Abstract
Lake Manzala is the largest northern lake in Egypt and receives significant quantities of wastewater. This study was conducted in 2015 and 2022 (before and after dredging) to assess changes in physicochemical parameters [transparency, salinity, dissolved oxygen (DO), chemical oxygen demand (COD), biological oxygen demand (BOD), and nutrients] as well as the community composition of zooplankton in relation to the dredging process. Water quality parameters, particularly salinity, classified the different lake sites into three groups (north, middle, and south) in 2015 and two groups (north-middle and south) in 2022. The highest values for transparency, salinity, and DO were recorded in the northern sector, while the highest values for BOD, COD, and nutrients were found in the southern sector. A total of 43 zooplankton species were identified in 2015, compared to 31 species in 2022. Notably, the number of saline species increased in 2022 to 12 species, and their distribution extended into the northern and middle sectors. Additionally, principal component analysis (PCA) revealed that zooplankton species could be divided into saline and freshwater groups. The study concluded that the chemical parameters and zooplankton composition in 2022 differed significantly from those in 2015 due to dredging, which altered the lake’s ecology by increasing salinity and reducing nutrient levels, particularly in the northern and middle regions.
Similar content being viewed by others
Introduction
The northern coastline of Egypt features five lakes connected to the Mediterranean Sea. These lakes serve as a significant source of fish production, contributing over 50% of Egypt’s total fisheries output in the 1980s. However, between 2015 and 2020, this contribution declined to less than 11%1. Lakes are particularly vulnerable to rapid environmental changes resulting from various human activities, which lead to decreased water quality and increased eutrophication. This situation is evident in the Mediterranean lakes of Egypt, which receive substantial amounts of untreated industrial, domestic, and agricultural wastewater2,3,4,5,6. As a result of these human activities, these coastal lakes have suffered severe ecological damage, leading to a decline in fish production. Lake Manzala is the largest lake among the northern coastal lakes of Egypt, covering a surface area of approximately 572.41 km27,8. It is recognized as one of the most significant wintering and nesting sites for various species of migratory birds9. Additionally, Lake Manzala is expected to play a vital role in mitigating the impacts of climate change. It is also essential for protecting coastal cities from storm surges and flooding. Furthermore, Lake Manzala is invaluable to the biodiversity of the Mediterranean region10. Moreover, it functions as a naturally occurring oxidation basin, acting as a buffer zone to prevent saline coastal water from infiltrating groundwater and agricultural fields, while also serving as a natural barrier between the drainage systems of the Mediterranean Sea and the Nile Delta11.
There are two main sources of water for Lake Manzala: (1) Saline water enters the lake from the Mediterranean Sea to the north through narrow channels, specifically the Al-Gamil, Ashtoum al-Gamil, and al-Sofara outlets12. Seawater flows into the lake through the al-Sofara outlets, located in the northwest corner, via several openings, with El-Boghdady being the most significant. (2) Freshwater sources enter the lake through numerous drains and pumping stations to the south, particularly Bahr al-Baqar, Hadous, Al-Mataria, Faraskur, and Al-Serw6. Untreated wastewater, laden with various pollutants—including heavy metals, pesticides, PCBs, high concentrations of nutrients, and organic matter—constitutes approximately 98% of the annual inflow to Lake Manzala. These pollutants have led to an increase in vegetation area while simultaneously decreasing water quality and fish productivity13. Additionally, the lake contains a substantial number of islands, which cover about 23% of its total area3. Since surface water is a complex mixture of soluble and insoluble chemicals, water quality encompasses all characteristics of a waterbody influenced by surrounding environmental factors. Waterbodies deteriorate to varying degrees due to the direct impact of dissolved and insoluble contaminants on surface water characteristics14. Overall, water pollution is a global issue that affects most waterways15 as a result of human activities and the ongoing, steady increase in pollution16.
The Egyptian government embarked on a massive project to restore the lake to its natural habitat. This initiative includes a large wastewater treatment project in Bahr El Baqar. Additionally, the lake restoration and treatment plan encompass an extensive dredging operation, which began in 2017 and is scheduled for completion in late 2022. The dredging process aims to increase lake depths in addition to removing islands and dense aquatic vegetation. Dredging is a lake restoration technique that involves the removal of surface-bottom layers containing pollutants, thereby controlling their release and nutrient bioavailability17,18,19. Sediment treatment is often necessary to enhance water quality and other ecosystem components20. Currently, dredging is the most widely used method for addressing pollutant-rich sediments. However, the positive and negative effects of dredging remain subjects of ongoing debate, particularly regarding its role in the ecosystem debate22,23,24,25,26,27. In several studies, dredging activities have been shown to cause significant changes in various components of lake ecosystems. Numerous investigations have documented the negative environmental impacts of dredging26,27,28,29,30,31,32,33. Among these negative impacts are increases in nitrate, phosphorus, ammonia, nitrogen, alkalinity, and conductivity following dredging19,34. Furthermore, the adverse consequences of dredging can lead to alterations in the structure and distribution of biota within a lake. In this context, Rehman et al.25 concluded that dredging had modified the water quality in Dal Lake, Kashmir, India, resulting in a dramatic shift in the zooplankton community structure. Factors such as predation and competition became influential in shaping the zooplankton community. Conversely, dredging may also serve as an effective strategy for improving lake environments by reducing internal nutrient loads, which can promote the dominance of less eutrophic zooplankton species and decrease the presence of indicator species associated with high eutrophication. Consequently, changes also occur in the composition of zooplankton communities24. Therefore, the current study aims to conduct a preliminary assessment of the environmental state of Lake Manzala by monitoring chemical changes and the community composition of zooplankton with the dredging process in its final stages. This study is characterized by the integration of chemical and biological assessments of water quality at different time intervals to evaluate the impact of dredging on the physicochemical parameters and zooplankton community structure of Lake Manzala, Egypt, by comparing data collected before (2015) and after (2022) the dredging process.
