Abstract
Autosomal-dominant hypocalcified amelogenesis imperfecta (ADHCAI) is caused by mutations in the FAM83H gene. Mutated FAM83H genes encode truncated FAM83H proteins with amino acid lengths between amino acids 1–286 and 1–693, in contrast to wild-type FAM83H (1–1179). Deletion of the C-terminus of FAM83H results in its subcellular translocation from the cytoplasmic compartment to the nuclear speckles, where splicing factors accumulate. However, the amino acid region of FAM83H required for nuclear speckle localization has not yet been determined, and whether all FAM83H-truncated proteins associated with ADHCAI localize to nuclear speckles remains unknown. Here, we examined the subcellular localization of FAM83H mutant proteins with truncations or deletions at various amino acid positions. Deletions within residues 1–300, which corresponds to the DUF1669 domain (17–281), attenuated or abolished the nuclear speckle localization of FAM83H. Meanwhile, some ADHCAI-related FAM83H-truncated proteins did not localize to nuclear speckles, despite the presence of the DUF1669 domain. These results suggest that the DUF1669 domain is required, but not sufficient, for nuclear speckle localization of FAM83H, demonstrating that nuclear speckle localization is not a common feature among FAM83H-truncated proteins related to ADHCAI.
Similar content being viewed by others
Introduction
FAM83H (FAMily with sequence similarity 83 H) belongs to the FAM83 family of genes, which consists of eight members (A–H)1. The FAM83H gene is the causative gene of autosomal-dominant hypocalcified amelogenesis imperfecta (ADHCAI)2. A heterozygous truncation mutation in FAM83H has been previously detected in patients with ADHCAI2,3. FAM83H mutant genes encode truncated FAM83H proteins with amino acid lengths between 1–286 and 1–693, in contrast to the wild-type FAM83H, which is composed of 1179 amino acids3. However, the mechanism through which FAM83H-truncated proteins cause ADHCAI remains unclear.
The molecular and cellular functions of FAM83H have been reported in the field of oncology, as increased expression of FAM83H has been observed in a variety of cancer types4,5. Previous studies have suggested that increased FAM83H expression promotes cancer cell invasion, proliferation, and survival6,7,8,9,10. FAM83H shows subcellular localization in keratin filaments of colorectal cancer and ameloblastoma cells6,11. FAM83H regulates the subcellular localization of the CK1 kinase family members, which are involved in diverse cellular processes12. FAM83H recruits CK1α to keratin filaments6. It has been suggested that FAM83H and CK1α are involved in the organization of the keratin cytoskeleton6. An aberrant keratin cytoskeleton has been observed in cancer cells overexpressing FAM83H in human colorectal cancer tissues6. The subcellular localization of FAM83H markedly changes in some cancer cells that lose the expression of keratin proteins due to cellular events, such as epithelial-to-mesenchymal transition (EMT)13. In RKO and WiDr colorectal cancer cells, which do not express keratin proteins, FAM83H localizes to nuclear speckles, where a variety of splicing factors accumulate13. The nuclear speckle localization of FAM83H subsequently leads to the recruitment of CK1α, δ, and ε to the nuclear speckles13. CK1α is associated with and phosphorylates splicing factors known as SR proteins and a nuclear poly(A) polymerase, Star-PAP, in nuclear speckles20,21. CK1α is involved in the synthesis of Star-PAP target mRNAs21. Nuclear speckle localization of FAM83H and the subsequent recruitment of CK1α may affect mRNA metabolism. In addition, FAM83H has been reported to regulate the expression of various proteins, such as cyclin D1, cyclin E1, MMP2, p53, p27, SCRIB, and β-catenin, in cancer cells7,8,9. NCK1/2 and SEC16A have been identified as FAM83H interactors3,14. A recent study reported that transcripts of Csnk1a1, Csnk1e, Ctnnb1, Fgf10, Ambn, Mmp20, and Dspp were significantly reduced in Fam83h knockout-mice15. Proteins encoded by Ambn, Mmp20, and Dspp, are known to participate in amelogenesis16,17.
Some studies have found that the expression of the FAM83H-truncated proteins influences the Wnt/β-catenin signaling pathway, in which CK1 members play a critical role22. In the mouse ameloblast cell line LS8, the stable transfection of the FAM83H-truncated protein with amino acid residues 1–395 was found to result in the activation of the Wnt/β-catenin signaling18. On the other hand, the inhibition of the Wnt/β-catenin signaling was observed in primary osteoblasts obtained from Fam83hQ396*/ Q396* knock-in mice, which also expressed the FAM83H-truncated protein 1–39519. Although the reason for the discrepancy between these studies is unknown, researchers have hypothesized that aberrant Wnt/β-catenin signaling may be involved in the mechanism of ADHCAI.
