Abstract
This study investigates drought resistance-growth trade-offs in dominant tree species across elevationally stratified habitats (depression, middle slope, hilltop) of Nonggang karst seasonal rainforest in southwestern China. Analysis of 38 species along a 190-meter aridity gradient reveals drought resistance enhancement through foliar modifications: 56.79% increase in cuticle thickness, 56.42% denser palisade mesophyll thickness, and 36.48% expansion in spongy mesophyll thickness, accompanied by 45.67% growth reduction. Structural equation modeling confirms a significant drought resistance-growth trade-off (β = − 0.504), indicating carbon allocation constraints. Hilltop species employ an anisohydric strategy combining stomatal optimization (4.45% shorter length, 27.17% wider apertures achieving 33.10% increased opening) with 15.52% reduced upper epidermis cell width and cuticle thickening, enhancing hydraulic efficiency despite 111.63% elevated dark respiration rates. This high-resistance/low-growth strategy contrasts with depression-adapted species showing superior low-light performance: 30.35% higher quantum yield and 63.44% lower light compensation points compared to hilltop counterparts. Environmental driver analysis identifies soil moisture (β = − 0.706) and bedrock exposure (β = − 0.594) as key factors shaping trait divergence. Drought suppresses growth through dual pathways: direct resource limitation (β = − 0.667) and indirect metabolic costs of resistance traits (β = − 0.469). Coordinated leaf trait evolution reduces niche overlap via drought-growth trade-offs, establishing habitat filtration-mediated biodiversity maintenance in karst ecosystems. These results provide a multidimensional framework for balancing stress resistance and productivity in tropical karst vegetation restoration.
Similar content being viewed by others
Introduction
The adaptive strategies of plants to the environment are primarily manifested through the coupling and trade-off relationships among their key functional traits1,2. Among these functional traits, plant leaf functional traits are particularly sensitive to environmental changes, demonstrating strong plasticity and diverse adaptive strategies under varying conditions3,4. For instance, under drought conditions, plant leaves develop various morphostructural traits that confer drought resistance. These traits include thicker and smaller leaves, thicker cuticles and palisade tissues, denser arrangement of mesophyll tissue, and xylem vessels with smaller diameter and thicker walls4,5,6. Collectively, these adaptations enhance water retention and improve water use efficiency5,6. Nonetheless, these adaptive features incur costs. Thicker leaves may impede light penetration, thereby affecting the absorption and utilization of light energy7. Dense mesophyll tissue increases the diffusion resistance of CO2 within the leaf, resulting in reduced photosynthetic rates and diminished carbohydrate synthesis capacity8. These negative effects may ultimately adversely impact plant growth, resulting in a trade-off between plant drought resistance and growth rate may exist, constrained by leaf structure.
Research has indicated that water status is the most crucial factor influencing the functional adaptability of plants9. In drought environments, plants are forced to evolve a series of unique morphological features and physiological mechanisms to effectively prevent water loss and enhance survival ability. These adaptive features are particularly evident in leaf structure, manifesting as leaf miniaturization, thickening, and densification10,11. Leaf veins, mesophyll tissue, and stomata, as core components of leaf water management, exhibit high sensitivity to water limitations12. As environmental water deficit increases, adaptive changes in plant leaf veins occur, include reductions in vessel diameter and increases in vessel wall thickening coefficients, thereby enhancing leaf embolism resistance5. Similarly, under drought conditions, the thicknesses of leaves and internal tissues (palisade tissue, spongy tissue, upper epidermis, and lower epidermis) of angiosperms such as Fraxinus mandshurica and Tilia amurensis significantly increase, accompanied by a corresponding decrease leaf area. This represents the plant’s optimization of water use efficiency by adjusting the leaf area to thickness ratio8,13. Tree species such as Populus euphratica exhibit modular adjustment strategies for leaf traits, prioritizing the optimization of structural traits such as leaf thickness, cuticle thickness, and midvein vascular bundle area to cope with varying drought intensities. These traits are more crucial than chemical stoichiometric traits in assessing drought resistance14. Furthermore, stomatal traits are closely related to water status, exhibiting complex regulatory mechanisms such as isohydric and anisohydric regulation15. Currently, there is no consensus on how stomatal density and size respond to water status, highlighting their complex adaptability to environmental changes16,17. When assessing plant drought resistance, using the membership function method to comprehensively consider multiple trait indicators can more fully reveal the plant’s adaptation strategies and survival ability under drought conditions. These indicators include, but are not limited to, leaf thickness, cuticle thickness, epidermis thickness, palisade mesophyll thickness, spongy mesophyll thickness, palisade tissue and spongy tissue ratio, and leaf density, which together constitute the evaluation system for plant drought resistance18,19.
The karst region in southwestern China, which is the largest and most intensely developed karst area globally, exhibits unique and complex geomorphic features20,21. The long-term erosion of soluble carbonate rocks has gradually evolved into diverse surface landscapes such as peak clusters and depressions, along with complex subsurface structures like caves and fissures, forming a unique double-layered structure of surface and subsurface22. This heterogeneous habitat provides ample space for the evolutionary diversity among vegetation species. consequently, the region boasts diverse forest community structures, rich tree species compositions, and numerous endemic species23. Among these, karst plants, unique to this region, utilize their rock-dwelling and drought-resistant adaptations to thrive in barren rock soils and extreme water conditions, making them a vital component of the region’s biodiversity23. Their presence also establishes the area as one of the key regions for global biodiversity conservation. However, it is precisely this unique landform that causes surface precipitation to rapidly converge into the underground water flow system through rock fissures and dolines, resulting in a scarcity of surface water resources and difficulties in utilizing groundwater resources24,25. Coupled with pronounced seasonal drought, the region faces severe geological and seasonal drought issues, demonstrating typical and significant ecological vulnerability26,27. In the context of frequent global extreme drought events, karst plants may suffer more severe drought stress compared to non-karst plants28,29. To cope with this unique habitat, karst plants exhibit a series of drought resistance strategies that are superior to those of non-karst plants. These include thicker leaves and upper and lower epidermis, high tissue density, conservative resource utilization30, higher photosynthetic rates, hydraulic conductivity, mechanical resistance31, and enhanced water use efficiency32. Additionally, karst landforms are primarily composed of limestone and clay, characterized by low rainwater infiltration rates on a small scale. Rainwater on mountaintops or hillsides that does not infiltrate through rock fissures or dolines forms surface runoff, which flows towards depressions29. This runoff path is also a journey of soil loss, transporting large amounts of soil to deposit in depressions or areas with lower slopes33. Consequently, soil thickness and continuity in karst peak-cluster depressions gradually decrease with increasing elevation. The rock exposure in hilltop areas typically exceeds 90%. Concurrently, there are gradient differences in available soil water resources, with depressions exhibiting relatively abundant water resources compared to hillsides and hilltops33. So, in the heterogeneous habitat of karst, especially in the face of gradient changes in water resources, what adaptive variations will the leaf functional traits of dominant tree species exhibit? And do these adaptive variations act synergistically to achieve a trade-off between plant drought resistance and growth rate?
