Abstract
Spread of insecticides resistance threatens the control of malaria. In this context, biological control using an endosymbiotic bacterium Wolbachia is being explored as a complementary method for its control. However, for optimal use of this bacterium in biocontrol strategies, it is imperative to characterize it. So, Anopheles gambiae complex mosquitoes were collected, morphologically identified, then blood fed and gravid female mosquitoes oviposited individually. After oviposition, the species of parent was molecularly determined, along with their w-Anga infection status. Additionally, we performed 16SrRNA gene sequencing of w-Anga-positive mosquitoes to determine their phylogeny. Finally, we amplified gene encoding the circumsporozoite protein to determinate their Plasmodium falciparum infection status and assessed the stability of w-Anga transmission of positive females and their offspring. From the results obtained, our w-Anga strains cluster with other Wolbachia Supergroup B strains. However, the prevalence of Plasmodium falciparum infection was lower in Wolbachia-infected females (4.59%) than in those uninfected (22.02%). Furthermore, the transmission frequency of this bacterium in infected Anopheles coluzzii females of the F0 generation to F1 offspring was 10.64% and 16.67% from infected females of the F1 generation to F2 offspring. This study results will serve as preliminary data for the possible use of Wolbachia in malaria control.
Similar content being viewed by others
Introduction
Wolbachia is an alphaproteobacterium, non-spore forming and Gram-negative common to several arthropod species including 70% of insects, as well as some nematodes1. It is an endosymbiotic bacterium currently being tested in biological vector control approaches1.
These control approaches, based on mosquito population replacement or suppression strategies, possess two essential properties: cytoplasmic incompatibility (CI) and the ability to inhibit pathogens, particularly blocking viral replication.
Cytoplasmic incompatibility is a property of Wolbachia that enables its spread within uninfected mosquito populations through a sterility syndrome. This occurs when a Wolbachia-infected male mates with an uninfected female (unidirectional CI) or when both individuals are infected with different Wolbachia strains (bidirectional CI), initially reducing wild mosquito populations (a suppression strategy employed through the Sterile Insect Technique (SIT)). This reduction provides a selective advantage to Wolbachia-infected females, which produce numerous infected offspring, ultimately replacing the wild mosquito population.
Indeed, previous studies involving field releases of Aedes aegypti mosquitoes trans-infected with different strains of Wolbachia (wMelPop, wAlbB, wAu) carried out in Australia, Malaysia and Brazil have shown stability and high prevalence of the bacterium in Aedes sp. populations. This led to a reduction in the incidence of Dengue fever cases in these localities, suggesting the use of this strategy in the eradication of this arbovirosis2,3,4. The success in exploiting the potential of Wolbachia against Aedes aegypti mosquito led some researchers to consider the use of this bacterium against Anopheles mosquitoes, the malaria vector. Unfortunately, the results of these studies were not fruitful and led the authors to consider that Anopheles mosquitoes cannot carry Wolbachia infection5,6. However, this view was reversed in 2014 when the first evidence of natural Wolbachia infections was found in Anopheles gambiae and Anopheles coluzzii collected in Burkina Faso: this bacterial strain was named “w-Anga” and belongs to a new potential phylogenetic supergroup specific to Anopheles, which is related but distinct from supergroups A and B associated with arthropods and evolutionarily linked7. Subsequently, similar evidence of natural infections of Anopheles with Wolbachia was observed across several countries in Africa8,9. In 2017 a natural Wolbachia strain different from that in Burkina Faso was identified in An. gambiae complex mosquitoes of Mali8. Furthermore, in 2019, a study carried out in Gabon confirmed the presence of natural Wolbachia infections in sixteen mosquito species, including all the main malaria vectors in Central Africa (An. gambiae, An. coluzzii, An. funestus, An. nili and An. moucheti)10 .
In order to use these natural Wolbachia strains in vector control strategies against Anopheles mosquitoes, it would be necessary to have knowledge on the phylogeny of w-Anga strain used and the stability of its transmission over the generations of mosquitoes. It would also be necessary to assess the potential impact of the bacterium on the prevalence of Plasmodium in Anopheles mosquitoes.
