Abstract
Hyperlipidemia, characterized by dysregulated lipid metabolism, is a major risk factor for cardiovascular diseases and is often accompanied by oxidative stress. This study aimed to investigated the protective effects and underlying mechanisms of Marein, a primary active flavonoid from Coreopsis tinctoria, in an H2O2-induced oxidative stress model using HepG2 cells. HepG2 cells was exposed to H2O2 to induce oxidative stress and lipid accumulation, followed by Marein intervention. Cell viability, reactive oxygen species (ROS) levels, and lactate dehydrogenase (LDH) release was assessed using CCK-8, fluorescence microscopy, and ELISA, respectively. Oxidative stress markers, including malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px), as well as lipid profiles (TC, TG, LDL-C, and HDL-C), were measured. The expression levels of SIRT1, Nrf2, and lipid metabolism-related genes (HMGCR, LDLR) were determined via RT-qPCR and Western blot analysis. The results revealed that Marein treatment significantly restored cell viability, reduced LDH release, and improved antioxidant capacity by lowering ROS and MDA levels while enhancing SOD and GSH-Px activities. Additionally, Marein intervention significantly mitigated lipid accumulation, evidenced by reduced by TC, TG, and LDL-C levels and increased HDL-C levels. Mechanistically, Marein activated the Sirtuin-1 (SIRT1)/Nuclear factor-erythroid-2-related factor 2 (Nrf2) signaling, which was confirmed by the reversal of its protective effects upon treatment with EX-527 (a specific SIRT1 inhibitor). These findings suggested that Marein exerted its antioxidative and lipid-lowering effects via the SIRT1/Nrf2 signaling, highlighting its potential as a therapeutic candidate for hyperlipidemia and related metabolic disorders.
Similar content being viewed by others
Introduction
Hyperlipidemia is a systemic lipid metabolism disorder characterized by elevated levels of low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and total cholesterol (TC), often accompanied by reduced levels of high-density lipoprotein cholesterol (HDL-C) in plasma1. This metabolic imbalance significantly increases the risk of cardiovascular disease (CVD), including atherosclerosis, myocardial infarction, and stroke, and has become one of the leading causes of morbidity and mortality worldwide2. Epidemiological studies have shown that individuals with hyperlipidemia are approximately twice as likely to develop CVD compared to those with normal lipid profiles, highlighting its critical role as a modifiable risk factor3. The rising prevalence of hyperlipidemia, driven by overnutrition, sedentary lifestyles, and obesity, is particularly concerning among younger populations, underscoring the urgent need for effective prevention and treatment strategies4. While statins are the first-line pharmacological agents for managing hyperlipidemia, their long-term use poses risks of liver and kidney damage, necessitating safer and more effective therapeutic alternatives5.
Traditional Chinese medicine has the advantages of non-toxicity and no side effects in the treatment of existing diseases, and its application is more and more extensive6 Coreopsis tinctoria Nutt., a wild alpine medicinal plant, is known for its flavonoid-rich profile and demonstrated pharmacological effects, including anti-inflammatory, anti-hypertensive, antioxidant, and lipid-lowering, properties7,8,9. Marein, a primary chalcone glucoside derived from Coreopsis tinctoria Nutt., has shown potential in mitigating oxidative stress and modulating lipid metabolism10,11,12,13. However, its specific role and mechanisms in hyperlipidemia remain poorly understood.
Oxidative stress, driven by excessive reactive oxygen species (ROS), disrupts lipid homeostasis and aggravates hyperlipidemia14,15,16,17. Sirtuin-1 (SIRT1) belongs to the nicotinamide (NAD+)-dependent class III histone deacetylase sirtuin family, the activation of which can greatly decrease ROS level and promote cell survival18. Nuclear factor-erythroid-2-related factor 2 (Nrf2) is a transcription factor involved in cell defense against oxidative stress, and it is also an activation target downstream of SIRT119. It has been revealed that the activation of SIRT1/Nrf2 pathway could reduce mitochondrial dysfunction and lipid accumulation in hepatocytes20. Although Marein has been reported to inhibit ROS generation, its ability to activate the SIRT1/Nrf2 pathway and alleviate lipid accumulation requires further exploration.