The N-terminal amino acid residues 17–281 of FAM83H form the DUF1669 (Domain of Unknown Function 1669) domain, which is conserved among the FAM83 family members1. The DUF1669 domain interacts with CK1 members, including CK1α, δ, and ε6,14,19,23. We previously reported that the C-terminal amino acid region around residues 1134–1139 is involved in the keratin localization of FAM83H24. The deletion of this C-terminal region results in the translocation of FAM83H from keratin filaments to nuclear speckles24. Importantly, FAM83H-truncated proteins related to ADHCAI do not have the C-terminal amino acid residues required for keratin localization. Although the detailed subnuclear localization has not been determined, nuclear localization of some FAM83H-truncated proteins related to ADHCAI has been reported14,19. Based on these findings, another hypothesis involving ADHCAI has emerged, positing that FAM83H-truncated proteins may acquire new functions that affect enamel formation by translocation to nuclear speckles. In this study, we investigated the amino acid region of FAM83H required for nuclear speckle localization in order to understand the localization of ADHCAI-related FAM83H mutant proteins to nuclear speckles.
Results
Subcellular localization of FAM83H-truncated proteins in WiDr cells
To identify the amino acid region of FAM83H required for its localization to nuclear speckles, we examined the subcellular localization of FAM83H-truncated proteins with various amino acid lengths. Plasmids encoding C-terminal FLAG-tagged FAM83H-truncated proteins were introduced into WiDr cells, which do not express keratin proteins13, and transiently expressed. The subcellular localization of these proteins was visualized by immunofluorescence. Nuclear speckle localization in the FAM83H mutants was determined by co-localization with the nuclear speckle protein SON13. Full-length FAM83H (1–1179) localized to the nuclear speckles in WiDr cells (Fig. 1 and Fig. S1). Similarly, nuclear speckle localization was observed for FAM83H-truncated proteins 1–1000, 1–900, 1–800, 1–700, 1–603, 1–500, and 1–400 (Fig. 1). FAM83H-truncated proteins 1–370, 1–350, and 1–330 showed gradual attenuation of nuclear speckle localization accompanied by shortening of amino acid length (Fig. 1). Moreover, a loss of nuclear speckle localization was observed in FAM83H-truncated proteins 1–296 and 1–286 (Fig. 1 and Fig. S1). In DLD1 cells expressing keratin proteins24, full-length FAM83H was localized in the cytoplasmic compartment, whereas FAM83H-truncated proteins 1–1000, 1–900, 1–800, 1–700, 1–603, 1–500, and 1–400 showed nuclear speckle localization (Fig. S2). As observed in WiDr cells (Fig. 1), attenuation or loss of nuclear speckle localization was shown by FAM83H-truncated proteins 1–370, 1–350, 1–330, 1–296, and 1–286 (Fig. S2). These results suggested that the amino acid region upstream of residue 400 in FAM83H is required for nuclear speckle localization.
Subcellular localization of FAM83H-truncated proteins in WiDr cells. WiDr cells were transfected with plasmids encoding C-terminal FLAG-tagged FAM83H-truncated proteins of the indicated amino acid lengths, and analyzed by immunofluorescence using antibodies against FLAG (green) and SON (red). Nuclei were visualized using DAPI (blue). Magnified images of the nuclei are shown. Scale bars, 10 μm.
Subcellular localization of CK1α in WiDr cells expressing FAM83H-truncated proteins
The expression of full-length FAM83H induced the nuclear speckle localization of CK1α in WiDr cells (Fig. 2), as previously reported13. We evaluated whether the nuclear speckle localization of CK1α was induced by FAM83H-truncated proteins in WiDr cells. FAM83H-truncated proteins 1–1000, 1–900, 1–800, 1–700, 1–603, 1–500, and 1–400 induced nuclear speckle localization of CK1α (Fig. 2). The induction of nuclear speckle localization of CK1α was gradually attenuated in WiDr cells expressing FAM83H-truncated proteins 1–370, 1–350, and 1–330, accompanied by shortening of the amino acid length of the FAM83H mutants. FAM83H-truncated proteins 1–296 and 1–286 were unable to induce nuclear speckle localization of CK1α. Similar results were observed in DLD1 cells expressing FAM83H mutants (Fig. S3). However, in DLD1 cells expressing full-length FAM83H, CK1α was localized in the cytoplasmic compartment because full-length FAM83H localized to the cytoplasmic compartment (Fig. S3). These results suggest that the nuclear speckle recruitment of CK1α requires an amino acid region upstream of residue 400 of FAM83H.