Results
Frequency of natural w-Anga infections according to localities, collection periods and mosquito species
The current study showed that the overall frequency of w-Anga infection among the collected mosquitoes was 13.84%. This frequency varied according to localities, collection periods and mosquito species. Also, the frequency of natural infection with w-Anga was 3.88% (8/206) for Soumousso, 13.93% (51/366) for VK5 “Vallée du Kou Sector 5” and 24.23% (47/194) for VK7 “Vallée du Kou Sector 7”. However, this frequency was statistically higher for mosquitoes originating from VK7 compared to those originating from VK5 (P = 0.00676) and Soumousso (P < 0.0001). Furthermore, the prevalence of w-Anga infection varied from 2.4% (3/125) for June, 4.41% (19/431) for July, 1.52% (1/66) for August and 57.64% (83/144) for September over all study sites. However, this frequency of infection was higher for September compared to June, July and August with a statistical value of P < 0.001. Among the mosquitoes collected during our study, An. coluzzii was the most infected species with w-Anga at 12.79% (98 /766) frequency, followed by An. gambiae with a frequency of 1.04% (8/766). However, no infection with the bacterium was recorded in An. arabiensis. Due to the high frequency of w-Anga within wild An. coluzzii, we performed the rest of the experiment on this species.
Phylogenetic analysis of w-Anga strains
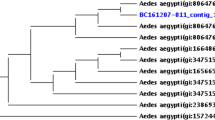
Phylogenetic analysis of 16SrRNA sequences used in our study was based on 33 sequences from NCBI, 4 from our studies and a positive control of the Wolbachia strain wAlbB. This resulted in a midpoint-rooted phylogenetic tree (Fig. 1). This tree showed that the 16 S sequences of our Wolbachia strains cluster with other Supergroup B strains such as wAnga VK5 3.1b for our sample wAnga03, wAns for wAnga02 and wAnCa for wAnga02 & wAnga04 (97–99% nucleotide identity).
Mosquito samples collected have been to 16SrRNA Nested PCR to obtain amplicons positive for Wolbachia strain w-Anga. These amplicons underwent 16SrRNA gene sequencing by Sanger technology using GENEWIZ/AZENTA’s internal formulation of BigDye V3 chemistry on an ABI3730xl sequencer. The sequences obtained were subjected to phylogenetic analysis, which involved aligning them with data available in the GenBank database using BLAST. Following the BLAST alignment, we established a phylogenomic relationship between our samples (five strains, including four derived from our 16SrRNA amplicons and a positive control of wAlbB strain) and 33 Wolbachia strains from NCBI by constructing a phylogenetic tree using the “Fasttree” program in the “Jupyter Notebook” application, version 4.2.4. This phylogenetic tree was visualized and annotated using “ITOL v6.9.1.” The assembly names on the phylogenetic tree were color-coded based on the identity of the supergroups (A-F) and other 16SrRNA sequences.
Impact of natural w-Anga infections on Plasmodium falciparum parasite presence within wild caught Anopheles coluzzii mosquitoes
From our analysis, it appears that the prevalence of P. falciparum infection was lower in w-Anga positive An. coluzzii females compared to w-Anga negative females. Indeed, only 5/109 (4.59%) w-Anga positive females were infected with P. falciparum compared to 24/109 (22.02%) w-Anga negative females that were infected with P. falciparum. Furthermore, the correlation test showed a significant negative correlation (P = 0.0003309) between natural w-Anga infection and P. falciparum infection (Fig. 2).
Plasmodium falciparum infection status based on w-Anga infection within wild caught Anopheles coluzzii. The proportions of Anopheles coluzzii mosquito females infected with Plasmodium among w-Anga-positive females (5/109) and among w-Anga-negative females (24/109) are shown in red (with a statistically significant difference between the two proportions, P = 0.0003309).
