Abstract
Endophytic fungi produce a diverse range of bioactive secondary metabolites with potential applications in biopesticide development. This study investigates the nematicidal and antifeedant properties of ethyl acetate extracts from endophytic fungi isolated from wild Arabidopsis thaliana populations in Spain. The extracts were tested against the plant-parasitic nematode Meloidogyne javanica, and two common insect pests, Myzus persicae and Spodoptera littoralis. Nine of the 13 extracts demonstrated significant nematicidal and/or antifeedant activity, indicating their potential as biopesticides. The active extracts were derived from six genera: Alternaria (3 isolates), Dydimella (1), Dothiora (1), Pleiochaeta (1), Penicillium (1), and Fusarium (2). Five extracts exhibited nematicidal activity above 90%, with three reducing the total number of M. javanica second-stage juveniles hatched from egg masses by 22–37%. Four extracts showed strong settling inhibition (> 70%) against M. persicae, and three exhibited feeding inhibition against S. littoralis. Chemical analysis by GC-MS and LC-MS revealed a wide array of unique secondary metabolites in the active extracts, reflecting substantial chemical diversity, regardless of the fungal origin. This study highlights the potential of fungal endophytes from A. thaliana as sources of novel biopesticides, paving the way for future research focused on harnessing these fungi for biopesticide development.
Similar content being viewed by others
Introduction
Plant protection products (PPPs) are key agricultural inputs to ensure plant health, crop productivity and food security, since they protect crops from pests and pathogens. However, the inappropriate uses of harmful chemical PPPs can have a negative impact on ecosystems biodiversity and plant, animal and human health. Regulation is nowadays focused on the sustainable and safe production of crops, what has led to a gradual withdrawal of harmful pesticides. In the last decades, alternative approaches to plant protection have been encouraged, such as the use of biopesticides, which tend to pose fewer risks than conventional PPPs1. Biopesticides are PPPs derived from naturally occurring compounds or agents obtained from biological sources like animals, plants, and microorganisms2. Among microbial sources, fungi play a significant role in biopesticide development due to their production of biologically active secondary metabolites3,4. In particular, fungal endophytes - fungi that naturally colonize internal plant tissues without causing disease symptoms5,6,7, are recognized as valuable reservoirs of bioactive compounds, with terpenoids and polyketides being among the most commonly isolated ones. The potential of these metabolites for medicinal and agricultural applications has also been emphasized, highlighting the need for further research in this field8. Protection against pests and herbivores is one of the multiple benefits that these microorganisms can naturally provide to the host plant9. These microorganisms enhance plant resistance by producing secondary metabolites that deter or inhibit a wide range of pests and herbivores, including microbial pathogens, insects, and nematodes10,12. A well-known example is the endophyte Neotyphodium sp. which protects plants against herbivores by the production of alkaloids13. Given the pressing need for sustainable pest control solutions, increasing attention has been directed toward the bioprospecting of fungal endophytes as potential sources of novel biopesticides14.
Plant-parasitic nematodes pose a major threat to global agriculture, causing hundreds of billion annual losses worldwide11. Among them, root-knot nematodes are particularly destructive, attacking nearly all vascular plants15. Meloidogyne javanica is one of the most economically important species, serving as a model for studying plant-nematode interactions16. Its second-stage juveniles (J2) are the responsible for infecting the plants by penetrating the roots. Infected plants show reduced plant growth and wilting, severely impacting crop productivity11. Due to environmental concerns, traditional nematicides such as methyl bromide have been banned or restricted, necessitating the development of sustainable control alternatives17.
In addition to nematodes, insect pests such as aphids and lepidopterans pose serious threats to agricultural production. Aphids, including Myzus persicae, cause significant economic losses through direct feeding and virus transmission, leading to severe yield reductions19. M. persicae is particularly problematic due to its ability to infest a wide variety of crops and its resistance to multiple insecticide classes, including organophosphates and neonicotinoids20. Consequently, biological control methods, including entomopathogens and biological compounds, are being actively explored20.
Lepidopteran pests, including Spodoptera spp., pose an additional challenge to agriculture21. S. littoralis is a highly invasive species which damages over 40 plant families, including key crops like wheat, maize, rice, cotton, and vegetables22. Its larvae strip leaves and bore into fruits, significantly reducing crop yields21. The control of Spodoptera spp. requires the massive use of insecticides, since these insects have acquired resistance to all chemical families, including organophosphates, carbamates, and pyrethroids, as well as a more recent family, diamides23.This growing resistance further underscores the need for alternative, sustainable pest management strategies.
In this context, the bioprospection of endophytic fungal cultures isolated from different plant species has yielded a wide variety of promising bioactive compounds against nematodes and insect pests24. For example, 4-hydroxybenzoic acid, indole-3-acetic acid (IAA) and gibepyrone D produced by the fungal endophyte Fusarium oxysporum strain 162 isolated from tomato plants showed high antagonistic effect against the root-knot nematode Meloidogyne incognita25. In another study, free fatty acids (oleic, linoleic, palmitic and stearic) present in an extract from the endophytic fungus Trichoderma sp. EFI 671, isolated from Laurus sp., showed strong antifeedant effects against the aphid Myzus persicae26. Recent reports on biocidal compounds from fungal endophytes include nematicidal and antifeedant dioxolanones from Phyllosticta sp. (YCC4) isolated from Persea indica111, aphid antifeedant stempholones from Stemphylium sp.27 or acaricidal mellein from Aspergillus sp.28 , both isolated from Bethencourtia palmensis.
Arabidopsis thaliana, a well-established model in plant research, has greatly advanced our understanding of plant–microbe interactions29,30. However, studies on its natural fungal endophytes remain scarce. Among the natural associations of this model plant, one notable example is the fungal endophyte Colletotrichum tofieldiae. C. tofieldiae establishes a mutualistic relationship with the plant that promotes plant growth and fertility under phosphate-starved conditions31. Beyond Arabidopsis, this endophyte also colonizes tomato and maize, improving their growth and yield32. Additionally, C. tofieldiae has demonstrated biocontrol potential by reducing the prevalence of mycotoxigenic Aspergillus spp. and lowering aflatoxin contamination in maize grains33. Other studies have identified additional fungal isolates from Arabidopsis that provide benefits under stress conditions, including isolates of Macrophomina, Sordaria, Phaeosphaeria, Chaetomium, and Truncatella34. Despite these promising findings, research on the natural fungal endophytes of A. thaliana remains limited, particularly regarding their secondary metabolites and potential as bioactive agents against plant pests29.
In this study, we explored the biopesticidal potential of fungal endophytes isolated from wild A. thaliana plants collected in the central Iberian Peninsula. Culture-filtrate extracts from these endophytes were screened for their nematicidal activity against M. javanica and their antifeedant effects on M. persicae and S. littoralis. The diverse nematicidal and antifeedant effects observed among the extracts emphasize the rich chemical diversity within these endophytes. Our study provides a foundation for exploring endophytes as biotechnological sources of biopesticides, opening new doors for eco-friendly and effective pest management solutions.