This study aimed to investigate the biological effects of Marein on oxidative stress and lipid metabolism in an H2O2-induced HepG2 cell model. We hypothesize that Marein exerts protective effects by activating the SIRT1/Nrf2 signaling pathway, providing novel insights into its potential as a therapeutic agent for hyperlipidemia.
Materials and methods
Cell culture and treatment
Human hepatoma cell line HepG2 was purchased from Cell Bank of the Chinese Academy of Sciences (Beijing, China) and cultured in DMEM medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin at 37 °C and 5% CO2. After reaching 60% confluence, HepG2 cells were exposed to 500 μM H2O2 for 6 h to induce oxidative stress. Besides, HepG2 cells was incubated with 5 μM Marein (≥ 96% purity, Shanghai Yuanye Bio-Technology Co., Ltd., China) for 24 h10. For inhibitor experiments, cells were pre-treated with SIRT1 inhibitor EX-527 (100 nM, #A10377, AdooQ BioScience, Nanjing, China) for 2 h before Marein treatment.
Cell viability assay
HepG2 cells were seeded in 96-well plates (1 × 104 cells/well). After treatments, each group was added with 10 μL Cell Counting Kit-8 (CCK‐8, #CK04, Dojindo Laboratories, Kumamoto, Japan) and incubated for 2 h. OD450 value was measured utilizing a microplate reader (Bio_Rad, Hercules, CA, USA).
Lactate dehydrogenase (LDH) release assay
LDH diagnostic kit (#A020-2-2, Jiancheng Bioengineering, Nanjing, Jiangsu, China) was adopted to assess LDH activity according to manufacturer’s protocol. In brief, HepG2 cells (1 × 105/well) were seeded in 24-well plates. After different treatment, cell supernatants were collected for LDH measurement at 450 nm utilizing a microplate photometer (Perkin Elmer, Waltham, MA, USA).
ROS detection
Intracellular ROS was determined by fluorescent probe DCFH-DA (Sigma, St. Louis, MO, USA). Briefly, HepG2 cells were harvested, washed with PBS, and incubated with 10 μM DCF-DA solution for 30 min at 37 °C in the dark. Subsequently, cell images were carried out under a fluorescence microscope (Olympus, Tokyo, Japan). Fluorescence was quantified using Image J (NIH, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Glutathione peroxidase (GSH-Px) detection kits were acquired from Nanjing JianCheng Bioengineering Institute, Nanjing, China) for the measurement of corresponding factor according to manufacturer’s protocols. Triacylglycerol (TG), total cholesterol (TC), HDL cholesterol (HDL-C), and LDL cholesterol (LDL-C) levels in cell supernatant were assayed utilizing enzymatic assay kits (JianCheng Bioengineering Institute). Each experiment was conducted in triplicate.
Quantitative reverse transcription PCR (RT-qPCR)
Total RNA from cells was extracted by TRIzol (#15596018CN, Invitrogen, USA). Complementary DNA was synthesized by conversion of total RNA utilizing a cDNA Synthesis Kit (#6210, TaKaRa, Dalian, China). RT-qPCR was conducted utilizing QuantiTect SYBR-Green PCR kit (#204,143, Qiagen, CA, USA) on an ABI-7500 real-time PCR system (ABI, Warrington, UK). The 2−∆∆Ct method was adopted to calculate gene expression, with GAPDH as internal control. Primers used in this study was listed in Table 1.
Western blot
Protein lysate was obtained using RIPA lysis buffer (#89,901, Thermo Fisher Scientific, MA, USA). Protein concentration was measured utilizing a BCA Kit (#23,227, Thermo Fisher Scientific, MA, USA). An equal amount of protein samples was loaded on 10% SDS-PAGE gel and transferred to PVDF membranes (Roche, Basel, Switzerland). After blockage with 5% fat-free milk, membranes were probed with primary antibodies at 4 °C overnight: HMGCR (#PA5-37,367, 1:1000, Invitrogen), LDLR (#ab52818, 1:1000, Abcam), SIRT1 (#ab189494, 1:1000, Abcam), Nrf2 (#ab62352, 1:1000, Abcam). After TBST washing, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (#7074, 1:1,000, Cell Signaling Technology) for 1 h, followed by detection of protein bands with enhanced chemiluminescence (ECL) kit (Thermo Fisher Scientific). Gray value was calculated by ImageJ software (NIH, MD, USA). β-actin was probed as internal reference.