Impact of natural w-Anga infections on fecundity and fertility of wild caught Anopheles coluzzii mosquitoes
The impact of natural w-Anga infections on the fecundity and fertility of 107 An. coluzzii females (divided into 34 w-Anga-infected females and 73 uninfected females) was investigated by comparing the mean number of eggs and larvae of w-Anga-infected females with those of uninfected females. Thus, the mean number of eggs laid by w-Anga infected females was statistically higher (P = 0.019) than that of the uninfected. It was 56.63 ± 4.82 eggs for the infected and 42.42 ± 2.55 eggs for the uninfected. The mean number of larvae was statistically higher (P = 0.0022) in w-Anga infected females than in uninfected females. The average number of larvae was 39.81 ± 4.90 for infected females and 23.66 ± 1.91 for uninfected females (Fig. 3).
Stability of w-Anga over generations in Anopheles coluzzii mosquito lines
Out of the 223 females that laid eggs individually, only 32 (14.35%) were infected with w-Anga (F0 generation). From the F1 offspring of infected females, we obtained 329 mosquitoes, including 169 males and 160 females. For this F1 generation, w-Anga was detected in 35 mosquitoes, including 9 males and 26 females. The frequency of transmission of w-Anga from infected females of the F0 generation to F1 offspring was therefore relatively low (10.64%; Table 1). The F2 offspring of infected F1 females produced 18 mosquitoes, divided into 12 males and 6 females. For this generation, w-Anga was detected in 3 mosquitoes, including 1 male and 2 females, with a transmission frequency of 16.67% (Table 1). However, there was no statistically significant difference between the frequency of w-Anga infection in the F1 generation and that in the F2 generation (P = 0.696).
Discussion
The detection of 16SrRNA sequences specific to w-Anga bacterial strain in two of the three An. gambiae sensus lato species, on different ecological sites and during the rainy in Western Burkina Faso suggests that wild Anopheles mosquitoes naturally harbor Wolbachia. This suggests that more efforts should be made to exploit this infection in malaria control. However, the frequencies of w-Anga infection recorded in the two collection sites were relatively low compared to frequencies found previously in the same localities11. Thus, these low infection frequencies could be due to the presence of extremely low densities of Wolbachia in An. gambiae complex mosquitoes in nature. Furthermore, the variation of w-Anga infection frequencies within An. gambiae sensus lato in our study over the collection period may be caused by ecological factors including temperature variations. Indeed, previous studies have reported a reduction in Wolbachia density in Aedes albopictus and Aedes aegypti following a temperature increase12,13. Referring to the initially isolated strains from one of our sites and considering their potential evolution and bacterial diversity, a deeper analysis of the genetic identity of our Wolbachia strains is crucial to determining their phylogenetic relationships with previously identified Wolbachia strains.
The different Wolbachia strains isolated in our study from An. gambiae complex mosquitoes, particularly from An. coluzzii, one of the species within this complex, belong to supergroup B.
Furthermore, referring to Wolbachia strain ‘’wAnga VK5 3.1b’’ which was previously isolated in 2014 from Anopheles mosquitoes at one of our collection sites ‘’VK5’’7, we report identity percentages of 99.20% for our sample wAnga01, 99.74% for wAnga 02, 99.73% for wAnga03 and 99.19% for wAnga 04. These facts allow us to assert that the wAnga strains isolated from our collected mosquitoes are similar to those isolated in 2014 in the same locality with the difference of a few nucleotides. This slight nucleotide variation between wAnga strains could be explained by the occurrence of mutations over time. Our w-Anga strain is similar to ‘’wAnga VK5 3.1b’’ strain, what about its transmission within this species under laboratory conditions?
