimulatory effects of leachates were also recorded in the leaves of wheat plants treated with 1% DLL and 1% DFL (Fig. 3B, C) in which activity of AlaAT is enhanced by 14.78 and 31% and AsAT activity by 23.02 and 17.76% as compared to control.
More or less similar trend was found in flag leaves of wheat plants raised using soil substrates under field conditions both at pre-flowering (Fig. 4B, C) and flowering stages (Fig. 4G, H). However, the greater activity of aminotransferases was recorded in older plants i.e., at flowering stage. Plants treated with higher concentration of leachate treatments 10% DLL and 10% DFL showed reduction in the activity of AlaAT by 19.04 and 10.86% and AsAT by 20.23 and 31.37% as compared to control (untreated) plants at flowering stage but, the enhancement in the activity of AlaAT by 9.02 and 6.18% and AsAT by 6.15 and 18.47% was noticed in plants receiving lower concentration of leachates i.e., 1% DLL and 1% DFL (Fig. 4G, H).
Activity of glutamate synthase (GOGAT) and glutamate dehydrogenase (GDH)
Glutamate synthase (GOGAT) and glutamate dehydrogenase (GDH) activity of wheat plants showed significant decrease with increase in the concentration of leachates of fresh and dry parts of marigold used as treatments. Experiments showed maximum reduction in the activity of GOGAT i.e., by 43.07 and 37.94% in wheat plants receiving 30% FLL and 30% FFL treatments (Fig. 2D) and by 60.59 and 63.54% in 10% DLL and 10% DFL treated plants respectively (Fig. 3D). Slight increase in the activity of GOGAT i.e. 30.25 and 19.48%, when fed with 5% FLL and 5% FFL leachates (Fig. 2D) and by 13.79 and 5.41% when fed with 1% DLL and 1% DFL leachates (Fig. 3D) was noticed. Similarly, 34.29 and 49.23% decline in GDH activity was recorded in wheat plants subjected to 30% FLL and 30% FFL treatments (Fig. 2E) and 36.42 and 52.49% decrease was found in 10% DLL and 10% DFL treated plants respectively (Fig. 3E). Activity of GDH also showed 9.60 and 14.17% increase when exposed to 5% FLL and 5% FFL treatments (Fig. 2E) and 20.63 and 21.19% while using 1% DLL and DFL treatments (Fig. 3E).
More or less similar trend was observed in flag leaves of wheat plants raised using soil substrates under field conditions at pre-flowering and flowering stages. Up to 42.40 and 37.17% reduction in the activity of GOGAT and 50.84 and 49.61% of GDH was recorded at pre-flowering (Fig. 4D, E) and up to 21.15 and 29.32% of GOGAT and 34.10 and 30.50% of GDH at flowering stage subjected to 10% DLL and 1treated with 10% DLL and 10% DFL.
Effect of marigold leachates on uptake of essential mineral elements like sodium, potassium, calcium and nitrogen
There was an increase in sodium and potassium contents in different parts of wheat plants raised under the treatment of higher concentration of leachates of dry parts particularly at flowering stage (Tables 5, 6) which may also have contributed to the alteration in growth/productivity parameters. Potassium contents in stem and root are not affected much with treatments of dry leachates which indicate efficient transport of potassium to leaves which ultimately restricts the sodium.
Calcium and nitrogen contents of wheat plants registered decrease both at vegetative as well as at flowering stage with increase in concentration of leachates applied. Maximum decline in the contents of calcium and nitrogen was observed in plants which receive dry leachates (10% w/v) of both plant parts (leaves and flowers) of marigold. The flag leaves accumulate greater calcium and nitrogen contents than stem and root. Nitrogen contents showed significant increase with the age of the plant however, no such significant increase was noticed in calcium content of treated wheat plants (Tables 7, 8).
Discussion
Allelopathic stress has been one of the major factors restricting crop distribution, growth and yield worldwide. Allelochemicals produced in plant tissues can control the survival and reproduction of other plants while disseminating into the environment through leaching, volatilization and root exudation41. Mechanisms mediating it through alterations in the physiological and biochemical pathways have not been studied so well. The study of allelopathy has been more feasible when residues of allelopathic plants were extracted/leachates prepared and subjected to the test crop plants. In the present study leachates of dry and fresh marigold leaves and flowers were applied to wheat. The results revealed a decrease in photosynthetic pigments of wheat plants exposed to higher concentration of leachates (fresh/dry parts) of marigold in all experimental sets, which may probably be the effect of allelochemicals present in these leachates. Photosynthesis is a vital process for plant growth and development which sustains plant life on earth. Extreme environmental conditions decrease photosynthetic efficiency of plants by inducing oxidative stress42, which decreases the activity of Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) and hampers the process of photosynthesis44,45. Under such conditions the assimilation of CO2 through Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) pathway is prevented46,47.