Immunofluorescence staining
The nuclear translocation of Nrf2 was evaluated using immunofluorescence staining. HepG2 cells were seeded on glass coverslips in 24-well plates (1 × 105 cells/well) and treated as described above. After treatment, cells were fixed with 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS for 10 min. Subsequently, cells were blocked with 5% bovine serum albumin (BSA) in PBS for 30 min, followed by incubation with primary anti-Nrf2 antibody (ab62352, 1:200 dilution, Abcam, USA) at 4 °C overnight. The next day, cells were washed with PBS and incubated with an Alexa Fluor 488-conjugated secondary antibody (1:500 dilution, Invitrogen, USA) in the dark for 1 h at room temperature.
DAPI (4',6-diamidino-2-phenylindole) was used to counterstain nuclei for 5 min. Coverslips were mounted onto glass slides using antifade mounting medium (Thermo Fisher Scientific, USA). Images were captured using a fluorescence microscope (Olympus, Tokyo, Japan), and fluorescence intensity was analyzed using ImageJ software (NIH, MD, USA).
Statistical analysis
Data were analyzed utilizing SPSS 19.0 software (IBM, Armonk, NY, USA) and expressed as standard deviation (SD).Differences between two groups were evaluated with Student’s t-test. Comparisons between multiple groups were performed with one-way ANOVA followed by Tukey’s post-hoc tests. p < 0.05 was considered statistically significant. All experiments were repeated at least three independent times.
Results
Marein alleviated H2O2-induced oxidative stress in HepG2 cells
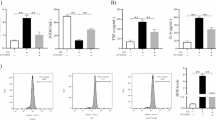
We first investigated whether Marein could mitigate oxidative stress induced by H2O2 in HepG2 cells. CCK-8 assay showed that H2O2 exposure significantly inhibited cell viability (p < 0.01), while LDH release, measured as an indicator of cell membrane integrity and necrotic cell death, was markedly increased (p < 0.05), indicating oxidative damage. Marein treatment effectively reversed these effects, restoring cell viability and reducing LDH release (p < 0.05) (Fig. 1A,B). Additionally, ELISA assay demonstrated that H2O2 elevated MDA level while suppressing the antioxidant enzymes SOD and GSH-Px (p < 0.01). However, these changes were dramatically overturned by Marein (p < 0.05) (Fig. 1C–E). Moreover, DCFH-DA staining revealed a substantial increase in intracellular ROS levels in response to H2O2 treatment, which was markedly ameliorated after Marein intervention (p < 0.001) (Fig. 1F). These data suggested that Marein effectively alleviated oxidative stress induced by H2O2, indicating its potent antioxidant properties in HepG2 cells.
Marein administration alleviated H2O2-induced oxidative stress in HepG2 cells. HepG2 cells were exposed 500 μM H2O2 condition for 24h, and then administrated by 5 μM Marein for another 24h. (a) CCK-8 detection was used to examine cell viability. (b) LDH content was detected to examine oxidative stress injury. (c–e) MDA (c), SOD (d), and GSH-Px (e) levels were detected by ELISA. (f) DCFDA detected ROS level. Data are represented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Marein suppressed H2O2-induced lipid metabolism disorders in HepG2 cells
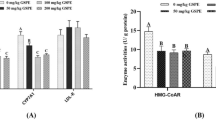
Next, we examined whether Marein could alleviate lipid metabolism disorders induced by H2O2 in HepG2 cells. Compared with the control group, H2O2 treatment led to significant increases in TC, TG, and LDL-C levels (p < 0.01 or p < 0.05), along with a decrease in HDL-C level (p < 0.001), indicating disrupted lipid metabolism. However, Marein administration reversed these effects by reducing TC, TG, and LDL-C levels and increasing HDL-C levels to near-normal levels (p < 0.05) (Fig. 2A–D). At the molecular level, H2O2 treatment increased the mRNA and protein expression of lipid metabolism-related genes including HMGCR and LDLR compared to the control group (p < 0.01 or p < 0.001), while Marein significantly overturned these trends (p < 0.05) (Fig. 2E–G). The results demonstrated that Marein effectively alleviated H2O2-induced lipid metabolism disorders by reducing lipid accumulation and modulating lipid-related gene expression in HepG2 cells.