The stable transmission of w-Anga infection is theoretically expected to reach a frequency of 100% across generations14. However, in our study, we observed low frequencies, indicating that the transmission of w-Anga infection was not stable between successive generations (F0–F1 and F1–F2) of An. coluzzii mosquitoes under laboratory conditions. Several hypotheses may explain this instability. The first hypothesis is based on the presence of residual genomic DNA from dead bacteria in certain females classified as positive, which would limit the transmission of bacterial infection to their offspring15,16. A second hypothesis could be contamination of the midgut lumen of An. coluzzii females by the Wolbachia bacterium or traces of its DNA17. Such contamination might originate either from plants previously fed on by insects carrying Wolbachia17 or from the cohabitation of Anopheles and Aedes mosquitoes infected with Wolbachia at collection sites, for example, in water storage containers where the simultaneous presence of larvae from both species is associated with the detection of Wolbachia18. In such cases of contamination, Wolbachia would not be present in the germ cells of mosquitoes, preventing transovarian transmission within Anopheles lineages19. Finally, another hypothesis could involve mutual exclusion between Wolbachia and certain bacterial genera, such as Asaia spp., in the reproductive organs of vector mosquitoes, a phenomenon recently demonstrated in a study20. Moreover, given the instability of w-Anga infection transmission in our An. coluzzii populations, an essential question arises: what is the impact of this bacterium on the fecundity and fertility of these mosquito populations? From the study of the impact of w-Anga on the fecundity of An. coluzzii in the laboratory, we found that the average number of eggs was statistically higher in infected females than in uninfected ones. A similar fact was observed in Drosophila melanogaster by Eva Fast et al.21. They found that Wolbachia infected flies laid four times more eggs than uninfected flies. They explained this phenomenon by the stimulation of germline stem cell division in infected females by Wolbachia, but also by the reduction of “programmed” death by Wolbachia in the organ where the eggs develop21. Furthermore, our study on An. gambiae complex mosquitoes as well as that of Dobson et al., on Aedes albopictus have shown that the presence of Wolbachia affects the fertility of their host22. This is because the average number of larvae hatched from the eggs of Wolbachia infected females was statistically higher than that of uninfected females.
Beyond its impact on fecundity and fertility of An. coluzzii, w-Anga may impact the development of Plasmodium in the mosquito as we found that the prevalence of P. falciparum infection was lower in Wolbachia-infected females than in those uninfected. Thus, this impact could be the inhibition of Plasmodium by w-Anga. Based on this hypothesis, we wonder about the mechanisms by which this inhibition could be achieved. One of these mechanisms would be the induction of potent anti-pathogenic effects following the activation of the immune system by the w-Anga bacterium. This activation of the immune system could then lead to an inhibitory effect on Plasmodium infection in the mosquito23,24. Another mechanism of inhibition would be the establishment of competition between w-Anga bacterium and Plasmodium parasite for some cellular components, such as cholesterol and other fatty acids of the host that are essential for them25. Also, competition would occur between w-Anga and Plasmodium for nutrient resources contained in the blood meal of the mosquito. Indeed, following a blood meal, large quantities of resources such as lipoproteins are circulated. These same lipoproteins are necessary for Plasmodium to escape the mosquito’s immune system26. However, their potential diversion by Wolbachia could lead to increased rates of Plasmodium destruction by the immune system. In addition, bacteria from the mosquito microbiome could compete with Wolbachia following a blood meal27 and divert the development resources of the Plasmodium. It is not clear whether the negative correlation we observed between Wolbachia and Plasmodium infection is driven by Wolbachia or Plasmodium prevalence. However, Wolbachia is typically received vertically, and so would be present before female mosquitoes are challenged with Plasmodium following maturation and blood-feeding.
Methods
Field mosquito collection, morphological identification and molecular differentiation of Anopheles gambiae complex species
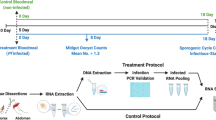
Mosquito collections were carried out monthly during the rainy season from June to September 2021. They consisted of capturing male Anopheles as well as blood fed and gravid female using the Residual Fauna Capture method in Bama “Vallée du Kou” (11°23’N, 4°24’W) and in Soumousso (11°04’N, 4°03’W); two localities located in Western Burkina Faso (Fig. 4)28. “Vallée du Kou” is a rice-growing area with an annual rainfall of about 1200 mm/year. It is characterized by highly productive and almost permanent mosquito breeding grounds due to the presence of the Kou River. Malaria transmission in this locality is essentially ensured by An. gambiae sensus lato and secondarily by An. funestus28.