There are reports which indicate that allelochemicals can harm the performance of stomatal control of CO2 supply, electron transport and the carbon reduction cycle48. Allelochemicals like Gramine decrease chlorophyll a, β-carotene, phycocyanin, allophycocyanin, phycoerythrin and total phycobilin pigments in cyanobacterium49. Allelochemicals benzoxazolin-2(3H)-one (BOA) and cinnamic acid (CA) inhibit PSII photochemistry and protein contents of 1-month-old C3 plant species Dactylis glomerata, Lolium perenne, and Rumex acetosa50. These stressors reduce chlorophylls, carotenoids and protein contents in Capsicum annuum L. upon application of aqueous leachates of Achillea biebersteinii51. Trichodesma africanum L. affects the chlorophyll and total carbohydrate contents in Portulaca oleracea L52. Bidens pilosa drastically affects both photo-system I and photo-system II activity of Pteris multifida. The damage was caused by root exudates and was experimentally found that invasive plants were responsible as compared to the non invasive ones for the harm to photosynthesis in local species53.
Besides, decrease in photosynthetic pigments of wheat plants while exposing to higher concentrations of marigold leachates, the results of the present study showed some enhancement in chlorophyll pigments of wheat plants when exposed to lower concentrations of marigold leachates. The positive impact of allelochemicals has also been reported in other plants. Seedlings of Triticum aestivum L. fed with dry leachates of leaves and ovary walls of Jatropha curcas were inhibited at higher concentration (1/10 w/v) whereas, treatments of lower concentration (1/100 w/v) of leachates was stimulatory to seedling growth14. Growth of pepper plants as reflected in plant height, chlorophyll contents was stimulatory at low concentration (pepper/garlic ratio 1:1 or 1:2) whereas, high garlic concentration (pepper/garlic ratio 1:4) led to damaging effects i.e. increasing MDA level due to lipid peroxidation in pepper leaves54. Fresh leaf extracts and leaf litter leachates of Chromolaena odorata inhibited germination, chlorophylls and seedling growth of Salvadora persica55.
Nitrate reductase (NR) is an important component of nitrogen metabolism. The activity of NR showed decrease in wheat seedlings subjected to higher concentration of marigold leachates and the roots of wheat seedlings are more affected as compared to shoots. However, the activity of nitrate reductase increases in wheat seedlings supplemented with lower concentration of treatments under laboratory conditions and a more or less similar trend was observed in field experiments (using sand and soil substrates). Nitrate reductase is the key enzyme catalyzing reduction of nitrate to nitrite for further assimilation. Nitrate reductase is also associated with the production of the signalling molecule nitric oxide (NO), which plays an important role in the regulation of plant growth and resistance under biotic and abiotic stresses56,57,58,59. The decline in the activity of nitrate reductase in different plants exposed to different environmental stresses like water stress60, salinity stress34,61,62, heavy metals63,64 and even under allelopathic stress has been reported earlier also13,14,15. Similarly, the activity of GDH and GOGAT showed significant increase in leaves of wheat plants subjected to lower concentration of leachates of fresh/dry leaves and flowers of marigold. This increased GDH activity may contribute towards enhanced synthesis of important amino acids like glutamate; however, the decrease in GDH and GOGAT activity at higher concentration of treatments reflect reduction in growth and ultimately yield loss. The concentration dependent effect on the activity of GS, GOGAT and GDH has been reported in Daucus carota L. treated with coumarin65, Lactuca sativa upon application of phytotoxic lignans like dihydro coniferyl alcohol and lariciresinol66 and Acorus calamus L. upon exposure to different concentrations of microcystins67. Nitrogen metabolism controls various cellular processes in plants by providing proteins and nucleic acids. Nitrogen uptake and metabolism play a crucial role in plant resistance by responding differently to different abiotic stresses at morphological, physiological and transcriptional levels68.