Methods
Site description
Lake Manzala is a rectangular, brackish, shallow, and turbid water basin. Lake Manzala is a rectangular, brackish, shallow, and turbid water basin. As of 2022, the lake measures approximately 43.1 km in length and has a mean width of 13.1 km, with depths ranging from less than 0.5 m to more than 2 m. The shallowest depths are found in the southern zone, while the deepest areas are located near the entrance to the Mediterranean Sea. The lake is situated between latitudes31° 07′ 03.2ʺʹ N and 31° 23′ 53.7ʺ N and longitudes 31° 47′ 45.4ʺ E and 32° 14′ 35.0ʺ E35. As previously mentioned, the lake receives saline water from the Mediterranean Sea through various outlets and fresh water (brackish water) from several drains that carry a significant amount of wastewater. Additionally, Lake Manzala is connected to the Suez Canal via a bit of exploring channel known as Al-Qabouti. Accoeding to Elshemy3, Khedr et al.8, and Abd Ellah9 this canal, referred to as the El-Raswa Canal, links the Suez Canal to an isolated pond in the northeastern part of the lake. This isolated pond serves as a treatment reservoir for the sewage from Port Said City. Lake Manzala is of great importance both nationally and globally; it contributed approximately 16.8% of Egypt’s natural fish production and 3.6% of the total fish production in 20218,36. Over the past few decades, the surface area of Manzala Lake has steadily decreased from 1709 km2 in 1907 to 565.91 km2 in 2016, primarily due to illegal land reclamation and aquaculture practices. Furthermore, the overgrowth of aquatic vegetation and the proliferation of islets have reduced the open water area, which shrank from over 70% to approximately 45% of the total lake area between 1986 and 2016. However, due to extensive government efforts, the lake area increased to 572.41 km2 in 2020, and the open water area rose to about 75% of the total lake area9,10. According to Abd Ellah35, the depth of Lake Manzala increased significantly between 2016 and 2022 as a result of dredging operations, where the volume of water increased from 378.67 MCM in 2016 to 903.64 MCM in 2022 with about an increase of 524.94 MCM in the water volume. For example, the area with a depth of more than 2 m increased from 9.41 km2 in 2016 to 166.49 km2 in 2022.
Sampling sites
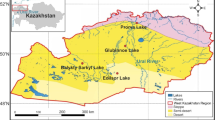
Samples were collected during the winter and summer seasons of 2015 and 2022 from 12 sites, dividing the lake into three sections: north, middle, and south/southeast. The northern section includes sites 1, 2, 3, 11, and 12, and extends along the Mediterranean coast and is connected to it by several canals. The middle section is represented by sites 4 and 5, located at the center of the lake. The southern and southeast sections comprise stations 6, 7, 8, 9, and 10, which are characterized by receiving significant volumes of water discharged from various domestic, agricultural, and industrial sources (Fig. 1 and Table 1).
Map of Manzala Lake showing sampling sites after Khedr et al.12 (The map was generated by ArcGis pro 3.1.0, https://experience.arcgis.com/experience/968e183c67774fa786298483a001438d/page/ArcGIS-License-request//).
Sampling program and analyzing technique
Triplicate subsurface water samples were collected using a Ruttner Water Sampler (2L) and transferred into polyvinyl chloride Van Dorn plastic bottles, which were stored in an ice box until analysis. Biological oxygen demand (BOD) samples were collected in glass-stoppered oxygen bottles, which were carefully filled with water samples. The physicochemical parameters, including temperature, electrical conductivity (EC), dissolved oxygen (DO), and pH, were measured in situ using the Multiparameter Hydrolab model (MultiSet 430i WTW, Germany) after calibration.
The transparency (penetration of light) was measured using the Secchi Disk (30 cm diameter). Total dissolved solids (TDS) were carried out using evaporation of known volumes37. BOD was carried out using the 5-day method. COD was measured using the potassium permanganate method. Nutrients were measured by colorimetric methods using a double beam UV/visible spectrophotometer (model Jenway 680, United Kingdom) at wave lengths of 543 nm "formation of reddish purple azo-dye for nitrite (NO2) and nitrate (NO3) after Hg reduction", 640 nm "phenate method for ammonia (NH4)", and 880 nm "ascorbic acid molybdate method for orthophosphate (PO4)"37. Total nitrogen (TN) and total phosphorus (TP) were quantified as NO3 and PO4, respectively, following persulfate digestion38. The analysis of triplicate samples for each site was conducted to ensure the precision of the analytical methods employed.
Zooplankton samples were collected by filtering 50 L of surface water through a plankton net with a mesh size of 20 μm. The samples were then concentrated to a final volume of 50 mL. One milliliter from each of five subsamples was examined using a binocular research microscope at magnifications of 100× and 400×. Zooplankton were identified to the lowest possible taxonomic level (species) using references from the taxonomic keys39,40,41,42,43,44,45. Zooplankton abundance was calculated according to APHA37 and expressed as individuals per cubic meter (ind./m3).
Statistical analysis
The coefficient of variation (CV) percentage was calculated using Microsoft Excel 2019. Discriminant analysis was employed to categorize similar sites based on chemical and zooplankton data using XLSTAT 2016 software. The normality of the examined data was evaluated using the Shapiro–Wilk test (p > 0.05), and homogeneity of variance was assessed with Levene’s test (p > 0.05). One-way ANOVA (α = 0.05), performed using XLSTAT 2016 software, was used to determine differences in water quality parameters among the lake’s sectors (north, middle, and south) in 2015 and 2022. Additionally, one-way ANOVA was conducted to compare different groups of the lake in 2015 and 2022 based on the abundance and number of saline species, freshwater species, total zooplankton, total rotifers, total copepods, total protozoa, and total cladocerans. Principal component analysis (PCA) was performed to illustrate the relationship between the most abundant zooplankton species and the main chemical parameters using XLSTAT 2016 software.
Results
Water quality
The results presented in Table 2 indicate that water salinity varied widely, ranging from 1.28 to 22.52‰ in 2015 and from 1.20 to 39.06‰ in 2022. The highest salinity values were recorded in the northern sector during the summer season at sites 3 and 12, respectively. Conversely, the lowest salinity values were found in the southern sector at sites 8 and 9. Similarly, the highest dissolved oxygen (DO) levels, measuring 12.22 mg/L and 12.7 mg/L, were observed in the northern sectors during the summer at sites 3 and 12, while the lowest DO levels of 0.39 mg/L and 0.66 mg/L, were recorded in the southern sector at sites 8 and 6 in 2015 and 2022, respectively. Additionally, nutrient levels, particularly ammonium (NH4), total nitrogen (TN), phosphate (PO4), and total phosphorus (TP), along with biochemical oxygen demand (BOD) and chemical oxygen demand (COD), exhibited the highest concentrations in the southern sector, whereas the lowest concentrations were observed in the northern and middle sectors.