This study investigated three karst habitats (depression, middle slope, hilltop) with distinct soil moisture gradients in Southwest China’s seasonal rainforests, focusing on 38 dominant tree species. By integrating multidimensional functional traits (leaf anatomy, photosynthetic physiology, growth dynamics), we developed a drought resistance evaluation system using membership function analysis. Redundancy analysis revealed relationships between environmental factors (soil moisture, rock exposure rate) and plant traits, while structural equation modeling quantified interactions among environmental conditions, drought resistance, and growth rates. This work systematically elucidated ecological adaptation strategies and revealed resource trade-offs between drought resistance and growth rates in heterogeneous karst environments. The findings support vegetation optimization for rocky desertification control, guide species selection in ecological restoration, and enhance conservation efforts in the fragile tropical karst regions. This integrated methodology offers a novel framework for studying biodiversity maintenance in global karst ecosystems.
Results
Variation in leaf anatomical traits of dominant tree species across elevational gradients
The study on the adaptive correlation between leaf anatomical traits of dominant tree species and elevational gradients in karst peak-cluster depression areas revealed systematic adaptive characteristics in leaf structure within the elevation range of 184–374 m (Fig. 1; Table 1). Mechanical support-related traits exhibited a significant positive correlation with elevation (p < 0.01): leaf thickness (LT), cuticle thickness (CT), palisade mesophyll thickness (PT), and spongy mesophyll thickness (ST) increased by 41.11%, 56.79%, 56.42%, and 36.48%, respectively, across the elevational gradient. Epidermal tissues thickened synchronously but with smaller increments, with lower epidermis thickness (LET) and upper epidermis thickness (UET) increasing by 10.21% and 5.06%, respectively. In contrast, cell morphological characteristics showed opposite trends: upper epidermis cell width (UECW), lower epidermis cell width (LECW), and palisade tissue cell width (PCW) significantly decreased with rising elevation (p < 0.01), declining by 15.52%, 8.08%, and 6.98%, respectively, reflecting a denser cell arrangement in high-elevation habitats.
The relationship between leaf anatomical traits, drought resistance (represented by MFVD), and elevation in dominant tree species. (A) Upper epidermis thickness (UET); (B) Upper epidermis cell length (UECL); (C) Upper epidermis cell width (UECW); (D) Lower epidermis thickness (LET); (E) Lower epidermis cell length (LECL); (F) Lower epidermis cell width (LECW); (G) palisade mesophyll thickness (PT); (H) Palisade cell length (PCL); (i) Palisade cell width (PCW); (J) spongy mesophyll thickness (ST); (K) Spongy cell length (SCL) ; (L) Spongy cell width (SCW) ; (M) Leaf thickness (LT) ; (N) Cuticle thickness (CT); (O) Membership function value for drought resistance (MFVD). Blue, black and red dots indicate species living in a depression, on a middle slope, and at a hilltop respectively. The red line indicates a significant positive correlation, the blue line indicates a significant negative correlation, and the black line indicates no correlation.
In terms of stomatal trait responses, the research uncovered differential adaptation strategies of leaf stomatal characteristics to elevational gradients and rock exposure rates (15–95%) (Fig. 2; Table 1). Stomatal length (SL) displayed dual negative responses, decreasing by 4.45% across the elevational gradient and 24.24% across the rock exposure gradient. Conversely, stomatal width (SW) showed significant positive correlations with both environmental factors, increasing by 27.17% with elevation and 30.34% with rock exposure rate. Stomatal density (SD) demonstrated stronger environmental sensitivity, increasing by 41.31% across the elevational gradient and 90.98% across the rock exposure gradient. Analysis of derived parameters related to stomatal morphology revealed that stomatal area increased by 21.51% with elevation but slightly decreased by 1.26% with rock exposure, while stomatal aperture (SA) exhibited positive responses to both gradients, increasing by 33.10% with elevation and 72.04% with rock exposure rate. These results indicate that plants adjust stomatal characteristics multidimensionally to adapt to composite environmental stresses.
The relationship between leaf stomatal traits and rock exposure rate in dominant tree species. (A) Stomatal length (SL); (B) Stomatal width (SW); (C) Stomatal density (SD); (D) Stomatal length (SL); (E) Stomatal width (SW); (F) Stomatal density (SD). Blue, black and red dots indicate species living in a depression, on a middle slope, and at a hilltop respectively. The red line indicates a significant positive correlation, the blue line indicates a significant negative correlation, and the black line indicates no correlation.
Evaluation of drought resistance of dominant tree species in different habitats
Principal component analysis (PCA) was conducted on 11 leaf anatomical traits (Table 2). By extracting principal components with eigenvalues greater than 1, the first four principal components (denoted as X1, X2, X3, and X4) were identified, explaining a cumulative variance of 86.673%, which sufficiently accounts for the variability in the original traits. X1 was associated with spongy ratio (SR), leaf palisade to spongy mesophyll thickness ratio (P/S), cell tense ratio (CTR), and spongy mesophyll thickness (ST); X2 with palisade mesophyll thickness (PT), leaf thickness (LT), cuticle thickness (CT), and upper epidermis thinkness (UE); X3 with stomatal aperture (SA) and stomatal density (SD); and X4 with lower epidermis thickness (LE). These components effectively summarized the original traits for drought resistance analysis.
