Abstract
Solute carrier family 26 member 4 (SLC26A4) plays an essential role in the progression of pathological cardiac hypertrophy. This study aimed to examine the involvement of SLC26A4 in cardiac hypertrophy by regulation of autophagy and activation of the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome. Cardiomyocytes were treated with 200 µmol/L phenylephrine (PE) to induce cardiac hypertrophy, followed by treatment with 10 mmol/L INF39, an NLRP3 inhibitor. Furthermore, the pLL3.7 lentiviral vector was used to construct a sh-SLC26A4 interference plasmid and a PLL3.7-cardiomyocytesv-SLC26A4 overexpression plasmid to intervene in PE-induced cardiac hypertrophy. Quantitative reverse transcription polymerase chain reaction and Western blotting were performed to measure the expression of ANP, BNP, β-MHC, SLC26A4, NLRP3, apoptosis associated speck-like protein containing a C-terminal caspase recruitment domain (ASC), and caspase-1. Immunofluorescence was used to detect the level of α-smooth muscle actin (α-SMA) to indicate the cardiomyocyte area. The expression levels of the autophagy proteins LC3, beclin-1, and p62 were determined by Western blotting. Finally, an SD rats model of cardiac hypertrophy was established using transverse aortic constriction (TAC) surgery. SLC26A4, NLRP3, IL-1β, ACS and caspase-1 were further examined at gene and protein levels. SLC26A4 expression was associated with cardiac hypertrophy in cell experiments. SLC26A4 promoted autophagy and activation of the NLRP3 inflammasome pathway, regulating the gene and protein expression of LC3, beclin-1, p62, ACS, NLRP3, and caspase-1. Similar results were observed in the TAC rat model, in which SLC26A4 expression was associated with cardiomyocyte enlargement and cardiac interstitial and perivascular fibrosis. SLC26A4 was involved in cardiac hypertrophy by promoting autophagy and activating the NLRP3 inflammasome pathway. Targeting the expression of SLC26A4 may provide a new treatment option for cardiac hypertrophy.
Similar content being viewed by others
Introduction
Pathological cardiac hypertrophy is an independent risk factor for cardiovascular diseases. It can cause myocardial ischemia, decreased myocardial compliance and arrhythmia, ultimately leading to serious consequences, such as heart failure and sudden death1,2. Although many genes and signaling pathways are found to be involved in the pathogenisis of cardiac hypertrophy, the underlying mechanisms have yet to be fully understood.
Interleukin-lβ (IL-1β) is upregulated in the cardiac hypertrophy model of cell and rats3,4. Overexpression of the Il-1β gene resulted in cardiomyocyte hypertrophy and increased atrial natriuretic peptide (ANP) and β-myosin heavy chain (β-MHC) expression in mice5. The splicing and maturation of IL-1β precursors are mediated by the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome6.
The solute carrier family 26 member 4 (SLC26A4) gene usually encodes the ion transporter-associated protein pendrin, which transports a variety of mono- and divalent ions, such as SO42−, HCO3−, I−, and Cl−, and plays an important role in the maintenance of ionic homeostasis. It is highly expressed in the inner ear as well as in the heart, kidneys, and liver7. Han et al. reported that SLC26A4-AS1 enhances SLC26A4 expression by sponging miR-301a-3p or miR-301b-3p, thereby exacerbating angiotensin II-induced cardiac hypertrophy8. In addition, in our previous study9, we found that silencing SLC26A4 ameliorated pathological cardiac hypertrophy, which may be related to the regulation of autophagy. However, whether the role of SLC26A in regulating autophagy is associated with NLRP3 inflammasome activation remains unknown.
In this study, we further investigated the involvement of SLC26A4 in pathological cardiac hypertrophy by constructing a model of cardiomyocyte hypertrophy and that of transverse aortic constriction (TAC) rats. We examined whether SLC26A4 regulates autophagy and activates NLRP3 inflammasome in cardiac hypertrophy.
Materials and methods
Cell culture and treatments
H9C2 cardiomyocytes were obtained from Sebachem Biologicals (China) and cultured in DMEM supplemented with 10% fetal bovine serum in an incubator at 37 °C with 5% CO2 and saturated humidity. After the cells were stabilized, phenylephrine (PE) (200 µmol/L)9 was used to induce pathological hypertrophy of cardiomyocytes, and intervened with NLRP3 inhibitor INF39 (10 mmol/L)10 to observe the changes of NLRP3 pathway. In addition, SLC26A4 interference plasmid and SLC26A4 overexpression plasmid were constructed with pLL3.7 lentiviral vector. H9C2 cells were divided into control group, PE group, overexpression lentiviral control group (OE-control), SLC26A4 overexpression lentiviral group (OE-SLC26A4), OE-SLC26A4 + PE group, OE-SLC26A4 + PE + 3-MA (an autophagy inhibitor) group, sh-SLC26A4 lentiviral control group(sh-NC), sh-SLC26A4 group, sh-SLC26A4 + PE group and sh-SLC26A4 + PE + autophagy activator Rapamycin (sh-SLC26A4 + PE + Rapa).