Materials and methods
Fungal strains
The 13 fungal isolates used in this work (Table 1) were isolated in 2010 and 2011 from surface sterilized A. thaliana plants from different wild populations of the central Iberian Peninsula described by García et al.35.
The identification of each isolate was conducted by sequencing the internal transcribed spacer (ITS) region with the primer pairs ITS1/ITS436. The fungal isolates were restored in potato-dextrose-agar plates (PDA, Difco™) and incubated at room temperature for seven days. Total fungal genomic DNA was extracted from mycelial fragments scraped from fresh fungal culture plates with CTAB method37. The PCR conditions were set as follows: 2 min at 94 °C for initial denaturation step (1 cycle); 30 s at 94 °C, 30 s at 52 °C and 1 min at 72 °C for amplification cycles (35 cycles); and a final cycle of extension of 10 min at 72 °C. Species was assigned based on the best hit of a blast search against Mycobank Database (https://www.mycobank.org/). ITS sequences were aligned with MAFFT v7.525 (Multiple Alignment Fast Fourier Transform)38 and trimmed with trimmAl v2.039. Phylogenetic tree was calculated with FastTree v2.1.1140 and plotted with iTOL v641.
Culture conditions and liquid-liquid extraction
The fungal isolates were restored in PDA (Difco™) and incubated at room temperature for seven days. Restored colonies were transferred to a new PDA plate and incubated under the same conditions for additional seven days. Spores were scrapped with sterile deionized water from fresh cultures and concentration was determined using a Neubauer Hemocytometer.
The fungal isolates were grown in Malt-Peptone liquid medium (10 g/L Malt Extract, Merck, 2.5 g/L Mycological Peptone, Oxoid™). For each fungal isolate, six 100 mL flasks with a volume of 49 mL of medium each, were inoculated with 1 mL of the fungal spore suspension at the concentration of 5 × 105 spores/mL, to reach a final concentration of 104 spores/mL of medium. In total, 300 mL of inoculated medium was used per isolate. The flasks were incubated in darkness at 25ºC and 120 rpm for 21 days. After incubation, the mycelium was removed from the culture by filtration through a double layer of filter paper with the help of an extraction pump.
Each culture filtrate was subjected to liquid-liquid extraction using ethyl acetate (EtOAc) three times. The volume of the culture filtrate was first measured and the sample was transferred to a separating funnel. An equal volume of EtOAc was added to the funnel, which was then sealed and vigorously shaken to promote mixing. After shaking, the funnel was allowed to stand until phase separation occurred due to the density difference between the aqueous and organic layers. The two phases were then carefully separated by decanting. The EtOAc fraction was subsequently evaporated at 40ºC using a rotary evaporator. The dried extract was weighed, and the fraction was stored at 4ºC for further use.
Screening
For the initial screening, the extracts were tested in bioassays against M. javanica (see Sect. 2.4.1), M. persicae (see Sect. 2.5.2), and S. littoralis (see Sect. 2.5.3), as described below. Extracts exhibiting mortality rates greater than 90% and inhibiting settling (for M. persicae) or feeding (for S. littoralis) by more than 70% were selected for further analysis at lower doses. For the M. javanica egg hatching assay, the three extracts with the lowest LC50 values were chosen (see Sect. 2.4.2).
Nematicidal activity
Nematicidal bioassay
The population of M. javanica was obtained from Instituto de Ciencias Agrarias (ICA), CSIC in Madrid, Spain as described by Moo-Koh et al.42. Egg masses of M. javanica were handpicked from infected tomato roots. Second-stage juveniles (J2) were obtained from hatched eggs by incubating handpicked egg masses in a water suspension at 25ºC for 24 h. The inoculum, was adjusted to a final concentration of 100 J2 nematodes per 95 µL of distilled water. Then, 5 µL of the dissolved extracts or the control solution (DMSO:0.6% Tween 20) were added to four wells of a 96-well plate containing 95 µL of the nematode suspension, achieving a final extract concentration of 1 µg/µL. Four replicates per treatment were included. The plates were sealed with parafilm to prevent evaporation and were incubated in a growth chamber at 25 ± 1 ºC in the dark. Dead J2s were counted at two experimental times (48 and 72 h) using a binocular microscope and mortality rate (M%) was calculated and corrected with Schneider-Orelli’s formula43.
Extracts with a nematicidal activity (M% >90%) were selected for subsequent trials at lower doses (0.5 µg/µL, 0.25 µg/µL and 0.125 µg/µL) to calculate effective lethal doses (LC50 and LC90) at 72 h by probit analysis (software Statgraphics 19, Statgraphics Technologies, Inc.).
Egg hatching inhibition assay
Egg hatching assay was performed as described by Andrés et al.44, with the three most active extracts (LC50 < 0.5), which were isolates 10034, 10070 and 110040. Four replicates of three egg masses each (a total of 12 egg masses) were tested for each treatment. The three egg masses of each replicate were placed in a well of a 24-well plate and exposed to a total volume of 400 µL of the extracts diluted in DMSO:0.6% Tween 20 at the concentration of 1 µg/µL or DMSO:0.6%Tween 20 for controls. Plates were sealed with parafilm to prevent evaporation and maintained in a growth chamber in darkness, at 25 ± 1 ºC and 70% relative humidity. After five days (day 0) the number of juveniles hatched out of the egg masses was recorded. The test solutions were subsequently removed and the wells with egg masses were washed and filled with sterilized distilled water. Egg hatching was monitored for 4 weeks, until hatching was complete in the control treatment, and then the relative hatching percentages (compared to controls) were recorded.
Antifeedant activity
Maintenance of M. persicae and S. littoralis colonies
M. persicae and S. littoralis were reared on bell pepper (Capsicum annuum L.) plants and artificial diet45, respectively. Host plants together with insect colonies were maintained at 21 ± 2ºC, 60–70% relative humidity, and 16 h light : 8 h dark in a growth chamber.
Bioassay of settling inhibition of the aphid M. persicae
Pepper leaf disks of 2 cm2 were cut into two even pieces (1 cm2 each). The two leaf sections were set on water-agar (1%) coating the bottom of a ventilated plastic box (3 × 3 × 1.5 cm). The dry extracts were redissolved in ethanol at an initial concentration of 10 µg/µL. In each box, one leaf section was treated by spreading 10 µL of the fungal extract over its surface, while the other served as a control, receiving 10 µL of ethanol. Once ethanol evaporated, 10 apterous aphids (24–48 h old) were placed in each plastic box. A total of 20 replicates (boxes) per extract were included in this experiment. The percentage of aphids that settled on each leaf section was recorded after 24 h (at the environmental conditions described above), as described by González-Coloma et al.46. Settling inhibition index was calculated at an initial concentration of 100 µg/cm2, using the following equation:
Where:
T = percentage of aphids on the treated section.
C = percentage of aphids on the control section.
Extracts with a SI index higher than 70% were considered active and selected for further assays at lower doses (50 µg/cm2, 25 µg/cm2). EC50 was calculated using a logarithmic regression model with the software Statgraphics 19 (Statgraphics Technologies, Inc.).