The membership function value for drought resistance (MFVD) varied significantly among species from different habitats. Table 3 shows that Pittosporum pulchrum (SP37) at the hilltop had the highest MFVD (0.508), followed by Dracaena cochinchinensis (SP25) (0.462), Memecylon scutellatum (SP38) (0.441), Sinosideroxylon pedunculatum (SP36) (0.441), Decaspermum gracilentum (SP32) (0.439), Tirpitzia ovoidea (SP28) (0.428), Garcinia paucinervis (SP24) (0.421), and Diospyros siderophylla (SP31) (0.418). Species with high MFVD were predominantly found on hilltop or middle slope above 315 m. MFVD increased by 24.23% along the 184–374 m elevation gradient (p < 0.001; Fig. 1O).
Growth and photosynthetic characteristics of dominant tree species in different habitats
Significant differences were noted in the Growth rates (GR) of dominant tree species across habitats, with values ranging from 0.019 to 0.403 cm·a−1 (Table 3). Pittosporum pulchrum (SP37) at the hilltop exhibited the lowest GR, while Baccaurea ramiflora (SP1) in the depression showed the highest. Growth rates declined markedly with elevation, showing a 45.67% reduction over the 184–374 m range (linear regression, p < 0.001) (Fig. 3A).
The relationship between growth rate, light response parameters, and elevation in dominant tree species. (A) Growth rate (GR); (B) apparent quantum efficiency (AQE); (C) maximum net photosynthetic rate (Pnmax) ; (D) light compensation point (LCP); (E) light saturation point (LSP); (F) dark respiration rate (Rd). Blue, black and red dots indicate species living in a depression, on a middle slope, and at a hilltop respectively. The red line indicates a significant positive correlation, the blue line indicates a significant negative correlation, and the black line indicates no correlation.
Significant differences were observed in the light response characteristic parameters of dominant tree species across different habitats (Table 4). The apparent quantum efficiency (AQE), reflecting the efficiency of light energy conversion in plant photosynthesis, was lowest in Boniodendron minus (SP30) on the hilltop (0.063 µmol·m−2·s−1) and highest in Ficus hispida (SP2) in the depression (0.086 µmol·m−2·s−1) (Table 4). The AQE decreased by 23.28% along the elevation gradient (linear regression, p < 0.001), while depression-adapted species exhibited higher AQE by 30.35% than that of hilltop species (Fig. 3B). The maximum net photosynthetic rate (Pnmax) ranged from 3.209 µmol·m−2·s−1 to 13.429 µmol·m−2·s−1. Linear regression analysis indicated that the relationship between elevation and Pnmax was not significant, suggesting similar maximum photosynthetic potential under saturating light conditions across habitats(Fig. 3C).
The light compensation point (LCP), which measures a plant’s ability to utilize low light, with a lower value indicating stronger ability, increased by 173.55% with elevation (p < 0.001). In contrast, depression-adapted species exhibited a 63.44% lower LCP than hilltop species, suggesting reduced adaptation to low light at higher elevations. This suggests that plants on the hilltop require higher light intensities to achieve a balance between photosynthesis and respiration, while tree species in depression can achieve this balance at lower light intensities, thus utilizing low light more effectively. Our study found that the light saturation point (LSP), which indicates a plant’s ability to utilize high light intensity, showed no significant correlation with elevation, suggesting similar capacities among tree species from different habitats. Lastly, dark respiration rates (Rd) increased by 111.63% with increasing elevation (184–374 m), as shown by linear regression (p < 0.001). This indicating that tree species on hilltop had notably higher dark respiration rates than those in depression (Fig. 3D-F).
Aridity gradient-driven trade-offs in plant drought resistance and growth
The results of redundancy analysis (RDA) revealed that the first two axes collectively explained 81.63% of the total variation, with the first axis contributing 66.95%. This suggests significant correlations between the functional traits of dominant tree species and habitat environmental factors. The principal component loadings of the red arrows demonstrated that soil water conent (SWC), slope (SLO), rock exposure rate (RER) and elevation (ELE) are the primary environmental factors influencing key functional traits, including the principal components X2 and X3 of leaf anatomical traits, MFVD and GR of the tree species. Further analysis demonstrated that both principal components X2 and X3 of leaf anatomical traits were significantly positively correlated with MFVD and negatively significantly correlated with GR, while MFVD was also negatively significantly correlated with GR. These results uncover that dominant tree species achieve a trade-off between drought resistance and growth rate through coordinated variation in leaf functional traits. Additionally, Fig. 4 clearly illustrates distinct distributions of tree species from three habitats along the SWC, SLO, RER, and ELE gradients, emphasizing their crucial role in shaping the distribution patterns of karst tree species.
Redundancy Analysis (RDA) on the functional traits of dominant tree species and environmental factors. ELE, elevation; SLO, slope; ASP, transformed aspect; RER, Rock exposure rate; SWC, soil water content; AAC, altitude above channel; CON, convexity; GR, growth rate; MFVD, membership function value for drought resistance. X1, X2, X3 and X4 represent the first four principal components of leaf anatomical traits. Blue, black and red dots indicate species living in a depression, on a middle slope, and at a hilltop respectively.
Quantitative analysis based on Structural Equation Modeling (SEM) revealed a significant negative correlation between plant drought resistance (DrR) and growth rate (GrR) (standardized path coefficient β = − 0.504), indicating that enhanced drought resistance corresponds to reduced growth rates (Fig. 5). Drought resistance (DrR) exhibited positive correlations with three leaf functional traits: thickened cuticle (CT, β = 0.763) showed the strongest association, followed by enhanced spongy tissue (ST, β = 0.511) and increased stomatal aperture (SA, β = 0.406). Environmental drought gradient (Env) suppressed plant growth through dual pathways: the direct effect (Env→GrR, β = − 0.667) accounted for 58.7% of the total inhibitory effect, while the indirect pathway (Env→DrR→GrR) further reduced growth by upregulating drought resistance, with a calculated path effect of 0.930 × (− 0.504) = − 0.469, contributing 41.3% of the total suppression. Environmental drought gradient (Env) demonstrated significant positive correlations with elevation (ELE, β = 0.708) and rock exposure rate (RER, β = 0.594), coupled with a strong negative correlation with soil water content (SWC, β = − 0.706), confirming that water scarcity is the primary driver of environmental heterogeneity.