Marein suppressed H2O2-induced lipid metabolism disorders in HepG2 cells. HepG2 cells were exposed 500μM H2O2 condition for 24h, and then administrated by 5 μM Marein for another 24h. (a–d) Determination of TC (a), TG (b), LDL-C (c), and HDL-C (d) by ELISA. (e, f) Detection of lipid metabolism related genes HMGCR (e) and LDLR (f) by RT-qPCR. (g) Western blot analysis of HMGCR and LDLR protein levels. Values were expressed as mean ± SD of three separate determinations. *P < 0.05, **P < 0.01, ***P < 0.001.
Marein activated SIRT1/Nrf2 signaling in H2O2-induced HepG2 cells
To investigate the mechanism underlying Marein’s protective effects, we assessed its role in activating SIRT1/Nrf2 signaling in H2O2-treated HepG2 cells. RT-qPCR analysis revealed that H2O2 significantly reduced the mRNA expression levels of SIRT1 and Nrf2 compared to the control group (p < 0.01), whereas Marein treatment markedly restored their expression (p < 0.05) (Fig. 3A,B). Similarly, Western blot analysis revealed that the protein levels of SIRT1 and Nrf2 were downregulated following H2O2 exposure (p < 0.01 or p < 0.001), and Marein administration significantly upregulated these protein levels (p < 0.05) (Fig. 3C).
Marein restored SIRT1/Nrf2 signaling in H2O2-induced HepG2 cells. HepG2 cells were exposed 500μM H2O2 condition for 24h, and then administrated by 5 μM Marein for another 24h. (a, b) mRNA expression levels of SIRT1 (a) and Nrf2 (b) were assessed using RT-qPCR. (c) Western blot analysis of SIRT1 and Nrf2 protein levels. (d) Immunofluorescence staining of Nrf2 nuclear localization (scale bar = 25 μm). Data are represented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Furthermore, immunofluorescence staining (Fig. 3D) revealed that Nrf2 was predominantly localized in the nucleus under normal conditions, indicating its active role in oxidative stress defense. However, upon H2O2 stimulation, Nrf2 localization shifted to the cytoplasm, suggesting impaired nuclear translocation and reduced transcriptional activity. Notably, Marein treatment facilitated Nrf2 nuclear translocation, restoring its nuclear presence to levels comparable to the control group.
Collectively, these results indicated that Marein exerted its antioxidant properties by activating the SIRT1/Nrf2 signaling in H2O2-triggered HepG2 cells.
Inhibition of SIRT1/Nrf2 reversed the antioxidant effects of Marein in H2O2-induced HepG2 cells
To address whether Marein could alleviate H2O2-induced oxidative stress in HepG2 cells through SIRT1/Nrf2 signaling, we used a specific inhibitor of SIRT1 (EX-527) to selectively inactivate SIRT1/Nrf2 signaling. Compared with H2O2 group, Marein treatment increased cell viability and reduced LDH release (p < 0.05), while the biological function of Marein was significantly eliminated after inhibiting SIRT1/Nrf2 pathway using EX-527 (p < 0.05) (Fig. 4A,B). Additionally, compared with H2O2 group, Marein treatment reduced MDA and ROS levels and increased SOD and GSH-PX activities (p < 0.01 or p < 0.001), which were greatly reversed after SIRT1/Nrf2 pathway inhibition (p < 0.05) (Fig. 4C–F). These results suggested that SIRT1/Nrf2 signaling was essential for the antioxidant effects of Marein in HepG2 cells.
Inactivation of SIRT1/Nrf2 reversed the antioxidant ability of marein in H2O2-induced HepG2 cells. HepG2 cells were subjected to different groups: control, H2O2, H2O2 + Marein, H2O2 + Marein + EX-527 (SIRT1 inhibitor). (a) CCK-8 detection was used to examine cell viability. (b) LDH content was detected to examine oxidative stress injury. (c–e) MDA (c), SOD (d), and GSH-Px (e) levels were detected by ELISA. (f) DCFDA detected ROS level. Data are represented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
SIRT1/Nrf2 signaling was critical for Marein’s regulation of H2O2-triggered lipid accumulation
To explore whether marein’s regulation of lipid metabolism under oxidative stress is mediated by the SIRT1/Nrf2 signaling, HepG2 cells were treated with H2O2, Marein, and the SIRT1 inhibitor EX-527. ELISA results showed that H2O2 significantly increased the levels of TC, TG, and LDL-C while decreasing HDL-C levels compared to the control group (p < 0.01 or p < 0.05, Fig. 5A–D). Marein treatment significantly reversed these changes (p < 0.05), indicating its protective role in lipid metabolism. However, the co-treatment with EX-527 treatment ameliorated Marein’s effects (p < 0.05) (Fig. 5A–D).