Materials and reagents
PE and INF39 were purchased from MCE (USA); TRIzol, a miRNA 1st Strand cDNA Synthesis Kit (by stem‒loop), Hifair® II 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus), Hieff® qPCR SYBR Green Master Mix (No Rox), and DEPC-Treated Water were purchased from Yeasen Bio (China). DMEM and fetal bovine serum were obtained from Gibco (USA). RIPA lysis buffer, skim milk powder, Phenylmethanesulfonyl fluoride (PMSF), a BCA protein quantification kit, an SDS-PAGE gel preparation kit, 5× protein lysis buffer, and enhanced chemiluminescence (ECL) reagent were purchased from Beyotime Bio (China). Tris, glycine, SDS, and protein staining markers were obtained from Solarbio (China). Anhydrous ethanol, xylene, hematoxylin and eosin (HE) staining solution, PBS, trichloromethane (chloroform), isopropyl alcohol, and anhydrous ethanol were purchased from Sinopharm Chemical Reagent (China).
RNA extraction and real-time quantitative polymerase chain reaction (RT-qPCR)
After extraction of total RNA from different subgroups of cardiomyocytes (5 samples per group), the purity and concentration of the RNA were determined, and the RNA was reverse-transcribed to cDNA using the Revertaid First Strand cDNA Synthesis Kit (Thermo). cDNA, primers, and DEPC water were added to a 20-µL system and mixed, and automated sample amplification was performed using the SsoAdvance Universal SYBR Green SuperMix (Bio-Rad) system to amplify the samples. The cycling conditions were (i) pre-denaturation (1 cycle at 95 °C for 30 s), (ii) cycling reaction (40 cycles at 95 °C for 10 s and 60 °C for 30 s ), (iii) dissolution profile (95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s for 1 cycle). Results were calculated using Bio-Rad CFX Manager 3.1 software. Finally, the relative expression levels of the target genes were determined using the 2-ΔΔCt method (GAPDH as reference gene). The primers used were listed in Table 1.
Western blotting analysis
Total proteins were extracted from the treated cells by adding an appropriate amount of RIPA lysis buffer (Beo Tianmei Biotech, China). Quantification was performed using the BCA method. The proteins were separated by SDS-PAGE, transferred to PVDF membranes, and blocked with 5% skim milk. The PVDF membrane was then cut and incubated overnight at 4 °C with primary antibody and then incubated with secondary antibody for 1 h at room temperature. The PVDF membrane was washed for 30 min after antibody binding and developed with enhanced ECL reagent, and the results were detected using a gel imaging system. The following antibodies were used: LC3 (1:1000, Sangon Biotechnology, China), beclin1 (1:1000, Shanghai Sangon Biotechnology Co., Ltd.), P62 (1:1000, Sangon Biotechnology, China), GAPDH (1:5000, Atagenix, China), anti-NLRP3 (1:1000, Sangon Biotechnology, China), anti-ASC (1:1000, Sangon Biotechnology, China), and anti-caspase-l (1:1000, Sangon Biotechnology, China). The secondary antibody used was goat anti-rabbit secondary antibody (1:5000). The relative protein expression was calculated by the ImageJ system using GAPDH as a reference.
Immunofluorescence and confocal microscopic assays
Cardiomyocytes were analyzed by α-SMA immunofluorescence staining, and images were visualized by confocal fluorescence microscopy. The specific procedure was described in detail in our previous study9.
Construction of a lentiviral vector for SLC26A4 interference and overexpression, and autophagy flux assay
After an NCBI search for the sequence of SLC26A4, the primer fragments were inserted into the lentiviral vector to construct the shRNA-SLC26A4 interference plasmid and the SLC26A4 overexpression plasmid. After successful primer embedding, plasmid amplification and extraction were performed. Finally, the constructed plasmid was used to generate amplified lentiviral in 293T cells. The packaged lentiviral was used to infect cardiomyocytes to test the infection effect and for subsequent studies.
Autophagy flux assay
H9C2 cells were cultured into 6-well plates with 2 × 105 cells per well. We infected cells with 20 µl mRFP-GFP-LC3 lentivirus. We replaced the medium at 24 h after cell infection and observed the infectious efciency at 48 h after cell infection. We added puromycin with 2.0 µg/ml and screened stable cell line after 48 h, then reduced puromycin to 0.5 µg/ml for screening in 2 weeks, and surviving cells were observed under the fuorescence microscope for further research.