Activity of aminotransferases decreases in both roots and shoots of wheat seedlings treated with higher concentration of marigold leachates. Roots of wheat seedlings exhibited greater alanine and aspartate aminotransferase activity as compared to shoots in almost all treatments under laboratory conditions. In pot experiments (using sand and soil substrates) the activity of alanine and aspartate aminotransferases decreases when fed with higher concentration of fresh/dry leachates of marigold parts. Enhancement in the activity of aminotransferases in wheat plants upon application of treatments of lower concentrations of leachates of marigold is also found in all experimental sets. Aminotransferases play an important role in nitrogen assimilation catalyzing the reversible transamination of keto-acids into amino acids resulting in enhanced biosynthesis of essential amino acids69,70. These results reflect the trend similar to that reported by Tomar et al.14 i.e. concentration dependent decrease in activity of aminotransferases in wheat seedlings up on exposure to Jatropha curcas leachates.
Sodium and potassium contents showed concentration dependent increase in flag leaves as well as in stem and roots of wheat plants treated with different concentrations of dry leaf and flower leachates of marigold however, the contents of calcium and nitrogen showed a decline with increasing concentration of leachates used as treatments both at vegetative and flowering stages. Mineral nutrients play a very important role during environmental stresses60,71. It has been reported earlier also that plants facing allelopathic stress showed increased or decreased accumulation of different nutrient contents depending on the concentration of treatments applied. Kobza and Einhellig72 found accumulation of different nutrient contents by sorghum plants treated with different concentrations of ferulic acid to be concentration dependent. Al-Hawas and Azooz73 have reported pea plants treated with different concentrations of Artemisia monosperma and Thymus vulgaris leaf extracts to show concentration dependent sodium, potassium, calcium and magnesium uptake/accumulation. Similarly, a significant increase or decrease in P, K, Ca, Mg and S contents in roots and shoots of Scots pine (Pinus sylvestris) and Norway spruce (Picea abies) seedlings treated with lichen metabolite usnic acid has been reported74. In most of the cases the effect was seen more pronounced in the wheat plants treated with flower leachates as compared to leaf leachates.
Conclusion
Conclusively, it can be said that decrease in chlorophyll and carotenoid contents and down-regulation of the activity of nitrogen metabolizing enzymes like nitrate reductase, alanine and aspartate aminotransferases, glutamate dehydrogenase and glutamate synthase was evident due to application of higher concentrations of marigold leaf and flower leachates. Nevertheless, slight stimulatory effects on the growth and the enzyme activities of seedlings/plants were noticed due to treatments of lower concentration. More or less similar trend was observed in all experimental sets. Further studies may be helpful to understand the underlying mechanism of allelochemicals of marigold leachates influencing photosynthesis and nitrogen assimilation of treated plants and to understand the expression levels of related genes that could be a valuable tool to improve the nitrogen metabolism under such extreme conditions of soil and environment.
Data availability
All data generated or analysed during this study are included in this published article. Data shall be made available on request from corresponding author; Mohammad Abass Ahanger (ahangerma@gmail.com).
References
Reigosa, M. J., Sanchez Moreiras, A. M. & Gonzalez, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 18, 577–608 (1999).
Bertin, C. & Yang, X. Weston LA The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256, 67–83 (2003).
Chou, C. H. Roles of allelopathy in plant biodiversity and sustainable agriculture. Crit. Rev. Plant Sci. 18, 609–636 (1999).
Singh, H. P., Batish, D. R. & Kohli, R. K. Allelopathy in agroecosystems: an overview. J. Crop Prod. 4, 1–41 (2001).
Singh, H. P., Batish, D. R. & Kohli, R. K. Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit. Rev. Plant Sci. 22, 239–311 (2003).
Callaway, R. M. & Ridenour, W. M. Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2, 436–443 (2004).
Wardle, D. A., Karban, R. & Callaway, R. M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 26, 655–662 (2011).
Zhang, Z., Liu, Y., Yuan, L., Weber, E. & van Kleunen, M. Effect of allelopathy on plant performance: a meta-analysis. Ecol. Lett. 24, 348–362 (2020).
Yu, J. Q., Ye, S. F., Zhang, M. F. & Hu, W. H. Effects of root exudates, aqueous root extracts of cucumber (Cucumis sativus L.) and allelochemicals on photosynthesis and antioxidant enzymes in cucumber. Biochem. System. Ecol. 31, 129–139 (2003).