Inactivation of SIRT1/Nrf2 restrained protective role of marein in H2O2-triggered lipid accumulation. HepG2 cells were subjected to different groups: control, H2O2, H2O2 + Marein, H2O2 + Marein + EX-527 (SIRT1 inhibitor). (a–d) Determination of TC (a), TG (b), LDL-C (c), and HDL-C (d) levels by ELISA. (e, f) Detection of lipid metabolism related genes HMGCR (e) and LDLR (f) by RT-qPCR. (g) Western blot analysis of HMGCR and LDLR protein levels. Values were expressed as mean ± SD of three separate determinations. *P < 0.05, **P < 0.01, ***P < 0.001.
At the molecular level, RT-qPCR analysis revealed that Marein significantly downregulated the expression of HMGCR and LDLR (p < 0.01), but these effects were abolished upon co-treatment with the pathway inhibitor EX-527 (p < 0.01) (Fig. 5E,F). Consistent with the mRNA results, Western blot analysis demonstrated that Marein reduced the protein expression of HMGCR and LDLR, which was also reversed by EX-527 (p < 0.05, Fig. 5G). These findings confirmed that SIRT1/Nrf2 signaling was a critical mediator of marein’s ability to mitigate lipid metabolism disorders under oxidative stress.
Discussion
This study demonstrates that Marein, a primary flavonoid from Coreopsis tinctoria Nutt., effectively alleviates oxidative stress and lipid metabolism disorders induced by H2O2 in HepG2 cells. These protective effects are mediated by the activation of the SIRT1/Nrf2 signaling pathway, providing new insights into the therapeutic potential of marein for hyperlipidemia.
Given the central role of oxidative stress in hyperlipidemia-related lipid metabolism disorders, HepG2 cells were selected as a well-established hepatic model for this study. HepG2 cells retain many of the metabolic functions of hepatocytes, including lipid metabolism, oxidative stress responses, and antioxidant defense mechanisms, making them widely used in studies investigating oxidative stress-induced hepatic dysfunction21,22. Moreover, H2O2-induced oxidative damage in HepG2 cells is a well-characterized model for mimicking oxidative stress-related lipid metabolic disturbances23.
Hyperlipidemia is a lipid metabolic disease caused by abnormal lipid metabolism. Excessive energy intake, obesity, and a series of obesity-associated factors results in an increase of oxidative stress17. Likewise, oxidative stress was also proved to be an important factor that led to abnormal lipid metabolism and lipid accumulation. Seo et al. described that ROS was gradually accumulated with aging, which accompanied by cholesterol and glucose intake, as well as cholesterol synthesis related genes (GLUT2, GK, SREBP2, HMGCR, and HMGCS) in vitro, and H2O2 exposure has been shown to promote glycolysis and lipid synthesis in vitro24, a process closely associated with SREBP1c activation17.
Several natural compounds have demonstrated lipid-lowering and antioxidative effects through targeting oxidative stress-mediated metabolic dysfunctions. Inonotus hispidus ameliorated hyperlipidemia through inhibiting oxidative stress and inflammation via Nrf2/NF-κB signaling in high fat diet fed mice25. Kaempferol-3-O-Glucuronide inhibited cholesterol-diet-induced non-alcoholic steatohepatitis via repressing oxidative stress26. In the current study, our findings indicated that treatment with Marein effectively protected HepG2 cells from H2O2-induced oxidative stress, reducing ROS levels and preventing lipid accumulation, which is consistent with a recent study reporting Marein’s lipid-lowering effects and its role in modulating lipid metabolism27. Notably, while both studies highlight Marein’s potential in regulating lipid accumulation, our research provides novel mechanistic insights by demonstrating that Marein exerts its effects through SIRT1/Nrf2 activation in an oxidative stress-induced model. Basis on these findings, we concluded that the protective roles of marein on lipid accumulation might be relied on the inhibition of oxidative stress induced by H2O2.