Figure 2 illustrates the discriminant analysis based on water quality parameters from 2015. Notably, the sampling sites of the lake were categorized into three groups: A, B, and C. Group A comprised the northern sites (St. 2, 3, and 12) and was characterized by elevated levels of salinity, electrical conductivity (EC), pH, dissolved oxygen (DO), and transparency (Table 3). Groups B and C exhibited some overlap. Group B included the southern sites (St. 6, 7, 8, 9, and 10), along with St. 1 in the northeast. This group was distinguished by high mean values of TN, TP, NH4, NO3, NO2, PO4, BOD, and COD. Conversely, Group C consisted of St. 11 (northwest) and St. 4 and St. 5 (located in the middle of the lake). This group was characterized by mean values of all parameters that fell between those of the other two groups. In a similar context, the 2022 discriminant analysis based on water quality parameters classified the various sites of the lake into two groups: A and B. Group A included the northern and middle sites (St. 1, 2, 3, 4, 5, 11, and 12) and was characterized by high mean values of salinity, DO, EC, pH, NO2, NO3, and transparency. In contrast, Group B encompassed the southern sites (St. 6, 7, 8, 9, and 10) and was characterized by elevated levels of TN, TP, NH4, PO4, BOD, and COD, as depicted in Fig. 3.
Zooplankton
In 2015, a total of 43 zooplankton species were identified, including 30 species of Rotifera, 4 species of Copepoda, 6 species of Protozoa, and 3 species of Cladocera, along with larval forms of Polychaeta, Cirripedia, and Nematoda (Meroplankton). The discriminant analysis of zooplankton species habitats categorized the various sites of the lake into two groups: A and B. Group A was distinguished by the presence and abundance of saline species, which included sites 2, 3, and 12. The saline species were represented by protozoan Favella serrata (Möbius, 1887), Helicostomella subulate (Ehrenberg, 1833), Euplotes minuta, rotifer Brachionus plicatilis (Müller, 1786), and copepod Paracartia latisetosa (Kričagin, 1873), as well as meroplanktonic larvae of Cirripidae and Polychaetes. B. plicatilis were the most abundant species of zooplankton. It formed 22.7% of the total zooplankton count, with the highest density of 976,507 ind./m3 at St. 3. Group B was characterized by the presence and abundance of freshwater species in sites 4, 5, 6, 7, 8, 9, 10, and 11 (Fig. 4). Notably, B. plicatilis was the only saline-brackish species recorded in group B sites, but it was recorded with low densities at St. 4, 5, 10, and 11. Brachionus angularis (Gosse, 1851) and B. calyciflorus (Pallas, 1766) were the dominant freshwater rotifers; they contributed 13.4 and 9.9% of total zooplankton abundance, respectively. B. angularis attained the highest density (682,500 ind. /m3) at St. 6 and 7, while B. calyciflorus was more abundant at St. 7 and 9, with average densities of 90,000 and 85,000 ind./ m3, respectively. Also, Filinia longiseta (Ehrenberg, 1834), Keratella tropica (Apstein, 1907), Philodina roseola (Ehrenberg, 1832), Lecane luna (Müller, 1776), L. bulla (Gosse, 1851), and Polyarthra vulgaris (Carlin, 1943) were abundant in group B sites. Acanthocyclops trajani (Petkovski, 1989) was the most abundant copepod (0.8% of total zooplankton abundance); it was recorded at all sites in Group B. The cladoceran Moina brachiata (Jurine, 1820) was the most abundant species; it attained the highest density of 10,500 ind. /m3 at St. 4. Vorticella campanula (Ehrenberg, 1838) was an abundant protozoan species. It shared 0.3% of the total zooplankton count; it was only recorded at group B. sites.
Discriminant analysis of the distribution of zooplankton species in Manzala Lake in 2015, (a) clustered the lake sites into two groups according to the distribution of zooplankton species, and (b) clustered the dominant zooplankton species into two groups; (Keratella quadrata (K. q), Lecane luna (L. l), Filinia opoliensis (F. o), Polyarthra vulgaris, Centropyxis aculeata (C.o), Daphnia magna (D. m), Keratella tropica (K. t), Brachionus calyciflorus (B. c), Lecane bulla (L. b), Brachionus angularis (B. a), Vorticella campanula (V. c), Asplanchna priodonta (A. p), Mesocyclops leuckarti (M. l), Filinia longiseta (F. l), Acanthocycl opstrajani (A. t), Moina brachiata (M. b), Trichocerca stylata (T. b), Thermocyclops sp. (T. sp.), Nematoda larvae (N. l), Philodena roseola (P. r), Polychaeta larve (P. l), Brachionusplicatilis (B.p), Paracartia latisetosa (P. lat), Helicostomella subulate (H. s), Euplotes minuta (E. m), Favella serrata (F. s), Cirripidea larvae (C. l)).
In 2022, zooplankton was represented by 31 species, including 16 species of Rotifera, 6 species of Copepoda, 7 species of Protozoa, and 2 species of Cladocera, in addition to the larval forms of Polychaeta, Cirripedia, and Nematoda (Meroplankton). The lake sites were categorized into two groups (A and B) based on discriminant analysis (Fig. 5). Group A comprised the northern and middle sites (1, 2, 3, 4, 5, 11, and 12), while Group B included only the southern sites (6, 7, 8, 9, and 10). The saline protozoan species Favella ehrenbergii (Claparède & Lachmann, 1858) and H. subulate were only recorded at group A sites. However, the saline protozoan Euplotes minuta (Yocum, 1930) was recorded at group A and B sites. It was the dominant protozoan species (3.1% of total zooplankton abundance). The rotifer B. plicatilis and Synchaeta calva (Lamarck, 1816) were the saline species recorded. B. plicatilis was the second most abundant species (25.7%) of total zooplankton abundance, and it was recorded at all sites, but it attained the highest density of 1752 ind/m3 at site 11. The other 14 rotifer species were freshwater, and they were constricted to group B sites. Among 11 freshwater rotifers, B. calyciflorus and Brachionus angularis (Gosse, 1851) were dominant (27.4 and 21.9% of the total zooplankton count, respectively). They were recorded at sites 6, 7, 8, 9, and 10. Oithona nana (Giesbrecht, 1892), Acartia clausii (Giesbrecht, 1889), Paracalanus parvus (Claus, 1863), and Euterpina acutifrons (Dana, 1847) were the saline species of Copepod. They were only recorded at group A sites. The saline copepod Paracartia latisetosa (Kričagin, 1873) was recorded at sites 1 and 10. Additionally, the freshwater copepod Acanthocyclops trajani (Petkovski, 1989) was recorded at sites 1, 4, 6, 7, and 9. Oithona nana was the most abundant copepod species (1.3% of total zooplankton abundance). It was observed at sites 2, 3, 4, 10, and 11. Moina rectirostris (Leydig, 1860) and Daphnia longispina (O.F. Müller, 1776) were the only cladoceran species; they were recorded at sites 7 and 8.