As for Soumousso, it is a Savannah area with annual rainfall between 1000 and 1200 mm. It is characterized by the presence of a semi-permanent stream that feeds mosquito breeding grounds. In this locality, the main species ensuring the transmission of malaria are An. coluzzii, An. gambiae, An. arabiensis, An. funestus and An. nili, with An. gambiae majority year round28.
A total of 766 mosquitoes were collected from dwellings and enclosures at the study sites. Wild caught mosquitoes were brought to the IRSS/Centre Muraz insectarium and identified morphologically using the Gillies & Coetzee dichotomous key. Females of the An. gambiae complex were selected and oviposited individually. The genomic DNA of those that laid eggs was extracted with Cetyl Trimethyl Ammonium Bromide (CTAB) on whole mosquitoes following the protocol of (Myriam & Céline (2003)). Subsequently, the DNA extracts were amplified by a SINE 200X PCR approach following the protocol of (Santolamazza and al., (2008)), using a pair of primers (S200 × 6.1 F: TCGCCTTAGACCTTGCGTTA; S200 × 6.1R: CGCTTCAAGAATTCGAGATAC) in order to identify their species. This SINE 200X PCR was performed under the following conditions: 10 min at 94 °C for denaturation followed by 35 cycles of 94 °C during 30 s, 54 °C during 30 s, and 72 °C during 1 min, with a final extension step at 72 °C during 10 min. Following amplification, electrophoresis was performed on a 2% agarose gel and bands were observed at 479 bp for An. coluzzii, 249 bp for An. gambiae sensus stricto and 223 bp for An. arabiensis.
Molecular detection of the w-Anga strain in wild caught An. gambiae complex mosquitoes
The detection of w-Anga was performed by a Nested PCR technique targeting a variable region of the conserved Wolbachia 16 S rRNA gene. For this amplification, two primer pairs were used (W-Spec F: CATACCTATTCGAAGGGATAG; W-Spec R: AGCTTCGAGTGAAACCAATTC) for the first phase of the amplification and (16SNF: GAAGGGATAGGGTCGGTTCG; 16SNR: CAATTCCCATGGCGTGACG) for the second primer set. Nested PCR was performed under the following conditions: 5 min at 95 °C for denaturation followed by 2 cycles of 2 min at 95 °C (a), 1 min at 60 °C (b), 1 min at 72 °C (c), followed by 30 s at 95 °C, 1 min at 60 °C, 45 s at 72 °C and repeat a, b and c in 40 cycles, with a final extension step at 72 °C for 5 min for the first primer set and 15 min at 95 °C for denaturation, followed by 35 cycles of 15 s at 95 °C, 15 s at 60 °C and 25 s at 72 °C, with a final extension step at 72 °C for 5 min for the second primer set. Electrophoresis of the second primer set amplicons was performed on a 1% agarose gel and bands were obtained at 412 bp for bacterial positive samples.
16SrRNA gene sequencing and phylogenetic tree realization
The phylogenetic tree realization of w-Anga positives samples was performed on conserved 16SrRNA sequences using Sanger technology. To do this, ten w-Anga PCR products were purified using Wizard® SV Gel and PCR Clean-Up purification kit, (Promega, USA). After purification, the samples have been sent to GENEWIZ from Azenta to perform 16SrRNA Sanger sequencing using an GENEWIZ-internal formulation of BigDye V3 chemistry on a ABI3730xl sequencer. At the end of sequencing, the data obtained were compared with the data available in the GenBank database (www.ncbi.nlm.nih.gov) using the BLAST (Basic Local Alignment Search Tool) search under default parameters and multiple sequence comparison. This alignment was used to confirm that the reads produced were the Wolbachia 16SrRNA gene. As a result of the BLAST alignment, we selected 30 Wolbachia sequences belonging to supergroups A to H and 03 other 16SrRNA sequences with which we created the phylogenetic tree using the web application Jupyter Note Book version 4.2.4. On Jupyter Note Book, all of these sequences were used as imput into the program Mafft version v7.526 and aligned using the default parameters. After alignment, the phylogenetic tree was generated using Fasttree, visualized and annotated by ITOL v6.9.1.