SIRT1 is a key metabolic sensor that modulates antioxidant defense, mitochondrial function, and lipid homeostasis, making it a crucial target for studying the protective effects of marein24. Oxidative stress is a well-recognized driver of lipid metabolism disorders, and SIRT1 has been extensively studied for its role in alleviating oxidative damage and improving lipid homeostasis. Studies have found that overexpression of SIRT1 enhanced the phosphorylation of AMPK and further inhibited lipid accumulation25 In addition, SIRT1 up-regulated FoxO1 and PGC-1α to increase liver antioxidant function28. In the context of oxidative stress-induced hepatic dysfunction, SIRT1 plays a critical role in reducing ROS levels and promoting cell survival. Previous studies have demonstrated that SIRT1 activation protects HepG2 cells from H2O2-induced oxidative damage, supporting its relevance as a mechanistic target in this study29. Furthermore, Nrf2, a key regulator of cellular oxidative stability, is a well-established downstream target of SIRT1, linking SIRT1 activity to antioxidant defense mechanisms19. Evidence from Nrf2 knockout models has shown that ROS levels were significantly increased in the liver and spleen, exacerbating oxidative damage, while inhibition of the Nrf2/HO-1 axis has been associated with lipid accumulation and metabolic dysfunction30,31
Given that Marein is hypothesized to function as an antioxidant compound, it is crucial to determine whether its effects are mediated through SIRT1-driven Nrf2 activation, as this pathway is essential for triggering antioxidant defense mechanisms. In addition, it was reported that Nrf2 activation promotes nuclear translocation and binding to small Maf proteins, which subsequently trigger antioxidant/electrophile response elements (ARE) and upregulate detoxification enzymes such as HO-1 and NQO132. This signaling cascade plays a key role in counteracting oxidative stress-induced lipid dysregulation, further highlighting the significance of the SIRT1/Nrf2 signaling in metabolic homeostasis.
Consistent with these findings, our results demonstrated that H2O2 stimulation significantly reduced SIRT1 and Nrf2 expression in HepG2 cells, leading to increased oxidative stress and lipid accumulation. Treatment with Marein effectively restored SIRT1 and Nrf2 levels, mitigating oxidative damage and preventing lipid deposition. Furthermore, co-treatment with EX-527, a selective SIRT1 inhibitor, abolished Marein’s protective effects, confirming that SIRT1 activation is essential for Marein’s antioxidant and lipid-lowering properties. These results suggest that SIRT1 acts as a critical upstream regulator of Nrf2 in this model, and its activation by Marein enhances cellular antioxidant capacity and lipid homeostasis. Moreover, the observed downregulation of HMGCR and LDLR following Marein treatment suggests that it may influence lipid metabolism not only through oxidative stress modulation but also via regulation of cholesterol synthesis and uptake pathways. Future studies are warranted to investigate whether Marein modulates other metabolic pathways to provide a more comprehensive understanding of its lipid-lowering effects.
The rising prevalence of hyperlipidemia and oxidative stress-related metabolic disorders underscore the urgent need for safer and more effective therapeutic options. While statins remain the effective lipid-lowering agents, their long-term use is often limited by adverse effects such as hepatotoxicity and myopathy32. Marein, as a natural flavonoid with minimal toxicity, presents a promising alternative or adjunctive therapy. Given its ability to activate the SIRT1/Nrf2 pathway, Marein may not only ameliorate oxidative stress but also regulate lipid metabolism and mitochondrial function, making it a potential candidate for treating non-alcoholic fatty liver disease (NAFLD), hyperlipidemia, and cardiovascular diseases. Future studies should evaluate marein’s efficacy in vivo, particularly in animal models of diet-induced hyperlipidemia, to validate its therapeutic potential. Moreover, exploring its pharmacokinetics, bioavailability, and long-term safety profile will be crucial for clinical translation.