Animals and treatments
Forty-five healthy SD rats were purchased from Hangzhou Medical College (China). SD rats were fed in a clean environment with sufficient food and water, 5 rats per cage, and observed daily for activity and feeding. SD rats weighing 250 ± 20 g were randomly divided into a sham operation group (sham), an TAC operation group (TAC), TAC + INF39 treatment group, TAC + SLC26A4 overexpression lentiviral control group(TAC + OE-Control), TAC + SLC26A4 overexpression lentiviral group(TAC + OE-SLC26A4), TAC + OE-SLC26A4 + autophagy inhibitor 3-MA group (TAC + OE-SLC26A4 + 3-MA), TAC + SLC26A4 interference lentiviral control group (TAC + sh-NC), TAC + SLC26A4 interference lentiviral group(TAC + sh-SLC26A4), and TAC + SLC26A4 interference lentiviral + autophagy activator rapamycin (TAC + sh-SLC26A4 + Rapa) group, each comprising 5 rats. In the TAC surgery group, a pathological cardiac hypertrophy model was established via lateral TAC surgery. During surgery, SD rats were injected intraperitoneally with a mixture of anesthetics, including ketamine (8 mg/100 g), xylazine (2 mg/100 g), and atropine (0.06 mg/100 g), and respiration was controlled at a tidal volume of 2–3 mL and a frequency of 90–110 breaths/min. After cleaning the operative area, an incision was made in the median of the anterior thorax extending to the second rib. The thymus and aortic arch were exposed, and the appropriate needle cushion was selected according to the diameter of the aortic arch (1.0–1.1 mm) and punctured with a 26–27 G needle cushion (0.4–0.6 mm in diameter). The aorta was narrowed after the right common carotid artery branch with a 5-gauge wire. After firm ligation, the pad and needle were withdrawn, and the incision was closed layer by layer. After recovery of spontaneous respiration was observed, the SD rats were extubated and transferred to cages. The rats were fed and checked regularly to prevent infection. The rest of the procedures in the sham-operated group were the same as those in the operated group, except for the group without aortic arch ligation. The model (TAC) + INF39 treatment group was injected intraperitoneally with the NLRP3 inhibitor INF39 every 2 days along with regular feeding, the model group was injected with saline as a control, and the SD rats were sacrificed after 4 weeks.
The experiment manager knows the detailed experimental protocol and records the experimental data. Animals will be removed from the experiment and anaesthetised (1% pentasorbital sodium (40 mg/kg)) and bled to death if they develop a disease such as infection or do not conform to the experimental specimen before the end of the experiment. Rats were anaesthetised and bled to death (The criteria was developed in accordance with the guidelines of the IACUC) at the end of the experiment. This study protocol was reviewed and approved by Zhejiang Academy of Agricultural Sciences Ethics Committee for the Welfare of Laboratory Animals, approval number [2021ZAASLA46]. Furthermore, all methods were performed in accordance with the relevant guidelines and regulations; and this study was reported in accordance with ARRIVE guidelines.
Immunohistochemical analysis
Rat heart tissue was fixed in 4% paraformaldehyde. Twenty-four h later, the tissues were dehydrated and embedded. After the wax was solidified, the myocardial tissue was cut into 4 μm thick sections on a paraffin microtome and deparaffinized. The nuclei were stained with hematoxylin. After 5–10 min, the cytoplasm was stained with eosin. One to three min later, the sections were dehydrated and sealed in 95% ethanol I for 5 min, 95% ethanol II for 5 min, absolute ethanol I for 5 min, absolute ethanol II for 5 min, xylene I for 5 min, and xylene II for 5 min and then sealed with neutral gum. Finally, photographs were taken using a Nikon eclipse Ci-e microscope (Olympus, Japan) and quantitatively analyzed using ImageJ software11.
Statistical analysis
All the data were statistically analyzed using GraphPad Prism software (Graph Pad Software, Inc., USA). The measurement data are expressed as the means ± standard deviations. Comparisons between two groups were made using a t test (normally distributed information), and multiple comparisons were made using one-way ANOVA (normal distribution with chi-square variance). Nonparametric tests were used if the data were not normally distributed or if the variance was not homogeneous. The results were considered statistically significant at a P value < 0.05.
Results
NLPR3 Inflammasome-related proteins were associated with SLC26A4 expression in PE-induced cardiomyocyte hypertrophy model
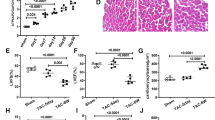
To verify the role of SLC26A4 in cardiac hypertrophy, we used 200 µmol/L PE to induce a cardiomyocyte hypertrophy model. We first validated the construction of cardiomyocyte hypertrophy model by detecting the expression of ANP, BNP and β-MHC. Compared with the control group, the mRNA expression levels of ANP, BNP and β-MHC were significantly increased in the PE group (Fig. 1A and C), which indicated that our cardiac hypertrophy model was successfully constructed. Immunofluorescence and confocal microscopy revealed that the expression of α-SMA was significantly greater in the myocardial tissues of the rats with PE-induced cardiac hypertrophy than in those of the control group (Fig. 1D). The cell surface area increased after PE treatment (P < 0.001) and significantly decreased after INF39 treatment (Fig. 1D, P < 0.05). We examined the SLC26A4 gene and protein, as well as ASC, NLPR3 and caspase-1 gene and protein expression levels. The mRNA expression of SLC26A4, ASC, NLPR3 and caspase-1 was significantly upregulated in the PE group (P < 0.001), but these genes expression significantly decreased after INF39 treatment (Fig. 1E and H, P < 0.01). Western blotting revealed similar results (Fig. 1I). These data suggested that SLC26A4 is associated with the NLRP3 inflammasome pathway, which may induce cardiac hypertrophy.