Bioassay of feeding inhibition of S. littoralis
Four pepper leaf disks (1 cm2) were placed at equal distances on a water-agar (1%) petri dish (9 cm diameter). Two leaf disks were treated with 10 µL of the fungal extract redissolved in ethanol and the other two with 10 µL of the ethanol, as control. After solvent evaporation, two newly molted S. littoralis L6 larvae were allowed to feed on the leaf disks at room temperature, until the consumption of either the treated or control disks reached 75%. A total of six replicates (petri dishes) per extract were included in this experiment.
Non-consumed leaf disk area was measured on their digitalized images with the software Image J version 1.53k47. Feeding inhibition index was calculated at an initial concentration of 100 µg/cm2, using the following equation:
Where:
T = non-consumed areas of treated leaf disks.
C = non-consumed areas of control leaf disks.
Extracts with a FI index higher than 70% were considered active and selected for further assays at lower doses (50 µg/cm2, 25 µg/cm2). EC50 was calculated using a logarithmic regression model with the software Statgraphics 19 (Statgraphics Technologies, Inc.).
Chromatography
Gas chromatography – mass spectrometry
The extracts were dissolved in dichloromethane (DCM) and analyzed by gas chromatography mass spectrometry (GC-MS) using a Shimadzu GC-2010 gas chromatograph coupled to a Shimadzu GCMS-QP2010 Ultra mass detector (electron ionization, 70 eV), equipped with a 30 m × 0.25 mm i.d. capillary column (0.25 μm film thickness) Teknokroma TRB-5 (95%) Dimetil- (5%) diphenylpolisiloxane. Sample injections (1 µl) were carried out by an AOC-20i autosampler. Working conditions were as follows: split ratio (20:1), injector temperature 300ºC, temperature of the transfer line connected to the mass spectrometer 250 ºC, initial column temperature 110 ºC, then heated to 290 ºC at 7 ºC/min and a Full Scan was used (m/z 35–450). Electron ionization mass spectra and retention data were used to assess the identity of compounds by comparing them with those found in the Wiley 229 and NIST 17 Mass Spectral Database. All extracts (4 µg/µl) were dissolved in 100% DCM for injection.
Liquid chromatography – mass spectrometry
The fungal extracts were analyzed by liquid chromatography coupled to mass spectrometry (LC-MS) in a Shimadzu apparatus equipped with LC-20AD pump and a CTO-10AS VP column oven coupled to a mass spectrometer with triple quadrupole as analyzer (LCMS-8040), with an electrospray ionization source (ESI). An ACE 3 C18 column (150 mm × 4.6 mm, 3 μm particle size) with an ACE3 C18 analytical pre-column was used for the separation. The compounds were eluted with MiliQ water with 0.1% acetic acid (A) and methanol (HPLC-MS grade) with 0.1% acetic acid (B). The solvent gradient started at 38% B reaching 100% in 45 min and 100% during 10 min and then 38% B for 7 min before the next injection, with a flow rate of 0.5 mL/min. The nitrogen flow (drying gas for solvent evaporation) was 15 L/min. The potential for the electrospray capillary was + 4.50 kV and a Full Scan was used in positive mode (m/z 110–850) used the Q3 quadrupole with a potential of 1.98 kV and a capillary temperature of 250ºC. The heat block temperature was 400ºC. The stock solutions of the extracts were injected at 0.5 µg/µl with 5 µl injection through an automatic injector (SIL-20 A XR). All extracts (0.5 µg/µl) were dissolved in 100% methanol for injection.
Hierarchical clustering
Hierarchical clustering of extracts based on GC-MS and LC-MS profiles (% peak area) were calculated based on squared euclidean distance and the complete linkage method with the software Statgraphics 19 (Statgraphics Technologies, Inc.).
Results
Extraction yields
Different isolates exhibited varying extraction yields of their culture filtrate in ethyl acetate (Supplementary Table S1). Yields (mg of extract per mL of culture filtrate) ranged from 2.6 mg/mL for A. terricola (isolate 10034) to 10.4 mg/mL for D. maculans (isolate 110040), with an average yield of 5.3 mg/mL across all extracts. Notably, yield differences were observed even among closely related isolates. For instance, Fusarium isolates 10027 and 10050 differed by 4.6 mg/mL. Penicillium isolates 10006 and 10062 had similar yields (6.9 and 7.8 mg/mL, respectively), whereas isolate 110145 exhibited a lower yield of 2.9 mg/mL (Supplementary Table S1). These findings suggest that extraction yield is isolate-specific rather than genus-dependent.
Nematicidal activity
A total of five extracts out of the 13 tested showed activity (> 90% M at 72 h) against M. javanica (Tables 2 and 3). Extract 10034 (A. terricola) exhibited the earliest effect causing a mortality rate of 77% at 48 h, while 10033 (Alternaria sp.) and 110040 (D. maculans) showed the highest effect at 72 h with a 100% of mortality. The most effective extract in terms of lethal dose at 72 h was 10,070 (A. alternata) with LC50 of 0.05 mg and LC90 of 0.4 mg. Extract 10034 showed the lowest LC50 at 72 h with 0.04 mg, however LC90 was the second highest.
The active extracts with the lowest LC50 values (10034, 10070, and 110040) were selected to assess their effect on egg hatching. The three treatments resulted in a reduction in the total number of J2 that hatched from egg masses in comparison to controls, being the effect stronger during the first seven days (Fig. 1 and Supplementary Table S2), with reductions between 11% and 62% at day 0 (after five days of exposition to extract) and between 41% and 52% at day 7. Extract 10070 showed the highest total hatching inhibition rate with 37%, followed by 110040 with 28% and 10034 with 22%.
Egg hatching inhibition assay. (A) Temporal series of the number of second-stage juveniles (J2) hatched out of three egg masses. (B) Total number of J2 hatched out at the end of the experiment and total inhibition rate of the most active extracts, from isolates 10034 (Alternaria terricola), 10070 (Alternaria alternata) and 110040 (Dothiora maculans). Egg masses were exposed for five days to the extracts diluted in DMSO:0.6% Tween 20 or DMSO:0.6% Tween 20 for controls. The number of J2 hatched out of the egg masses was recorded at day 0 (after five days of exposition to extracts) and at 7, 14, 21 and 28 days immersed in water. Data (n = 4) shows means ± standard deviations.
Antifeedant activity
The extracts from the 13 endophytic fungi were screened for their biocontrol properties against the insect pests M. persicae and S. littoralis. Four fungal extracts (10006, 10027, 10050 and 10070), representing 31% of the tested extracts, showed high (> 70%) SI effect against M. persicae at a starting concentration of 100 µg/cm2 (Table 2). With these active extracts we calculated EC50 by conducting the assays at lower concentrations (Table 4). Extract from isolate 10050 (F. spartum) exhibited the lowest EC50 at 20 µg/cm2, followed by 10006 (P. solitum) with a EC50 of 30 µg/cm2. The latter was also the extract showing the highest SI rate at 100 µg/cm2 with 93.6%.
Three fungal extracts (10006, 10013 and 10070) exhibited high FI effect against S. littoralis (Table 2). The extract from isolate 10070 (A. alternata) was the most effective with 99.4% FI at 100 µg/cm2 and a EC50 of 42 µg/cm2 (Table 4).