Structural equation model depicting relationships among environmental factors (Env), plant drought resistance (DrR), and plant growth rate (GrR). Circles denote latent variables inferred from observed variables, while boxes represent directly observed variables. Single-headed arrows (→) represent direct effects between variables, while double-headed arrows (↔) denote covariances. All standardized path coefficients (β) are numerically labeled with color-coded indicators: green for positive effects and red for negative effects. Abbreviations: Elevation (ELE); Rock exposure rate (RER); Soil water content (SWC); Growth rate (GR); Stomatal aperture (SA); spongy mesophyll thickness (ST); Cuticle thickness (CT).
Discussion
Adaptive variations in leaf anatomical traits and drought resistance of tree species
In the karst seasonal rainforests of southwestern Guangxi, China, habitat heterogeneity intensifies with elevation, marked by stronger light exposure, reduced soil moisture, thinner soil layers, and increased rock outcrops31. Comparative analysis reveals that tree species inhabiting hilltop develop thicker, denser leaves with more open stomata compared to those in depression. Key anatomical traits (including leaf thickness, cuticle thickness, palisade mesophyll thickness, and stomatal aperture) demonstrate significant elevation-dependent increases. These structural modifications reflect adaptive responses to the progressively drier and harsher conditions from lower to higher elevations. Over evolutionary timescales, karst tree species have developed xeromorphic leaf features: thickened leaves and cuticles enhance water retention6, while compact palisade tissues and dense mesophyll improve light-use efficiency and water regulation34. Such adaptations underscore the plants’ capacity to exploit karst microhabitats and optimize survival strategies under environmental stress. Leaf anatomical traits are widely recognized as indicators of plant adaptation to drought and extreme habitat35,36. By evaluating multiple traits through the membership function method, this study confirms that drought resistance in karst tree species strengthens with elevation, with hilltop species exhibiting superior resilience. These findings align with prior research emphasizing the linkage between xeromorphic traits and habitat-driven selection pressures6,34.
This study challenges the classical “small aperture-high density” paradigm in xerophytic plants17 by uncovering a unique “high-aperture, compact-type” stomatal configuration in montane plants, achieved through enlarged stomatal apertures combined with shortened stomatal morphology. This strategy relies on the synergistic effects of thickened cuticles, dense palisade tissues, and cavitation-resistant structures to mitigate water loss risks associated with large stomatal apertures. The adaptive mechanism aligns temporally with the Karst habitat: it maximizes CO₂ uptake during nocturnal periods when rock condensation creates a moist window, while structural protections counteract daytime drought stress, decoupling water use from carbon fixation 35. Phenotypic trade-off analyses reveal that investments in drought-resistant traits significantly suppress growth rates in Karst ecosystems, underscoring the strong environmental filtering of adaptive traits36,37,38. Divergent trait strategies among species may reduce ecological niche overlap. These findings establish a novel theoretical framework for mountain vegetation restoration, demonstrating how plants transcend traditional stomatal regulation theories through multidimensional trait coordination.
Adaptive variations in growth rate and photosynthetic characteristics of tree species
Dominant tree species exhibited significant adaptive variations in growth rates (GR) across karst habitats. GR declined markedly with elevation, with hilltop species showing the slowest growth, contrasting sharply with depression-dwelling species. Drought inhibited hilltop growth by reducing stomatal conductance and metabolic activity39, limiting photosynthesis and carbohydrate supply41, confirming water’s critical role in karst ecosystems.
Light-response analyses revealed decreasing apparent quantum efficiency (AQE) with elevation. Depression-dwelling species had higher AQE (> 0.07) than hilltop species (0.04–0.07), enabling superior light-energy utilization under identical conditions. For example, hilltop specialists Huangli wood and Maoye Tie Lan showed AQE values of 0.063 and 0.066, within the typical range (0.04–0.07)42,43. In contrast, depression and middle slope species exceeded this range, reflecting divergent strategies: while hilltop species prioritized drought resistance over light efficiency, tolerating arid, high-light-intensity, rocky habitats despite reduced competitiveness, depression-dwelling species maximized light-use efficiency to thrive in moist, shaded, deep-soil environments .
Significant differences in light compensation point (LCP) and dark respiration rate (Rd) further highlighted habitat adaptations. Depression-dwelling species had lower LCP and Rd values, optimizing low-light capture and minimizing energy loss, adaptations to stable, resource-rich conditions. Hilltop species exhibited higher LCP and elevated Rd values, indicating metabolic costs of sustaining drought-resistant traits (e.g., thickened cuticles) under water stress, consistent with drought-induced physiological adjustments44,45. While light saturation point (LSP) showed no habitat differences, hilltop drought likely constrained strong-light-intensity utilization, reducing photosynthetic efficiency—a pattern aligning with soil drought’s known effects on elevating LCP and lowering LSP46.
Maximum photosynthetic capacity (Pnmax) remained conserved across habitats, but ecological divergence arose from light-use strategies. Depression-dwelling species’ high AQE and low LCP supported efficient carbon fixation in shaded understories, driving faster growth. Hilltop species’ high LCP and Rd reflected trade-offs favoring drought resistance over growth, diverting carbon allocation to metabolic maintenance. Thus, habitat-specific balances between light-capture efficiency and respiratory costs ultimately shaped actual growth rates.
Trade-off strategies between drought resistance and growth rate of tree species
In karst ecosystems, environmental factors such as soil water content, slope gradients, bedrock exposure, and elevation collectively shape tree species’ drought resistance (DrR) and growth rate (GrR) through their influence on leaf anatomical traits. The heterogeneous topography creates contrasting microhabitats: hilltops with limited soil moisture and higher light intensity versus sheltered depressions with greater water retention and reduced irradiance. This spatial variation drives niche differentiation among dominant tree species along environmental gradients47, supporting the niche differentiation hypothesis. Soil-plant interactions further modulate trait expression, where reduced water availability constrains cellular turgor pressure5, while nutrient gradients in organic carbon and nitrogen influence foliar stoichiometry48. The unique rock fissure networks in karst systems facilitate deep root penetration and hydraulic redistribution, partially compensating for surface aridity49.