Pyroptosis-related proteins are associated with SLC26A4 expression in PE-induced cardiac hypertrophy. (A–C) The mRNA expression of ANP (A), β-MHC (B) and BNP (C) was detected in PE-induced cardiomyocyte by RT-qPCR. (D) representative images of cardiac hypertrophy, as shown by α-SMA immunofluorescence. The nucleus was stained with DAPI (blue); the scale bar represents 100 μm. (E–H) The mRNA expression of SLC26A4 (E), ASC (F), NLRP3 (G) and caspase-1 (H) was detected in PE-induced cardiomyocyte by RT-qPCR. I: The protein expression of ACS, NLRP3, and caspase-1 was detected in PE-induced cardiac hypertrophy by Western blotting (*P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group; Results were analyzed by one-way ANOVA, Protein bands were cut from different sites).
SLC26A4 activated autophagy and NLRP3 inflammasome in a PE-induced cardiomyocyte hypertrophy model
To examine the mechanism underlying SLC26A4-induced cardiomyocyte hypertrophy. We examined the expression of SLC26A4 after the transfection of cardiomyocytes with overexpression and interference plasmids. RT-qPCR (Fig. 2A) and Western blotting (Supplementary Fig. 1) revealed that SLC26A4 expression was significantly upregulated after the lentiviral transfection of the SLC26A4 overexpression plasmid (P < 0.001), whereas the expression of SLC26A4 after the lentiviral transfection of shRNA-SLC26A4 was significantly suppressed (P < 0.001), indicating successful plasmid embedding. In addition, we observed that the mRNA expression of ANP, BNP, β-MHC, SLC26A4, ASC, NLRP3, and caspase-1 was significantly greater in the PE group than in the control group (P < 0.001). After the transfection of cardiomyocytes with the SLC26A4 plasmid, the mRNA expression of SLC26A4, ACS, NLRP3, and caspase-1 was significantly upregulated in the OE-SLC26A4 + PE group compared with the control group (P < 0.001). SLC26A4, ACS, NLRP3, and caspase-1 gene expression were significantly reduced after treatment with the autophagy inhibitor 3-MA (P < 0.001). However, after sh-SLC26A4 lentiviral transfection of cardiomyocytes, the expression of SLC26A4, ACS, NLRP3, and caspase-1 was significantly suppressed in the sh-SLC26A4 group (P < 0.01); a similar effect was observed in the sh-SLC26A4 + PE group (p < 0.05). After treatment with the autophagy activator rapamycin, there was no significant difference between the sh-SLC26A4 + PE + Rapa group and the control group (Fig. 2B and H).
SLC26A4 activated autophagy, leading to NLRP3 inflammasome activation in a PE-induced cardiomyocyte hypertrophy model. (A): The mRNA expression of SLC26A4 after SLC26A4 overexpression or interference in cardiomyocytes. (B–H): The mRNA expression of ANP (B), BNP (C), β-MHC (D), SLC26A4 (E), ACS (F), NLRP3 (G), and Caspase-1 (H) was detected in PE-induced cardiomyocyte by RT‒qPCR. (I–P): Western blotting was used to detect the protein expression of LC3 (J), beclin1 (K), p62 (L), NLRP3 (M), ASC (N), Caspase-1 (O) and SLC26A4(P) in PE-induced cardiomyocyte. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group; Results were analyzed by one-way ANOVA, Protein bands were cut from different sites).
Furthermore, autophagy- and NLRP3 inflammasome-associated proteins were further examined, we observed that the expression of LC3 and beclin1 was upregulated in the PE group (P < 0.001), and the expression levels of ASC, NLRP3, and caspase-1 were also significantly greater (P < 0.01), in contrast to the significantly lower expression level of p62 (P < 0.01). The expression levels of LC3, beclin1, ASC, NLRP3, and caspase-1 were more significantly increased (P < 0.001), but those of p62 were decreased (P < 0.001) after SLC26A4 lentiviral transfection. After treatment with the autophagy inhibitor 3-MA, protein expression of LC3, beclin1, ASC, NLRP3, caspase-1and p62 showed the opposite trend (P < 0.001). We also observed that after cardiomyocytes were transfected with sh-SLC26A4 lentiviral, the expression levels of LC3, beclin1, ASC, NLRP3, and caspase-1 were significantly decreased (P < 0.001), whereas the expression of p62 was significantly increased (P < 0.001). Upon treatment with rapamycin, the protein expression of LC3, beclin1, p62, ASC, NLRP3, and caspase-1 was mildly inhibited or not significantly altered (Fig. 2I and P).