Metabolomic analysis
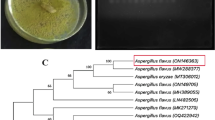
As a result of the GC-MS analysis, the presence of 117 compounds (>%1 abundance) was revealed, among which only 42 (36%) could be identified (Supplementary Table S3). The number of compounds in each extract ranged from 9 (isolate 10006, P. solitum), to 25 (isolate 110013, P. setosa), and the presence of the different compounds was mostly specific of each isolate, with just one compound (No. 100: unidentified) present in the extracts of all nine isolates and three compounds (No. 34: 2,10 bisaboladien-1-one (IUPAC: (2E,10E)-bisabola-2,10-dien-1-one); No. 48: 9-Octadecen-1-ol, (Z)- (IUPAC: (Z)-9-octadecen-1-ol), and No. 69: Tributyl acetylcitrate, IUPAC: Trinbutyl 2acetoxypropane-1,2,3tricarboxylate) shared by the extracts of five or more isolates. Namely, compounds (No. 34) 2,10-bisaboladien-1-one and (No. 69) Tributyl acetylcitrate were present in all three extracts with activity against M. persicae (Supplementary Table S3). Samples could be grouped in three clusters according to their volatile-lipid composition (Fig. 2A), with cluster 3 divided further in three subgroups. Clusters GC1 and GC2 grouped the extracts from isolates from Polán population (roots and rosette respectively). Cluster GC3 grouped extracts from isolates from the two populations and different plant organs. The grouping did not correlate with the taxonomy of the isolates or the activity (Fig. 2C), indicating that the volatile-lipidic composition of the extracts was not specific for these traits.
Sample clustering according to metabolomic profiles. Hierarchical clustering of fungal extracts based on their (A) GC-MS (Gas-Chromatography and Mass – Spectrometry) and (B) LC-MS (Liquid Chromatography and Mass – Spectrometry) profile. (C) Phylogenetic tree of the fungal isolates based on ITS sequences and classification based on activity of the extracts and the clustering of chromatographic profiles.
A non-target LC-MS analysis showed the presence of at least 28 metabolites, grouped by their retention time, with a range of 7 to 12 metabolites per extract (Supplementary Table S4). Three of the metabolites (No. 130, 134 and 136) were present in all the extracts and two metabolites were absent in just one (No. 132 and 139). The clustering of the results (Fig. 2B) showed a profile that could be related to the taxonomical proximity of the isolates. A total of four groups could be established, with three including one species: LC1 (110040, D. maculans); LC2 (110013, P. setosa); and LC4 (10006, P. solitum), and one including three genera, which could be divided into two: LC3.1 (10070-10033-10034, Alternaria spp. and 10013, D. macrostoma) and LC3.2 (10027–10050, Fusarium spp.).
Discussion
In nature, the preeminent model plant A. thaliana hosts a high variety of fungal endophytes35. Characterizing its natural endophytic mycobiota offers valuable opportunities to deepen our understanding of plant-endophyte interactions. However, characterization of endophytes isolated from A. thaliana is relatively limited, particularly in the context of secondary metabolites biosynthesis and their potential biotechnological applications. In this work, we obtained extracts from 13 A. thaliana fungal endophytes and screened them for their activity against the phytoparasitic nematode M. javanica and the plant insect pests M. persicae and S. littoralis. To our knowledge, this is the first study that explores the potential nematicidal and antifeedant effects of secondary metabolites derived from fungal endophytes of A. thaliana.
Our findings show that 9 out of the 13 (69%) extracts exhibited nematicidal and/or antifeedant activity, what highlights the enormous potential of fungal endophytes of this model plant to produce bioactive compounds of agricultural interest. The nine isolates with bioactive extracts have different origins regarding population (Polán or Menasalbas) and plant tissue (root, rosette and floral stalk), so their activity is independent from the geographical site or plant organ of provenance. Notably, there where isolates from the roots with activity against leaf pests (i.e. 10006 or 10050) and isolates from the rosette with activity against root nematodes (i.e.10033, 10034 or 110040). Even, the extract from isolate 10070 from the floral stalk was active to both leaf and root attackers. Therefore, the tissue of origin of the isolate did not determine the possible activity of a fungal endophyte.
Nematicidal activity
Isolates 110040 and 110013 from the species D. maculans and P. setosa, respectively, and the three isolates of Alternaria (10033-10034-10070) produced extracts with nematicidal activity. Dothiora species are found on plants in terrestrial habitats as saprobes and weak pathogens on stressed plants48,49,50. Previous studies reported the production of hormonemates, compounds with cytotoxic activity against tumoral cells, by endophytic Dothiora sp. isolated from Launaea arborescens51. P. setosa is an important pathogenic necrotrophic fungi of grain legumes52. The only compound described for this species is setosol, which has been shown to inhibit the growth of different fungal phytopathogens, such as the fungus Magnaporthe oryzae53. However, this is the first report on the nematicidal effects of an organic extract from endophytic species belonging to these two genera.
The fungal genus Alternaria is a diverse group of ascomycete fungi which occupy different ecological niches ranging from saprobes to endophytes and pathogens. It is widespread in nature, commonly found in a wide range of hosts and substrates, such as soil, plants, organic matter, wood or textiles54,55. From a human health perspective, Alternaria is notable due to its airborne spores, which are among the most prevalent allergens56. As plant pathogens, Alternaria species can cause significant pre- and post-harvest diseases, sometimes leading to mycotoxin accumulation and serious economic losses57. Nonetheless, several Alternaria species have gained attention for their ability to produce secondary metabolites with a variety of bioactive properties, positioning them as valuable candidates for biotechnological applications in the pharmaceutical and agricultural industries58,59. In this work, the three Alternaria isolates showed nematicidal activity. Lou et al.60 already showed the potential nematicidal effect of endophytic Alternaria sp. isolated from Salvia miltorrhiza. The bioactive compound was alternariol methyl ether, which was toxic to the model organism Caenorhabditis elegans and the plant parasitic nematode Bursaphelenchus xylophilus. However, although there is promising evidence of the nematicidal effects of secondary metabolites produced by Alternaria, this field remains still underexplored.
Antifeedant activity
Isolate 10006 identified as P. solitum, produced an extract with antifeedant activity against M. persicae and S. littoralis. P. solitum is known for its role in the spoilage of pome fruits during storage61. In addition, this fungus has been isolated from extremophilic environments, such as the acidic waters of the Berkeley Pit Lake62 and Antarctica63. There is no direct evidence in the literature supporting the use of P. solitum metabolites as biopesticides. However, various secondary metabolites from this fungus have exhibited potential bioactive properties. One example is compactin (also referred to as mevastatin), a precursor for the cholesterol-lowering drug pravastatin64,65. Additionally, viridicatol, a quinoline alkaloid, exhibited moderate anti-tumor activities against certain cancer cell lines and potent anti-food allergic effects in vitro66. Nonetheless, many studies have explored the metabolites of other Penicillium species for biopesticide purposes. Non-endophytic species of this genus are known producers of insecticidal compounds such as tryptoquialanines67, indole diketopiperazine alkaloids68, okaramine indole alkaloids69, meroterpenoids70, (-)-botryodiplodin71, yaequinolones72 or the terpenoid-pyridine oxalicines, active against S. frugiperda73. The closely related species P. crustosum, produces neurotoxic penitrems, which have exhibited insecticidal activity74. Furthermore, an endophytic Penicillium isolated from Derris elliptica also was shown to produce the antifeedant compound rotenone against the lepidopteran Plutella xylostella and the aphid Lipaphis erysimi75. Still, this is the first report documenting the insecticidal potential of secondary metabolites from P. solitum in agriculture.