Subcellular localization of CK1a in WiDr cells expressing FAM83H-truncated proteins. WiDr cells were transfected with plasmids encoding C-terminal FLAG-tagged FAM83H-truncated proteins of the indicated amino acid lengths, and analyzed by immunofluorescence using antibodies against FLAG (green) and CK1α (red). Nuclei were visualized using DAPI (blue). Magnified images of the nuclei are shown. Scale bars, 10 μm.
Amino acid residues 300–400 of FAM83H are not required for the nuclear speckle localization
To determine whether amino acid residues 300–400 of FAM83H are required for nuclear speckle localization, we tested the subcellular localization of FAM83H mutants with deletions in the range of amino acid residues 300–400. WiDr cells were transfected with a vector encoding FAM83H mutants with a deletion of amino acid residues 300–330 (Δ300–330), 300–350 (Δ300–350), 300–370 (Δ300–370), and 300–400 (Δ300–400), and examined by immunofluorescence using antibodies against FAM83H, CK1α, and nuclear speckle protein SC-35. Because WiDr cells do not express endogenous FAM83H13, images visualized using the anti-FAM83H antibody showed the subcellular localization of the FAM83H mutants (Fig. 3). Unexpectedly, all FAM83H mutants were localized to nuclear speckles and recruited CK1α to the nuclear speckles (Fig. 3). These results suggest that amino acid residues 300–400 of FAM83H are not essential for nuclear speckle localization or recruitment of CK1α to nuclear speckles. Alternatively, these results led to the hypothesis that amino acid residues 1–300 of FAM83H, which approximately correspond to the DUF1669 domain (residues 17–281), are required for the nuclear speckle localization and nuclear speckle recruitment of CK1α.
Subcellular localization of FAM83H mutants with a deletion mutation in the range of amino acid residues 300–400 in WiDr cells. WiDr cells were transfected with plasmids encoding C-terminal FLAG-tagged FAM83H-mutants with deletion of the indicated amino acid residues and analyzed by immunofluorescence using antibodies against FAM83H (red), CK1α (green), and SC-35 (violet). Nuclei were visualized using DAPI (blue). Scale bars, 10 μm.
The DUF1669 domain of FAM83H is required but not sufficient for its nuclear speckle localization and the nuclear speckle recruitment of CK1α
Next, we assessed the requirement of the DUF1669 domain of FAM83H for nuclear speckle localization and nuclear speckle recruitment of CK1α. FAM83H mutants with deletion mutations in the amino acid residues 1–300 were expressed in WiDr cells, and the subcellular localization of the mutants and CK1α was examined by immunofluorescence (Fig. 4). Nuclear speckle localization of the FAM83H mutant lacking the N-terminal 49 residues (50–1179) was attenuated compared to that of full-length FAM83H (Fig. 4). The FAM83H mutant with a deletion of residues 51–100 (Δ51–100) showed a more severe attenuation of nuclear speckle localization (Fig. 4). FAM83H mutants with deletions of residues 101–150 (Δ101–150), 151–200 (Δ151–200), 201–250 (Δ201–250), and 251–300 (Δ251–300) completely disappeared from the nuclear speckles (Fig. 4). Nuclear speckle localization of CK1α was not observed in any of the deletion mutants (Fig. 4). These results suggest that the DUF1669 domain of FAM83H is required for nuclear speckle localization and recruitment of CK1α to nuclear speckles.
Subcellular localization of FAM83H mutants with a deletion mutation in the range of amino acid residues 1–300 in WiDr cells. WiDr cells were transfected with plasmids encoding C-terminal FLAG-tagged FAM83H-mutants with deletion of the indicated amino acid residues and analyzed by immunofluorescence using antibodies against FAM83H (red), CK1α (green), and SC-35 (violet). Nuclei were visualized using DAPI (blue). Scale bars, 10 μm.