In addition, to determine whether SLC26A4 promoted autophagy, we intervened with a lysosomal inhibitor (bafilomycin A1) on a PE model, detected the expression levels of autophagy-related proteins, and And autophagic flux was detected. As demonstrated in Fig. 3A–E, autophagy was significantly increased after SLC26A4 overexpression. In addition, we intervened using siRNA ATG5 (Supplementary Fig. 1C) on PE-induced model to further validate the relationship between autophagy and NLRP3 inflammasome. The expression of NLRP3, ASC and Caspase-1 was significantly reduced after knockdown of ATG5 gene (Fig. 3F–I). Combined with the above findings, in the PE model, SLC26A4 over-activated autophagy and NLRP3 inflammasome were activated, and when the autophagy factor was knocked down, the activation of NLRP3 inflammasome was inhibited. Taken together, these data suggest that SLC26A4 overactivates autophagy, leading to NLRP3 inflammasome activation in a PE-induced cardiomyocyte hypertrophy model.
SLC26A4 regulates autophagy protein expression in a PE-induced cardiomyocyte hypertrophy model. (A–C): The protein expression of LC3 and P62 after SLC26A4 overexpression or interference in Baf.A1. (D–E): An autophagy flux assay. (F–I): Western blotting was used to detect the protein expression of NLRP3 (G), ASC (H), and Caspase-1 (I) after ATG5 gene knockdown. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group; Results were analyzed by one-way ANOVA, Protein bands were cut from different sites).
Expression of SLC26A4 was associated with PE-induced changes in the cardiomyocyte area
To further validate the role of SLC26A4, we used immunofluorescence and confocal microscopy to examine the relationship between SLC26A4 expression and PE-induced changes in cardiomyocytes. We observed a significant increase in cell surface area after the intervention used PE (P < 0.001), which further increased after transfection with SLC26A4 lentiviral (P < 0.001), but decreased after intervention with the autophagy inhibitor 3-MA (P < 0.05). In contrast, the cell surface area significantly decreased after transfection with the sh-SLC26A4 lentiviral (P < 0.01), and the autophagy activator rapamycin prevented the effects of the sh-SLC26A4 plasmid (P < 0.01) (Fig. 4). This finding suggested that SLC26A4 expression is associated with PE-induced changes in cardiomyocytes.
α-SMA immunofluorescence of cardiomyocytes. (A): α-SMA immunofluorescence showing images of SLC26A4-transfected H9C2 cardiomyocyte hypertrophy model. Nuclei were stained with DAPI (blue), α-SMA staining (green) was used to measure the relative cell surface areaThe scale bar is 10 μm. (B): Statistical analysis of α-SMA protein expression levels. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group; Results were analyzed by one-way ANOVA).
SLC26A4 expression led to cardiac structural changes in TAC model rats
Based on the results of the in vitro experiments, we explored the changes in the cardiac structure of TAC model rats after OE-SLC26A4 and sh-SLC26A4 lentiviral transfection. We first detected mRNA expression of ANP, BNP, and β-MHC in rat heart tissues and calculated the rat heart weight/body weight ratio. As shown in Fig. 5A and D, ANP, BNP, and β-MHC mRNA expression levels significantly increased, and rat heart weight/body weight ratio also increased accordingly in the model group compared to the control group, which suggested our animal model was successfully constructed. Furthermore, HE staining demonstrated a normal cardiac morphology of the sham-operated group (sham), the cardiomyocytes were neatly arranged with clear cell gaps. Compared with the model (TAC) group, cardiomyocyte enlargement and cardiac interstitial and perivascular fibrosis were significantly greater in the TAC + INF39 and TAC + sh-SLC26A4 groups. In contrast, the cardiomyocytes in TAC + OE-SLC26A4 group was disarranged, and the myocardial fibers were significantly thickened (Fig. 5E). This finding suggested that SLC26A4 expression is associated with structural changes of the heart.
Animal model validation and HE staining observation of heart tissue. (A–C): The relative expression of ANP (A), BNP (B) and β-MHC (C) in SD rat heart tissues was detected by RT-qPCR; (D): Heart-to-weight ratio in SD rats; (E): HE staining observation of heart tissue. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group; Five rats were used in each group, and the results were analyzed by one-way ANOVA).