Despite the promising findings, this study has several limitations. First, the experiments were conducted solely in an in vitro model using HepG2 cells, which may not fully replicate the complex physiological environment in vivo. Future studies should validate these findings in normal hepatocytes or additional liver cancer cell lines to improve the translatability of the results. Second, the study lacks a hyperlipidemic model, limiting its ability to assess Marein’s lipid-lowering effects under pathological conditions. Future research should incorporate free fatty acid-induced lipid accumulation models or high-fat diet-induced hyperlipidemic animal models to better evaluate marein’s metabolic fate, bioavailability, and therapeutic potential. Third, only a single concentration of Marein was used, necessitating a dose–response analysis to determine its optimal range. Fourth, the absence of both a Marein-only group and a positive control for SIRT1/Nrf2 activation limits the ability to assess Marein’s baseline effects in the absence of oxidative stress and to validate its mechanistic role. Future studies should include Marein-only and positive control groups for comparison. Additionally, while the study focuses on SIRT1/Nrf2 activation, it does not comprehensively assess downstream targets such as HO-1, NQO1, or lipid metabolism regulators (e.g., SREBP-1c, PPARα, CPT1), which would also provide deeper mechanistic insights. Future research should further explore additional metabolic pathways, pharmacokinetics, and potential synergistic effects with existing lipid-lowering agents to enhance its clinical relevance.
In conclusion, this study suggested that Marein could rescue oxidative stress damage and lipid accumulation caused by H2O2 in HepG2 cells, possibly through activating SIRT1/Nrf2 pathway. These findings not only provide novel mechanistic insights into Marein’s pharmacological actions but also suggest its potential as a therapeutic candidate for hyperlipidemia and related metabolic disorders. Future studies should focus on validating these results in vivo and exploring its clinical applications.
Data availability
All data generated or analyzed are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- DAPI:
-
40,6-Diamidino-2-phenylindole
- AREs:
-
Antioxidant response elements
- CVD:
-
Cardiovascular disease
- CCK-8:
-
Cell Counting Kit-8
- ECL:
-
Chemiluminescence
- ELISA:
-
Enzyme-linked immunosorbent assay
- HDL-C:
-
HDL cholesterol
- HRP:
-
Horseradish peroxidase
- IF:
-
Immunofluorescence staining
- Keap1:
-
Kelch-like ECH-associated protein 1
- LDH:
-
Lactate dehydrogenase
- LDL-C:
-
LDL cholesterol
- MDA:
-
Malondialdehyde
- Nrf2:
-
Nuclear factor-erythroid-2-related factor 2
- ROS:
-
Reactive oxygen species
- SIRT1:
-
Sirtuin-1
- SD:
-
Standard deviation
- TC:
-
Total cholesterol
- TG:
-
Triacylglycerol
References
Stewart, J. et al. Hyperlipidemia. Pediatr. Rev. 41(8), 393–402 (2020).
Yao, Y. S., Li, T. D. & Zeng, Z. H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 19(1), 23 (2020).
Liu, T., Zhao, D. & Qi, Y. Global trends in the epidemiology and management of dyslipidemia. J. Clin. Med. 11(21), 6377 (2022).
Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Managed Care 23(9 Suppl), S139 (2017).
Singh, S. et al. Insights into the pivotal role of statins and its nanoformulations in hyperlipidemia. Environ. Sci. Pollut. Res. 29(51), 76514–76531 (2022).
Wang, J., Wong, Y. K. & Liao, F. What has traditional Chinese medicine delivered for modern medicine?. Expert. Rev. Mol. Med. 20, e4 (2018).
Du, D. et al. Protective effects of flavonoids from Coreopsis tinctoria Nutt. on experimental acute pancreatitis via Nrf-2/ARE-mediated antioxidant pathways. J. Ethnopharmacol. 224, 261–272 (2018).
Li, Y. et al. Flavonoids furom Coreopsis tinctoria adjust lipid metabolism in hyperlipidemia animals by down-regulating adipose differentiation-related protein. Lipids Health Dis. 13, 1–8 (2014).
Ren, Z. et al. Coreopsis tinctoria modulates lipid metabolism by decreasing low-density lipoprotein and improving gut microbiota. Cell. Physiol. Biochem. 48(3), 1060–1074 (2018).
Jiang, B. et al. Protective effects of marein on high glucose-induced glucose metabolic disorder in HepG2 cells. Phytomedicine 23(9), 891–900 (2016).
Jiang, B. et al. Marein protects against methylglyoxal-induced apoptosis by activating the AMPK pathway in PC12 cells. Free Radic. Res. 50(11), 1173–1187 (2016).
Niu, G. et al. Marein ameliorates Ang II/hypoxia-induced abnormal glucolipid metabolism by modulating the HIF-1alpha/PPARalpha/gamma pathway in H9c2 cells. Drug Dev. Res. 82(4), 523–532 (2021).