The C-terminal FLAG-tagged FAM83H-truncated proteins 1–286 and 1–296 did not localize to the nuclear speckles despite the presence of the DUF1669 domain (Fig. 1). We hypothesized that the DUF1669 domain is not sufficient but is required for the nuclear speckle localization of FAM83H, or that the C-terminal FLAG-tag may affect the function of the DUF1669 domain of FAM83H 1–286 and 1–296. To evaluate the second possibility, N-terminal FLAG-tagged FAM83H-truncated proteins 1–286 and 1–296 were used to test their subcellular localization. N-terminal FLAG-tagged full-length FAM83H localized to nuclear speckles and induced the nuclear speckle localization of CK1α (Fig. S4), whereas the N-terminal FLAG-tagged FAM83H-truncated proteins 1–286 and 1–296 did not show nuclear speckle localization or CK1α recruitment to the nuclear speckles (Fig. S4). These results suggested that the delocalization of FAM83H-truncated proteins 1–286 and 1–296 from the nuclear speckles was not due to the artificial effect of the FLAG-tag. In summary, we conclude that the DUF1669 domain of FAM83H is required for nuclear speckle localization but is not sufficient.
FAM83A and F do not localize to nuclear speckles
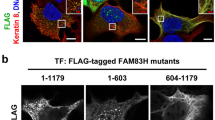
The DUF1669 domain is conserved among FAM83 family members1. We tested whether FAM83A and F were localized to nuclear speckles. C-terminal FLAG-tagged FAM83A and F (Fig. 5a) were expressed in WiDr cells, and their subcellular localization was analyzed by immunofluorescence. FAM83A and F showed neither the nuclear speckle localization nor the recruitment of CK1α to nuclear speckles (Fig. 5b). These results suggest that nuclear speckle localization is not a common feature of the FAM83 family members.
Subcellular localization of FAM83A and F in WiDr cells. (a) The amino acid lengths of FAM83H, A, and F are shown in light gray columns. Yellow columns indicate the DUF1669 domain. Dark-gray columns indicate the FLAG-tag. (b) WiDr cells were transfected with plasmids encoding FLAG-tagged FAM83H, A, or F and analyzed by immunofluorescence using antibodies against FLAG (red), CK1α (green), and SON (violet). Nuclei were visualized using DAPI (blue). Scale bars, 10 μm.
Discussion
In this study, we examined the subcellular localization of FAM83H mutants to determine the amino acid region required for nuclear speckle localization (Fig. 6). Deletions within the DUF1669 domain of FAM83H were found to either attenuate (50–1179 and Δ51–100) or abolish (Δ101–150, Δ151–200, Δ201–250, and Δ251–300) its nuclear speckle localization; which suggests that the DUF1669 domain is required for FAM83H to localize nuclear speckles. In addition, this study provides important information regarding the mechanism by which FAM83H mutations cause ADHCAI. The FAM83H mutant genes related to ADHCAI encode FAM83H-truncated proteins with amino acid lengths between amino acids 1–286 and 1–693. In this study, nuclear speckle localization was revealed to not be a common feature of FAM83H-truncated proteins with amino acid lengths ranging between 1–286 and 1–700. FAM83H-truncated proteins 1–400, 1–500, 1–600, and 1–700 were localized to nuclear speckles, whereas the nuclear speckle localization of 1–286, 1–296, 1–330, 1–350, and 1–370 was attenuated or abolished. These results suggest that the translocation of FAM83H-truncated proteins to nuclear speckles is not the cause of ADHCAI.
The FAM83H mutants used in the study. Full-length FAM83H comprises 1179 amino acid residues. The amino acid lengths of the FAM83H mutants used in this study are shown in light gray columns. Yellow columns indicate the DUF1669 domain. Dark-gray columns indicate the amino acid residues of the FLAG-tag (F). Red lines indicate the positions of keratin localization residues (K, 1134–1139). Green columns indicate GFP. The ○, △, and × marks respectively indicate the positive, attenuated, and negative localization of FAM83H mutants or CK1α to nuclear speckles (NS) in WiDr cells expressing the indicated mutants.
Although we have shown that the DUF1669 domain of FAM83H is required for nuclear speckle localization, nuclear speckle localization of FAM83H-truncated proteins with amino acid lengths between 1–286 and 1–370 was attenuated or abolished despite the presence of the DUF1669 domain. The attenuated or absent nuclear speckle localization in these mutants suggested that the DUF1669 domain was insufficient for nuclear speckle localization. FAM83H-truncated proteins with amino acid lengths greater than 1–400 are normally localized in nuclear speckles. Therefore, the DUF1669 domain may require approximately 100 additional amino acid residues to flank its C-terminus. However, the FAM83H mutants Δ300–330, Δ300–350, Δ300–370, and Δ300–400 were localized in the nuclear speckles. These results suggest that the C-terminal flanking amino acid residues required for the DUF1669 domain must be of appropriate length but do not need to be in a specific sequence order. To confirm this hypothesis, we examined the subcellular localization of FAM83H 1–286 fused with the C-terminal enhanced green fluorescent protein (GFP) comprising 246 amino acids (Fig. 6). FAM83H 1–286-GFP did not localize to the nuclear speckles (Fig. S5). However, the results did not support this hypothesis. Further studies are required to elucidate the mechanism by which FAM83H localizes to nuclear speckles.