Discriminant analysis of the distribution of zooplankton species in Manzala Lake in 2022 (a) clustered the lake sites into two groups according to the distribution of zooplankton species, and (b) clustered the dominant zooplankton species into two groups; (Brachionus budapestinensis (B.b), Lecane bulla (L. b), Asplanchina sp. (A. sp.), Centropyxis aculeata (C.o), Brachionus calyciflorus (B. c), Brachionus angularis (B. a), Filinia longiseta (F. l), Moina rectirostris (M. r), Polyarthra vulgaris (P. v), Brachionus plicatilis (B.p), Acartia clausii (A. c), Polychaeta larve (P. l), Favella ehrenbergi (F. e), Oithona nana (O. n), Paracalanus parvus (P. p), Helicostomella subulate (H. s), Synchaeta calva (S. c), Euterpina acutifrons (E. a).
An analysis of variance (ANOVA) was conducted to compare groups A and B in 2015 and 2022, focusing on the abundance and number of saline species, freshwater species, total zooplankton, total rotifers, total Copepoda, total protozoa, and total Cladocera. The comparison between winter and summer was also evaluated based on the aforementioned criteria. Each criterion showed significant differences (P < 0.05) between the two group sites (A and B) in 2015, except for the number of saline species, copepod diversity, cladoceran abundance, and diversity (Table 4). In 2022, the variance between the two group sites was significant across all comparison criteria, except for protozoan diversity and cladoceran diversity. Furthermore, the two years exhibited significant differences across all criteria, except for the abundance and diversity of Rotifera and the abundance of Cladocera.
The relationship of water quality parameters and zooplankton species
The PCA analysis (Fig. 6) indicated that salinity was the most influential parameter for zooplankton species, effectively categorizing them into two distinct groups. It exhibited a positive correlation with F. ehrenbergi, H. subulate, E. minuta, B. plicatilis and S. calva, O. nana, A. clausii, P. parvus, and E. acutifrons. while demonstrating a negative correlation with other species. Additionally, electrical conductivity (EC) and transparency were positively correlated with saline species. TN and TP showed significant positive correlations with B. calyciflorus, B. angularis, P. roseola, P. vulgaris, and A. trajani.
Discussion
Water quality
Dredging operations are among the most prevalent methods for rehabilitating water bodies. Although the final benefits of dredging can vary significantly, it remains one of the most effective solutions for improving water quality by removing pollutants that have accumulated in the sediments of lakes, rivers, streams, canals, ponds, and other bodies of water21. In the past decade, Egypt has developed a national strategy aimed at rehabilitating the northern lakes and restoring their natural state. A key component of this strategy includes the dredging and cleansing of lakes, with Lake Manzala being one of the most significant projects. The objectives of the dredging operations extend beyond merely increasing water depth; they also focus on eliminating contaminants from the sediments. Notably, one of the primary goals, particularly in areas adjacent to the Mediterranean Sea, is to facilitate the influx of large volumes of high-quality seawater while simultaneously reducing the amount of wastewater discharged into the lake. This study was conducted in 2015 (prior to dredging and cleansing) and again in 2022 (at the conclusion of the dredging), highlighting the changes in the physicochemical properties of the water that occurred during this period. Regarding our findings, we observed significant increases in the values of water parameters such as transparency, EC, salinity, and DO that indicate an improvement in the water quality, as illustrated in Fig. 7. Conversely, we noted reductions in pollution indicator parameters, such as BOD and COD (Fig. 8), along with decreases in nutrient levels, including ammonia, phosphate, total nitrogen, and total phosphorus.
The highest values of salinity in 2015 (22.50 ‰) and 2022 (38.06 ‰) were recorded in sites close to the sea outlets at St. 3 and St. 12, respectively. Furthermore, the mean salinity levels increased from 4.23, 8.86, and 6.54 ‰ to 5.91, 15.16, and 10.53 ‰, reflecting percentage increases of 39.67%, 71.14%, and 60.96% during winter, summer, and the annual average, respectively. It is noteworthy that water salinity rose to varying degrees across different sectors of the lake. The annual average salinity increased by 39.22%, 223.92%, and 60.23% in the northern, middle, and southern sectors, respectively. The significant increase in salinity in the middle sector indicates a greater incursion and spread of seawater into the lake. Additionally, summer exhibited a higher percentage increase in salinity (71.14%) compared to winter (39.67%). Whereas the percentage increase in salinity was up to 48.72, 276.66, and 72.63% and 15.28, 157.43, and 47.12% in the northern, middle, and southern sectors during summer and winter, respectively (Fig. 7a). Also, the present study recorded higher salinity levels than previous measurements of 5.37, 2.53, 1.1–22.5, 1.1–17.3, and 1.39–21.9% recorded in 1985, 2001, 2004, 2017, and 2020, respectively46,47,48,49. These findings confirm that the dredging processes have successfully facilitated the entry of substantial amounts of seawater deep into the lake and into regions far from the El-Boughazes outlets. According to several prior studies1,6, s seawater is of higher quality than lake water, which is influenced by the influx of various wastewater sources. This ultimately led to a noticeable improvement in the quality of lake water.
Water transparency is one of the most important properties affected by dredging operations. Generally, transparency decreases while turbidity increases during the dredging activities and in the short period following their completion27. However, the results of the current study contradict this observation, as water transparency increased during both the winter and summer seasons, specifically during dredging process and its final stages. The use of modern dredging equipment may have contributed to this outcome by preventing an increase in turbidity and a decrease in transparency. But the most crucial factor in enhancing transparency is the increased influx of seawater, which is clearer and more transparent, and its distribution in varying proportions within the lake.