Three interconnected pathways govern the DrR-GrR trade-off: (1) Stomatal optimization strategy: Hilltop tree species evolve smaller stomata with higher opening ratios to maximize nocturnal CO₂ uptake during transient condensation events, balancing reduced daytime gas exchange efficiency. This reflects adaptive adjustments to intermittent water availability. (2) Resource allocation constraints: Structural investments such as thickened cuticles and denser palisade tissues enhance drought resistance but divert metabolic resources required for other physiological processes, aligning with Chapin’s growth-defense allocation theory. Hilltop specimens exhibit significantly reinforced leaf structures compared to depression-dwelling counterparts. (3) Dual environmental stress pathways: Growth limitation arises both directly through water scarcity suppressing cellular turgor pressure and indirectly through energy expenditure associated with drought-resistance traits. Compared to non-karst forests, karst ecosystems demonstrate stronger DrR-GrR trade-offs, reflecting intensified selection pressures for resource utilization efficiency in extreme habitats.
Conclusion
This study systematically elucidates the ecological adaptation mechanisms underlying plant trade-offs between drought resistance and growth through multidimensional trait coordination across karst habitat gradients. The results demonstrate that hilltop species develop a coordinated strategy involving stomatal morphological optimization and enhanced leaf structural reinforcement, establishing a “compact stomata-high openness” regulatory pattern. While this adaptive approach significantly improves water use efficiency, it requires prioritized resource allocation to sustain drought-resistance adaptations, thereby limiting growth performance relative to depression-dwelling species. Depression-adapted species optimize photosynthetic efficiency under low-light conditions through refined photon utilization and reduced energy compensation thresholds, whereas hilltop species maintain carbon balance in arid environments through integrated physiological adjustments and modified respiratory metabolism. These findings provide a critical theoretical framework for implementing stratified vegetation restoration strategies (“depression-slope-hilltop zonation”) in karst rocky desertification control, while offering novel insights into understanding carbon sequestration stability mechanisms in lithospheric ecosystems.
Materials and methods
Study site
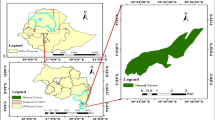
The Guangxi Nonggang National Nature Reserve (The Nonggang Reserve), situated in the eastern Longzhou County and northern Ningming County, Guangxi Zhuang Autonomous Region, China (106° 42′ 28″−107° 04′ 54″ E, 22° 13′ 56″− 22° 33′ 09″ N), encompasses a total area of 10,080 ha and is distributed in a northwest-southeast elongated strip. Located at the northern edge of the tropics, the Nonggang Reserve experiences an annual average temperature of 22 °C and is characterized by a tropical monsoon climate with distinct wet and dry seasons. Annual rainfall ranges between 1150 and 1550 mm, with 76% of precipitation occurs from May to September, leading to seasonal water shortages and vegetation stress due to drought. The Nonggang Reserve features exposed karst landforms, including multiple peak-cluster depressions and peak-forest valleys. Over a short elevation change of 250–300 m from the depression bottoms to the hilltops, significant variations in soil moisture and fertility, light intensity, rock exposure, and vegetation community types are observed. Notably, the ecological distribution patterns in peak-cluster depressions are similar across different locations50,51. Based on environmental factors and vegetation characteristics, the Nonggang Reserve can be classified into three habitats. (1) Depression (elevation < 230 m): These areas are shaded by surrounding mountains, receiving weak sunlight, and maintaining a consistently humid environment with potential flooding during the rainy season. Rock exposure is approximately 10%, and the soil layer is relatively thick, with a depth of up to 60 cm. The dominant plant species include moisture-loving and shade-tolerant species like Parashorea chinensis, Saraca dives, and Ficus hispida, with a canopy layer height of approximately 35–45 m. (2) Middle slope (230 m < elevation < 310 m): These areas receives ample sunlight and has moderate soil moisture; rock exposure is about 80%, with a thin and discontinuous soil layer ranging from 30 to 60 cm in thickness. Dominant plants include Excentrodendron tonkinense, Garcinia paucinervis, and Diplodiscus trichospermus, with a canopy layer height of 20–30 m. (3) Hilltop (elevation > 310 m): These areas experience direct sunlight throughout the day and are relatively arid, with summer surface temperatures exceeding 60 °C. Rock exposure is as high as 95%, and soil is scarce, with a thickness less than 30 cm. Dominant species include drought-resistant and light-loving plants such as Sinosideroxylon pedunculatum, Boniodendron minus, and Pistacia weinmanniifolia. Due to environmental constraints, the forest height is only 5–6 m, featuring crooked tree trunks, extremely small leaves, and hard textures50.
This study was conducted within a 15-hectare monitoring plot in the Nonggang Karst Seasonal Rainforest (hereinafter referred to as the “Nonggang Plot”). The Nonggang Plot spans 500 m east to west and 300 m north to south, featuring a central depression surrounded by steep slopes, thereby constituting a holistic karst ecosystem with heterogeneous habitats encompassing “depression-middle slope-hilltop” terrain (Fig. 6). Following the global forest biodiversity monitoring protocol established by the Center for Tropical Forest Science (CTFS), the Nonggang Plot has been thoroughly divided and marked51. The plot was divided into 1,500 quadrats of 10 m×10 m, with each corner of each quadrat permanently marked with aluminum tubes. For more detailed research, each 10 m×10 m quadrat was further subdivided into four 5 m×5 m sub-quadrats. Field surveys were conducted using the 10 m×10 m quadrat as the basic unit. During these surveys, each tree individual (Diameter at breast height (DBH) ≥ 1 cm) was tagged, surveyed, and located. The survey content included tree species number, species name, DBH, tree height, crown width, spatial coordinates, and growth dynamics. Additionally, various environmental factors surrounding each tree individual were measured and calculated, including elevation (ELE), slope (SLO), transformed aspect (ASP), convexity (CON), rock exposure rate (RER), soil water content (SWC) and altitude above channel (AAC) etc. The initial survey of the Nonggang Plot was completed in 2011, followed by two additional surveys in 2016 and 2021, respectively.
Selection of dominant tree species
Within the Nonggang Plot, a continuous slope encompassing three habitats of “depression-middle slope-hilltop” was selected for the study. The top 10 dominant tree species in terms of importance value from each habitat were chosen as study subjects. Specifically, there were 12 species in the depression habitat, and 13 species in both the middle slope and hilltop habitats (Table 5). For each species, 10 healthy mature sample trees with DBH ≥ 10 cm were selected for leaf collection.