Nuclear speckle localization of FAM83H depends on SON13. Interactome analysis has previously highlighted interactions between FAM83H and SON13. Because the DUF1669 domain is required for nuclear speckle localization, this DUF1669 domain could be involved in the interaction between FAM83H and SON. The interaction between FAM83H and CK1 members is mediated by the DUF1669 domain6,23; thus, the DUF1669 domain may be involved in the link between CK1 members and SON to recruit them to nuclear speckles. In a previous study, proteins interacting with FAM83H-truncated protein 1–286 were compared to those interacting with full-length FAM83H by proteomic analysis of their co-immunoprecipitates13. CK1 members were detected in the co-immunoprecipitates of the full-length protein and the 1–286 mutant, whereas SON was detected only in the co-immunoprecipitates of the full-length protein, suggesting that the FAM83H-truncated proteins 1–286 can interact with CK1 but not with SON. These results suggest that the delocalization of FAM83H-truncated protein 1–286 from the nuclear speckles may be due to their inability to interact with SON. Therefore, the DUF1669 domain may not be a SON-binding site. Alternatively, the DUF1669 domain may play a critical role in regulating interactions between FAM83H and SON.
Among the ADHCAI-related mutants of FAM83H with different amino acid lengths between 1–286 and 1–693, their common features may be involved in the mechanism of ADHCAI. Thus, the identification of their common features contributes to the understanding of the etiology of ADHCAI. A common structural feature among the ADHCAI-related FAM83H mutants is the presence of a DUF1669 domain. The truncation mutation in the shortest mutant, residues 1–286, occurs at an amino acid residue close to the DUF1669 domain (residues 17–281). No patient with ADHCAI harboring deletions within the DUF1669 domain of FAM83H has been reported to date. Circumstantial evidence has indicated that the DUF1669 domain, which mediates CK1 binding, is required for FAM83H mutations to cause ADHCAI. The binding of FAM83H-truncated mutants to CK1 members through the DUF1669 domain may aberrantly affect the functions of CK1 members related to amelogenesis. FAM83H-truncated proteins have been reported to alter subcellular localization of CK1α23,24. Translocation of CK1α affects its function because CK1α phosphorylates different substrates at different subcellular localizations12. A previous study has reported that FAM83H-truncated proteins recruit CK1α to nuclear speckles24, which raises the question as to whether the recruitment of CK1α to nuclear speckles triggers ADHCAI. However, this hypothesis was refuted in the present study by showing that the recruitment of CK1α to nuclear speckles is not a common event induced by all ADHCAI-related FAM83H mutants. Another hypothesized mechanism of ADHCAI is the dominant-negative inhibition of CK1α. A previous study reported that murine FAM83H-truncated protein 1–395 induced the activation of the Wnt/β-catenin signaling pathway in mouse ameloblast cell line LS818. CK1α negatively regulates the Wnt/β-catenin signaling pathway by phosphorylating β-catenin in the cytoplasmic compartment22. Binding of FAM83H-truncated protein 1–395 to CK1α may prevent the phosphorylation of β-catenin by sequestrating CK1α from β-catenin. However, another study showed conflicting results by showing that the Wnt/β-catenin signaling was suppressed in primary osteoblasts obtained from Fam83hQ396*/ Q396* knock-in mice, which also expressed the murine FAM83H-truncated protein 1–39519. Further studies are needed to dissolve this discrepancy and to elucidate whether all ADHCAI-related FAM83H mutants exert a common effect on the Wnt/β-catenin signaling pathway. Although the present study eliminated the hypothesis that the nuclear speckle localization of FAM83H mutants and CK1α is involved in ADHCAI, our knowledge is still not sufficient to establish a reliable hypothesis regarding the mechanism of ADHCAI. In addition to CK1α, the involvement of other CK1 members in the mechanism of ADHCAI is also needed to be explored because the DUF1669 domain interacts not only with CK1α but also with CK1δ and ε6,13,23. To understand the mechanism of ADHCAI, future studies are needed to determine the functions in which CK1 members are affected by FAM83H-truncated proteins with specific amino acid lengths between 1–286 and 1–693.