Our findings indicate the water transparency of Manzal Lake fluctuated between 10–60 cm in 2015 and 25–65 cm in 2022, reflecting the lake’s turbidity. However, following the dreading process in 2022, transparency improved by 7.14%, 58.06%, and 28.78% during the winter, summer, and annual average, respectively. Consistent with the rising trend of salinity, the rate of transparency increase was more pronounced in the summer with recorded improvements of 60%, 83.33%, and 40% in the northern, middle, and southern sectors, respectively, compared to the winter values of 3.13%, 4.35%, and 13.79%. Additionally, the increase in transparency was most significant in the middle (31.43%), northern (30.65%), and southern (24.49%) sectors, confirming the enhanced incursion and spread of seawater into the lake (Fig. 7b).
In the same trend, DO levels increased by 17.02%, 26.87%, and 21.49% during the winter, summer, and annual averages, respectively. However, the southern sector exhibited a significant increase rate of 9.75% and 78.67% compared to the northern sector, which recorded increases of 23.21% and 1.78%, and the middle sector, which showed increases of 7.2% and 33.92% during winter and summer, respectively (Fig. 7c). It is important to note that the southern region recorded low oxygen values during 2015 and 2022, reaching 0.39 and 0.68 mg/L, respectively. These values are significantly lower than the internationally permissible limits for fish and aquatic organisms, which are 5.5 mg/L for freshwater and 8 mg/L for marine water50. These results are consistent with previous studies, which attributed the severe depletion of oxygen in the southern part of the lake to the disposal of large quantities of various types of waste.
In contrast to the fluctuating patterns of oxygen concentration in Lake Manzala during the two study periods, BOD and COD, which are indicators of organic pollution, decreased significantly from average values of 29.36 mg/l and 67.09 mg/l in 2015 to 23.26 mg/l and 58.12 mg/l in 2022, representing a reduction rate of − 20.78% and − 8.79%, respectively. Additionally, the percentage changes in BOD and COD during the summer (− 32.22% and − 27.15 mg/l) were greater than those observed in winter (− 8.45% and − 11.0%), as illustrated in Fig. 8a, b. Furthermore, the most significant reductions were observed in the middle region of the lake, followed by the southern and northern areas. Overall, BOD and COD levels in Lake Manzala remain elevated, exceeding international permissible limits, which indicates a deterioration in the lake’s environmental status. Despite some relative improvement following dredging efforts, BOD and COD values continue to surpass the permissible thresholds necessary for the healthy existence of aquatic organisms. This situation is primarily attributed to the inflow of wastewater laden with organic materials, particularly from the Bahr al-Baqar drain in the southern sector. Consistent with the trends observed in BOD and COD, nutrient levels also exhibited a notable decrease in 2022 compared to 2015 and previous studies3,10,47,48,50. The decline rates of the concentrations of ammonia (Fig. 8c), nitrate, total nitrogen, reactive phosphate (Fig. 8d), and total phosphorus were recorded as 10.84%, 19.15%, 35.52%, 12.77%, 41.70%, and 18.97% in winter and 40.58%, − 0.23%, 46.75%, 40.89%, 44.43%, and 18.98% in summer, respectively. Nevertheless, nutrient levels remain high, for example, the levels of ammonia exceeded the permissible limits10 in many sites, ranging from 0.21 mg/l to 11.19 mg/l in 2015 and from 0.11 mg/l to 9.23 mg/l in 2022.
The increase in water quality scale in Manzal Lake during 2022 compared to 2015 is confirmed by the discriminant analysis data. Figure 2 shows that the selected sites in Manzala Lake are divided into 3 groups: A-northern (2, 3, and 12), B-southern (sites 6–10), in addition to St. 1 in the northeast, and C-middle (sites 4 and 5) and St.11 in the northwest. The water in the northern sites was of the best quality, followed by the middle sites, then the south. However, it is worth noting that the middle group tends towards and joins the southern group, which indicates an increase in the deterioration of its water properties. It is notable that station 1, which is located in the northwest, belongs to Group B, which has the worst water quality, as a result of the impact of wastewater discharged from the Port Said Wastewater Treatment Plant (Port Said WWTP). This is despite the relative proximity of station 1 of the sea outlet, which indicates the entry of small amounts of seawater and its weak effect within the lake in this area. This may be supported by station 11 (in the northwest) belonging to group C (the middle sector stations). While Fig. 3 shows the division of the stations in 2022 into two groups only, the stations in the middle sector (stations 4 and 5) belonged to the northern group, becoming one group that includes stations (1–5, 11, and 12), which greatly illustrates the clear improvement in the characteristics of the lake’s water in general and especially the middle sector due to the dredging processes. From a general environmental perspective, despite the relative improvement in water quality parameters in 2022, Lake Manzala still faces major challenges as a result of high values of pollution indicators such as BOD, COD, and ammonia in addition to TN and TP. This indicates beyond doubt that it is necessary to reduce the quantity of wastewater and its treatment before discharge into the lake.
Zooplankton
A total of 43 and 31 zooplankton species were identified in 2015 and 2022, respectively. They included Rotifera (30 and 16 species), Copepoda (4 and 6 species), Protozoa (6 and 7 species), and Cladocera (3 and 2 species), in addition to the larval forms of Polychaeta, Cirripidea, and Nematoda (Meroplankton). Among the 43 zooplankton species, Favella serrata, Helicostomella subulate, Euplotes minuta, Brachionus plicatilis, and Paracartia latisetosa, as well as the meroplanktonic larvae of Cirripedes and Polychaetaes, were the only saline taxa recorded in 2015. Furthermore, they were restricted to st. 2, 3, and 12. While the number of saline taxa increased to 12 taxa in 2022, F. ehrenbergi, H. subulate, E. minuta, B. plicatilis and S. calva, O. nana, A. clausii, P. parvus, P. latisetosa, and E. acutifrons, in addition to meroplanktonic larvae of Cirripidaes and Polychaetaes, were the saline indicators identified in 2022. In comparison with the 2015 and previous studies, the saline species were relatively increased, and they extended in the northern and middle parts of the lake51. recorded two adult saline species in the lake: B. plicatilisand P. latisetosa, as well as Polychaete larvae and Cirriped nauplius. While P. latisetosaand Cirriped nauplius were the only saline forms recorded by McLaren52. Khalifa and Mageed50 .recorded B. plicatilis and O. nana, as well as Cirriped nauplius. Also, Mageed53 listed B. plicatilis, P. parvus, O. nana, Nitocra lacustris, Polychaete larvae, and Cirriped nauplius as saline forms in Make Manzala. Nevertheless, they were restricted to the northern part of the lake. Abdel Mola and El-Rashid54 did not record any saline species in the lake, but this study was conducted in the southern part of the lake. The current study assumed that the increase in the saline species number, especially in the northern and middle parts, may be related to the rise in salinity due to the dredging process. Thus, the lake sites were also grouped into two groups (saline and freshwater) according to the discriminant analysis. Furthermore, the variance between the two groups’ sites was significant according to most comparison criteria based on the ANOVA test. In addition, the PCA analysis showed that salinity was the most effective parameter for zooplankton species; it divided the species into two groups (saline and freshwater). It was positively correlated with F. ehrenbergi, H. subulate, E. minuta, B. plicatilis and S. calva, O. nana, A. clausii, P. parvus, and E. acutifrons. Among the saline species, B. plicatilis was frequently recorded in all sites, but it was more abundant in high-salinity sites. B. plicatilis can live in a wide range of salinity. Therefore, it was the dominant rotifer species in the Egyptian Mediterranean brackish lakes, such as El-Manzala and El-Burullus Lakes5,54. It was also the dominant species in saline lakes such as Qarun and Magic Lakes, Egypt55,56. However, a salinity of up to 20% is the optimum for its growth and reproduction5.