Yao, M. et al. Marein protects human nucleus pulposus cells against high glucose-induced injury and extracellular matrix degradation at least partly by inhibition of ROS/NF-κB pathway. Int. Immunopharmacol. 80, 106126 (2020).
Bai, J. et al. Oxidative stress contributes to abnormal glucose metabolism and insulin sensitivity in two hyperlipidemia models. Int. J. Clin. Exp. Pathol. 8(10), 13193–13200 (2015).
Frijhoff, J. et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 23(14), 1144–1170 (2015).
Newsholme, P. et al. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 473(24), 4527–4550 (2016).
Sekiya, M. et al. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 375(4), 602–607 (2008).
Hori, Y. S. et al. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS ONE 8(9), e73875 (2013).
Chen, Z. et al. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem. Pharmacol. 177, 113951 (2020).
Chen, Q. & Lou, Y. G protein-coupled receptor 39 alleviates mitochondrial dysfunction and hepatocyte lipid accumulation via SIRT1/Nrf2 signaling. J. Bioenerg. Biomembr. 55(1), 33–42 (2023).
Yu, Q. et al. Aged Pericarpium Citri Reticulatae “Chachi” attenuates oxidative damage induced by tert-Butyl Hydroperoxide (t-BHP) in HepG2 cells. Foods 11(3), 273 (2022).
Peng, S. et al. Alleviating effect of lipid phytochemicals in seed oil (Brassica napus L.) on oxidative stress injury induced by H(2)O(2) in HepG2 cells via Keap1/Nrf2/ARE signaling pathway. Nutrients 16(17), 2820 (2024).
Zhang, A. et al. Peptides derived from casein hydrolyzed by Lactobacillus: Screening and antioxidant properties in H2O2-induced HepG2 cells model. J. Funct. Foods 117, 106221 (2024).
Seo, E. et al. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell 18(2), e12895 (2019).
Zhang, Y. et al. Inonotus hispidus protects against hyperlipidemia by inhibiting oxidative stress and inflammation through Nrf2/NF-κB signaling in high fat diet fed mice. Nutrients 14(17), 3477 (2022).
Deng, Y. et al. Kaempferol-3-O-glucuronide ameliorates non-alcoholic steatohepatitis in high-cholesterol-diet-induced larval zebrafish and HepG2 cell models via regulating oxidation stress. Life (Basel) 11(5), 445 (2021).
Zhang, P. P. et al. Marein reduces lipid levels via modulating the PI3K/AKT/mTOR pathway to induce lipophagy. J. Ethnopharmacol. 312, 116523 (2023).
Zang, Y. et al. Improvement of Lipid and Glucose metabolism by capsiate in palmitic acid-treated HepG2 cells via activation of the AMPK/SIRT1 signaling pathway. J. Agric. Food Chem. 66(26), 6772–6781 (2018).
Tian, L. et al. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 139(4), 352–360 (2019).
Hu, Y. et al. Protective efficacy of carnosic acid against hydrogen peroxide induced oxidative injury in HepG2 cells through the SIRT1 pathway. Can. J. Physiol. Pharmacol. 93(8), 625–631 (2015).
Han, K. et al. Nrf2 knockout altered brain iron deposition and mitigated age-related motor dysfunction in aging mice. Free Radic. Biol. Med. 162, 592–602 (2021).
Suzuki, T. & Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 88(Pt B), 93–100 (2015).
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
This study is supported by Social Charity Science and Technology Research Project of Zhongshan (2022B1162), the Science and Technology Development Fund of the Macau SAR (SKL-QRCM(UM)) and the Open Research Project of Macau Institute for Translational Medicine and Innovation of the University of Macau (No.SKL-QRCM-MITMI-ORP2302).
Author information
Authors and Affiliations
Contributions
X.C., and L.Zhang. conceived and designed the research; R.L., Y.K. and Y.C. performed the research and acquired the data, Y.X., L.Zhao., and L.Zhang. analyzed and interpreted the data. All authors were involved in drafting and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, L., Liu, R., Kang, Y. et al. Marein from Coreopsis tinctoria Nutt. alleviates oxidative stress and lipid accumulation via SIRT1/Nrf2 signaling. Sci Rep 15, 18761 (2025). https://doi.org/10.1038/s41598-025-97964-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97964-7