Methods
Plasmids
The p3xFLAG-CMV14 vectors encoding C-terminal FLAG-tagged FAM83H 1–1179 (full-length), 1–603, 1–296, and 1–286 were previously generated6,24. The vectors encoding C-terminal FLAG-tagged FAM83H 1–1000, 1–900, 1–700, 1–500, 1–400, 1–370, 1–350, 1–330, and 50–1179, N-terminal FLAG-tagged FAM83H 1–1179 (full length), 1–296, and 1–286, and C-terminal GFP-fused FAM83H 1–1179 were generated using an In-Fusion HD Cloning Kit (Takara Bio Inc., Shiga, Japan) or the Seamless Ligation Cloning Extract (SLiCE) method25. The insert sequences were amplified by PCR using p3xFLAG-CMV14-FAM83H (full-length) as a template and appropriate primers (Table S1). The PCR products were inserted into the p3xFLAG-CMV14-FAM83H vector digested with BamHI and XhoI (1–1000 and 1–900), the p3xFLAG-CMV14 vector digested with BamHI and EcoRI (1–700, 1–500, 1–400, 1–370, 1–350, and 50–1179), the pcDNA3.1(+)-FLAG/myc-His vector digested with EcoRI and BamHI (N-terminal FLAG-tagged 1–1179, 1–296, and 1–286), or the pEGFP-N1 vector digested with BamHI and HindIII (C-terminal GFP-fused FAM83H 1–1179). The pcDNA3.1(+)-FLAG/myc-His vector was generated by inserting a FLAG-tag sequence (ACCATGGACTACAAAGACGATGATGACAAA) into the pcDNA3.1(+)/myc-HisA vector digested with HindIII and BamHI. The p3xFLAG-CMV14-FAM83H 1–800 vector was generated by the SLiCE method using the p3xFLAG-CMV14-FAM83H vector digested by BamHI and XhoI and a DNA duplex with the sense sequence 5’-GCGCCG CAGCCTCG AGAGCTG CCTGCTGG ACCTGCGC GACTCC TTTGCACA GCAGGG ATCCCGG GCTGACT-3'. The p3xFLAG-CMV14 vectors encoding C-terminal FLAG-tagged FAM83H with a deletion mutation Δ51–100, Δ101–150, Δ151–200, Δ201–250, Δ251–300, Δ300–330, Δ300–350, Δ300–370, and Δ300–400 and the pEGFP-N1 vector encoding C-terminal GFP-fused FAM83H 1–286 were generated by the site-directed mutagenesis (SDM) method using PrimeSTAR GXL (Takara Bio Inc.), KOD plus Neo (Toyobo, Osaka, Japan), or KOD One DNA polymerases (Toyobo) in accordance with the concept of a PrimeSTAR Mutagenesis Basal Kit (Takara Bio Inc.). PCR for SDM was performed using the p3xFLAG-CMV14-FAM83H (full-length) vector or pEGFP-N1-FAM83H (full-length) as a template and appropriate primers (Table S2). p3xFLAG-CMV14 vectors encoding C-terminal FLAG-tagged FAM83A and F were generated using the SLiCE method. The insert sequences were amplified by PCR using plasmids containing FAM83A or F cDNA as templates and the appropriate primers (Table S3). The PCR products were inserted into the p3xFLAG-CMV14-FAM83H vector and digested with BamHI and EcoRI. Plasmids containing FAM83A (IRAK047L06) or F (IRAK048L18) cDNA26,27,28,29 were provided by RIKEN BRC (Ibaragi, Japan) through the National BioResource Project of MEXT, Japan. The inserted DNA sequences of all generated plasmids were verified by Sanger sequencing.
Cell culture and transfection
The WiDr and DLD1 colorectal cancer cell lines were obtained from the American Type Culture Collection (ATCC; VA, USA). Cells were cultured at 37 °C in 5% CO2 in Iscove’s modified Dulbecco’s medium (IMDM) (Nacalai Tesque, Kyoto, Japan) supplemented with 5% fetal bovine serum (FBS) (Biowest, Nuaillé, France). Plasmid transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific).