On the other hand, the freshwater species, mainly rotifers, were more abundant in the southern sites, while they decreased or disappeared in the north and middle parts in 2022. It may be associated with the high eutrophication in the south, which may decrease in the north and the middle part due to the dredging. According to Abde-Aziz and Aboul-Ezz57 and Zakaria et al.58, freshwater rotifers in the northern lakes in Egypt have increased markedly as a result of eutrophication. Thus, TN and TP were significantly correlated positively with B. calyciflorus, B. angularis, P. roseola, P. vulgaris, and A. trajani, while these species were negatively correlated with salinity. Ramdani et al.59 reported that nutrients and salinity have the greatest influence on the annual and spatial composition of zooplankton changes in Lake Manzal. Zhang et al.24 suggested that the low abundance of Brachionus budapestinensis, B. angularis, B. diversicornis, and Synchaeta spp. is associated with the decline of the trophic state in a shallow eutrophic lake by dredging. It is known that the high densities of Brachionus spp. are biological evidence for high eutrophication60. In this respect, the flourishing of B. calyciflorus in the southern sites in 2015 and 2022 is associated with high eutrophication due to the discharging of several drains54. Also, the species was recorded in the Rosetta branch of the river Nile as an indicator species for pollution61. B. angularis is cosmopolitan and has a wide distribution in waters that have a high percentage of eutrophication. Its growth is influenced by salinity and temperature62.
Finally, the available zooplankton studies in Lake Manzal are scarce; in addition, these studies have some differences in zooplankton composition. The difference in taxa composition from one study to another may be due to differences in the number of samples, methodology, and inclusion or exclusion of coastal species53. However, our results regarding zooplankton composition and distribution in 2022 were markedly different from 2015 and a few previous studies, meaning that dredging may have played a role in changing the ecology of the lake by increasing salinity and reducing nutrients in the northern and middle parts of the lake. Therefore, we can assume that dredging is the main factor influencing zooplankton composition and environmental properties in 2022.
Conclusion
Lake Manzala is the largest Egyptian Mediterranean lake. Unfortunately, the lake has been exposed to many violations and encroachments over the past decades, such as the shrinking of the area and increased pollution due to receiving huge amounts of a variety of sewage. However, the lake rehabilitation project that began in 2017 through dredging operations hopes to improve its general environmental condition. The current study, which compares the lake status in 2015 (before the dredging) and 2022 (after the dredging), aims to clarify the extent to which dredging operations affect the environmental status of the lake through chemical characteristics and zooplankton communities. The study showed a significant improvement in the water quality and environmental features of Lake Manzala, especially in the northern sector and in the middle of the lake, as a result of the increase in depth due to the dredging process, which led to the entry and spread of seawater to deeper areas within the lake. The results showed an increase in values of salinity, transparency, and dissolved oxygen. In contrast, there were reductions in the values of pollution indicator parameters such as BOD and COD, as well as nutrients (ammonia, phosphate, total nitrogen, and total phosphorus). On the other hand, the results for zooplankton revealed that the saline species were relatively increased in 2022 compared with the 2015 and previous studies, and they extended in the northern and middle parts of the lake. Therefore, we can assume that dredging is the main factor influencing zooplankton composition and environmental properties in 2022. However, from a general environmental perspective, despite the relative improvement in water quality parameters in 2022, Lake Manzala still faces major challenges as a result of high values of pollution indicators such as BOD, COD, and ammonia in addition to TN and TP. This indicates beyond doubt that it is necessary to reduce the quantity of wastewater and its treatment before discharge into the lake. Our study focused on the changes between 2015 and 2022 due to the dredging processes; however, more future studies are planned to determine the long-term changes in the impact of dredging on the environmental condition of the lake.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Mehanna, S. F., Fattouh, S. A., Mehassab, A. F. & Koleib, Z. M. A comparative study of fish production from Lake Manzalah before and during the cleansing and development operations based on surplus production modeling approach. Egypt. J. Aquat. Biol. Fish. 26(5), 613–624 (2022).
Ahmed, M. H., Donia, N. & Fahmy, M. A. Eutrophication assessment of Lake Manzala, Egypt using geographical information systems (GIS) techniques. J. Hydroinform. 8(2), 101–109 (2006).
Elshemy, M. Water quality assessment of Lake Manzala, Egypt: a comparative study. Int. J. Sci. Res. Environ. Sci. 4(6), 11 (2016).
Goher, M. E., Abdo, M. H., Bayoumy, W. A. et al. Some heavy metal contents in surface water and sediment as a pollution index of El-Manzala Lake, Egypt. J. Basic Environ. Sci. 2, 210–225 (2017).
Hegab, M. H., Ahmed, N. M., Kadry, S. M., ElSayed, R. A. & Goher, M. E. Impact of heavy metals on the food web in the Mediterranean lagoon, Lake Burullus, Egypt. Oceanol. Hydrobiol. Stud. 49(3), 215–229 (2020).
Hegab, M. H., Goher, M. E., Mohamed, N. A., El-Sham, A. S. & ElSayed, S. M. An integrated trophic state assessment of a Mediterranean lagoon (Lake Manzala, Egypt) using chemical and rotifer indices. Egypt. J. Aquat. Res. https://doi.org/10.1016/j.ejar.2024.02.001 (2024).