Farhoudi, R. & Lee, D. J. Allelopathic effects of barley extract (Hordeum vulgare) on sucrose synthase activity, lipid peroxidation and antioxidant enzymatic activities of Hordeum spontoneum and Avena ludoviciana. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 83, 447–452 (2013).
Li, J. et al. Allelopathic effect of Artemisia argyi on the germination and growth of weeds. Sci. Rep. 11, 4303 (2021).
Shankar, R. M., Veeralakshmi, S., Sirajunnisa, A. R. & Rajendran, R. Effect of allelochemicals from leaf leachates of Gmelina arborea on inhibition of some essential seed germination enzymes in green gram, red gram, black gram, and chickpea. Int. Sch. Res. Notices 1, 108682 (2014).
Singh, N. B. Allelopathic stress produced by bitter gourd (Momordica charantia L.). J. Stress Physiol. Biochem. 10, 5–14 (2014).
Tomar, N. S., Sharma, M. & Agarwal, R. M. Phytochemical analysis of Jatropha curcas L. during different seasons and developmental stages and seedling growth of wheat (Triticum aestivum L) as affected by extracts/leachates of Jatropha curcas L. Physiol. Mol. Biol. Plants 21, 83–92 (2015).
Mozdzen, K. et al. The allelopathic potential of Rosa blanda Aiton on selected wild-growing native and cultivated plants in Europe. Plants 10, 1806 (2021).
Suntia Roa, N. B. & Singh, S. Allelopathic effects of Hyptis suaveolens L. on growth and metabolism of pea seedlings. Scientia 12, 171–176 (2015).
Mamude, C. & Asfaw, Z. Allelopathic effects of Oldeania alpina (K Schum.) Stapleton leaf aqueous extract on seed germination and initial seedling growth of two selected crops. Adv. Bamboo Sci. 4, 100034 (2023).
Gibson, L. & Liebman, M. A laboratory exercise for teaching plant interference and relative growth rate concepts. Weed Technol. 17, 394–402 (2003).
Duke, S. O. Allelopathy : Current status of research and future of the discipline: A commentary. Allelopathy J. 25, 17–30 (2010).
Saha, D., Marble, S. C. & Pearson, B. J. Allelopathic effects of common landscape and nursery mulch materials on weed control. Front. Plant Sci. https://doi.org/10.3389/fpls.2018.00733 (2018).
Abugre, S. & Sam, S. J. Q. Evaluating the allelopathic effect of Jatropha curcas aqueous extract on germination, radicle and plumule length of crop. Int. J. Agr. Biol. 12, 769–772 (2010).
Sodaeizadeh H, Hosseini Z. Allelopathy an environmentally friendly method for weed control. Int Conf Appl Life Sci. 10-12, 387–392. (2012).
Yoneyama, K. & Natsume, M. Allelochemicals for plant–plant and plant–microbe interactions. Compr. Nat. Prod. II 4, 539–561 (2013).
Singh, H. P., Batish, D. R. & Kohli, R. K. Allelopathy in agroecosystems: an overview. J Crop Prod 4, 1–41 (2001).
LA Inderjit, W. & Duke, S. O. Challenges, achievements and opportunities in allelopathy research. J Plant Interact. 1, 69–81 (2005).
Macias, F. A., Chinchilla, N., Varela, R. M. & Molinillo, J. M. G. Bioactive steroids from Oryza sativa L. Steroids 71, 603–608 (2006).
Cheng, F. & Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 6, 1020 (2015).
Raghava, S. P. S., Singh, K. P. & Dantuluri, V. S. R. Marigold. In Ornamental Plants for Gardening (eds Chopra, V. L. & Singh, M.) 257–267 (Scientific Publishers, 2013).
Mir, R. A., Ahanger, M. A. & Agarwal, R. M. Marigold: From mandap to medicine and from ornamentation to remediation. Am. J. Plant Sci. 10, 309–338 (2019).
Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. Technical Communication No. 22, 547. Commonwealth Bureau, London. (1966).
Mir, R. A., Argal, S., Ahanger, M. A., Jatav, K. S. & Agarwal, R. M. Differential activity of wheat antioxidant defense system and alterations in the accumulation of osmolytes at different developmental stages as influenced by marigold (Tagetes erecta L.) leachates. Front. Plant Sci. 13, 1001394 (2022).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 24, 1–15 (1949).