Isolate 10013, D. macrostoma, exhibited antifeedant effects against S. littoralis. D. macrostoma (formerly Phoma macrostoma) is a fungal species with diverse ecological roles. It has been recognized as a plant pathogen, notably causing calyx-end rot in pears during cold storage76. However, it has recently shown potential as a biocontrol agent against rapeseed clubroot, significantly reducing disease severity and improving crop yields77. The genus Didymella is well known for producing a variety of secondary metabolites with phytotoxic and cytotoxic activities, which have garnered attention for their potential as bioherbicides. For instance, an endophytic Didymella isolated from mangroves synthesizes cytotoxic ascomylactams (macrocyclic alkaloids), didymetone78 and phomapyrrolidones79. Additionally, cytotoxic naphthalenones and didymelol have been isolated from the endophytic fungus D. glomerata, found in Saussurea laniceps80. Another example is D. pinodes, an aggressive isolate from pea (Pisum sativum), which produces pinolidoxin, a phytotoxin affecting several plant species81. Despite these findings, this study marks the first report of insect antifeedant effects from an organic extract of endophytic Didymella. The compound duroquinone, identified in the GC-MS profile of the extract of isolate 10013, may contribute to this effect. Duroquinone has previously been reported to inhibit survival, growth, and pupation in the Black Cutworm (Agrotis ipsilon) by reducing ingestion and the efficiency of food conversion82. However, further studies are required to confirm its role in the antifeedant activity observed against S. littoralis.
Two species of Fusarium (isolates 10027–10050) produced active extracts against M. persicae. The fungal genus Fusarium is a large and significant group of filamentous fungi, primarily found in soil and associated with plants. While certain Fusarium species are notorious for causing plant diseases, such as Fusarium wilt and root rot in crops like cereals83,84,85, legumes86, and bananas87,88, as well as for producing harmful mycotoxins89, the majority of strains are saprotrophs or endophytes. In fact, Fusarium is one of the most abundant endophytic fungal genera, with certain species providing benefits to the plant90,91,92. Moreover, Fusarium is a rich source of bioactive compounds from various chemical classes, including those with insecticidal properties93,94. Specifically, F. sambucinum from Nicotiana tabacum produced the prenylated indole alkaloids sclerotiamides and notoamide with potent insecticidal activity against Helicoverpa95. Moreover, several species of Fusarium have been discovered as entomopathogenic in aphids96, which could be related with the antifeedant effect observed in their organic extracts.
Apart from the nematicidal effect, extract from isolate 10070, identified as A. alternata, also showed antifeedant activity against M. persicae and S. littoralis. Previous studies have reported that A. alternata strains isolated from Azadirachta indica gave extracts with antifeedant and toxic effects against S. litura97. Also, altenuene, an acetyl cholinesterase inhibitor isolated from an endophytic A. alternata strain of Catharantus roseus, exhibited insecticidal effect against S. litura98. The extract from the isolate 10070 was the only one showing activity against all three plant pests examined in this study, indicating its broad-spectrum efficacy and underscoring its potential as a versatile source of biopesticide products. Additional research will be necessary to identify the specific molecules responsible for this effect.
Analysis of chromatographic profiles
The extracts, analyzed by GC-MS and LC-MS, exhibited a highly diverse composition, with most metabolites being unknown and unique to each extract. Our results did not show a direct correlation between the secondary metabolite profiles and nematicidal or antifeedant activity. Nonetheless, we could observe that two compounds— (No. 34) 2,10-bisaboladien-1-one and (No. 69) tributyl acetylcitrate—were present in all three extracts that demonstrated activity against M. persicae (Supplementary Table S3). While acetyltributylcitrate is commonly used as a plasticizer99, recent studies have reported it as a naturally occurring component in antimicrobial extracts100,101. For example, it has been identified as a bioactive compound in crude extracts of actinomycetes with antibacterial and antifungal properties101, as well as in the fungicidal compounds from Michelia champaca bark extract101. However, its use as a biopesticide remains unexplored. The compound 2,10-bisaboladien-1-one is already known for its antifeedant effects on M. persicae, significantly reducing both probing activity and the number of intracellular penetrations102. This suggests that 2,10-bisaboladien-1-one may be one of the key compounds responsible for the observed bioactivity.
In addition, no clear relationship between the chemical composition of the extracts and the taxonomical proximity of the isolates was observed. The analysis of the less polar fraction (DCM-soluble) of the extracts, containing the lipid fraction, did not show a taxonomy-dependent distribution. On the other hand, the secondary metabolites in the methanol-soluble fraction (analyzed by LC-MS) revealed chemical profiles that could be related to taxonomy, though only to a limited extent. This lack of a clear pattern could be expected, as secondary metabolite profiles in fungi are complex traits influenced by mono- and polyphyletic factors. Although certain metabolites in fungal groups like the Xylariaceae family strongly correlate with phylogenies103, the inconsistent distribution of secondary metabolites across the fungal kingdom and the great influence of environmental factors in their biosynthesis makes difficult its correlation with phylogeny104. Comparative genomic studies have shown that homologous genes and gene clusters related to secondary metabolism are distributed across a wide phylogenetic range of species105. In some cases, this distribution is consistent with inheritance from a common ancestor, as seen with the ergot alkaloid gene cluster in Claviceps and Metarhizium106. In other cases, the presence of large, complex gene clusters in distantly related taxa is thought to result from horizontal gene transfer107. Within-species genomic comparisons have revealed polymorphisms in secondary metabolite gene clusters, such as gene gain/loss, cluster mobility, and allelic variations, which might explain this evolutionary divergencies106,108,109.
In summary, our study underscores the significant potential of fungal endophytes as sources of bioactive compounds with biocontrol capabilities against agricultural pests. The diverse composition of extracts from different fungal isolates does not correlate with the nematicidal and antifeedant activities observed, suggesting similar activities for different metabolites. Nevertheless, identifying the bioactive compounds is essential for gaining a deeper understanding of the mechanisms underlying the observed effects. Future research will focus on bioguided fractionation of selected extracts for the identification of the active compounds. Our results also show that a model host as A. thaliana, routinary found in many different anthropic ecosystems in temperate regions34,35,110, hosts fungal endophytes with the ability to produce high diversity of compounds that are bioactive. The use of a model plant gives opportunities to better study the relationship of the plant with these endophytes and the conditions in which the secondary metabolites of interest are produced in the interaction with the plants.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Chandler, D. et al. The development, regulation and use of biopesticides for integrated pest management. Philosophical Trans. Royal Soc. B: Biol. Sci. 366, 1987 (2011).