Al-Agroudy, N. & Elmorsi, R. Progressive improvement of the water quality criteria of Lake Manzala, Egypt. Egypt. J. Aquat. Biol. Fish. 26(3), 31–43 (2022).
Khedr, A. I., et al. Assessment of PAH pollution in mediterranean lakes: Distribution, sources, and health implications for fish and consumers, case study Manzala Lake, Egypt. Water Cycle. (2024) (in press).
Abd Ellah, R. An extensive nationwide program for developing the Egyptian lakes, Lake Manzalah: From an ambiguous to a bright future. Egypt. J. Aquat. Res. 47, 337–343. https://doi.org/10.1016/j.ejar.2021.11.002 (2021).
Mahmoud, A., Flefil, N., El Sayed, S., Tahoun, U. & Goher, M. Phytoplankton and bacterial dynamics related to the physicochemical characteristics of Manzala Lake Water, Egypt. Egypt. J. Bot. 62(3), 879–899. https://doi.org/10.21608/EJBO.2022.145176.2019 (2022).
Shaltout, K., El-Bana, M. & Galal, T. Coastal lakes as hot spots for plant diversity in Egypt Egyptian Coastal Lakes and Wetlands, Part II. Clim. Change Biodiver. 72, 129–146. https://doi.org/10.1007/698201780 (2017).
Khedr, A. I., Goher, M. E., Soliman, Y. A. & Abd El-Aziz, G. S. Risk index profile of polycyclic aromatic hydrocarbons in sediments of Manzala lake for protection of benthic life. Egypt. J. Aquat. Biol. Fish. 27(4), 77–94 (2023).
Hossen, H. et al. Forecasting future changes in Manzala Lake surface area by considering variations in land use and land cover using remote sensing approach. Arab. J. Geosci. 11, 1–17 (2018).
Liu, X, et al. Estimation of the key water quality parameters in the surface water middle of Northeast China based on gaussian process regression. Remote Sens. 14(24), 6323. https://doi.org/10.3390/rs14246323 (2022).
Al-Shaibah, B. et al. Modeling water quality parameters using landsat multispectral images: A case study of Erlong Lake Northeast China. Remote Sens. 13(9), 1603. https://doi.org/10.3390/rs13091603 (2021).
Al-Hameedi, W. M. M. et al. Remote sensing-based urban sprawl modeling using multilayer perceptron neural network Markov Chain in Baghdad Iraq. Remote Sens. 13(20), 4034. https://doi.org/10.3390/rs13204034 (2021).
Søndergaard, M. et al. Lake restoration: successes, failures and long term effects. J. Appl. Ecol. 44(6), 1095–1105 (2007).
Chen, M. et al. Successful control of internal phosphorus loading after sediment dredging for 6years: a field assessment using high-resolution sampling techniques. Sci. Total Environ. 616–617, 927 (2018).
Choppala, G. et al. Dissolution and redistribution of trace elements and nutrients during resuspension of iron monosulfide enriched sediments. Chemosphere 201, 380. https://doi.org/10.1016/j.chemosphere.2018.01.164 (2018).
Hadnagy, E. et al. Pilot-scale evaluation of an in situ amendment delivery and mixing device for contaminated sediment remediation applications. J. Soils Sediments 15, 480–489 (2015).
Gustavson, K. E. et al. Evaluating the effectiveness of contaminated-sediment dredging. Environ. Sci. Technol 42, 5042–5047 (2008).
Lohrer, A. M. & Wetz, J. J. Dredging-induced nutrient release from sediments to the water column in a southeastern saltmarsh tidal creek. Mar. Pollut. Bull. 46(9), 1156–1163 (2003).
Zhong, J. C. & Fan, C. Advance in the study on the effectiveness and environmental impact of sediment dredging. J. Lake Sci. 19(1), 1–10 (2007).
Zhang, S. et al. Effects of sediment dredging on water quality and zooplankton community structure in a shallow of eutrophic lake. J. Environ. Sci. 22(2), 218–224 (2010).
Rehman, M. et al. Dredging induced changes in zooplankton community and water quality in Dal Lake, Kashmir, India. Afr. J. Environ. Sci. Technol. 10(5), 141–149 (2016).
Jing, L. et al. Dredging project caused short-term positive effects on lake ecosystem health: A five-year follow-up study at the integrated lake ecosystem level. Sci. Total Environ. 686, 753–763. https://doi.org/10.1016/j.scitotenv.2019.05.133 (2019).
Pereira, A. C. & Mulligan, C. N. Practices for eutrophic shallow lake water remediation and restoration: A critical literature review. Water 15(12), 2270. https://doi.org/10.3390/w15122270 (2023).
Voie, O. A., Johnsen, A. & Rossland, H. K. Why biota still accumulates high levels of PCB after removal of PCB contaminated sediments in a Norwegian fjord. Chemosphere 46, 367–372 (2002).
Weston, D. P., Jarman, W. M., Cabana, G., Bacon, C. E. & Jacobson, L. A. An evaluation of the success of dredging as remediation at a DDT-contaminated site in San Francisco Bay, California, USA. Environ. Toxicol. Chem. 21, 216–224 (2002).
Hoover, J. J., Boysen, K. A., Beard, J. A. & Smith, H. Assessing the risk of entrainment by cutterhead dredges to juvenile lake sturgeon (Acipenser fulvescens) and juvenile pallid sturgeon (Scaphirhynchus albus). J. Appl. Ichthyol. 27, 369–375 (2011).
Drabble, R. Projected entrainment of fish resulting from aggregate dredging. Mar. Pollut. Bull. 64, 373–381 (2012).
Erftemeijer, P. L. A. et al. Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar. Pollut. Bull. 64, 1737–1765 (2012).
Todd, V. L. G. et al. A review of impacts of marine dredging activities on marine mammals. ICES J. Mar. Sci. 72(2), 328–340. https://doi.org/10.1093/icesjms/fsu187 (2015).
Kundangar, M. R. D. & Abubakr, A. Post dredging changes and comparative limnology of Dal Lake Kashmir. Pollut. Res. 20(4), 539–547 (2001).
Abd Ellah, R. G. Using single-beam bathymetric data technique to estimate dredging: a case study in Lake Manzala (Egypt). Arab. J. Geosci. 15, 1649. https://doi.org/10.1007/s12517-022-10937-2 (2022).