Antibodies
The following antibodies were used: anti-FLAG (F1804; Sigma-Aldrich, MO, USA), anti-SON (HPA023535; Sigma-Aldrich), anti-FAM83H (HPA024604; Sigma-Aldrich), anti-CK1α (sc-6477; Santa Cruz Biotechnology), and anti-SC-35 (S4045; Sigma-Aldrich). Alexa Fluor 488-conjugated donkey anti-mouse IgG (A-21202), Alexa Fluor 568-conjugated donkey anti-mouse IgG (A10037), anti-rabbit IgG (A10042), Alexa Fluor 594-conjugated donkey anti-goat IgG (A-11058), and Alexa Fluor 647-conjugated donkey anti-mouse IgG (A-31571) antibodies were purchased from Thermo Fisher Scientific. Alexa Fluor 488-conjugated donkey anti-goat IgG (705-545-147) and Alexa Fluor 647-conjugated donkey anti-rabbit IgG (711-605-152) were purchased from Jackson ImmunoResearch Laboratories (PA, USA).
Immunofluorescence
Cells were fixed with 100% MeOH at ‒20 °C for 2 min, blocked with Blocking One (Nacalai Tesque) on ice for 30 min, and sequentially incubated with primary and secondary antibodies at room temperature for 1 h. The DNA was stained with 100 ng/ml of 4′-6-diamidino-2-phenylindole (DAPI). The stained samples were viewed under an FV1000 confocal microscope equipped with the objective lens UPlan SApo 100×/1.40 Oil (Olympus, Tokyo, Japan) or an LSM900 Airyscan2 equipped with the objective lens Plan-Apochromat 63x/1.40 Oil DIC (Carl Zeiss AG, Oberkochen, Germany). All the images shown in the figures are single confocal planes of representative cells. Composite images were prepared using Photoshop CS5 (Adobe, San Jose, CA, USA). Nuclear speckle localization of FAM83H and CK1α was determined by comparing their immunofluorescence intensities in the region co-localized with nuclear speckle markers with those in the nucleoplasm by the eye. Positive nuclear speckle localization was defined as stronger immunofluorescence intensities in the nuclear speckles than in the nucleoplasm (Fig. S1). The judgement was performed independently by two participants.
Data availability
The materials and data used during the current study available from the corresponding author on reasonable request.
References
Bozatzi, P. & Sapkota, G. P. The FAM83 family of proteins: from pseudo-PLDs to anchors for CK1 isoforms. Biochem. Soc. Trans. 46, 761–771 (2018).
Kim, J. W. et al. FAM83H mutations in families with autosomal-dominant hypocalcified amelogenesis imperfecta. Am. J. Hum. Genet. 82, 489–494 (2008).
Wang, S. K. et al. FAM83H and autosomal dominant hypocalcified amelogenesis imperfecta. J. Dent. Res. 100, 293–301 (2021).
Bartel, C. A., Parameswaran, N., Cipriano, R. & Jackson, M. W. FAM83 proteins: fostering new interactions to drive oncogenic signaling and therapeutic resistance. Oncotarget 7, 52597–52612 (2016).
Snijders, A. M. et al. FAM83 family oncogenes are broadly involved in human cancers: an integrative multi-omics approach. Mol. Oncol. 11, 167–179 (2017).
Kuga, T. et al. A novel mechanism of keratin cytoskeleton organization through casein kinase Iα and FAM83H in colorectal cancer. J. Cell. Sci. 126, 4721–4731 (2013).
Kim, K. M. et al. FAM83H is involved in the progression of hepatocellular carcinoma and is regulated by MYC. Sci. Rep. 7 https://doi.org/10.1038/s41598-017-03639-3 (2017).
Kim, K. M. et al. The expression patterns of FAM83H and PANX2 are associated with shorter survival of clear cell renal cell carcinoma patients. Front. Oncol. 9, 14. https://doi.org/10.3389/fonc.2019.00014 (2019).
Hussein, U. K. et al. FAM83H and SCRIB stabilize β-catenin and stimulate progression of gastric carcinoma. Aging 12, 11812–11834 (2020).
Jeong, T. Y., Lee, H. I., Park, M. S., Seo, M. Y. & Jang, K. Y. Individual and Co-Expression Patterns of FAM83H and SCRIB at Diagnosis Are Associated with the Survival of Colorectal Carcinoma Patients. Diagnostics. 12, 1579 (2022). https://doi.org/10.3390/diagnostics12071579
Kuga, T. et al. FAM83H and casein kinase I regulate the organization of the keratin cytoskeleton and formation of desmosomes. Sci. Rep. 6, 26557. https://doi.org/10.1038/srep26557 (2016).