Kumar, J., Ramlal, A., Mallick, D. & Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021. 10 (Page 1185 10), 1185 (2021).
Macías-Rubalcava, M. L. & Sánchez-Fernández, R. E. Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World J. Microbiol. Biotechnol. 33, (2017).
Hashem, A. H. et al. Bioactive compounds and biomedical applications of endophytic fungi: a recent review. Microb. Cell. Fact. 22, 1–23 (2023).
Hardoim, P. R. et al. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 79, 293–320 (2015).
Lugtenberg, B. J. J., Caradus, J. R. & Johnson, L. J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 92, fiw194 (2016).
Yan, L. et al. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 103 3327–3340 (2019). https://doi.org/10.1007/s00253-019-09713-2
Mousa, W. K. & Raizada, M. N. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front. Microbiol. 4, 44840 (2013).
Ratnaweera, P. B. & de Silva, E. D. Endophytic Fungi: A remarkable source of biologically active secondary metabolites, in endophytes: crop productivity and protection (eds. Maheshwari, D., Annapurna, K.) 191–212Springer, (2017).
Bamisile, B. S., Dash, C. K., Akutse, K. S., Keppanan, R. & Wang, L. Fungal endophytes: beyond herbivore management. Front. Microbiol. 9, 544 (2018).
Agrios, G. Plant Pathology: Fifth Edition (Elsevier, 2005).
Kusari, S., Hertweck, C. & Spiteller, M. Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem. Biol. 19, 792–798 (2012).
Schardl, C. et al. Currencies of mutualisms: sources of alkaloid genes in vertically transmitted Epichloae. Toxins (Basel). 5, 1064–1088 (2013).
Segaran, G. & Sathiavelu, M. Fungal endophytes: A potent biocontrol agent and a bioactive metabolites reservoir. Biocatal. Agric. Biotechnol. 21, 101284 (2019).
Castagnone-Sereno, P., Danchin, E. G. J., Perfus-Barbeoch, L. & Abad, P. Diversity and evolution of root-knot nematodes, genus Meloidogyne: new insights from the genomic era. Annu. Rev. Phytopathol. 51, 203–220 (2013).
Abad, P. et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 26, 909–915 (2008).
Azlay, L., Boukhari, E., Mayad, M. E. M., Barakate, M. & E. H. & Biological management of root-knot nematodes (Meloidogyne spp.): a review. Org. Agric. 13, 99–117 (2022).
Chen, J. Xiang & SONG, B. An. Natural nematicidal active compounds: recent research progress and outlook. J. Integr. Agric. 20, 2015–2031 (2021).
Van Emden and Harrington. Aphids as Crop Pests (CABI, 2017).
Ali, J. et al. Peach–Potato aphid Myzus persicae: current management strategies, challenges, and proposed solutions. Sustainability 15, 11150 (2023).
Pasiecznik, N. M. et al. CABI/EPPO distribution maps of plant pests and plant diseases and their important role in plant quarantine. EPPO Bull. 35, 1–7 (2005).
Cheng, T. et al. Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 1, 1747–1756 (2017).
Hilliou, F., Chertemps, T., Maïbèche, M. & Le Goff, G. Resistance in the genus Spodoptera: key insect detoxification genes. Insects 12, 544 (2021).
Andrés, M. F., Diaz, C. E., Giménez, C. & Cabrera, R. González-Coloma, A. Endophytic fungi as novel sources of biopesticides: the Macaronesian Laurel forest, a case study. Phytochem. Rev. 16, 1009–1022 (2017).
Bogner, C. W. et al. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb. Biotechnol. 10, 175–188 (2017).
Kaushik, N. et al. Chemical composition of an aphid antifeedant extract from an endophytic fungus, Trichoderma sp. EFI671. Microorganisms 8, 420 (2020).
Diaz, C. E. et al. Antifeedant, antifungal and nematicidal compounds from the endophyte Stemphylium solani isolated from Wormwood. Sci. Rep. 14, 1–10 (2024).
Reyes Castillo, N. et al. Optimization of fungicidal and acaricidal metabolite production by endophytic fungus Aspergillus sp. SPH2. Bioresour Bioprocess. 11, 1–12 (2024).
Poveda, J., Díaz-González, S., Díaz-Urbano, M., Velasco, P. & Sacristán, S. Fungal endophytes of Brassicaceae: molecular interactions and crop benefits. Front. Plant. Sci. 13, 932288 (2022).
Koornneef, M. & Meinke, D. The development of Arabidopsis as a model plant. Plant J. 61, 909–921 (2010).
Hiruma, K. et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165, 464–474 (2016).
Díaz-González, S. et al. Mutualistic fungal endophyte Colletotrichum tofieldiae Ct0861 colonizes and increases growth and yield of maize and tomato plants. Agronomy 10, 1493 (2020).
Díaz-González, S. et al. Plant growth promoting fungal endophyte Colletotrichum tofieldiae Ct0861 reduces mycotoxigenic Aspergillus fungi in maize grains. BioRxiv 2025.01.17.633531 https://doi.org/10.1101/2025.01.17.633531 (2025).
Mesny, F. et al. Genetic determinants of endophytism in the Arabidopsis root mycobiome. Nat. Commun. 12 (1 12), 1–15 (2021).
García, E., Alonso, Á., Platas, G. & Sacristán. The endophytic mycobiota of Arabidopsis thaliana. Fungal Divers. 60, 71–89 (2013).
Bokulich, N. A. & Mills, D. A. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl. Environ. Microbiol. 79, 2519–2526 (2013).
Doyle, J. J. & Doyle, J. L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. PHYTOCHEMICAL Bull. (1987).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. TrimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2 - Approximately maximum-likelihood trees for large alignments. PLoS One. 5, e9490 (2010).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v6: recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 52, W78–W82 (2024).
Moo-Koh, F. A. et al. In vitro assessment of organic and residual fractions of nematicidal culture filtrates from thirteen tropical Trichoderma strains and metabolic profiles of most-active. J. Fungi. 8, 82 (2022).
Schneider-Orelli, O. Entomologisches Praktikum: Einführung in Die Land und Forstwirtschaftliche Insektenkunde. (1947).
Andrés, M. F., González-Coloma, A., Sanz, J., Burillo, J. & Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 11, 371–390 (2012).
Poitout, S. & Bues, R. Rearing of several species of Lepidoptera Noctuidae on a rich artificial medium and a simplified artificial medium. Ann. De Zool. Ecologie Anim. Ale. 2, 79–91 (1970).
Gonzalez-Coloma, A. et al. Structure- and species-dependent insecticidal effects of neo-clerodane diterpenes. J. Agric. Food Chem. 48, 3677–3681 (2000).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to imageJ: 25 years of image analysis. Nat. Methods. 9, 671–675 (2012).
Crous, P. W. & Groenewald, J. Z. They seldom occur alone. Fungal Biol. 120, 1392–1415 (2016).