CAPMAS, Statistical Yearbook, Annual Bulletin of Statistics Fish Production, produced by Central Agency for Public Mobilization and Statistics. https://www.capmas.gov.eg/Pages/StatisticsOracle.aspx?Oracle_id=1634&page_id=5104&YearID=23537 (2023).
American Public Health Association (APHA) (Standard Methods for the examination of water and wastewater. 23rd ed., 1546 (2017)
Valderrama, J. C. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 10(2), 109–122 (1981).
Koste, W. Rotatoria, die Ra˜dertiereMitteleuropas. (Gebru¨derBorntraeger, 1978).
Shiel, R. & Koste, W. Rotifera recorded from Australia. Trans. R. Soc. S. Aust. 103, 57–68 (1979).
Ruttner-Kolisko, A. Problems in taxonomy of rotifers, exemplified by the Filinia longiseta terminalis complex. In Rotifer Symposium V 291–298 (Springer, 1989).
Abou Zaid, M. M., Hellal, A. M. Tintinnids (Protozoa: Ciliata) from the coast of Hurghada Red Sea, Egypt. Egypt. J. Aquat. Res. 38(4), 249–268 (2012).
Bledzki, L. A., Rybak, J. I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis 918 (Springer International Publishing, 2016).
Conway, D. V. Marine Zooplankton of Southern Britain-Part 1: Radiolaria, Heliozoa, Foraminifera, Ciliophora, Cnidaria, Ctenophora, Platyhelminthes, Nemertea, Rotifera and Mollusca. Occasion. Publ. Mar. Biol. Assoc. 25 (2011).
Yousif, A. Y. F., Skryabin, V., Gubanova, A., Khvorov, S. & Prusova, I. Marine zooplankton practical guide for the northwestern Arabian gulf. Kuwait Inst. Sci. Res. Kuwait 2, 1–194 (2011).
El-Araby, A. The occurrence and biological characteristics of marine fishes in Lake Manzalah at the area of the lake sea connection. M.Sc. Thesis. Fac. Sc. Alex. Univ., 104 (1990).
Ali, M. H. H. Assessment of some water quality characteristics and determination of some heavy metals in Lake Manzalah, Egypt. Egypt. J. Aquat. Biol. Fish. 12(2), 133–154. https://doi.org/10.21608/ejabf.2008.1998 (2008).
Elmorsi, R. R., Hamed, M. A. & Abou-El-Sherbini, K. S. Physicochemical properties of Manzala Lake, Egypt. Egypt. J. Chem. 60(4), 519–535 (2017).
CCME (Canadian Council of Ministers of the Environment) Canadian Water Quality Guidelines for the protection of aquatic life. Summary Tabls. https://ccme.ca/en/summary-table [last visit 9 March 2024]. (Canadian Water Quality Guidelines, 2024).
Khalifa, N. & Mageed, A. Some ecological aspects on the zooplankton in Lake Manzala, Egypt. Egypt. J. Zool 38, 293–307 (2002).
El-Maghraby, A.M., Wahby, S.D., Shaheen, A.H. The ecology of zooplankton in Lake Manzala. Notes and Memoirs, No. 70 (1963).
MacLaren, Engineers, Planners and Scientific Inc. Lake Manzala study. EGY/76/001-07, Final Report to Arab Republic of Egypt, Ministry of Development and New Communities and UNDP Office for Projects Execution. Toronto, Canada (1982).
Mageed, A. A. A. Distribution and long-term historical changes of zooplankton assemblages in Lake Manzala (south Mediterranean Sea, Egypt) (2007).
Abdel Mola, H., El-Rashid, A. Effect of drains on the distribution of zooplankton at the southeastern part of Lake Manzala, Egypt. Egypt. J. Aquat. Biol. Fish. 16(4), 57–68. https://doi.org/10.21608/ejabf.2012.2142 (2012).
El-Shabrawy, G. M., Anufriieva, E. V., Germoush, M. O. et al Does salinity change determine zooplankton variability in the saline Qarun Lake (Egypt)?. Chin. J. Oceanol. Limnol. 33(6), 1368–1377 (2015).
Anufriieva, E. V., Goher, M. E., Abd Ellatif, M. H., et al. Ecosystems of artificial saline lakes. A case of Lake Magic in Wadi El-Rayan depression (Egypt). Knowl. Manag. Aquat. Ecosyst. 421, 31. https://doi.org/10.1051/kmae/2020024 (2020)
Abdel-Aziz, N. E., Aboul-Ezz, S. M. The structure of zooplankton community in Lake Maryout, Alexandria, Egypt. Egypt. J. Aquat. Res. 30(A),160–170 (2004).
Zakaria, H. Y., Ahmed, M. H. & Flower, R. J. Environmental assessment of spatial distribution of zooplankton community in Lake Manzalah, Egypt. Acta Adriat. 48, 161–172 (2007).
Ramdani, M. et al. Environmental influences on the qualitative and quantitative composition of phytoplankton and zooplankton in North African coastal lakes. Hydrobiologia 622, 113–131 (2009).
Attayde, J. L., Bozelli, R. L. Assessing the indicator properties of zooplankton assemblages to disturbance gradients by canonical correspondence analysis. Can. J. Fish. Aquat. Sci. 55(8), 1789–1797 (1998).
Hegab, M. H. & Khalifa, N. Applicability of using biological indices to assess water quality of the Nile Branches, Egypt. Pak. J. Biol. Sci. PJBS 24(3), 383–393 (2021).
Anitha, P. S., Sabu, A. S., George, R. M. Effects of salinity, temperature, feed type and feed concentration on the reproductive rate of Brachionus angularis. Int. J. Fauna Biol. Stud. 3, 10–17 (2015).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Designed the field study: all authors, field works and samples collection: S.M.E., M.H.H. and M.E.G., Experimental laboratory work: S.M.E., M.S.A.H. Performed the statistical analysis of the data: M.H.H., Wrote the manuscript: all authors, review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
All authors voluntarily agree to participate in this research study.
Consent to publish
All authors voluntarily approved the publication of this research study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Sayed, S.M., Hegab, M.H., Abdelhameed, M.S. et al. Evaluating the restoration of Lake Manzala after dredging using water quality parameters and zooplankton changes. Sci Rep 15, 16306 (2025). https://doi.org/10.1038/s41598-025-98069-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98069-x