Determination of the spatio-temporal frequency of the bacterial strain w-Anga in Anopheles gambiae complex mosquitoes
The spatio-temporal frequency of w-Anga was determined by performing a molecular screening of the bacterium from June to September 2021 at different study sites. This screening consisted of determining the proportion of w-Anga using the Nested PCR technique for the detection of the said bacterium in An. gambiae complex mosquitoes collected at VK5, VK7 (Two localities of Vallée du Kou) and Soumousso. Subsequently, the species of these mosquitoes was determined by SINE 200X PCR technique.
Molecular detection of Plasmodium falciparum between wild caught of Anopheles coluzzii mosquitoes infected and uninfected to w-Anga
The impact of w-Anga on the presence of wild P. falciparum was assessed by determining the status of P. falciparum infection between wild caught An. coluzzii females carrying w-Anga and those not carrying the bacterium. The detection of P. falciparum consisted of the search for sporozoites by a classical PCR technique using a pair of primers (Pf1: GGAATGTTATTGCTAACAC; Pf2: AATGAAGAGCTGTGTATC) targeting the gene coding for the parasite-specific circumsporozoite protein (CSP). This PCR was performed under the following conditions: 3 min at 94 °C for denaturation, followed by 35 cycles of 30 s at 94 °C, 1 min 15 s at 56 °C and 1 min at 68 °C, followed by 10 min at 68 °C. Electrophoresis of the amplicons was performed on a 2% agarose gel and bands were observed at 501 bp for sporozoite positive samples.
Determination of the impact of natural w-Anga infections on fecundity and fertility of female mosquitoes Anopheles coluzzii
The impact of natural infection with w-Anga on fecundity and fertility of An. coluzzii females was assessed by comparing fecundity and fertility parameters in w-Anga positive F0 females versus females without w-Anga. For the assessment of fecundity, egg counts of w-Anga positive and w-Anga negative females were carried out using a hand-held counter by observation with a hand magnifying glass. For fertility, a count of larvae hatched from eggs (previously counted) of w-Anga infected females as well as uninfected females was carried out. This count was carried out using a transfer pipette.
Laboratory monitoring of the transmission stability of natural w-Anga infection in Anopheles coluzzii mosquito populations
The transmission of w-Anga in An. coluzzii mosquitoes was monitored in the laboratory (in a controlled enclosure under conditions of 26 ± 2℃ temperature and 80 ± 2% relative humidity) over two generations to verify the stability of the bacterium’s transmission in wild-caught mosquitoes. This activity, based on the determination of the generational infection rate (F0 females to F1 offspring and F1 to F2) to the bacterium, involved selecting cups containing the eggs of w-Anga-positive An. coluzzii females and hatching them. On emergence, a total of 329 offspring were obtained for the F1 generation and 18 mosquitoes for the F2 generation. All offspring from these two generations were tested for w-Anga using Nested PCR to verify the stability of this bacterial transmission.
Data analysis
In this study, data analysis and graphs realization were conducted using R (R Core Team, 2021), RStudio (Rstudio Team, 2021), and the packages reshape2 (Wickham, 2007), multcomp (Hothorn, 2008) tidyverse (Wickham, 2019), ggplot2 (Wickham, 2016), scales (Wickham, 2020), broom (Robinson, 2022), and ggpubr (Kassambara, 2020). The tables were made with Microsoft Word 2016. The Chi-squared Pearson and Wilcoxon tests were used for comparisons. p-values < 0.05 were considered statistically significant.
Conclusion
The development of new approaches to malaria vector control is imperative. For this purpose, Wolbachia bacteria could be a promising alternative for vector control. This study shows that Anopheles mosquitoes from “Vallée du Kou” and Soumousso harbor w-Anga infection at a fairly high frequency, and that bacterial strains isolated from “Vallée du Kou” belong to supergroup B. However, under laboratory conditions, the transmission of this infection was unstable in An. coluzzii mosquitoes. Moreover, a negative correlation was observed between w-Anga infection and P. falciparum infection in these mosquitoes. This correlation suggests that Wolbachia could potentially serve as a biological control tool.