Leaf collection and anatomical trait measurement
Leaf sampling was conducted during the growing season (September to October) on sunny days. Branches were harvested from five different orientations (east, south, west, north, and center) of each sample tree using high branch cutters, and 5 mature functional leaves were collected from each branch. To avoid the influence from the main vein, small pieces of 0.5 cm × 0.5 cm were cut from the middle of each leaf to the edge at the 1/2 position and immediately placed in test tubes containing glutaraldehyde fixative for fixation.
Subsequently, the samples were transported to the laboratory and processed through a series of steps, including gradual dehydration with ethanol, critical point drying, and gold plating. They were then observed and photographed under a vacuum electron scanning microscope (ZEISS EVO18). The AxioVision SE64 Rel. 4.9.1 scanning microscope software was utilized to measure multiple anatomical traits of the leaves, encompassing leaf thickness (LT), cuticle thickness (CT), upper epidermis thickness (UET), lower epidermis thickness (LET), palisade mesophyll thickness (PT), spongy mesophyll thickness (ST), stomatal length (SL), stomatal width (SW), and stomatal count (SC). Additionally, several indices were calculated, including leaf palisade to spongy mesophyll thickness ratio (P/S = PT/ST), cell tense ratio ( CTR = PT/LT ), spongy ratio (SR = ST/LT), stomatal aperture (SA = SW/SL), stomatal density (SD = number of stomata per unit area in the field of view), and the stomstal area (SAR=(Π × SL×SW)/4). Each anatomical trait was measured more than 5 times to ensure accuracy throughout the measurement process. Ultimately, a large dataset comprising leaf anatomical trait data was obtained. It included 38 tree species, with 10 sample trees per species, 5 leaves per sample tree, and 5 repetitions per leaf, totaling 10,250 data points.
Concurrently, the serial numbers of each sample tree within the Nonggang Plot were recorded, and the environmental factor data surrounding the sample trees were retrieved (Table 5). Within the Nonggang Plot, the changes in elevation gradient around individual trees reflect the comprehensive variations in water and heat conditions of their respective habitats, and there is a negative correlation between elevation and soil moisture content50,51.
Drought resistance evaluation
The drought resistance, which encompassing multiple traits, was evaluated using the membership function value (MFV) derived from fuzzy mathematics theory52. The evaluation steps are as follows: Firstly, a membership function is applied to assign a corresponding value within the closed interval [0,1] to each trait, termed as the “single-factor membership function value,” which serves to assess each trait individually. Secondly, a weighted arithmetic mean of all single-factor membership function values (MFVs) is computed, i.e. the “comprehensive membership function value (CMFV)”. The weights are determined by the ratio of each principal component’s eigenvalue to the sum of all eigenvalues obtained from the principal component analysis (PCA). Finally, the resulting “comprehensive membership function value (CMFV)” is denoted as the membership function value for drought resistance (MFVD) of the test material. A higher MFVD indicates greater overall drought resistance of the test material53,54.
The formula for calculating the MFVD of tree species is presented as follows:
In the formula, Uij represents the drought resistance membership function value of a certain leaf trait (j) of a tree species (i); DCij represents the drought resistance coefficient of a certain leaf trait (j) of a tree species (i); DCjmax represents the maximum drought resistance coefficient among all tree species for a certain leaf trait (j); DCjmin represents the minimum drought resistance coefficient among all tree species for a certain leaf trait (j); Ui represents the average drought resistance membership function value of all traits of a tree species (i); pm represents the weight coefficient of the m-th principal component; n represents the number of measured traits. A higher Ui indicates stronger drought resistance of a tree species (i).
Growth rate quantification
To accurately quantify the growth of individual tree species, the growth rate (GR) of each species is defined as the average annual increase in diameter at breast height (DBH) between two consecutive surveys, specifically between 2011 and 2016 and 2016–20217,55. The specific calculation formula is: GR = (DBHfinal–DBHinitial) / (tfinal–tinitial), where t represents the survey time. During the data processing stage, abnormal data points resulting from human or other external factors (where DBHinitial≥DBHfinal) were excluded to ensure the accuracy of the results. Ultimately, 310 individuals were retained for the subsequent statistical analysis, out of the original data comprised 38 species×10 individuals = 380 individuals.
Measurement of light response curves
Given the intricate terrain and sampling challenges of the Nonggang Plot, this study selected representative tree species for the measurement of photosynthetic physiological indicators. These measurements were conducted on sunny days between September and October, which corresponds to the growing season of the karst seasonal rainforest. Specifically, measurement were conducted from 9:00 AM to 12:00 PM, as this time period correspond to the most active photosynthesis. The LI-6400XT portable photosynthesis system (manufactured by LI-COR, Inc., USA) was utilized, which was equipped with a red-blue light source (Li-6400-02B). Fourteen decreasing light intensities, ranging from 2000 to 0 µmol·m−2·s−1 (specifically, 2000, 1800, 1500, 1200, 1000, 800, 500, 300, 200, 150, 100, 50, 20, and 0µmol·m−2·s−1) were set to measure the net photosynthetic rate of tree species leaves under different light intensities. During the measurement, the temperature was maintained at (25 ± 1) °C, relative humidity at (70 ± 5)%, and CO2 concentration at (400 ± 2) µmol·mol−1. For each tree species, measurements were taken from three sample trees. Subsequently, the obtained light response curves were fitted using the rectangular hyperbola modified model, enabling the derivation of key indicators such as apparent quantum efficiency (AQE), light saturation point (LSP), maximum net photosynthetic rate (Pnmax), light compensation point (LCP), and dark respiration rate (Rd).The modified rectangular hyperbola model is expressed as follows56:
In the formula: Pn denotes the net photosynthetic rate; I represents the light intensity; α represents the slope of the plant photosynthesis light response curve at I = 0 (dimensionless), representing the initial slope of the light response curve; Pnmax denotes the maximum net photosynthetic rate; β is termed the light inhibition term; γ which has a value equivalent to α/Pnmax, is referred to as the light saturation term ; Rd represents the dark respiration rate.
Data analysis
This study employed R statistical software to investigate elevational adaptation mechanisms in dominant tree species through integrated analytical workflows. Data collection involved photosynthetic light response curves fitted using Photosyn Assistant 4.1.157. Linear regression analysis revealed elevation-driven patterns across leaf anatomical traits, drought resistance indices, and growth parameters. We fitted the model using the GLS function in R’s nlme package58, incorporating the corAR1 function to account for temporal autocorrelation in trees’ repeated-measures data. Model parameters were used to analyze the magnitude of variation in leaf traits across elevational gradients quantitatively.
