Abstract
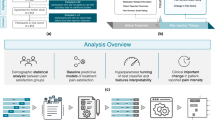
High-impact chronic pain (HICP) affects 8.5% of the population and is associated with higher healthcare utilization and costs. Sparse data exist on pain treatment response differences between HICP vs. lower-impact chronic pain (LICP). We conducted a secondary analysis of a randomized controlled trial in a diverse community sample (N = 1067) with chronic low back pain who were demographically diverse (female: 77%; non-Caucasian: 32%; high school or less education: 19%; mean age: 50.8) and clinically severe (baseline pain intensity = 6.6, baseline pain interference = 6.2, and 42% severe/completely disabled). We compared HICP vs. LICP treatment responses for an 8-week Skills-Based Virtual Reality program at end-of-treatment and at 12-months for pain intensity and pain interference (multi-primary outcomes). MMRM analysis (multiplicity corrected) revealed significantly larger reductions (and clinically meaningful reductions) for HICP than LICP for both primary outcomes at both post-treatment time points. End-of-treatment reduction in pain interference among HICP reclassified 70% of them as LICP, and this improvement held at 12-months (67%). Significantly larger reductions were found for HICP vs LICP for the secondary outcomes Sleep Disturbance and the Oswestry Disability Index, but not for Depression. No differences were found for HICP vs. LICP for device engagement or usability scores.
Trial registration: ClinicalTrials.govNCT05263037.
Similar content being viewed by others
Introduction
High-impact chronic pain (HICP) is defined as pain that persists for at least 3 months with at least one major activity restriction, such as being unable to work outside the home, attend school, or perform household chores1,2,3,4. In 2023 nearly one-quarter of adults reported having chronic pain in the past 3 months, and 8.5% of the population reported having chronic pain that frequently limited life or work activities (referred to as high-impact chronic pain)3,5. Compared to those with lower-impact chronic pain (LICP), people with HICP have higher healthcare utilization and costs, greater likelihood for opioid prescription6 and have 5 times the morphine equivalent daily dose. Those with HICP are 3 times more likely to have surgery and emergency room visits3,6. The U.S. Department of Health and Human Services called for increased focus on HICP in the 2016 National Pain Strategy7. Yet, to date, no published reports exist that describe behavioral pain treatment efficacy in HICP, or that compare HICP vs. LICP response to pain treatment. Increased pain treatment and utilization patterns underscores a specific need to investigate low-risk treatments in HICP. Secondary analyses of existing outcomes data from behavioral treatment studies may yield important insights on how to best meet the needs of this complex population.
To this end, we conducted a secondary analysis of a recently completed double-blind, randomized placebo-controlled trial that compared a Skills-Based virtual reality (VR) program with Sham (trial details to follow in the Methods section)8. We previously reported clinically meaningful reductions [Minimal Clinically Important Difference (MCID) ≥ 2 points]9 in pain intensity (average reduction = -2.0 ± 1.9, effect size = 1.02) and pain interference (average reduction = -2.3 ± 2.0, effect size = 1.04) at the end of treatment for the Skills-Based VR program with reductions superior to those for Sham (Skills-Based VR vs. Sham between group p = 0.0004 for intensity, p = 0.00001 for interference). Some regression to the mean was observed at 12 months post-treatment, though durably significant pain reductions remained10. In addition, the Skills-Based VR group had significantly larger reductions for PROMIS Depression, PROMIS Sleep Disturbance, and Oswestry Disability Index (ODI) (largest p = 0.004) compared to Sham at end-of-treatment8 and 12-months post-treatment10.
In the current study, we used the validated classification approach for Brief Pain Inventory pain interference score11,12 of ≥ 7 to classify participants at baseline as either HICP vs LICP, and at post-treatment time points to determine individual change in classification category13. Specifically, within the pain relief skills-based VR group we compared treatment response for HICP vs HICP at end-of-treatment and at 12 months post-treatment. For completeness, we also briefly summarize the same analyses in the Sham group.
Methods
Study design, participants and randomization
The Methods, CONSORT diagram and analyses at end-of-treatment and 12-months post-treatment for the primary decentralized randomized controlled trial are published8. Although the secondary analysis presented in this report focuses primarily on the Skills-Based VR therapy, for completeness, we briefly describe some of the trial details, including the Sham VR group. A double-blind, randomized placebo-controlled trial was conducted in a national community sample of 1,067individuals with self-reported chronic lower back pain [> 3 months and average Brief Pain Inventory12 pain intensity (0–10 pain rating scale) and pain interference of ≥ 4 for the past month]. The resulting sample was demographically diverse (female: 77%; non-Caucasian: 32%; high school or less education: 19%; mean age: 50.8) and clinically severe (baseline pain intensity = 6.6; baseline pain interference = 6.2, disability = 42%; severe/completely disabled). Following written consent, participants were randomized and received either: (1) an 8-week, immersive, 3-dimensional pain relief Skills-Based VR program; or (2) Sham VR (2-dimensional nature videos delivered in a VR headset with no pain relief skills content). 12Skills-Based VR offers a fixed sequence of 56 daily VR sessions that incorporates pain neuroscience education and evidence-based self-regulatory skills commonly acquired in evidence-based behavioral pain treatments such as cognitive behavioral therapy and mindfulness treatments. Sham VR was designed to mimic the VR device experience without 3D immersion, verbal and pain relief skills content. Sham VR involved a fixed sequence of 56 2D nature videos overlaid with neutral music. The study protocol was approved by the WCG Institutional Review Board (Puyallup, WA) in December 2021, and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. Study data were collected from January 31, 2022 to October 31, 2023 (12-months post-treatment) and will continue to 24-months post-treatment.
Self-report measures
Demographics
Age, gender, race, ethnicity, annual household income, height, weight, and pain comorbidities.
Brief pain inventory (BPI): Pain interference12.
The BPI Pain Interference measures how much pain interferes with seven domains of daily life (enjoyment of life, general activity, mood, normal work, relations with other people, sleep, and walking ability) over the last 24 h using a 0 – 10 numeric rating scale. The scores on the seven items are averaged to generate a global pain interference score. This measure was administered at baseline, end-of-treatment, 1-, 2-, 3-, 6-, and 12-months post-treatment. Five additional administrations of the BPI pain interference were included (one per day) during the 5 days following initial baseline administration, with a requirement that at least 2 be completed. These were averaged with the baseline BPI to obtain the overall baseline BPI interference scores.
Brief pain inventory (BPI): pain intensity12
The BPI Pain Intensity uses a single item to measure pain intensity over the last 24 h using a 0 – 10 numeric rating scale. This measure was administered at baseline, end-of-treatment, 1-, 2-, 3-, 6-, and 12-months post-treatment. Five additional administrations of the BPI pain intensity were included (one per day) during the 5 days following initial baseline administration, with a requirement that at least 2 be completed. These were averaged with the baseline BPI to obtain the overall baseline BPI intensity.
PROMIS sleep disturbance (version 8b14 )
The PROMIS Sleep Disturbance is an 8-item survey that assesses an individual’s perception of sleep quality, sleep depth, and restoration associated with sleep over the past 7 days. Raw scores on the PROMIS Sleep Disturbance survey are converted to T-scores, which range from 28.9—76.5. This measure was administered at baseline, end-of-treatment, 1-, 2-, 3-, 6-, and 12-months post-treatment.
PROMIS depression (version 8b 15)
The PROMIS Depression is an 8-item survey that assesses the frequency and severity of depressive symptoms over the past 7 days. It covers topics such as feelings of worthlessness, hopelessness, and loss of interest in activities. Raw scores on the PROMIS Depression survey are converted to T-scores, which range from 37.1–81.1. This measure was administered at baseline, end-of-treatment, 1-, 2-, 3-, 6-, and 12-months post-treatment.
Oswestry disability index (ODI) (version 2.1b16)
The ODI is a 10-item survey that assesses how low back pain affects one’s ability to manage in everyday life. Scores range from 0 – 100 and reflect the level of disability. This measure was administered at baseline, end-of-treatment, 1-, 2-, 3-, 6-, and 12-months post-treatment.
System usability scale17
The System Usability Scale is a 10-item survey that provides a global assessments of system usability (example item: “I thought the system was easy to use.”). Some items are reverse scored, a multiplier is applied to the sum, and total scores range from 0–100. This measure was administered at end-of-treatment.
Objective measure
Therapeutic engagement
Therapeutic engagement was objectively assessed as the number of sessions initiated with at least 45% of the session completed. The number of sessions completed by each participant was captured from the VR device after treatment and upon device return.
Statistical analysis
Continuous and categorical baseline variables were summarized using mean (SD) and count (percentage), respectively. The standardized mean differences between HICP and LICP groups were calculated. Between-group comparisons were conducted using t-tests (for continuous baseline variables) or Fisher exact tests (for categorical baseline variables). The modified intention-to-treat analysis set (i.e., all participants randomized who received a VR device) was used to investigate Skills-Based VR therapy effects in HICP vs LICP. To evaluate Skills-Based VR therapy effects in HICP vs LICP on the two primary endpoints (BPI pain interference and BPI pain intensity), separate mixed models for repeated measure (MMRM;18,19) analysis was conducted with the dependent variable being the relevant endpoint score (BPI interference or BPI intensity) and independent variables being group (baseline HICP vs LICP) and time (i.e., baseline, end-of-treatment or 12-months post-treatment). Group effects were summarized as the estimated between-group difference in endpoint score reductions from baseline to end-of-treatment or baseline to 12-months post-treatment and the associated 95% CI. Because the MMRM analysis included baseline endpoint scores in repeated measurements, the analysis adjusted for potential imbalance in baseline endpoint score by treating the baseline value as a covariate. Similar analyses examined the group effect for the four secondary endpoints (PROMIS Anxiety, PROMIS Sleep Disturbance, PROMIS Depression and ODI). For the primary endpoints, the Bonferroni adjusted statistical significance level for comparing groups was set at a 2-sided level of 0.025 to adjust for 2 comparisons. For the secondary endpoints, the Bonferroni adjusted statistical significance level for comparing groups was set at a 2-sided level of 0.0125 to adjust for 4 comparisons. For completeness, we conduct the same analysis in the Sham VR group.
Results
Participants
Table 1 displays the baseline demographic and clinical characteristics by pain impact group (LICP vs HICP) for the Skills-Based VR therapy participants. Thirty-four percent (N = 192) were classified as HICP. The HICP group reported greater racial diversity (p = 0.005), lower levels of household annual income (p = 0.0001), and higher BMI (p = 0.01) than the LICP group. For baseline clinical symptoms, the HICP group reported significantly greater levels of symptom severity across all variables with p-value group differences ranging from 0.01 to < 0.0001.
Skills-based VR therapy pain impact group differences
Primary endpoints
MMRM yielded a significant group x time interaction for BPI Pain Interference (p < 0.001) that was characterized by a significantly larger baseline to end of treatment Pain Interference reduction for HICP (-2.68, p < 0.001) vs. LICP (-2.00, p < 0.001) (0.73 [95% CI, 0.36 to 1.10; p < 0.001]) that was sustained to 12-months post-treatment (HICP reduction = -2.58, p < 0.001; LICP reduction = -1.56, p < 0.001; 1.02 [95% CI, 0.58 to 1.47; p < 0.001]). [See Table 2 for details.] Interestingly, at the two post-treatment time points, HICP participants’ average Pain Interference score was newly classified in the LICP range (end-of-treatment average = 5.39, 12-months post-treatment average = 5.52), with 70% of individual HICP participants at end-of-treatment (N = 114), and 67% of individual HICP participants at 12-months post-treatment (N = 104) newly reclassified as LICP.
MMRM revealed a significant group x time interaction for BPI Pain Intensity (p < 0.001) that was characterized by a significantly larger baseline to end of treatment Pain Intensity reduction for HICP (-2.20, p < 0.001) vs. LICP (-1.77, p < 0.001) (0.44 [95% CI, 0.07 to 0.81; p = 0.02]) that was sustained to 12-months post-treatment (HICP reduction = -2.08, p < 0.001; LICP reduction = -1.45, p < 0.001; 0.63 [95% CI, 0.22 to 1.05; p = 0.003]).
Secondary endpoints
MMRM yielded a significant group x time interaction for PROMIS Sleep Disturbance (p < 0.001) that was characterized by a significantly larger baseline to end of treatment Sleep Disturbance reduction for HICP (-6.12, p < 0.001) vs. LICP (-3.51, p < 0.001) (2.61 [95% CI, 1.25 to 3.97; p < 0.001]) that was sustained to 12-months post-treatment (HICP reduction = = -6.74, p < 0.001; LICP reduction = -4.10, p < 0.001; 2.64 [95% CI, 1.05 to 4.23; p = 0.001]). MMRM yielded a significant group x time interaction for the Oswestry Disability Index (p = 0.005) that was characterized by a significantly larger baseline to end of treatment Disability reduction for HICP (-10.53, p < 0.001) vs. LICP (-6.59, p < 0.001) (3.94 [95% CI, 1.57 to 6.32; p = 0.001]) that was sustained to 12-months post-treatment (HICP reduction = -12.12, p < 0.001; LICP reduction = -8.55, p < 0.001; 3.57 [95% CI, 0.51 to 6.63; p = 0.02]). MMRM yielded a non-significant group x time interaction for PROMIS Depression (p = 0.58). A significant reduction in Depression was found for the LICP, but not HICP group at end-of-treatment (LICP = -1.26, p = 0.001; HICP = -0.51, p = 0.50) and this was sustained for the LICP group at 12-months post-treatment (LICP = -1.91, p < 0.001; HICP = -1.15, p = 0.24).
Therapeutic engagement and VR device usability
Skills-Based VR engagement was high and did not differ between LICP and HICP groups (p = 0.65). LICP participants completed 39.0 ± 15.77 (4.9 experiences/week) and HICP participants completed 39.9 ± 16.83 (of 56) experiences (5.0 experiences/week) during the treatment. VR device usability was high and did not differ across LICP and HICP participants (p = 0.48). The device received a rating of 91.8 ± 9.56 and 91.3 ± 12.15 (of 100) for the LICP and HICP groups, respectively (both A + ratings on the System Usability Scale).
Sham VR therapy pain impact group differences
The LICP vs HICP analysis presented above was replicated in the Sham VR group. The results are displayed in Table 3 and are briefly summarized here. MMRM revealed a significant group x time interaction for BPI Pain Interference (p < 0.001) and BPI Pain Intensity (p = 0.002). For both measures, the pain reduction for HICP was numerically larger than for LICP, but this difference did not reach statistical significance for pain intensity from baseline to end-of-treatment (p = 0.47), and the difference for both pain measures was generally smaller than that observed for the Skills-Based VR program. MMRM revealed a significant group x time interaction for PROMIS Sleep Disturbance (p < 0.001), the Oswestry Disability Index (p = 0.002), but not PROMIS Depression (= 0.44). For Sleep Disturbance and the Oswestry Disability Index, the baseline to end-of-treatment and baseline to 12-months post-treatment reduction for HICP was significantly larger than for LICP (largest p = 0.007), and of similar magnitude to that for the Skills-Based VR program. For Depression, the baseline to end-of-treatment reduction for HICP was smaller than that for LICP and was non-significant (p = 0.82). This pattern reversed at 12-months post-treatment with an HICP reduction advantage, but the difference was still non-significant (p = 0.57). Sham VR usage was significantly lower in the HICP group (p = 0.03) with HICP participants completing on average 3.6 sessions per week, and LICP participants completing on average 4.1 sessions per week. VR device usability was high and did not differ across LICP and HICP participants (p = 0.59).
Discussion
Compared to individuals with LICP, those with HICP have higher medical utilization6, greater risks from various pain treatments, including adverse effects from medications, complications from invasive treatments, or potential misuse of pain medications20,21, and modest to negligible clinical improvements resulting from treatments22. Moreover, HICP is more common among women, older adults, those with lower socioeconomic status, and rural residents3,23. While it has long been known that the aforementioned groups disproportionately experience chronic pain, and at greater severity, the HICP classification provides a new and validated way to examine the impacts of pain, and a lens through which we may consider treatment efficacy and effectiveness.
Indeed, lower risk treatment approaches may better meet the needs of this complex patient population, yet behavioral treatment efficacy and effectiveness data are lacking for HICP. To help fill this important gap, we conducted a secondary analysis on data from a randomized study of a 56-session skills-based VR program and characterized the immediate and long-term treatment responses among diverse individuals based on their HICP vs. LICP status.
For our multi-primary outcomes, we found that the HICP group had superior and durable VR treatment response compared to LICP; meaning, the HICP group had significantly larger reductions in Pain Intensity and Pain Interference up to 1 year post-treatment. Moreover, durable reductions in Pain Interference led two-thirds of the HICP group to be reclassified as LICP at 1 year follow-up. A similar HICP pain intensity and pain interference advantage was observed for Sham, but this difference did not reach statistical significance for pain intensity at end-of-treatment, and for both pain measures, the magnitude of the difference was smaller for the sham than for the skills-based VR group. An HICP pain reduction advantage was not unexpected since the Sham has some therapeutic value in the form of pain distraction and relaxation24.
In terms of secondary outcomes, we found a similar pattern of results for Sleep Disturbance and Disability, with the HICP group evidencing superior improvements over the LICP, with durability of symptom reduction to 1 year follow-up. This was mirrored in the Sham group. Depression was the only outcome with divergent results: we found no significant group by time effect. Compared to HICP, the LICP had superior and statistically significant (but not clinically meaningful) reductions in Depression up to one year. This result is surprising given the direction of all other effects. Future work will investigate this anomalous result further.
Our findings have relevance within the context of several factors. New analyses from the Centers for Disease Control and Prevention suggest that rates of HICP are increasing across U.S. age groups and races5. The U.S. Department of Health and Human Services called for increased focus on HICP in the 2016 National Pain Strategy7. Yet, we found no published reports describing behavioral pain treatment efficacy in HICP, nor any studies comparing HICP vs. LICP response to pain treatment. While several active NIH and PCORI-funded projects seek to address this large data gap, our results contribute to a broader understanding about how to best treat HICP. Interestingly, our results suggest that the patients who are most impacted and symptomatic reaped the largest benefits from the pain relief VR program. Data show that HICP are often overmedicalized and undertreated with behavioral approaches25. Our evidence supports a HICP treatment approach that would apply behavioral medicine treatments first, or very early in the pain treatment process, specifically to reduce patient risks and optimize their pain and health outcomes. Indeed, our finding that two-thirds of HICP patients were reclassified as LICP at 1 year is noteworthy. Future research may examine whether the majority of HICP patients who reclassify to LICP also evidence expected reductions in healthcare utilization and associated medical treatment risks.
Previously, we reported that the clinical effectiveness of the pain relief skills VR program was invariant across age, gender, race/ethnicity and socioeconomic status26 – factors that commonly associate with HICP. Results from our current secondary analysis provide a more descriptive and nuanced analysis specific to HICP. Finally, we note that therapeutic engagement and device usability were high and equivalent among the HICP and LICP participants.
Skills-Based VR for chronic pain was constructed with a multimodal approach grounded in several self-regulatory techniques and therapies including cognitive behavioral skills, meditation, mindfulness, biofeedback experiences, education, and interoceptive awareness and control. Taken together, these results suggest that complex HICP patients benefit from this self-delivered therapy, and to a greater degree than those who are less impacted by pain.
Limitations
Limitations to consider when evaluating the study results include the following: First, although the HICP vs LICP classification is empirically driven, participants cannot be randomized to these groups and thus the results are still correlational in nature. Second, other potentially important factors not included in the analysis could be causal, such as pain-related and non-pain-related co-morbidities. Along those lines, a deeper understanding of the underlying diagnoses and conditions that define the HICP population (e.g., what percent might have trauma, are in disability, have cancer/palliative care, etc.), and what other treatments might be involved in a one-year period for that cohort of patients (e.g., how many are on opioid therapy, receiving injections, undergoing surgery) would be advantageous, and should be the focus of future research. Third, self-reported chronic lower back pain was not confirmed by healthcare professionals. Fourth, our study was specific to chronic lower back pain and results may not generalize to other populations.
Conclusion
A pain relief skills VR program was associated with clinically meaningful benefits to up to one-year post-treatment, with the greatest benefits found for patients with HICP. While further research is needed to understand how to best meet the needs of people with HICP, our results suggest that the home-based VR program tested here may be a scalable and effective treatment option.
Data availability
A data dictionary and de-identified participant data will be made available after publication and upon approved request of a detailed meta-analytic study proposal. Requests should be made to the corresponding author along with a study proposal and a signed data access agreement.
Abbreviations
- HICP:
-
High Impact Chronic Pain
- LICP:
-
Lower Impact Chronic Pain
- VR:
-
Virtual reality
References
Şentürk, I. A. et al. High-impact chronic pain: evaluation of risk factors and predictors. Korean J. Pain 36, 84–97 (2023).
Dahlhamer, J. et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 67, 1001–1006 (2018).
Pitcher, M. H., Von Korff, M., Bushnell, M. C. & Porter, L. Prevalence and profile of high-impact chronic pain in the United States. J. Pain 20, 146–160 (2019).
Vaegter, H. B. P., Due Bruun, K. M. P. & Bye-Møller, L. P. High-Impact Chronic Pain. International Association for the Study of Pain (2023).
Lucas, J. W. & Sohi, I. Chronic Pain and High-Impact Chronic Pain Among U.S. Adults, 2023. https://doi.org/10.15620/cdc/169630 (2024).
Herman, P. M., Broten, N., Lavelle, T. A., Sorbero, M. E. & Coulter, I. D. Health Care Costs and Opioid Use Associated with High-impact Chronic Spinal Pain in the United States. Spine (Phila Pa 1976) 44, 1154–1161 (2019).
National Pain Strategy: A Comprehensive Population Health-Level Strategy for Pain. U.S. Department of Health and Human Services (2016).
Maddox, T. et al. In-Home Virtual Reality Program for Chronic Lower Back Pain: A Randomized Sham-Controlled Effectiveness Trial in a Clinically Severe and Diverse Sample. Mayo Clinic Proceedings: Digital Health 1, 563–573 (2023).
Farrar, J. T., Young, J. P., LaMoreaux, L., Werth, J. L. & Poole, R. M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158 (2001).
Maddox, T. et al. 12-month Results for a Randomized Sham-Controlled Effectiveness Trial of An In-Home Skills-Based Virtual Reality Program for Chronic Low Back Pain. Pain Rep. 9(5), e1182 (2024).
Cleeland, C. The Brief Pain Inventory User Guide. The Brief Pain Inventory (2009).
Cleeland, C. S. & Ryan, K. M. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore vol. 23 Preprint at (1994).
Cook, K. F. et al. Threshold-scores for Levels of Chronic Pain Impact Derived from the Perspectives of Patients and Clinicians Based on Self-reported Pain Interference. Journal of Patient Reported Outcomes, revised and resubmitted, (2024).
Yu, L. et al. Development of short forms from the PROMISTM sleep disturbance and sleep-related impairment item banks. Behav. Sleep Med. 10, 6–24 (2011).
Cella, D. et al. The Patient-Reported outcomes measurement information System (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Med Care 45, S3–S11 (2007).
Fairbank, J. C. T. & Pynsent, P. B. The oswestry disability index. Spine (Phila Pa 1976) 25, 2940–2953 (2000).
Brooke, J. SUS -A quick and dirty usability scale Usability and context. Usability Evaluation Industry 189, 4–7 (1996).
Laird, N. M. & Ware, J. H. Random-effects models for longitudinal data. Biometrics 38, 963–974 (1982).
Cnaan, A., Laird, N. M. & Slasor, P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 16, 2349–2380 (1997).
Chou, R. et al. Opioid treatments for chronic. Pain https://doi.org/10.23970/AHRQEPCCER229 (2020).
Vane, J. R. & Botting, R. M. Mechanism of action of antiinflammatory drugs. Int. J. Tissue React. 20(1), 3–15. https://doi.org/10.3109/03009749609097226 (1998).
Bair, M. J., Robinson, R. L., Katon, W. & Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 163(20), 2433–2445. https://doi.org/10.1001/archinte.163.20.2433 (2003).
Prevalence and Profile of High Impact Chronic Pain. National Center for Complementary and Integrative Health (2018).
Hoffman, H. G., Patterson, D. R. & Carrougher, G. J. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: A controlled study. Clin. J. Pain 16, 244–250 (2000).
Falasinnu, T. et al. The Problem of pain in the United States: A Population-Based characterization of biopsychosocial correlates of high impact chronic pain using the national health interview survey. J0. Pain 24, 1094–1103 (2023).
Maddox, T. et al. Sociodemographic predictors of clinical effectiveness, therapeutic program engagement, and device usability for an in-home virtual reality program for chronic low back pain: Secondary analysis of a randomized controlled trial. J. Med. Extended Reality 1, 65–72 (2024).
Funding
AppliedVR, Inc. financially supported this study.
Author information
Authors and Affiliations
Contributions
TM, LO, CS and JS were involved in all aspects of the study. TA, KF, and RM were involved in study implementation, project and participant management. WL-Z and RM were involved in data analysis, and data presentation. RB served as medical monitor and helped interpret the findings. BD was involved in study design, data interpretation and manuscript preparation. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
Dr. Maddox, Roselani Maddox, and Takisha Adair are employees of AppliedVR, