Abstract
This study aims to explore the impact of fly ash (FA) on two types of free-floating aquatic plants, Eichhornia crassipes and Pistia stratiotes, growing in two different locations. The stress caused by FA has led to a significant biochemical alteration in several leaf properties, including ascorbic acid, relative water, and chlorophyll, as well as anatomical changes in leaf, petiole, and stolon in the growing plants at highly contaminated sites (HCS) relative to the low contaminated site (LCS). According to the study, HCS plants lose total chlorophyll overall, have shallower ascorbic acid levels, and have higher RWC than LCS plants. These findings imply that both species are highly resilient to pollution. The assessment of the shape and size of the epidermis, cortex, palisade cells, air space, bundle sheath, xylem cavity, phloem cells, vascular bundle, parenchyma, pith of the leaves, petioles, and stolon in the HCS is shorter than the LCS. The APTI values of E. crassipes (8.407%) and P. stratiotes (9.681%) are higher in HCS than the values of E. crassipes (7.729%) and P. stratiotes (9.077%) in LCS. These results suggest that both species exhibit greater APTI values in plants from HCS, indicating their tolerance to pollution. We target six water bodies in HCS and LCS to assess the FA-containing water quality. We calculated the water quality using WA-WQI and CCME-WQI. The higher WA-WQI scores indicate higher water pollution levels. The value of WA-WQI is higher in HCS sites included in the KTPP colony (93.94), Amalhanda (91.43), and Barunan Ghoshpara (89.07) than in LCS sites such as in Kashinathpur (88.59), but the CCME-WQI scores are 64.33, 76.09 and 75.71 respectively. The investigation highlights that both species are exceptionally suitable as stress-tolerant plants for fly ash and possess the potential to serve as an option for the restoration of water bodies impacted by fly ash. This study will enhance our comprehension of the potential advantages of these plants, particularly in the phytoremediation of polluted aquatic ecosystems.
Similar content being viewed by others
Introduction
Water pollution is now one of the most significant global threats due to the shortage and inconsistent availability of water sources1,2. Thermal pollution from thermal power disposal services seriously threatens aquatic and terrestrial habitats3. Cosgrove and Loucks state that these power facilities return high-temperature water into the same environment after cooling their equipment with cold water from water sources4. Jeremias et al.5 assert that constructing thermal power plants near saltwater may result in accidents and environmental problems, potentially leading to the extinction of species5. According to previous research, aquatic animals may suffer catastrophic consequences from a 1 °C temperature differential6. In addition, variations in water temperature may result in altered habitats within aquatic ecosystems, which can have detrimental impacts on aquatic creatures by changing the structure and function of the ecosystem7,8. According to Mondal et al.9, aquatic creatures may react differently to temperature fluctuations and have particular temperature needs. Furthermore, it is crucial to monitor the temperature of the water in the vicinity of power facilities10,11.
According to research by Rabbani et al.3, thermal power plants were a primary source of contaminants that looked at the effects of one in India from 1997 to 20043. In their 2009 study, Cuesta et al.12 examined the ecological footprint of US thermal power facilities using parametric and non-parametric methodologies1,12. The research revealed that although the methods produced different efficiency outcomes, they consistently ranked the power plants in the same order. The highlights above include decreasing their ecological footprint by limiting their freshwater consumption. Karmakar et al. suggested using saltwater rather than freshwater to cool thermoelectric power facilities13. Thermal power plants’ pollution rate fluctuates over time, according to Murty et al.14. Pan et al.15 suggest that lowering the activation of power plants in Canada could significantly reduce pollution emissions. Tian et al.16 developed a model for extracting water and waste from thermal power plants to mitigate pollution, establishing a scenario restricting water extraction and statewide electricity transport.
The study showed that electricity is produced by many sources, out of which coal-fired thermal power stations are significant contributors due to their high availability and low cost. Along with electricity production, these power plants produce fly ash in huge amounts, causing serious environmental threats. Fly Ash contaminates soil and groundwater17. Additionally, releasing power plant effluents—such as wastewater and thermal discharges—into water bodies puts aquatic plants, animals, and organisms in danger. KTPS, with a capacity of 1260 MW, is a major thermal power plant situated in Mecheda in Purba Medinipur district of West Bengal, about 55 km from Kolkata18. This power plant uses bituminous coal of low quality, low calorific value, and high fly ash content19. The flash and the effluents from the power plant impact the environment by decreasing the area’s air and water quality. In this research, we are studying the effects of the KTPS on water quality and the impact on the selected aquatic plants. The contaminated effluent adversely affects the biochemistry, morphology, and anatomy of the free-floating aquatic plants in the water bodies adjacent to the power station. For our study, we are selecting specific parameters to investigate the changes in water quality, anatomy, and biochemical properties of the selected species - Eichhornia crassipes and Pistia stratiotes.
The aquatic habitat of the KTPS exemplifies an ecosystem that is particularly vulnerable to environmental pollution. Different businesses, including thermal power plants, discharge wastewater into the aquatic environment, disrupting the ecosystem’s equilibrium by releasing contaminants and excessive heat20. Many aquatic species permanently leave the region due to their inability to adapt to the altered circumstances brought on by this heat pollution. The direct release of hot water from the KTPS into the drainage system harms the ecosystem. Given the importance of this issue, our study will examine KTPS thermal water outflow pollution. The study’s main objective is to investigate the changes in multiple parameters of selected plant species. This will facilitate our understanding of the potential benefits of these plants, especially in the phytoremediation of contaminated aquatic environments. We specifically focus on examining the environmental effects of E. crassipes and P. stratiotes in mitigating pollution from thermal power plants. We have developed a water quality model that helps determine the degree of contamination.
One of the primary advances in the study’s technology was using the datasets to examine the effects of thermal power plant pollution on the KTPS water network and the influence of the water quality in the area on aquatic life using the WQI methods. This study’s innovation is based on the use of sophisticated models for predicting water quality behavioural traits. Using these models for predicting water quality is an innovative approach, and they remain relatively recent in hydrological research. Researchers are intrigued by this study since it provides a novel and realistic method for predicting and assessing water quality parameters in aquatic ecosystems, especially in areas impacted by thermal power plants. These models to predict and evaluate the effects of water quality factors may enhance our understanding of the effects of thermal pollution on aquatic life.
Materials and methods
Study area
The Kolaghat CD Block, located in the Purba Medinipur district of West Bengal, is home to the census town of Kolaghat. It is located at 22°24′− 22°26′ North and 87°50′− 87°53′ East, at an elevation of roughly 9 m above sea level, covering an area of about 6.07 sq. km (Fig. 1). The summer temperatures in the region range between 26 °C and 43 °C. In contrast, the winter temperatures range from 10 °C to 19 °C. The monsoonal periods last from the end of September to the end of July, during which the southwest monsoons bring moisture from the Bay of Bengal, resulting in the majority of the yearly average rainfall of 175 cm occurring during this period.
Study of Kolaghat Thermal Power Plant Area. The red dot is sampling stations (https://www.arcgis.com/index.html).
We used ArcGIS 10.8.2 software for the study to prepare the sampling locations in Fig. 1. The current study selected five experimental sites in and around the KTPS surrounding areas to collect water parameters. The KTPS is located within 5 km of Santipur 1 No. We chose Gram Panchayat, Giripara, KTPS colony, Amalhanda, and Barnan Ghorapara as HCS due to fly ash production and effluent release into water bodies. We selected Kashinathpur, the sixth site, as the LCS due to its distance of approximately 85 km from Kolaghat and the absence of any pollution sources. Compared to the other five sites, data on air quality showed significantly lower concentrations of pollutants at the control site. The pH values of water observed at the six different experimental sites ranged from 7.65 to 8.05. We conducted the study from November to July to observe seasonal water and air pollution differences.
The production of FA and effluents from the thermal power plant significantly impacts the environment by reducing the air and water quality in the area. This pollution directly affects aquatic environments and leads to water contamination. To assess the impact of water pollution on plant species, we collected two of the most prevalent free-floating taxa, namely E. crassipes and P. stratiotes, from each location. Additionally, we collected water samples from the ponds where these selected plants grow and receive effluents from the thermal power plant.
Sectioning and microscopy
The different parts (leaf lamina, petiole, and stolon) of collected specimens were correctly cleaned and cut into transverse sections through freehand sectioning. We stained the section tissue with safranin to reveal the transparent tissue systems. Finally, the tissues were mounted with glycerine for better observation under the Dewinter DIGI510 phase contrast microscope.
Extraction of chlorophyll
We took 3 gm of fresh leaf samples and homogenized them properly with 10 ml of 80% acetone in a mortar pestle. The homogenate was centrifuged for 3 min at 2500 rpm. After the centrifugation, the absorbance was measured from the green supernatant with the help of the Shimadzu UV-VIS (UV- 1900i) spectrophotometer21,22 in Eqs. 1, 2, 3.
Where A663 = Absorbance at 663 nm; A645 = Absorbance at 645 nm; V = Total volume of solution and W = Weight of the leaf tissue.
Leaf extract pH
We used 2 gm of fresh leaves and mixed them well with 10 ml of double-distilled water. After filtering, we used a Global digital pH meter with a buffer solution of pH 7, and the readings were recorded23.
Relative water content of leaf (RWC)
1 gm of fresh leaves from each sample was immersed in distilled water overnight. The turgid leaves were collected from immersed samples and weighed. After that, the leaves were dried in a hot air oven at 70 ºC overnight and weighed19. The formula for calculation in Eq. 4.
The formula uses the abbreviations FW, DW, and TW to represent the weight of fresh, dried, and turgid leaves, respectively.
Ascorbic acid test and APTI
5 g of cleaned and fresh leaves of E. crassipes and P. stratioteswere mixed with 10 ml of 4% oxalic acid. Next, we properly homogenize the mixture using a mortar and pestle, resulting in a final volume of up to 25 ml. We determined ascorbic acid using the titration method and 2,6-dichlorophenol indophenol (DCPIP)24,25. Dye solution: 104 mg of 2,6 (DCPIP) and 84 mg of sodium bicarbonate were mixed with 400 mL of distilled water. The stock standard for ascorbic acid: We mixed 100 ml of 4% oxalic acid with 100 mg of ascorbic acid. The working standard for ascorbic acid involves diluting 100 ml of 4% oxalic acid with 10 ml of stock standard ascorbic acid. In contrast to the dye solution (V2), we titrated 5 ml of sample solution and 10 ml of 4% oxalic acid until a light pink hue developed and lasted for a short while. Similarly, we titrated 10 ml of 4% oxalic acid and 5 ml of working standard ascorbic acid against the dye solution (V1).
The formula for ascorbic acid calculation is below in Eq. 5.
Where V1 and V2 are the dyes absorbed by 0.5 mg of standard ascorbic acid and 5 ml of test material, respectively.
APTI formula was calculated by26 in Eq. 6.
Where A = ascorbic acid content, T = total chlorophyll content, P = leaf extract pH, and R = RWC.
Assays of proline contain
Estimated proline content using the modified methods of Bates et al.27. Fresh seedlings (0.5 g) were crushed in 10 mL of 3% sulfosalicylic acid. Proline was quantified using the ninhydrin reaction, followed by separation in toluene on ice, with absorbance measured at 520 nm. Concentrations were expressed as µmol/g fresh weight using the formula28:
Water quality index calculation
The WQI is a quantitative measure derived from several physical, chemical, and biological attributes. It evaluates the total water quality and is useful for assessing the status of different water bodies, including rivers, wetlands, and groundwater.
Weighted arithmetic index method
The Weighted Arithmetic (WA-WQI) is widely recognized and utilized for classifying water quality. It offers a straightforward and user-friendly approach by assigning weights to different water parameters based on their significance. This flexibility allows users to select and incorporate the specific parameters relevant to their water quality assessment process29. WA-WQI is calculated by using Eq. 730.
Equation 9 is used to determine the quality rating scale (Qi) for each parameter.
In the analysed water, V represents the estimated concentration of the ith parameter.
Vo represents the optimal value of this parameter in pure water. Vo = 0 (except pH = 7.0 and DO = 14.6 mg/L), and Si is the ith parameter’s suggested standard value. We use Eq. 9 to determine the unit weight Wi for each water quality metric.
Where K is the ratio constant and can be determined using Eq. 11.
On a scale of 0 to 100, the WA-WQI thoroughly evaluates water quality; greater values indicate higher pollution levels. The classification of water body quality is divided into five distinct classes: “Excellent,” “Good,” “Poor,” “Very poor,” and “Unsuitable.” (Table 1)31.
Canadian council of ministers of the environment (CCME- WQI)
The CCME-WQI was introduced in 1997 by the Canadian Council of Ministers of the Environment, building upon the British Columbia WQI32. Many countries utilize this index to evaluate water quality, making slight modifications to accommodate their specific calculation preferences and parameter selection. This allows for enhanced ease of calculation and flexibility in incorporating relevant parameters into the index assessment process. Based on the British Columbia WQI, the CCME-WQI in 199732.
Due to its simplicity of computation and versatility in terms of selecting the elements that go into its calculation, this index is utilized by many nations to evaluate the quality of their water. Three essential components make up the CCME-WQI: amplitude (F3), frequency (F2), and scope (F1). We compute the final CCME Index as a single, dimensionless number, ranging from 0 (very poor quality) to 100 (very good quality)33,34.
In brief, the above factors are calculated using Eqs. 11, 12.
The number of variables whose goals are not achieved is the scope factor F1.
The frequency factor F2 is the number of times the goals are not achieved. Equations 14, 15, 16 are used to compute the amplitude factor F3, which is the amount by which the goals are not reached, in three phases.
Finally, the calculation of the CCME -WQI is done using Eq. 16.
The final index score (0–100) divides the water quality index into the following five groups, with a higher score indicating better water quality: Poor: 0–44, Marginal: 45–64, Fair: 65–79, Good: 80–94, and Excellent: 95–100 (Table 1)35.
Results
Biochemical evaluation
Chlorophyll content
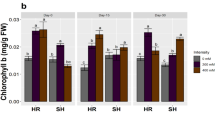
Chlorophyll content relates to a plant’s growth, development, and photosynthetic activity. A change in total chlorophyll is generally shown under stress conditions. The total chlorophyll content recorded in HCS was 0.699 ± 0.016 mg/L and 0.253 ± 0.008 mg/L in E. crassipes and P. stratiotes plant species, respectively. In contrast, the total chlorophyll content in LCS was higher, with 1.119 ± 0.011 mg/L in E. crassipes and 0.491 ± 0.006 mg/L in P. stratiotes. A loss of total chlorophyll is observed in plants from highly contaminated sites, i.e., the KTPS area (Fig. 2).
Leaf extract pH
pH is known to help during stressed conditions by influencing stomatal activity. Leaves with a neutral pH are known to be more pollution-tolerant. The pH of the leaf extract in E. crassipes and P. stratiotes was recorded as 6.58 ± 0.05 and 6.28 ± 0.06, respectively, in HCS. In contrast, in LCS, the leaf extract pH values were 6.30 ± 0.10 for E. crassipes and 6.52 ± 0.07 for P. stratiotes. These results show that both plants are pollution-tolerant to an extent. Out of these two plant species, E. crassipes is more resistant to pollution (Fig. 3).
Relative water content
Plants contain a sufficient amount of water to sustain all of their functions and withstand stress, which aids in maintaining the physiological balance under stressful circumstances. High RWC denotes a high level of pollution resistance. In the current investigation, the RWC in HCS was 81.00%± 2.65 for E. crassipes and 94.90%± 3.60 for P. stratiotes. In comparison, the RWC in LCS was 74.25%± 2.65 for E. crassipes and 88.20%± 4.45 for P. stratiotes. Here, both species show higher RWC in highly contaminated sites, showing their tolerance in response to pollution (Fig. 3).
Ascorbic acid content
Plants may withstand stress during adverse conditions due to the antioxidant ascorbic acid. Through the activation of many defense mechanisms in the plants, it promotes pollution tolerance. A rise in ascorbic acid concentrations indicates that plants can withstand pollution. In the present investigation, the ascorbic acid content in the HCS area was 0.326 ± 0.01 mg/g in E. crassipes and 0.291 ± 0.02 mg/g in P. stratiotes. In contrast, the ascorbic acid content in the LCS area was higher, measuring 0.411 ± 0.01 mg/g in E. crassipes and 0.367 ± 0.02 mg/g in P. stratiotes. The amount of ascorbic acid in plants from contaminated areas has decreased. Plants from highly contaminated sites had a shallower ascorbic acid concentration than plants from low contaminated sites, suggesting that both species strongly tolerate pollution (Table 2).
APTI
The APTI, an indicator of plants’ capacity to withstand pollution, is connected with the following parameters: relative water content, ascorbic acid concentration, total chlorophyll content, and leaf extract pH. When exposed to pollution, plants respond in different ways. The APTI values in E. crassipes and P. stratiotes were recorded as 8.407% and 9.681%, respectively, in HCS. In contrast, in LCS, the APTI values were 7.729% for E. crassipes and 9.077% for P. stratiotes. Plants in highly contaminated sites have elevated levels of APTI in E. crassipes and P. stratiotes (Table 2).
Proline content
Proline concentration determines the stress condition in plants. When the plant is in stress produces more proline amino acids. The concentration of proline in P. stratiotes at LCS site (2.07 µmol/g) is lower than the HCS area (7.74 µmol/g). The E. crassipes at LCS area (3.20 µmol/g) also shows a lower proline concentration than the HCS area (9.09 µmol/g). Proline concentration increased three times in HCS than LCS area plants (Fig. 4).
Water quality assessment
The amount of both biodegradable and non-biodegradable oxidizable contaminants is indicated by BOD and COD, two crucial indices that aid in determining the degree of contamination. The presence of calcium in water bodies is caused by sewage, industry, or rock effluents, all of which are dangerous for human health to consume. TDS is an acronym for TDS in water, which indirectly affects plant growth by influencing the soil. A key indicator of water quality is electrical conductivity, with higher conductivity indicating higher pollution levels. Compared to the water collected from Kashinathpur, the water from the Kolaghat area exhibits greater TDS, COD, BOD, electrical conductivity, calcium, and acidity, indicating a more contaminated water body (Table 3).
In accordance with WHO guidelines, the optimal pH range for drinkable water is between 6.5 and 8.5. pH of the majority of our study area locations is above 7.5. Giripara shows the highest pH value of 8.05, whereas Barnan Ghorapara has a pH of 7.36. Electrical conductivity is based on temperature and concentration of ions present in a sample, with a recommended limit of 300 µS/cm; our samples show a maximum of 2.46 µS/cm in the Santipur area and a minimum of 1.29 µS/cm in Amalhanda. None of our locations exceed the recommended limit of electrical conductivity (Table 3).
Dissolved solids primarily constitute inorganic salts dissolved in the water samples. According to WHO, TDS values above 1000 mg/L are unpleasant, although a recommended limit of 500–2000 mg/L is observed. Out of all the locations, only Santipur and Giripara have TDS values of 1154 mg/L and 1070 mg/L, respectively; the rest of the locations have TDS values lower than 1000 mg/L. Alkalinity in all the locations exceeds the permissible limit of 200 mg/L except for Santipur No. 1 Gram Panchayat, with a value of 180.60 mg/L (Table 3).
The water sample collected from Amalhanda has a value of 385 mg/L, showing maximum alkalinity. COD and BOD are recommended to be within a standard value of less than 3 mg/L and up to 5 mg/L, respectively. The BOD of the Santipur area is 7.64 mg/L, which exceeds the permissible limit. In contrast, all other locations are within the permissible limit, and the COD of every location exceeds the recommended limit with a maximum of 48.20 mg/L and a minimum of 11.94 mg/L in the KTPS colony and Santipur area, respectively (Table 3).
Anatomical assessment
Leaf lamina of E. crassipes
The plant sample from the low-contaminated sites had a thicker epidermal layer and was covered with a thin cuticle layer. The leaf lamina contains spongy and palisade mesophyll cells with significant air gaps. The air gaps from the highly contaminated sites are smaller, measuring 11.80 ± 2.60 μm, compared to those from low-contaminated sites, which measure 16.45 ± 3.43 μm. A layer of parenchyma cells encircles variable-length vascular bundles. The size of the vascular bundles in the highly contaminated sites is reduced to 13.58 ± 2.88 μm, compared to 15.12 ± 1.73 μm in the low-contaminated sites. There are black depositions in cells seen in plants from highly contaminated sites (Table 4; Fig. 5).
Petiole of E. crassipes
The plant sample collected from the highly contaminated sites shows a single-layered epidermis without a cuticle. Raphide crystals are more prevalent in the parenchyma cells of plants from highly contaminated sites. Sclereids are observed to emerge from aerenchyma cells. In comparison, plants from the low-contaminated sites exhibit larger air spaces. Furthermore, there is a notable reduction in the size of vascular bundles in the plants from the highly contaminated sites, measuring 14.35 ± 3.15 μm. In contrast, the plants from the low-contaminated sites have vascular bundles measuring 17.03 ± 1.69 μm. Additionally, plants from low-contaminated sites have larger phloem cells, measuring 1.57 ± 0.21 μm, while plants from highly contaminated sites have smaller phloem cells, measuring 1.29 ± 0.35 μm (Table 4; Fig. 5).
Stolon of E. crassipes
The plant samples from low contaminated sites display an epidermal layer followed by a multilayered cortex region, with a larger cortical region measuring 8.65 ± 1.28 μm. The airspaces are smaller, measuring 11.80 ± 2.60 μm in highly contaminated sites compared to low contaminated sites. In contrast, the plants collected from highly contaminated sites have a smaller pith and vascular bundles region measuring 5.25 ± 1.63 μm and 13.08 ± 1.24 μm, respectively. Depositions are observed in the airspaces, with more deposition in plants from the highly contaminated sites (Table 4; Fig. 5).
Leaf lamina of P. stratiotes
Single-layered epidermis with trichomes and paracytic stomata are seen. Both acicular and rosette types of crystals are present, with more found in highly contaminated sites. Vascular bundles are visible. The parenchyma layer is larger in samples from low contaminated sites, measuring 9.53 ± 0.78 μm, compared to 6.30 ± 0.87 μm in the highly contaminated sites. There are more trichomes in the highly contaminated sites. Larger idioblasts, measuring 9.61 ± 0.84 μm, are seen in the samples from low contaminated sites, whereas idioblasts of 8.38 ± 0.58 μm are seen in highly contaminated sites (Table 5; Fig. 5).
Petiole of P. stratiotes
Multicellular trichomes are observed on the epidermal layer and are more prevalent in plants from highly contaminated sites. Groups of vascular bundles are visible, with a larger size measuring 20.50 ± 1.24 μm in plants from low-contaminated sites. The parenchyma layer measures 5.79 ± 0.69 μm in highly contaminated sites and 6.37 ± 0.37 μm in low-contaminated sites. Additionally, rosette and acicular crystals are present in lower quantities in plants from the low-contaminated sites (Table 5; Fig. 5).
Stolon of P. stratiotes
The cortex of plants from low-contaminated sites is larger, measuring 5.68 ± 0.85 μm. An epidermal layer lines it and has trichomes on it. Aerenchyma is abundantly present, with a more significant size in plants from low-contaminated sites measuring 12.86 ± 1.62 μm and a slightly smaller size in plants from highly contaminated sites measuring 10.88 ± 1.99 μm. Numerous raphide crystals within parenchyma cells are observed in plants from highly contaminated sites compared to those from low-contaminated sites (Table 5; Fig. 5).
Micrographic anatomical view of E. crassipes (A-Leaf, B-Petiole, and C-Stolon) and P. stratiotes (D-Leaf, E-Petiole, and F-Stolon). ae = aerenchyma, as = air space, bs = bundle sheath, co = cortex, dcid = druse crystal idioblast, ep = epidermis, lp = lower palisade, p.a. = parenchyma, ph = phloem, pi = pith, rcid = raphide crystal idioblast, up = upper palisade, vb = vascular bundle, xy = xylem.
Surface water quality index (WQI) assessment
Additionally, the WA-WQI and CCME-WQI values for several sites are included in Table 6. The WA-WQI readings for all stations are under the “Very Poor” category, which indicates that the water quality is below acceptable standards. The CCME-WQI evaluations, on the other hand, present a different picture, with some stations being rated as “Good,” others as “Fair,” and one station being rated as “Marginal.”
For example, the Santipur GP and Giripara stations have WA-WQI ratings of 88.37 and 87.55, respectively, which are considered to be in the “Very Poor” category. The CCME-WQI deems their scores of 85.21 and 85.81, respectively, as “good.” Similar to the previous example, the WA-WQI values of the Amalhanda and Barnan Ghorapara stations are classified as “Very Poor.” However, their CCME-WQI scores of 76.09 and 75.71 are regarded as “fair.” The KTPS colony station stands out with the highest WA-WQI value of 93.94, placing it again in the “Very Poor” category. However, its CCME-WQI score of 64.33 places it in the “Marginal” category. On the other hand, the “low-contamination station” has a WA-WQI of 88.59, which places it inside the “Very Poor” category as well; nevertheless, its CCME-WQI of 86.22 is regarded as “highly recommended.”
Discussion
Industrialization poses a substantial influence on the ecosystem in numerous aspects. Many industries, such as power plants, release a large amount of heavy metals and toxic substances into the environment3,5,36,37. These pollutants can reach the water bodies through atmospheric deposition and surface run-off38,39. The leaching of soil and rocks produces naturally occurring pollutants, which are generally less harmful to the population than those resulting from human activities40. Every parameter was essential for calculating the WQI. The EC of a sample is a function of the TDS present41. The levels of TDS in the samples collected from various parts of Kolaghat exceeded the permissible limit. Santipur No. 1 Gram Panchayat had the highest TDS levels (Table 3). Most of the water samples had pH values above the allowable alkalinity limit, indicating an alkaline range. COD levels were extremely high in all 5 locations of Kolaghat, with the highest levels recorded in the KTPS colony. Apart from Santipur, most BOD values were within the permissible range.
In a polluted environment, plants tend to show a decrease in various photosynthetic pigments42. The thorough analysis of the data reveals a correlation between the pollution level and the reduction in total chlorophyll content. The plant samples we collected from the vicinity of the HCS study area exhibited a decrease in total chlorophyll content, indicating that pollution is impacting the chlorophyll levels in the plants. Studies have suggested that plants in HCS have lower chlorophyll levels, as supported by the previously reported data43. Study reveals that pH is another important factor that plays a significant role in plant species’ physiological reactions under stress conditions44. pH controls the stomatal activity of plant species, which in turn controls stress conditions. Studies have shown that plants with acidic soils tend to be harmed by pollutants, in contrast to those with neutral-pH leaves, which are more resistant to these stresses45. The pH values of the leaf extracts from both plant species in the present investigation are almost neutral, suggesting they are tolerant of pollution. Water is necessary for processes, including the movement of nutrients and minerals. People claim that plants with a high relative water content have a higher pollution tolerance, RWC determines the water availability and transpiration rate in the leaf, which maintains the physiological condition of the plant species in stress conditions43,46. According to our findings, both the plant species E. crassipes and P. stratiotes exhibit higher RWC in the HCS study area, demonstrating their effectiveness in tolerating pollution.
In the current investigation, in the HCS area, both the plant species, i.e., E. crassipes and P. stratiotes, have lower levels of ascorbic acid in comparison with the LCS area plant species. According to previously published articles, strong reductants, such as ascorbic acid, promote pollution tolerance47,48. As a result of pollution exposure, ascorbic acid levels decrease49. The recorded results are consistent with previously investigated data, confirming that these plant species act as pollution-tolerant organisms in the HCS study area. Previously reported data suggest that higher APTI values indicate higher resistance to pollution50,51,52. In the current observation, both E. crassipes and P. stratiotesexhibited higher APTI values at HCS sites compared to the LCS study area, indicating their greater tolerance to pollution. In this study, we found that proline concentration was higher in the HCS area than in the LCS area for both selected plant species. Similar observations have been reported in previous studies. Additionally, published research supports that plant species produce higher amounts of proline under stress conditions than in natural conditions55,56.
Our study also revealed a reduction in average cell size in plants from the polluted area, likely due to their slower growth in stressed environments, previously reported articles also state that plant growth is arrested in stressed environmental conditions53,54. Average cell sizes of the epidermis, bundle sheath, and vascular bundles in different parts of both E. crassipes and P. stratiotes showed a significant reduction in the samples collected from HCS, as shown in Tables 4 and 5. We observe the presence of raphide crystals in the parenchyma cells of different parts of the plants. These raphide crystals consist of calcium oxalate, which plays a role in metal detoxification57. Plants collected from the HCS have more raphide crystals than plants collected from the LCS. Water quality impacts plants’ growth, which influences their size. The water quality of the HCS retards the growth of plants. Our comparative study reveals a degree of pollution tolerance in both the plant species E. crassipes and P. stratiotes.
Conclusions and future aspects
The Kolaghat thermal power station has a significant negative environmental impact on the surrounding area. It produces a large amount of fly ash and nitrogen oxides during the energy generation process, which affects the quality of the land, water, and air and, consequently, the health of people, animals, and plants. Research indicates that E. crassipes and P. stratiotes can tolerate pollution to a certain extent and effectively scavenge and collect contaminants, making them suitable for phytoremediation. These plant species could be utilized to monitor pollution levels around the thermal power plant. Additionally, using fly ash for purposes such as manufacturing building materials and constructing roads can help reduce pollution. Implementing strategies involving these plant species and reusing fly ash can significantly reduce pollutant levels, benefitting the environment and living organisms. The comparison of water quality indices (WA-WQI and CCME-WQI) suggests that low-contaminated sites have better water quality than highly contaminated sites. To improve water quality at contaminated sites, it is crucial to identify sources of contamination and implement appropriate mitigating actions such as establishing wastewater treatment facilities, promoting environmentally responsible behavior, and implementing stricter pollution control systems. Community education and engagement are also key to maintaining and enhancing water quality through encouraging responsible water consumption and sustainable practices in business, agriculture, and residential areas. Regular monitoring and evaluation of water quality are essential to assess implemented processes’ effectiveness and progress.
The present investigation highlights the significant impact of FA on the selected plant species of the natural ecosystem, providing baseline information on anatomical and biochemical changes of that species. While extensive research exists, key questions remain: How does FA enter the cells? What are its species-specific concentrations? How can it be effectively removed? What are its effects on humans? Future research should focus on identifying toxic contaminants in FA, standardizing mitigation strategies, and optimizing extraction of valuable elements while ensuring safe disposal. Developing vegetation around FA disposal sites is crucial for ecosystem stability. Phytoremediation depends on pollutant bioavailability, necessitating soil amendments and genomic studies to enhance plant resilience. “Omics” tools are vital for understanding plant adaptation strategies in eco-restoration. Additionally, addressing public awareness, policy incentives, and regulatory support is essential for advancing green remediation technologies.
Data availability
Data is provided within manuscript.
Abbreviations
- APTI:
-
Air pollution tolerance index
- BIS:
-
Bureau of Indian Standard
- BOD:
-
Biological oxygen demand
- Ca:
-
Calcium
- CCME-WQI:
-
Canadian Council of Ministers of the Environment Water Quality Index
- Chl a:
-
Chlorophyll a
- Chl b:
-
Chlorophyll b
- COD:
-
Chemical oxygen demand
- CPCB:
-
Central Pollution Control Board
- DCPIP:
-
Dichlorophenol indophenol
- EC:
-
Electrical conductivity
- EPA:
-
Environmental Protection Agency
- FA:
-
Fly ash
- HCS:
-
Highly contaminated sites
- IS:
-
Indian Standard
- KTPS:
-
Kolaghat Thermal Power Station
- LCS:
-
Low contaminated sites
- RWC:
-
Relative Water Content
- TCh:
-
Total Chlorophyll Content
- TDS:
-
Total dissolved solids
- WA-WQI:
-
Weighted Arithmetic Water Quality Index
- WHO:
-
World Health Organization
- WQI:
-
Water Quality Index
References
Mokarram, M., Mokarram, M. J. & Najafi, A. Thermal power plants pollution assessment based on deep neural networks, remote sensing, and GIS: A real case study in Iran. Marine Pollution Bulletin, 192, p.115069. (2023).
Abbaspour, M., Javid, A. H., Moghimi, P. & Kayhan, K. Modeling of Thermal Pollution in Coastal Area and its Economical and Environmental Assessment2pp.13–26 (International Journal of Environmental Science & Technology, 2005).
Rabbani, M. et al. Assessing Thermal Power effluent-induced Air Quality and Associated Environmental Stress on Blumea lacera and Phyla nodiflora Using Chemometric, Remote Sensing and Machine Learning Approach1–19 (Geology, 2024).
Cosgrove, W. J. & Loucks, D. P. Water management: current and future challenges and research directions. Water Resour. Res. 51 (6), 4823–4839 (2015).
Jeremias, G. et al. Synthesizing the role of epigenetics in the response and adaptation of species to climate change in freshwater ecosystems. Mol. Ecol. 27 (13), 2790–2806 (2018).
Kumari, M., Sarma, K. & Sharma, R. Using Moran’s I and GIS to study the spatial pattern of land surface temperature in relation to land use/cover around a thermal power plant in Singrauli district, Madhya Pradesh, India15p.100239 (Society and Environment, 2019).
Zeng, G., Wu, Z., Cao, W., Wang, Y., Deng, X.,… Zhou, Y. (2020). Identification of anti-nociceptive constituents from the pollen of Typha angustifolia L. using effect-directed fractionation. Natural Product Research, 34(7), 1041–1045.
Logan, L. H. & Stillwell, A. S. Probabilistic assessment of aquatic species risk from thermoelectric power plant effluent: incorporating biology into the energy-water nexus. Appl. Energy. 210, 434–450 (2018).
Mondal, I. et al. Intra and Inter Annual Variability of Coastal Water Quality in Sundarban Mangrove Dominated Estuarine Ecosystem Using Remote Sensing and Hybrid Machine LearningElsevier, 140889 (Models Journal of Cleaner Production, 2024).
Mondal, I. et al. Variability of bio-optical Properties of Sundarbans Mangrove Estuarine Ecosystem Using Elemental Analysis, Sentinel 3 OLCI Imageries and Neural Network Models (Advances in Space Research, 2024b).
de Szechy, M. T. M., de Koutsoukos, V. S. & de Barboza, C. A. M. Long-term decline of brown algal assemblages from Southern Brazil under the influence of a nuclear power plant. Ecol. Indic. 80, 258–267 (2017).
Cuesta, R. A., Lovell, C. A. K. & Zofío, J. L. Environmental efficiency measurement with translog distance functions: a parametric approach. Ecol. Econ. 68, 2232–2242 (2009).
Karmakar, J. et al. Analyzing spatio-temporal variability of aquatic productive components in Northern Bay of Bengal using advanced machine learning models. Ocean & Coastal Management, Elsevier, 251, p.107074. (2024).
Murty, M. N., Kumar, S. & Dhavala, K. K. Munich Personal RePEc Archive Measuring Environmental Efficiency of Industry: A Case Study of Thermal Power Generation in India Measuring Environmental Efficiency of Industry (A Case Study of Thermal Power Generation in India*, 2006).
Pan, S. Y., Snyder, S. W., Packman, A. I., Lin, Y. J. & Chiang, P. C. Cooling water use in thermoelectric power generation and its associated challenges for addressing water-energy nexus. Water-Energy Nexus. 1, 26–41 (2018).
Tian, X., An, C., Nik-Bakht, M. & Chen, Z. Assessment of reductions in NO2 emissions from thermal power plants in Canada based on the analysis of policy, inventory, and satellite data. J. Clean. Prod. 341, 130859 (2022).
Sahoo, P. K., Kim, K., Powell, M. A. & Equeenuddin, S. M. Recovery of metals and other beneficial products from coal fly Ash: A sustainable approach for fly Ash management. Int. J. Coal Sci. Technol. 3 (3), 267–283 (2016).
Mondal, I., Maity, S., Das, B., Bandyopadhyay, J. & Mondal, A. K. Modeling of environmental impact assessment of Kolaghat thermal power plant area, West Bengal, using remote sensing and GIS techniques. Model. Earth Syst. Environ. 2, 1–12 (2016).
Maity, S., Mondal, I., Das, B., Mondal, A. K. & Bandyopadhyay, J. Pollution tolerance performance index for plant species using Geospatial technology: evidence from Kolaghat thermal plant area, West Bengal, India. Spat. Inform. Res. 25, 57–66 (2017).
Aboul-Enein, A. M. et al. Cytotoxic and antioxidant properties of active principals isolated from water hyacinth against four cancer cells lines. BMC Complement. Altern. Med. 14, 1–11 (2014).
CI, K. C. & Indira, G. Quantitative Estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of strobilanthes Kunthiana (Neelakurinji). J. Med. Plants. 4, 282–286 (2016).
Kumari, R. et al. Extraction and Estimation of chlorophyll content of seed treated lentil crop using DMSO and acetone. J. Pharmacognosy Phytochemistry. 7 (3), 249–250 (2018).
Agbaire, P. O. & Esiefarienrhe, E. Air pollution tolerance indices (apti) of some plants around Otorogun gas plant in delta State, Nigeria. J. Appl. Sci. Environ. Manage. 13, 11–14 (2009).
Denre, M. The determination of vitamin C, total phenol and antioxidant activity of some commonly cooking spices crops used in West Bengal. Int. J. Plant. Physiol. Biochem. 6 (6), 66–70 (2014).
Dinesh, B., Yadav, B., Reddy, R. D., Padma, A. S. & Sukumaran, M. K. Determination of ascorbic acid content in some Indian spices. Int. J. Curr. Microbiol. Appl. Sci. 4 (8), 864–868 (2015).
Javanmard, Z., Kouchaksaraei, M. T., Hosseini, S. M. & Pandey, A. K. Assessment of anticipated performance index of some deciduous plant species under dust air pollution. Environ. Sci. Pollut. Res. 27, 38987–38994 (2020).
Bates, L. S., Waldren, R. P. A. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207 (1973).
Reddy, S. H., Al-kalbani, H., Al-Qalhati, S., Al-Kahtani, A. A., Al Hoqani, U., Azmi,S. N. H., … Settaluri, V. S. (2024). Proline and other physiological changes as an indicator of abiotic stress caused by heavy metal contamination. Journal of King Saud University-Science, 36(8), 103313.
Iticescu, C. et al. Lower Danube water quality quantified through WQI and multivariate analysis. Water 11 (6), 1305 (2019).
Zotou, I., Tsihrintzis, V. A. & Gikas, G. D. Performance of seven water quality indices (WQIs) in a mediterranean river. Environ. Monit. Assess. 191, 1–14 (2019).
Tyagi, S., Sharma, B., Singh, P. & Dobhal, R. Water quality assessment in terms of water quality index. Am. J. Water Resour. 1 (3), 34–38 (2013).
Canadian Council of Ministers of the Environment (CCME). ‘Canadian Water Quality Guidelines for the Protection of Aquatic Life: CCME Water Quality Index 1.0’, Technical Report, Canadian Council of Ministers of the environment winnipeg, MB, Canada. (2001). Available at: http://www.ccme.ca/sourcetotap/wqi.html
Canadian Council of Ministers of the Environment (CCME). Canadian ‘Water Quality Guidelines for Protection of Aquatic Life Guidance for Site–Specific Application of Water Quality Guidelines in Canada and Procedures for Deriving Numerical Water Quality Objectives’, Canadian Council of Ministers of the Environment, Winnipeg, MB, (2003). Available at: http://www.ccme.ca/initiatives/water.html?category_id=41#77
Sutadian, A. D., Muttil, N., Yilmaz, A. G. & Perera B.J.C. Development of river water quality indices—a review. Environ. Monit. Assess. 188, 1–29 (2016).
Li, Y. et al. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 6, 64 (2018).
Joshi, P. C. & Swami, A. Physiological responses of some tree species under roadside automobile pollution stress around City of Haridwar, India. Environmentalist 27, 365–374 (2007).