SLC26A4 regulated NLRP3 inflammasome activation in vivo
We further explored the relationship between SLC26A4 expression and NLRP3 inflammasome activation. Immunohistochemical results suggested that SLC26A4 induced myocardial hypertrophy in TAC rats. The expression of NLRP3 and IL-1β was significantly greater in the TAC and INF39 intervention group than in the control group (P < 0.001). We also found that the expression of these genes was more pronounced in the TAC SLC26A4 lentiviral group (P < 0.001). However, protein expression levels of NLRP3 and IL-β were downregulated after treatment with the autophagy inhibitor 3-MA or transfection with sh-SLC26A4 lentiviral (Fig. 6).
Immunohistochemical analysis of NLRP3 and IL-1β protein expression levels. (A): Immunohistochemical analysis of NLRP3 and IL-1β protein expression levels. (B): The protein expression levels of NLRP3 and IL-1β. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group; Five rats were used in each group, and the results were analyzed by one-way ANOVA).
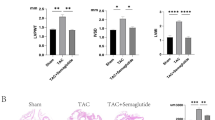
Then, we examined the expression levels of NLRP3 and IL-β mRNA and protein in rat heart tissues. RT-qPCR results (Fig. 7A and E) revealed that the expression levels of SLC26A4, ASC, NLRP3, caspase-1, and IL-1β in the TAC model group were significantly greater than those in the control group (P < 0.001), and they were more strongly increased after transfection with OE-SLC26A4 (P < 0.001). The SLC26A4, ASC, NLRP3, caspase-1, and IL-1β genes expression were reduced after 3-MA treatment (P < 0.001). However, after transfection with sh-SLC26A4 lentiviral, there was no significant difference in the expression of SLC26A4, ASC, NLRP3, caspase-1, or IL-1β between the TAC + sh-SLC26A4Mgroup and the control group (P > 0.05), and similar results were found for the TAC + sh-SLC26A4 + Rapa group (P > 0.05).
Expression levels of NLRP3 inflammasome pathway mRNAs and proteins. (A–E): The mRNA expression of SLC26A4 (A), ASC (B), NLRP3 (C), Caspase-1 (D) and IL-1β (E). (F–J): The protein expression levels of ASC (G), Caspase-1 (H), pro-Caspase-1 (I), and NLRP3 (J). (*P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group; Five rats were used in each group, and the results were analyzed by one-way ANOVA, Protein bands were cut from different sites).
We also observed that the protein expression levels of ACS, NLRP3, pro-caspase-1, and caspase-1 were more significant in the TAC model group than in the control group (P < 0.001). Treatment with 3-MA significantly decreased the expression of ACS, NLRP3, pro-caspase-1, and caspase-1 (P < 0.001). In addition, we observed that the expression levels of NLRP3 and pro-caspase-1 were significantly suppressed after sh-SLC26A4 lentiviral transfection (P < 0.001), whereas the expression levels of ASC and caspase-1 were not significantly different from those in the control group (P > 0.05). However, the protein expression of ASC and pro-caspase-1 was significantly increased after rapamycin treatment (P < 0.001), and that of NLRP3 was also significantly increased (P < 0.01) (Fig. 7F and J). In conclusion, these data suggest that SLC26A4 mediates the activation of the NLRP3 inflammasome to promote the progression of cardiac hypertrophy both in vivo and in vitro.
Discussion
As a classical signalling pathway protein that promotes pyroptosis, the NLRP3 inflammasome is one of the most intensively studied inflammasomes, consists of the NOD-like receptor, the adaptor protein (ASC), and the effector protein (caspase-1 [IL-1β-converting enzyme]). As reported in previous studies, caspase-1 splices and promotes the maturation of IL-1β, IL-18, and IL-3312. Various exogenous or endogenous factors can activate the NLRP3 inflammasome. The activation of the NLRP3 inflammasome by infection, ROS, injury, metabolites, and ATP promotes the activation of downstream target proteins that induce pyroptosis13,14,15,16. NLRP3 inflammasome may also be involved in the pathogenesis of cardiac hypertrophy; However, the molecular mechanisms by which the NLRP3 inflammasome mediate the development of cardiac hypertrophy remain unclear. In previous studies, autophagy and inflammasomes interact. Inflammasomes induce autophagy, similarly, autophagy also regulates inflammasome activation17,18,19,20.
Autophagy can negatively or positively regulate NLRP3 inflammasome activation. Additionally, the NLRP3 inflammasome reverses the effects of autophagy21,22. Liu et al. reported that acetylation of autophagy-related gene 5 (ATG5) inhibits autophagosome maturation23, whereas Sirtuin 3 (SIRT3) forms a complex with Atg5 in cells and inhibits the acetylation of endogenous Atg5, which promotes autophagosome maturation. However, the NLRP3 inflammasome was more abundant in SIRT3-deficient cells. These results suggest that autophagy negatively regulates NLRP3 inflammasome activation. Similarly, Chang et al.24 found that Resveratrol, a naturally occurring polyphenolic compound in plants, induces autophagy by activating the p38 gene, inhibiting the activation of the NLRP3 inflammasome in macrophages, and alleviating inflammatory responses. Zhou et al.25 found BBR inhibited the activation of the NLRP3 inflammasome through the upregulation of autophagy in macrophages. Reducing the level of beclin-1 in cells or adding an autophagy inhibitor significantly improved the inhibitory effect of autophagy on the NLRP3 inflammasome. However, Du Pont et al.26 found that autophagy positively regulates NLRP3 inflammasome activation. This study revealed that under starvation conditions, autophagy can induce caspase-1 activation through the Atg5-dependent nonclassical pathway, promote the activation of the NLRP3 inflammasome, and increase the maturation of proinflammatory cytokines, such as IL-1β and IL-18, which leads to pyroptosis. Moreover, the proinflammatory factors IL-1β and IL-18 are degraded due to the lack of a signal peptide, further exacerbating tissue inflammatory damage.