Nevertheless, to better understand the various properties of this bacterium and guide future research, it is important to consider the limitations of the present study. These include the lack of seasonal variability in sample collection, the restriction of collections to a single ecological niche (the Southern Sudanian Zone), and the use of a single mosquito species (An. coluzzii) for experiments. These factors limit the extrapolation of results to other ecosystems and other species within the An. gambiae complex. This underscores the need to expand the study by including data from different ecological niches and seasons while assessing the effects of w-Anga on malaria transmission by other significant vectors such as An. gambiae and An. arabiensis.
Despite these limitations, the study highlights two main key findings: the instability of w-Anga infection transmission over An. coluzzii mosquitoes’ generations, and the negative correlation between w-Anga infection and Plasmodium presence within these mosquitoes.
Data availability
The R code and data for all analyses in this article are available as supplementary files.
Abbreviations
- An. arabiensis:
-
Anopheles arabiensis
- An. coluzzii:
-
Anopheles coluzzii
- An. gambiae:
-
Anopheles gambiae
- CSP:
-
Circumsporozoite protein
- CTAB:
-
Cetyl Trimethyl Ammonium Bromide
- DNA:
-
DeoxyRibonucleic Acid
- PCR:
-
Polymerase Chain Reaction
- P. falciparum:
-
Plasmodium falciparum
- rRNA:
-
ribosomal Ribonucleic Acid
- SIT:
-
Sterile Insect Technique
- w-Anga :
-
Wolbachia strain originated from Anopheles gambiae mosquitoes.
- wAlbB :
-
Strain B of Wolbachia originated from Aedes albopictus mosquitoes.
- wAu :
-
Wolbachia from Australia.
- wMelPop :
-
Wolbachia from Drosophila melanogaster.
References
Nugapola, N. W. N. P., De Silva, W. A. P. P. & Karunaratne, S. H. P. P. Distribution and phylogeny of Wolbachia strains in wild mosquito populations in Sri Lanka. Parasit. Vectors. 10, 1–8 (2017).
Neill, S. L. O., Pettigrew, M. M., Sinkins, S. P. & Braig, H. R. In vitro cultivation of Wolbachia Pipientis in an Aedes albopictus cell line. Insect Mol. Biol. 6, 33–39 (1997).
Nazni, W. A. et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 29(24), 4241–4248 (2019).
Gesto, J. S. M. et al. Large-Scale deployment and establishment of Wolbachia into the Aedes aegypti population in Rio de Janeiro, Brazil. Front. Microbiol. ;12. (2021).
Rasgon, J. L. & Scott, T. W. An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J. Med. Entomol. 41(2), 255–257 (2004).
Kittayapong, P., Baisley, K. J., Baimai, V. & O’Neill, S. L. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 37(3), 340–345 (2000).
Baldini, F. et al. Evidence of natural Wolbachia infections in field populations of Anopheles Gambiae. Nat. Commun. 3985(5), 1–7 (2014).
Gomes, F. M. et al. Effect of naturally occurring Wolbachia in Anopheles gambiae s. l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. PNAS. 114(47), 12566–71 (2017).
Wong, M. L. et al. Natural Wolbachia infection in field – collected Anopheles and other mosquito species from Malaysia. Parasit. Vectors. 13, 1–15 (2020).
Ayala, D. et al. Natural Wolbachia infections are common in the major malaria vectors in central Africa. Evol. Appl. 12, 1583–1594 (2019).
Shaw, W. R. et al. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat. Commun. 7, 1–7 (2016).
Wiwatanaratanabutr, I. & Kittayapong, P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J. Invertebr Pathol. 102(3), 220–224 (2009).
Ross, P. A. et al. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLOS Pathog ;1–17. (2017).
Axford, J. K., Ross, P. A., Yeap, H. L., Callahan, A. G. & Hoffmann, A. A. Fitness of wAlbB wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am. J. Trop. Med. Hyg. 94(3), 507–516 (2016).
Walker, T. et al. Stable high-density and maternally inherited Wolbachia infections in Anopheles moucheti and Anopheles demeilloni mosquitoes. Curr. Biol. 31(11), 2310–2320 (2021).