Gupta, P., Vishwakarma, M. & Rawtani, P. M. Assessment of water quality parameters of Kerwa dam for drinking suitability. Int. J. Theoretical Appl. Sci. 1 (2), 53–55 (2009).
Subba Rao N. Groundwater quality from a part of Prakasam district, Andhra Pradesh, India. Appl. Water Sci. 8, 1–18 (2018).
Subba Rao, N. Controlling factors of fluoride in groundwater in a part of South India. Arab. J. Geosci. 10, 1–15 (2017).
Rashid, M. H.; Kamruzzaman, M.; Haque, A. N. A.; Krehenbrink, M. Soil Microbes for Sustainable Agriculture. In Sustainable Management of Soil and Environment; Meena, R., Kumar, S., Bohra, J., Jat, M., Eds.; Springer: Singapore, 339–382 (2019).
Ramakrishnaiah, C. R., Sadashivaiah, C. & Ranganna, G. Assessment of water quality index for the groundwater in Tumkur taluk, Karnataka State, India. J. Chem. 6 (2), 523–530 (2009).
Giri, B. et al. Composition and sources of organic tracers in aerosol particles of industrial central India. Atmos. Res. 120, 312–324 (2013).
Basar, S. K. & Mondal, A. K. Morphological and biochemical differentiations of plants in polluted and nonpolluted environments. Int. J. Env Tech. Sci. 4S, 8, 13 (2017).
Wilkinson, L. Statistical methods in psychology journals: guidelines and explanations. Am. Psychol. 54 (8), 594 (1999).
Tak, A. A. & Kakde, U. B. Assessment of air pollution tolerance index of plants: a comparative study. Int. J. Pharm. Pharm. Sci. 9 (7), 83–89 (2017).
Jyothi, S. J. & Jaya, D. S. Evaluation of air pollution tolerance index of selected plant species along roadsides in Thiruvananthapuram, Kerala. J. Environ. Biol. 31 (3), 379–386 (2010).
Rai, P. K., Panda, L. L., Chutia, B. M. & Singh, M. M. Comparative assessment of air pollution tolerance index (APTI) in the industrial (Rourkela) and Non industrial area (Aizawl) of India: an ecomanagement approach. Afr. J. Environ. Sci. Technol. 7 (10), 944–948 (2013).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of botany, 217037 (2012). (2012)(1).
Klumpp, G., Furlan, C. M., Domingos, M. & Klumpp, A. Response of stress indicators and growth parameters of tibouchina pulchra Cogn. Exposed to air and soil pollution near the industrial complex of Cubatão, Brazil. Sci. Total Environ. 246 (1), 79–91 (2000).
Sahu, C., Basti, S. & Sahu, S. K. Air pollution tolerance index (APTI) and expected performance index (EPI) of trees in Sambalpur town of India. SN Appl. Sci. 2 (8), 1327 (2020).
Gupta, K. Bioaccumulation potential of pistia stratiotes and its response to tannery effluent exposure. Int. J. Phytopharm Res. 5, 1–6 (2014).
Omosun, G., Edeoga, H. O. & Markson, A. A. Anatomical changes due to crude oil pollution and its heavy metals component in three mucuna species. Recent. Res. Sci. Technol. 1, 264–269 (2009).
Sukumaran, D. Effect of air pollution on the anatomy some tropical plants. Appl. Ecol. Environ. Sci. 2 (1), 32–36 (2014).
Nakata, P. A. Advances in our Understanding of calcium oxalate crystal formation and function in plants. Plant Sci. 164 (6), 901–909 (2003).
Kijowska-Oberc, J., Dylewski, L. & Ratajczak, E. Proline concentrations in seedlings of Woody plants change with drought stress duration and are mediated by seed characteristics: a meta-analysis. Sci. Rep. 13 (1), 15157 (2023).
Zhang, S., Shao, L., Sun, Z., Huang, Y. & Liu, N. An atmospheric pollutant (inorganic nitrogen) alters the response of evergreen broad-leaved tree species to extreme drought. Ecotoxicol. Environ. Saf. 187, 109750 (2020).
He, L., Shan, Y., Liu, C., Cao, H., Liu, X.,… Guo, Y. (2024). Prediction of bedload transport inside vegetation canopies with natural morphology. Journal of Hydrodynamics,36(3), 556–569. doi: https://doi.org/10.1007/s42241-024-0033-7.
Acknowledgements
The authors sincerely thank the authorities of Aliah University, New Town, Kolkata, for providing continuous support and all kinds of laboratory facilities. They are also grateful to the Water Testing Laboratory at the Vivekananda Institute of Biotechnology, Nimpith, for their valuable help with analytical work. Special thanks go to the research scholars of the Department of Biological Sciences, Aliah University, for their constant support and technical assistance during the study.
Funding
Specifically, no funding was provided for this research.
Author information
Authors and Affiliations
Contributions
“AU: Formal analysis; Investigation; Methodology; Software; Writing - original draft; MSH: Formal analysis; Investigation; Methodology; SSI: Formal analysis; Investigation; Methodology; Software; Writing - original draft; SKR: Formal analysis; Software; Methodology; AI: Methodology; Reviewing and Editing; IM: Formal analysis; Investigation; Methodology; EA: Methodology; Reviewing and Editing; SMAIS: Conceptualization; Formal analysis; Investigation; Methodology; Writing - original draft; Supervision.”
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Upadhyay, A., Hossain, M.S., Islam, S.S. et al. Fly ash pollution causes morpho-anatomical and biochemical changes in Eichhornia crassipes (Mart.) Solms and Pistia stratiotes L: demonstrating stress-tolerant activity. Sci Rep 15, 14154 (2025). https://doi.org/10.1038/s41598-025-97583-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97583-2
Keywords
This article is cited by
-
Assessing water parameters and spatial patterns of zooplankton distribution in relation to the water quality index: indicators of aquatic health and ecosystem safety
Proceedings of the Indian National Science Academy (2026)