In conclusion, autophagy bidirectionally regulates the activation of the NLRP3 inflammasome, which depends on the specific cellular environment. Autophagy regulates inflammatory responses27, and inflammatory factors can induce autophagy28,29. Many studies have elucidated the effects of SLC26A4 on cardiac hypertrophy8,9,30,31,32, and its mechanism may be related to the regulation of autophagy. Therefore, we constructed cellular and rat models of cardiac hypertrophy to investigate whether SLC26A4 induces pathological cardiac hypertrophy by regulating autophagy and inflammasome activation.
In this study, SLC26A4 expression was associated with cardiomyocyte hypertrophy and cardiac structural alterations, and SLC26A4 activated autophagy to regulate NLRP3 inflammasome activation, thereby exacerbating cardiac hypertrophy. Our findings deepen the understanding of the role of the NLRP3 inflammasome signaling pathway in the pathological mechanism of cardiac hypertrophy. SLC26A4 regulates autophagy and activates the NLRP3 inflammasome to mediate cardiomyocyte hypertrophy, which may provide new ideas for early diagnosing of cardiac hypertrophy and drug development. Furthermore, cardiomyocytes modulate resident macrophage-mediated inflammation and diastolic dysfunction in patients with cardiomyopathy33. However, myocardial inflammation and function were significantly alleviated after intervention with the selective oxidase subunit 4 (NOX4) inhibitor GKT13783133. Therefore, whether the mechanism of SLC26A4-mediated cardiac hypertrophy is related to NOX4 will be of interest for subsequent studies.
However, some issues remain to be resolved. For example, the specific mechanism by which SLC26A4 regulates the NLRP3 signaling pathway needs to be further explored. Whether targeting the NLRP3 inflammasome pathway is beneficial for treating cardiac hypertrophy remains unclear, and further studies are needed to verify whether SLC26A4 modulates the NLRP3 pathway to prevent cardiac hypertrophy. In addition, it remains unknown whether autophagy promotes the activation of the NLRP3 inflammasome directly or through other pathways. However, investigating the regulatory relationship between autophagy and inflammasomes may provide new ideas for the treatment and prognosis of cardiac hypertrophy.
In summary, the results of this study demonstrated that SLC26A4 expression is related to cardiomyocyte hypertrophy, and its mechanism may be associated with the activation of the NLRP3 inflammasome pathway caused by the excessive promotion of autophagy by SLC26A4. Therefore, targeting the expression of SLC26A4 and inhibiting the activation of autophagy and the NLRP3 inflammasome pathway may provide a basis for new treatment options for cardiac hypertrophy.
Data availability
Data Availability Statement: The datasets supporting the conclusions of this article are included within the article (Due to the principle of confidentiality of the article, all data are reasonably accessible to corresponding authors).
References
Shimizu, I. & Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 97245–97262 (2016).
Adzika, G. K. et al. Pathological cardiac hypertrophy: The synergy of adenylyl cyclases Inhibition in cardiac and immune cells during chronic catecholamine stress. J. Mol. Med. (Berl) 97(7), 897–907 (2019).
Qiu, Z. et al. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J. Diabetes Res. 2019(8), 151836 (2019).
Bai, Y. et al. Caspase-1 regulate AngII-induced cardiomyocyte hypertrophy via upregulation of IL-1β. Biosci. Rep. 38(2), e6554 (2018).
Sun, M. et al. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 115(11), 1398–1407 (2007).
Zhen, Y. & Zhang, H. NLRP3 inflammasome and inflammatory bowel disease. Front. Immunol., 10276 (2019).
Pelzl, L. et al. DOCA sensitive Pendrin expression in kidney, heart, lung and thyroid tissues. Cell. Physiol. Biochem. 30(6), 1491–1501 (2012).
Han, X. et al. SLC26A4-AS1 aggravates AngII-induced cardiac hypertrophy by enhancing SLC26A4 expression. Arq. Bras. Cardiol. 120(4), e20210933 (2023).
Tang, L. et al. Inhibiting SLC26A4 reverses cardiac hypertrophy in H9C2 cells and in rats. PeerJ, 8e8253 (2020).