Crous, P. W. & Groenewald, J. Z. The genera of fungi - G 4: Camarosporium and Dothiora. IMA Fungus. 8, 131–152 (2017).
Hyde, K. D. et al. The numbers of fungi: is the descriptive curve flattening? Fungal Diversity 103, 219–271 (2020).
Pérez-Bonilla, M. et al. Hormonemate derivatives from Dothiora sp., an endophytic fungus. J. Nat. Prod. 80, 845–853 (2017).
Agudo-Jurado, F. J., Reveglia, P., Rubiales, D., Evidente, A. & Barilli, E. Status of phytotoxins isolated from necrotrophic fungi causing diseases on grain legumes. Int. J. Mol. Sci. 24, 5116 (2023).
Okeke, B., Seigle-Murandi, F., Steiman, R. & Kaouadji, M. Setosol, a biologically active heptaketide-like metabolite from the Pleiochaeta setosa phytopathogen. Biosci. Biotechnol. Biochem. 58, 734–736 (1994).
Thomma, B. P. H. J. Alternaria spp.: from general saprophyte to specific parasite. Mol. Plant. Pathol. 4, 225–236 (2003).
Lawrence, D. P., Rotondo, F. & Gannibal, P. B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycological Progress. 15, 1–22 (2015).
Kasprzyk, I. et al. Air pollution by allergenic spores of the genus Alternaria in the air of central and Eastern Europe. Environ. Sci. Pollut. Res. 22, 9260–9274 (2015).
Pinto, V. E. F. & Patriarca, A. Alternaria species and their associated mycotoxins. Methods Mol. Biol. 1542, 13–32 (2017).
Zhao, S. et al. Secondary metabolites of Alternaria: A comprehensive review of chemical diversity and pharmacological properties. Front. Microbiol. 13, 1085666 (2023).
Lou, J., Fu, L., Peng, Y. & Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 18, 5891–5935 (2013).
Lou, J. et al. Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif01 and its bioactivities. Brazilian J. Microbiol. 47, 96–101 (2016).
Jurick, W. M. et al. Penicillium solitum produces a polygalacturonase isozyme in decayed Anjou Pear fruit capable of macerating host tissue in vitro. Mycologia 104, 604–612 (2012).
Stierle, D. B., Stierle, A. A., Girtsman, T., McIntyre, K. & Nichols, J. Caspase-1 and – 3 inhibiting drimane sesquiterpenoids from the extremophilic fungus Penicillium solitum. J. Nat. Prod. 75, 262–266 (2012).
Gonçalves, V. N. et al. Penicillium solitum: A mesophilic, psychrotolerant fungus present in marine sediments from Antarctica. Polar Biol. 36, 1823–1831 (2013).
Boruta, T., Przerywacz, P., Ryngajllo, M. & Bizukojc, M. Bioprocess-related, morphological and bioinformatic perspectives on the biosynthesis of secondary metabolites produced by Penicillium solitum. Process Biochem. 68, 12–21 (2018).
Frisvad, J. C. & Filtenborg, O. Terverticillate Penicillia: chemotaxonomy and mycotoxin production. Mycologia 81, 837–861 (1989).
He, Z. H. et al. Chemical constituents of the deep-sea-derived Penicillium solitum. Mar. Drugs. 19, 580 (2021).
Costa, J. H. et al. Monitoring Indole alkaloid production by Penicillium digitatum during infection process in citrus by mass spectrometry imaging and molecular networking. Fungal Biol. 123, 594–600 (2019).
Jia, B., Ma, Y., Chen, D., Chen, P. & Hu, Y. Studies on structure and biological activity of indole diketopiperazine alkaloids. Progress Chem. 30, 1067–1081 (2018).
Hayashi, H., Takiuchi, K., Murao, S. & Arai, M. Structure and insecticidal activity of new Indole alkaloids, Okaramines a and B, from Penicillium simplicissimum ak-40. Agric. Biol. Chem. 53, 461–469 (1989).
Geris, R., Rodrigues-Fo, E., du Silva, H. H. G. & da Silva, I. G. Larvicidal effects of fungal meroterpenoids in the control of Aedes aegypti L., the main vector of dengue and yellow fever. Chem. Biodivers. 5, 341–345 (2008).
Cabedo, N., López-Gresa, M. P., Primo, J., Ciavatta, M. L. & González-Mas, M. C. Isolation and structural elucidation of eight new related analogues of the mycotoxin (-)-botryodiplodin from Penicillium coalescens. J. Agric. Food Chem. 55, 6977–6983 (2007).
Uchida, R., Imasato, R., Tomoda, H., Omura, S. Yaequinolones New insecticidal antibiotics produced by Penicillium sp. FKI-2140. J. Antibiot. 59, 652–658 (2006).
Li, C., Gloer, J. B., Wicklow, D. T. & Dowd, P. F. Antiinsectan decaturin and oxalicine analogues from Penicillium thiersii. J. Nat. Prod. 68, 319–322 (2005).
Carmen González, M. et al. Insecticidal activity of penitrems, including penitrem G, a new member of the family isolated from Penicillium crustosum. J. Agric. Food Chem. 51, 2156–2160 (2003).
Hu, M. Y. et al. Insecticidal metabolites produced by Penicillium spp., an endophytic fungus in Derris elliptica Benth. Allelopathy J. 21, 349–360 (2008).
Wenneker, M., Pham, K. T. K. & Kots, K. First report of Didymella macrostoma causing calyx-end rot of Pear (Pyrus communis) in the Netherlands. Plant Dis. 107, 2855 (2023).
Li, G. et al. Biological control of rapeseed clubroot (Plasmodiophora brassicae) using the endophytic fungus Didymella macrostoma P2. Plant Dis. 1088, 2399–2409 (2024).
Yuan, Y., Wang, G., She, Z., Chen, Y. & Kang, W. Metabolites isolated from the Mangrove endophytic fungus Didymella sp. CYSK-4 and their cytotoxic activities. Fitoterapia 171, 105692 (2023).
Chen, Y. et al. Ascomylactams A-C, cytotoxic 12- or 13-Membered-Ring macrocyclic alkaloids isolated from the Mangrove endophytic fungus Didymella sp. CYSK-4, and structure revisions of phomapyrrolidones A and C. J. Nat. Prod. 82, 1752–1758 (2019).
Luo, G. et al. Naphthalenones and naphthols isolated from the Saussurea laniceps endophytic fungus Didymella glomerata X223. Chem. Biodivers. 17, e2000315 (2020).
Cimmino, A. et al. Pinolide, a new nonenolide produced by Didymella pinodes, the causal agent of ascochyta blight on Pisum sativum. J. Agric. Food Chem. 60, 5273–5278 (2012).
Reese, J. C. & Beck, S. D. Effects of allelochemics on the black cutworm, Agrotis ipsilon; effects of p-benzoquinone, hydroquinone, and duroquinone on larval growth, development, and utilization of food. Ann. Entomol. Soc. Am. 69, 59–67 (1976).
Yli-Mattila, T. Ecology and evolution of toxigenic Fusarium species in cereals in Northern Europe and Asia. J. Plant. Pathol. 92, 7–18 (2010).