Chrostek, E. & Gerth, M. Is Anopheles Gambiae a natural host of wolbachia ? Am. Soc. Microbiol. 10(3), 1–10 (2014).
Chrostek, E., Pelz-stelinski, K., Hurst, G. D. D. & Hughes, G. L. Horizontal transmission of intracellular insect symbionts via plants. Front. Microbiol. 8, 1–8 (2017).
Nilsson, L. K. J., Sharma, A., Bhatnagar, R. K., Bertilsson, S. & Terenius, O. Presence of Aedes and Anopheles mosquito larvae is correlated to bacteria found in domestic water-storage containers. FEMS Microbiol. Ecol. 94, 1–15 (2018).
Sasaki, T. & Ishikawa, H. Wolbachia infections and cytoplasmic incompatibility in the almond moth and the mediterranean flour moth. Zoological 744(16), 739–744 (1999).
Rossi, P. et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit. Vectors. 278(8), 1–10 (2015).
Fast, E. M. et al. Wolbachia enhance drosophila stem cell proliferation and target the germline stem cell niche. Sci. (80-). 334, 990–992 (2011).
Dobson, S. L., Rattanadechakul, W. & Marsland, E. J. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity (Edinb). 93, 135–142 (2004).
Kambris, Z. et al. Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles Gambiae. PLOS Pathog. 6(10), 1–9 (2010).
Hughes, G. L., Koga, R., Xue, P., Fukatsu, T. & Rasgon, J. L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles Gambiae. PLOS Pathog. 7(5), 3–10 (2011).
Naciri, M. La bactérie Wolbachia bloque l’infection des moustiques par différents pathogènes Humains. Med. Sci. 35, 584–585 (2019).
Rono, M. K., Whitten, M. M. A., Oulad-abdelghani, M., Levashina, E. A. & Marois, E. The major yolk protein vitellogenin interferes with the anti-Plasmodium response in the malaria mosquito Anopheles Gambiae. PLOS Biol. 8(7), 1–12 (2010).
Hughes, G. L. et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. ;111(34):2–7. (2014).
Dabiré, K. R. et al. Anopheles funestus (Diptera: Culicidae) in a humid Savannah area of Western Burkina Faso : bionomics, insecticide resistance status, and role in malaria transmission. J. Med. Entomol. 44(6), 990–997 (2007).
Acknowledgements
We are very grateful to our entire team, in particular Mr Kientega Mahamadi and Mr Bationo Richard for their contributions respectively in the creation of the phylogenetic tree and in the development of our laboratory and field work in particular.
Funding
This work was partially supported by the Burkina Faso government Master thesis Scholarship granted to ELD. The FNIH-Wellcome Trust International Training grant reference Ref: 218,771/Z/19/Z awarded to Dr. Etienne Bilgo and the ANTIVeC Pump Priming AV/PP0025/1 awarded to Prof. Abdoulaye Diabate supported purchasing some lab and fieldwork expenses during this study respectively.
Author information
Authors and Affiliations
Contributions
EB, AD, ELD designed the experiments; ELD, IS, EJG, BL and EB performed the experiments and analyzed the data. EB, ELD, MVM, BL, SS and AD wrote the manuscript. EB and AD are the guarantors of the study. All authors read and approved of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Ethical approval
Experiments with animals were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. In addition, Experiments followed the IRSS Animal Welfare Assurance A5926-01. Trained personnel and veterinarians cared for animals involved in this study and all efforts were made to minimize suffering. All work with w-Anga was performed under biosafety containment level II requirements.
Consent for publication
All authors have approved the final manuscript and consent for the publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Estelle, D.L., Jacques, G.E., Issiaka, S. et al. Unstable laboratory Wolbachia strain w-Anga is negatively correlated with Plasmodium falciparum in wild malaria vectors. Sci Rep 15, 17732 (2025). https://doi.org/10.1038/s41598-025-97288-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97288-6
Keywords
This article is cited by
-
The symbiotic Wolbachia in Anopheles and its role in reducing the transmission of Plasmodium: updates and prospects
Archives of Microbiology (2026)