One-way ANOVA detected interspecific differences in light response parameters across habitats, while Redundancy Analysis (RDA) implemented through the R vegan package quantified environmental drivers influencing functional traits. Structural Equation Modeling (SEM) comprised three sequential phases: (1) collinearity diagnosis using the R collinear package (VIF < 5 threshold)59, (2) variable selection retaining key environmental (Elv: elevation, BRR: bedrock ratio, SWC: soil water content) and drought-resistance indicators (CT: cuticle thickness, SA: stomatal area, ST: stomatal density), and (3) model construction via the R lavaan package using maximum likelihood estimation60. The SEM demonstrated excellent fit indices (χ2(11) = 10.408, p = 0.494; RMSEA = 0.032)61, confirming elevation-mediated drought adaptation trade-offs with growth constraints.
Data availability
All data generated or analysed during this study are included in this published article.
References
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Li, Y. et al. Leaf trait networks based on global data: Representing variation and adaptation in plants. Front. Plant. Sci. 12, 710530 (2021).
Jurik, T. W., Chabot, J. F. & Chabot, B. F. Effects of light and nutrients on leaf size, CO2 exchange, and anatomy in wild strawberry (Fragaria virginiana). Plant. Physiol. 70, 1044–1048 (1982).
Witkowski, E. & Lamont, B. Leaf specific mass confounds leaf density and thickness. Oecologia 88, 486–493 (1991).
Zhang, Q. W., Zhu, S. D., Jansen, S. & Cao, K. F. Topography strongly affects drought stress and xylem embolism resistance in Woody plants from a karst forest in Southwest China. Funct. Ecol. 35, 566–577 (2021).
Chartzoulakis, K., Patakas, A., Kofidis, G., Bosabalidis, A. & Nastou, A. Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Sci. Hort. 95 (1–2), 39–50 (2002).
Bhusal, N. et al. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 779, 146466 (2021).
Khan, A. et al. Limited acclimation in leaf morphology and anatomy to experimental drought in temperate forest species. Biology (Basel). 11, 1186 (2022).
Johnson, K. M., Jordan, G. J. & Brodribb, T. J. Wheat leaves embolized by water stress do not recover function upon rewatering. Plant. Cell. Environ. 41, 2704–2714 (2018).
Aritsara, A. N. A. et al. Divergent leaf and fine root pressure-volume relationships across habitats with varying water availability. Plant. Physiol. 190, 2246–2259 (2022).
Yan, C. L., Ni, M. Y., Cao, K. F. & Zhu, S. D. Leaf hydraulic safety margin and safety-efficiency trade-off across angiosperm woody species. Biol. Lett. 16, 20200456 (2020).
Anderegg, W. R. L. et al. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the Globe. Proc. Natl. Acad. Sci. USA. 113, 5024–5029 (2016).
Bhusal, N., Han, S. G. & Yoon, T. M. Impact of drought stress on photosynthetic response, leaf water potential, and stem Sap flow in two cultivars of bi-leader Apple trees (Malus × domestica Borkh). Sci. Hort. 246, 535–542 (2019).
Yao, S. Y. et al. Adaptation strategies of Populus euphratica to arid environments based on leaf trait network analysis in the mainstream of the Tarim river. Forests 15, 437 (2024).
Martinez-Vilalta, J., Poyatos, R., Aguade, D., Retana, J. & Mencuccini, M. A new look at water transport regulation in plants. New. Phytol. 204, 105–115 (2014).
Xu, Z. Z. & Zhou, G. S. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 59 (12), 3317–3325 (2008).
Conesa, M., Muir, C. D., Molins, A. & Galmés, J. Stomatal anatomy coordinates leaf size with Rubisco kinetics in the Balearic limonium. AoB Plants. 12, plz050 (2019).
Li, X. et al. Application of morphological and physiological markers for study of drought tolerance in lilium varieties. Horticulturae 8, 786 (2022).
Mitrofanova, I., Tsyupka, V. & Jain, S. M. Morpho-anatomical characterization of in vitro regenerated plants. In Advances in Plant Tissue Culture: Current Developments and Future Trends (eds Rai, A. C. et al.) 175–204 (Academic Press, 2022).
Li, Z. W. et al. Monthly sediment discharge changes and estimates in a typical karst catchment of Southwest China. J. Hydrol. 555, 95–107 (2017).
Wang, K. L. et al. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 34, 2743–2763 (2019).
Yao, C. H., Jiang, Z. C. & Yuan, D. X. Vegetation karst effects on the karst area of Southwest China. Acta Geoscientia Sinica. 22, 1–6 (2001).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A. B. & Kent J. Biodivers. Hotspots Conserv. Priorities Nat. 403, 853–858 (2000).
Wilcox, B. P. et al. Subsurface storm flow is important in semiarid karst shrublands. Geophys. Res. Lett. 35, L10403 (2008).
Liu, M., Xu, X., Wang, D., Sun, A. Y. & Wang, K. Karst catchments exhibited higher degradation stress from climate change than the non-karst catchments in Southwest China: An ecohydrological perspective. J. Hydrol. 535, 173–180 (2016).
Fu, P. L., Liu, W. J., Fan, Z. X. & Cao, K. F. Is fog an important water source for Woody plants in an Asian tropical karst forest during the dry season? Ecohydrology 9, 964–972 (2016).
Zhu, S. D., Chen, Y. J., Fu, P. L. & Cao, K. F. Different hydraulic traits of Woody plants from tropical forests with contrasting soil water availability. Tree Physiol. 37, 1469–1477 (2017).
Xu, C., McDowell, N. G., Fisher, R. A., Wei, L. & Middleton, R. S. Increasing impacts of extreme droughts on vegetation productivity under climate change. Nat. Clim. Change. 9, 948–953 (2019).
Vicente-Serrano, S. M., Quiring, S. M., Peña-Gallardo, M., Yuan, S. S. & Dominguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Sci. Rev. 201, 102953 (2020).
Geekiyanage, N., Goodale, U. M., Cao, K. & Kitajima, K. Leaf trait variations associated with habitat affinity of tropical karst tree species. Ecol. Evol. 8, 286–295 (2018).