Arata, A. F. et al. The richness of Fusarium species in maize tassels and their relationship with Fusarium stalk rot. Eur. J. Plant. Pathol. 168, 351–362 (2024).
Zuo, S. et al. Assessment of genetic diversity and the population structure of species from the Fusarium fujikuroi species complex causing fusarium stalk rot of maize. J. Fungi. 10, 574 (2024).
Sampaio, A. M., De Sousa Araújo, S., Rubiales, D. & Patto, M. C. V. Fusarium wilt manage. Legume Crops Agron. 10, 1073 (2020).
Dita, M., Barquero, M., Heck, D., Mizubuti, E. S. G. & Staver, C. P. Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant. Sci. 871, 398832 (2018).
Ploetz, R. C. Fusarium wilt of banana. Phytopathology 105, 1512–1521 (2015).
Ji, F. et al. Occurrence, toxicity, production and detection of Fusarium mycotoxin: a review. Food Production, Processing and Nutrition 1, 1–14 (2019).
Pappas, M. L. et al. The beneficial endophytic fungus Fusarium solani strain K alters tomato responses against spider mites to the benefit of the plant. Front. Plant. Sci. 9, 408405 (2018).
Vu, T., Hauschild, R. & Sikora, R. A. Fusarium oxysporum endophytes induced systemic resistance against radopholus similis on banana. Nematology 8, 847–852 (2006).
de Lamo, F. J. & Takken, F. L. W. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant. Sci. 11, 500488 (2020).
Ahmed, A. M., Mahmoud, B. K., Millán-Aguiñaga, N., Abdelmohsen, U. R. & Fouad, M. A. The endophytic Fusarium strains: a treasure trove of natural products. RSC Adv. 13, 1339–1369 (2023).
Toghueo, R. M. K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology 11, 1–21 (2020).
Zhang, P. et al. Angularly prenylated indole alkaloids with antimicrobial and insecticidal activities from an endophytic fungus Fusarium sambucinum TE-6L. J. Agric. Food Chem. 67, 11994–12001 (2019).
Santos, A. C. S., Diniz, A. G., Tiago, P. V. & de Oliveira, N. T. Entomopathogenic Fusarium species: a review of their potential for the biological control of insects, implications and prospects. Fungal Biol. Rev. 34, 41–57 (2020).
Kaur, T., Kaur, J., Kaur, A. & Kaur, S. Larvicidal and growth inhibitory effects of endophytic Aspergillus niger on a polyphagous pest, Spodoptera litura. Phytoparasitica 44, 465–476 (2016).
Bhagat, J. et al. Cholinesterase inhibitor (Altenuene) from an endophytic fungus Alternaria alternata: optimization, purification and characterization. J. Appl. Microbiol. 121, 1015–1025 (2016).
Chaos, A. et al. Tributyl citrate as an effective plasticizer for biodegradable polymers: effect of plasticizer on free volume and transport and mechanical properties. Polym. Int. 68, 125–133 (2019).
Awad, N. M., Rasmey, A. H. M., Elshamy, A. I. & Aboseidah, A. Antimicrobial activities of some Actinomycetes isolated from cultivated soil. Egypt. Front. Sci. Res. Technol. 8, (2024).
Bawa, I. G. A. G., Santi, S. R., Rita, W. S., Suryanadi, O. & Indyan, G. Active compounds of Michelia champaca bark extract against Curvularia verruculosa fungi causing leaf spot disease in rice (Oryza sativa L). J. Appl. Nat. Sci. 16, 420–426 (2024).
Gutiérrez, C., Fereres, A., Reina, M., Cabrera, R. & González-Coloma, A. Behavioral and sublethal effects of structurally related lower terrenes on Myzus persicae. J. Chem. Ecol. 23, 1641–1650 (1997).
Stadler, M. Importance of secondary metabolites in the Xylariaceae as parameters for assessment of their taxonomy, phylogeny, and functional biodiversity. Curr. Res. Environ. Appl. Mycol. 1, 75–133 (2011).
Frisvad, J. C., Andersen, B. & Thrane, U. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol. Res. 112, 231–240 (2008).
Spatafora, J. W. & Bushley, K. E. Phylogenomics and evolution of secondary metabolism in plant-associated fungi. Curr. Opin. Plant. Biol. 26, 37–44 (2015).
Young, C. A. et al. Genetics, Genomics and evolution of ergot alkaloid diversity. Toxins 7, 1273–1302 (2015).
Wisecaver, J. H., Slot, J. C. & Rokas, A. The evolution of fungal metabolic pathways. PLoS Genet. 10, e1004816 (2014).
Liu, L., Xi, Z. & Davis, C. Coalescent methods are robust to the simultaneous effects of long branches and incomplete lineage sorting. Mol. Biol. Evol. 32, 791–805 (2015).
Lind, A. L. et al. Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLoS Biol. 15, e2003583 (2017).
Thiergart, T. et al. Root microbiota assembly and adaptive differentiation among European Arabidopsis populations. Nat. Ecol. Evol. 4, 122–131 (2019).
Díaz, C.E., Andrés, M.F., Bolaños, P., González-Coloma, A. Nematicidal and Insecticidal Compounds from the Laurel Forest Endophytic Fungus Phyllosticta sp. Molecules 29 (19), 4568. https://doi.org/10.3390/molecules29194568 (2024)
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
S.D.-G was supported by a Margarita Salas Grant for junior doctors (RD 289/2021), funded by the Spanish Ministry of Science, Innovation and Universities (MCIN/AEI/https://doi.org/10.13039/501100011033) and the European Union – NextGenerationEU and PID2021-123697OB-I00 funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and “ERDF A way of making Europe”. C.G.-S was supported by grant PEJ-2020-AI/BIO-19,580 funded by Comunidad de Madrid and is currently supported by grants PRE2022-103983 and CEX2020-000999-S-20-3 funded by MCIN/AEI/ https://doi.org/10.13039/501100011033 and “ESF Investing in your future”. S.S. research is supported by grant PID2021-123697OB-I00 funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and “ERDF A way of making Europe”. S.D.-G., A.G.-C. and M.A. research was supported by grant PID2019-106222RB-C31/SRA (State Research Agency, https://doi.org/10.13039/501100011033 ).
Author information
Authors and Affiliations
Contributions
S.D.-G., S.S., A.G.-C. and M.A. conceptualized and designed the research. S.S. provided the fungal isolates. C.G.-S refreshed the isolates and conducted the ITS identification. S.D.-G. constructed the phylogenetic tree, obtained the fungal extracts, conducted the bioassays and prepared the first draft of the manuscript. S.D.-G., A.G.-C. and M.A. prepared the tables and figures. S.D.-G., S.S., A.G.-C. and M.A. contributed to the text of the main manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Díaz-González, S., Andrés, M.F., González-Sanz, C. et al. Nematicidal and antifeedant activity of ethyl acetate extracts from culture filtrates of Arabidopsis thaliana fungal endophytes. Sci Rep 15, 11332 (2025). https://doi.org/10.1038/s41598-025-94939-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94939-6