Fulcher, L. J. & Sapkota, G. P. Functions and regulation of the serine/threonine protein kinase CK1 family: moving beyond promiscuity. Biochem. J. 477, 4603–4621 (2020).
Kuga, T. et al. Casein kinase 1 is recruited to nuclear speckles by FAM83H and SON. Sci. Rep. 6, 34472. https://doi.org/10.1038/srep34472 (2016).
Tachie-Menson, T. et al. Characterisation of the biochemical and cellular roles of native and pathogenic amelogenesis imperfecta mutants of FAM83H. Cell. Signal. 72, 109632. https://doi.org/10.1016/j.cellsig.2020.109632 (2020).
Nasseri, S. et al. CRISPR/Cas9-Induced Fam83h Knock-out leads to impaired Wnt/β-Catenin pathway and altered expression of tooth mineralization genes in mice. Iran. J. Biotechnol. 21, e3673. https://doi.org/10.30498/ijb.2023.391902.3673 (2023).
Pugach, M. K. & Gibson, C. W. Analysis of enamel development using murine model systems: approaches and limitations. Front. Physiol. 5, 313. https://doi.org/10.3389/fphys.2014.00313 (2014).
Paine, M. L. et al. Dentin sialoprotein and dentin phosphoprotein overexpression during amelogenesis. J. Biol. Chem. 280, 31991–31998 (2005).
Yang, M. et al. Fam83h mutation inhibits the mineralization in ameloblasts by activating Wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 501, 206–211 (2018).
He, Z. et al. Fam83h mutation causes mandible underdevelopment via CK1α-mediated Wnt/β-catenin signaling in male C57/BL6J mice. Bone 172, 116756. https://doi.org/10.1016/j.bone.2023.116756 (2023).
Gross, S. D., Loijens, J. C. & Anderson, R. A. The casein kinase Ia isoform is both physically positioned and functionally competent to regulate multiple events of mRNA metabolism. J. Cell. Sci. 112, 2647–2656 (1999).
Gonzales, M. L., Mellman, D. L. & Anderson, R. A. CKIα is associated with and phosphorylates Star-PAP and is also required for expression of select Star-PAP target messenger RNAs. J. Biol. Chem. 283, 12665–12673 (2008).
Cruciat, C. M. Casein kinase 1 and Wnt/β-catenin signaling. Curr. Opin. Cell. Biol. 31, 46–55 (2014).
Fulcher, L. J. et al. The DUF1669 domain of FAM83 family proteins anchor casein kinase 1 isoforms. Sci. Signal. 11, eaao2341. https://doi.org/10.1126/scisignal.aao2341 (2018).
Kuga, T. et al. The conserved C-terminal residues of FAM83H are required for the recruitment of casein kinase 1 to the keratin cytoskeleton. Sci. Rep. 12, 11819. https://doi.org/10.1038/s41598-022-16153-y (2022).
Okegawa, Y. & Motohashi, K. A simple and ultra-low cost homemade seamless ligation cloning extract (SLiCE) as an alternative to a commercially available seamless DNA cloning kit. Biochem. Biophys. Rep. 4, 148–151 (2015).
Ota, T. et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat. Genet. 36, 40–45 (2004).
Otsuki, T. et al. Signal sequence and keyword trap in Silico for selection of full-length human cDNAs encoding secretion or membrane proteins from oligo-capped cDNA libraries. DNA Res. 12, 117–126 (2005).
Kimura, K. et al. Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes. Genome Res. 16, 55–65 (2006).
Itoh, M. et al. Constructing orfeome resources with removable termination codons. Biotechniques 41, 44–48 (2006).
Acknowledgements
This study was supported by the JSPS KAKENHI (grant numbers 24K12893, 21K09865, and 17K08293). We thank Dr. Hayashibara (RIKEN, Japan) and Prof. Sugano (Tokyo Univ., Japan) for providing the plasmids containing FAM83A and F cDNAs. We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
T.K. contributed to conception, design, data acquisition, analysis, and interpretation and drafted and critically revised the manuscript. M.S., S.H., S.Y., S.M., Y.F., Y.T., and S.H. contributed to data acquisition, analysis, and interpretation and critically revised the manuscript. Y.K. and N.Y. contributed to conception and design and critically revised the manuscript. All authors provided their final approval and agreed to be accountable for all aspects of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kuga, T., Saraya, M., Higuchi, S. et al. The DUF1669 domain of FAM83H is required for its localization to nuclear speckles. Sci Rep 15, 12301 (2025). https://doi.org/10.1038/s41598-025-96356-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96356-1