Ding, Z. et al. Total extract of Abelmoschus manihot L. alleviates uric acid-induced renal tubular epithelial injury via Inhibition of caspase-8/caspase-3/NLRP3/GSDME signaling. Front. Pharmacol. (8), e8253 (2020).
Di Cataldo, S., Ficarra, E. & Macii, E. Computer-aided techniques for chromogenic immunohistochemistry: Status and directions. Comput. Biol. Med. 42(10), 1012–1025 (2012).
Xie, Q. et al. Lipopolysaccharide/adenosine triphosphate induces IL–1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells. Int. J. Mol. Med. 34(1), 341–349 (2014).
Tartey, S. & Kanneganti, T. D. Differential role of the NLRP3 inflammasome in infection and tumorigenesis. Immunology 156(4), 329–338 (2019).
Minutoli, L. et al. ROS-Mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid. Med. Cell. Longev. 20162183026 (2016).
Zhang, Y. Z. et al. NLRP3 inflammasome and lipid metabolism analysis based on UPLC-Q-TOF-MS in gouty nephropathy. Int. J. Mol. Med. 44(1), 172–184 (2019).
Coll, R. C. et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome Inhibition. Nat. Chem. Biol. 15(6), 556–559 (2019).
Deretic, V., Saitoh, T. & Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13(10), 722–737 (2013).
Han, X. et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 15(11), 1860–1881 (2019).
Harris, J. et al. Autophagy and inflammasomes. Mol. Immunol., 8610–8615 (2017).
Tao, Y. et al. The role of autophagy and NLRP3 inflammasome in liver fibrosis. Biomed. Res. Int. 20207269150 (2020).
Biasizzo, M. & Kopitar-Jerala, N. Interplay between NLRP3 inflammasome and autophagy. Front. Immunol. 11591803 (2020).
Qiao, L. et al. Deficient Chaperone-Mediated autophagy promotes inflammation and atherosclerosis. Circ. Res. 129(12), 1141–1157 (2021).
Liu, P. et al. Sirtuin 3-induced macrophage autophagy in regulating NLRP3 inflammasome activation. Biochim. Biophys. Acta Mol. Basis Dis. 1864(3), 764–777 (2018).
Chang, Y. P. et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 230(7), 1567–1579 (2015).
Zhou, H. et al. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: A new mechanism linking Berberine to insulin resistance improvement. Biomed. Pharmacother, 89864–89874 (2017).
Dupont, N. et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. Embo J. 30(23), 4701–4711 (2011).
Wu, M. Y. & Lu, J. H. Autophagy and macrophage functions: inflammatory response and phagocytosis. Cells 9(1) (2019).
Hu, F. et al. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat. Commun. 12(1), 3651 (2021).
Jiang, G. M. et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer. 18(1), 17 (2019).
Kim, B. G. et al. Resistance to hypertension and high Cl(-) excretion in humans with SLC26A4 mutations. Clin. Genet. 91(3), 448–452 (2017).
Song, C. et al. Construction and analysis of cardiac hypertrophy-associated lncRNA-mRNA network based on competitive endogenous RNA reveal functional LncRNAs in cardiac hypertrophy. Oncotarget 7(10), 10827–10840 (2016).
Li, M. et al. Digenic inheritance of mutations in EPHA2 and SLC26A4 in Pendred syndrome. Nat. Commun. 11(1), 1343 (2020).
Vendrov, A. E. et al. Cardiomyocyte NOX4 regulates resident macrophage-mediated inflammation and diastolic dysfunction in stress cardiomyopathy. Redox Biol. 67102937 (2023).
Acknowledgements
Acknowledgments: The authors would like to thank the Joint Funds of the Zhejiang Provincial Natural Science Foundation of China Under Grant and Zhejiang Medical and Health Research Fund for their support, and all other authors for their contributions.
Funding
1.This research was supported by the Joint funds of the Zhejiang Provincial Natural Science Foundation of china Under Grant: No.BY21H250002. 2.Zhejiang Medical and Health Research Fund:2021KY057. 3.Zhejiang Medical and Health Research Fund:2022488544.
Author information
Authors and Affiliations
Contributions
L.T. conceived and designed the study. L.W., Q.T., conducted most of the experiments and data analysis, and wrote the manuscript. W.W. participated in collecting data and helped to draft the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Study approval statement: This study protocol was reviewed and approved by Zhejiang Academy of Agricultural Sciences Ethics Committee for the Welfare of Laboratory Animals, approval number [2021ZAASLA46].
Consent for publication
The authors declared that all participants consented to the publication of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, Lq., Wang, Ll., Tang, Qf. et al. SLC26A4 regulates autophagy and activates the NLRP3 inflammasome to mediate pathological cardiac hypertrophy. Sci Rep 15, 12511 (2025). https://doi.org/10.1038/s41598-025-97874-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97874-8