Chen, Y. J. et al. Water-use advantage for lianas over trees in tropical seasonal forests. New. Phytol. 205, 128–136 (2015).
Wang, J., Wen, X., Zhang, X. & Li, S. The strategies of water-carbon regulation of plants in a subtropical primary forest on karst soils in China. Biogeosciences 15, 4193–4203 (2018).
Hartmann, A., Goldscheider, N., Wagener, T., Lange, J. & Weiler, M. Karst water resources in a changing world: Review of hydrological modeling approaches. Rev. Geophys. 52, 218–242 (2014).
Aguraijuja, K., Klõśeiko, J., Ots, K. & Lukjanova, A. Effect of wood Ash on leaf and shoot anatomy, photosynthesis and carbohydrate concentrations in Birch on a cutaway peatland. Environ. Monit. Assess. 187, 444–444 (2015).
Anderegg, W. R. L. et al. Woody plants optimise stomatal behaviour relative to hydraulic risk. Ecol. Lett. 21, 968–977 (2018).
Chiaki, T. et al. Drought affects the fate of non-structural carbohydrates in Hinoki Cypress. Tree Physiol. 42 (4), 784–796 (2022).
Yang, Y. et al. Root carbon resources determine survival and growth of young trees under long drought in combination with fertilization. Front. Plant. Sci. 13, 929855 (2022).
Dong, R. K. et al. Carbon allocation in Picea Jezoensis: Adaptation strategies of a non-treeline species at its upper elevation limit. For. Ecosyst. 11, 100188 (2024).
Dreyer, E. Photosynthesis and drought in forest trees. In Trees-Contributions to Modern Tree Physiology (eds Rennenberg, H. et al.) 215–238 (Backhuys Publishers, 1997).
Li, C. N., Chen, S. X. & Wang, Y. Physiological and proteomic changes of castanopsis fissa in response to drought stress. Sci. Rep. 13, 12567 (2023).
Kaldenhoff, R. & Fischer, M. Aquaporins in plants. Acta Physiol. 187, 169–176 (2006).
Wu, F. Z., Bao, W. K. & Li, L. F. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of sophora Davidii seedlings. Photosynthetica 46, 40–48 (2008).
Xu, D. Q. Photosynthetic efficiency 2–52 (Shanghai Scientific and Technical Publishers, 2002).
Allen, C. D. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 259, 660–684 (2010).
Itam, M., Mega, R., Tadano, S., Abdelrahman, M. & Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 10, 17189 (2020).
Xu, Y. L., Liang, L. L. & Lisak, G. Blue-emissive antioxidant carbon dots enhance drought resistance of pea (Pisum sativum L). ACS Appl. Mater. Interfaces. 16, 39090–39103 (2024).
Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 19, 605–611 (2004).
Bai, K. D. et al. Leaf nutrient concentrations associated with phylogeny, leaf habit and soil chemistry in tropical karst seasonal rainforest tree species. Plant. Soil. 434, 305–326 (2019).
Cai, L. L. et al. Coexisting plants restored in karst desertification areas cope with drought by changing water uptake patterns and improving water use efficiency. J. Hydrol. 654, 132813 (2025).
Wang, B. et al. Species composition and Spatial distribution of a 15 ha Northern tropical karst seasonal rain forest dynamics study plot in Nonggang, Guangxi, Southern China. Biodivers. Sci. 22, 141–156 (2014).
Guo, Y. et al. Partitioning beta diversity in a tropical karst seasonal rainforest in Southern China. Sci. Rep. 8, 17408 (2018).
Zadeh, L. A. Fuzzy sets. Inf. Control. 8, 338–353 (1965).
Liu, C. Y., Yang, Z. Y. & Hu, Y. G. Drought resistance of wheat alien chromosome addition lines evaluated by membership function value based on multiple traits and drought resistance index of grain yield. Field Crop Res. 179, 103–112 (2015).
Li, B. et al. Physiological response to Low-Temperature stress and cold resistance evaluation of Ziziphus Jujuba Var. Spinosa clones from different provenances. Forests 15, 1130 (2024).
Toledo, M., Poorter, L., Peña-Claros, M., Alarcón, A. & Bongers, F. Climate is a stronger driver of tree and forest growth rates than soil and disturbance. J. Ecol. 99, 254–264 (2011).
Ye, Z. P. A new model for relationship between light intensity and the rate of photosynthesis in Oryza sativa. Photosynthetica 45, 637–640 (2007).
R Core Team R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria. (2024). https://www.R-project.org/
Pinheiro, J., Bates, D., Debroy, S. & Sarkar, D. R. Core team. Nlme: Linearand nonlinear mixed effects models. (2025). https://doi.org/10.32614/CRAN.package.nlme
Benito, B. collinear: R Package for Seamless Multicollinearity Management. (2023). https://blasbenito.github.io/collinear/
Rosseel, Y. Lavaan: An R package for structural equation modeling. J. Stat. Softw. 48 (2), 1–36 (2012).
Curran, P. J., Bollen, K. A., Paxton, P., Kirby, J. & Chen, F. The noncentral chi-square distribution in misspecified structural equation models: Finite sample results from a Monte Carlo simulation. Multivar. Behav. Res. 37 (1), 1–36 (2002).
Acknowledgements
This study was supported by National Natural Science Foundation of China (grant numbers 32271599 and 32260276), Guangxi Science and Technology Base and Talent Special Project (grant number Guike AD20325003), The special funding project for postdoctoral researchers in Guangxi (grant number 20230719007).
Author information
Authors and Affiliations
Contributions
Xiankun Li designed this study. Xiankun Li and Jing Wang conceived the manuscript. Denghui Wang, Yanping Dong, Jianxing Li, Fang Lu, Wanglan Tao, Yili Guo and Wusheng Xiang collected and processed the samples/data. Jing Wang and Bin Wang analyzed the data. Jing Wang wrote the manuscript and Meilan Wen revised and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experimental research and field studies involving plants (cultivated or wild), including plant material collection, were conducted in compliance with institutional, national, and international guidelines, as well as local legislation. We affirm our commitment to ethical standards, biodiversity conservation, and environmental protection.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Wang, B., Wang, D. et al. Trade-off strategies between drought resistance and growth rate of dominant tree species in karst forests within heterogeneous habitats. Sci Rep 15, 26381 (2025). https://doi.org/10.1038/s41598-025-97550-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97550-x