Srivastava, H. S. In vivo activity of nitrate reductase in maize seedlings. Indian J. Biochem. Biophys. 11, 230–232 (1974).
Ahanger, M. A. & Agarwal, R. M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 115, 449–460 (2017).
Reitman, S. & Frankel, S. A colorimetric method for the determination of serum glutamic oxalactic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28, 56–63 (1957).
Robinson, S. A. et al. The role of glutamate dehydrogenase in plant nitrogen metabolism. Plant Physiol. 95, 509–516 (1991).
Lea, P. J., Joy, K. W. & Blackwell, R. D. Biochemical and biophysical research communications. In Inorganic Nitrogen in Plants and Microorganisms 115–120 (Springer, 1990).
Ahanger, M. A., Agarwal, R. M., Tomar, N. S. & Shrivastava, M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent). J. Plant Int. 10, 211–223 (2015).
Jackson, M. L. Soil Chemical Analysis (Prentice Hall of India Pvt Ltd., 1973).
Iswaran, V. & Marwaha, T. S. A modified rapid kjeldahl method for determination of total nitrogen in agricultural and biological materials. Geobios 7, 281–282 (1980).
Chon, S. U. & Nelson, C. J. Allelopathic dynamics in resource plants. In Allelopathy: Current Trends and Future Applications 81–110 (Springer, 2012).
Ahanger, M. A., Mir, R. A., Alyemeni, M. N. & Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 147, 31–42 (2020).
Allen, D. J. & Ort, D. R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 6, 36–42 (2001).
Muhammad, I. et al. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 11, 615942 (2021).
Mir, R. A., Argal, S. & Ahanger, M. A. Role of osmolytes in regulation of growth and photosynthesis under environmental stresses. In Improving Abiotic Stress Tolerance in Plants 99–118 (CRC Press, 2020).
Kohli, S. K. et al. Synergistic effect of 24-epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turk. J. Biol. 41, 943–953 (2017).
Chauhan, J. et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 10, 100296 (2023).
Zhou, Y. H. & Yu, J. Q. Allelochemicals and photosynthesis. In Allelopathy: A Physiological Process with Ecological Implications (eds Reigosa, M. J. et al.) 127–139 (Springer, 2006).
Hong, Y., Hu, H. Y., Sakoda, A. & Sagehashi, M. Effects of allelochemical gramine on photosynthetic pigments of cyanobacterium Microcystis aeruginosa. Int. J. Environ. Ecol. Eng. 4, 579–583 (2010).
Hussain, M. I. & Reigosa, M. J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching, and heat energy dissipation in three C3 perennial species. J. Exp. Bot. 62, 4533–4545 (2011).
Abu-Romman, S. Allelopathic potential of Achillea biebersteinii Afan. (Asteraceae). World Appl. Sci. J. 15, 947–952 (2011).
El-Shora, H. M. & Abdel-Aal, I. F. F. Allelopathic potential of Trichodesma africanum L.: Effects on germination, growth, chemical constituents and enzymes of Portulaca oleracea L. Int. J. Curr. Microbiol. App. Sci. 4, 941–951 (2015).
Zhang, K. M. et al. Photosynthetic electron-transfer reactions in the gametophyte of Pteris multifida reveal the presence of allelopathic interference from the invasive plant species Bidens pilosa. J. Photochem. Photobiol. B 158, 81–88 (2016).
Ding, H., Cheng, Z., Liu, M., Hayat, S. & Feng, H. Garlic exerts allelopathic effects on pepper physiology in a hydroponic co-culture system. Biol. open 5, 631–637 (2016).
Laxman, D. U., Desai, N. M. & Krishna, G. D. Allelopathic potentials of Chromolaena odorata L. on growth and biochemical characteristics of Salvadora persica. Asian J. Biol. Sci. 12, 122–129 (2019).
Kolbert, Z., Bartha, B. & Erdei, L. Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J. Plant Physiol. 165, 967–975 (2008).
Chamizo-Ampudia, A., Sanz-Luque, E., Llamas, A., Galvan, A. & Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 22, 163–174 (2017).
Chauhan, P., Mir, R. A. & Khah, M. A. Ascorbate–glutathione cycle: nitric oxide and phytohormone interactions for plant stress tolerance. In Nitric Oxide in Plants: A Molecule with Dual Roles 148–178 (Wiley, 2022).
Fu, Y. F., Zhang, Z. W. & Yuan, S. Putative connections between nitrate reductase S-nitrosylation and NO synthesis under pathogen attacks and abiotic stresses. Front. Plant Sci. 9, 474 (2018).
Ahanger, M. A., Tittal, M., Mir, R. A. & Agarwal, R. M. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 254, 1953–1963 (2017).
Husen, A., Iqbal, M., Sohrab, S. S. & Ansari, M. K. A. Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.). Agric. Food Secur. 7, 1–14 (2018).
Zhang, Y., Zhang, L. & Hu, X. H. Exogenous spermidine-induced changes at physiological and biochemical parameters levels in tomato seedling grown in saline-alkaline condition. Bot. Stud. 1, 55–58 (2014).
Irfan, M., Ahmad, A. & Hayat, S. Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J. Biol. Sci. 21, 125–131 (2014).
Singh, P., Singh, I. & Shah, K. Reduced activity of nitrate reductase under heavy metal cadmium stress in rice: An In silico answer. Front. Plant Sci. 9, 1948 (2018).
Abenavoli, M. R., Sorgona, A., Sidari, M., Badiani, M. & Fuggi, A. Coumarin inhibits the growth of carrot (Daucus carota L. cv. Saint Valery) cells in suspension culture. J. Plant Physiol. 160, 227–237 (2003).
Carillo, P. et al. Effects of the allelochemicals dihydrodiconiferyl alcohol and lariciresinol on metabolism of Lactuca sativa. Open Bioact. Compd. J. 3, 18–24 (2010).
Chen, G., Li, Q., Bai, M. & Chen, Y. Nitrogen metabolism in Acorus calamus L. leaves induced changes in response to microcystin–LR at environmentally relevant concentrations. Bull. Environ. Contam. Toxicol. 103, 280–285 (2019).
Ye, J. Y., Tian, W. H. & Jin, C. W. Nitrogen in plants: from nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2(1), 4. https://doi.org/10.1007/s44154-021-00030-1 (2022).
Rocha, M. et al. Analysis of alanine aminotransferase in various organs of soybean (Glycine max) and in dependence of different nitrogen fertilisers during hypoxic stress. Amino Acids 39, 1043–1053 (2010).
de la Torre, F., Canas, R. A., Pascual, M. B., Avila, C. & Canovas, F. M. Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J. Exp. Bot. 65, 5527–5534 (2014).
Khalil, S. E. & Seleem, F. M. Role of biochar soil amendment for alleviation of adverse effects of water stress on Dimorphotheca ecklonis plants. Biosci. Res. 16, 1337–1353 (2019).
Kobza, J. & Einhellig, F. A. The effects of ferulic acid on the mineral nutrition of grain sorghum. Plant Soil 98, 99–109 (1987).
Al-Hawas, G. H. S. & Azooz, M. M. Allelopathic potentials of Artrmisia monosperma and Thymus vulgaris on growth and physio-biochemical characteristics of pea seedlings. Pak. J. Biol. Sci. 21, 187–198 (2018).
Piznak, M., Kolarcik, V., Goga, M. & Backor, M. Allelopathic effects of lichen metabolite usnic acid on growth and physiological responses of Norway spruce and Scots pine seedlings. S. Afr. J. Bot. 124, 14–19 (2019).
Acknowledgements
The authors are thankful to Head, School of Studies in Botany, Jiwaji University, Gwalior, for providing necessary facilities. Authors are also thankful to Jiwaji University, for financial assistance in the form of research grant for the said project.
Author information
Authors and Affiliations
Contributions
The manuscript holds part of the Ph D work of RAM. The study was conceived and designed by Prof. RMA. RAM performed the experiments and wrote the first draft of manuscript, and RMA crosschecked the results and manuscript. SA and MAA helped in the experiments. RPS helped in the survey of necessary literature.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mir, R.A., Argal, S., Ahanger, M.A. et al. Modulation of nitrogen metabolising enzymes and the photosynthetic pigments in wheat upon exogenous application of marigold leachates. Sci Rep 15, 31654 (2025). https://doi.org/10.1038/s41598-025-94721-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94721-8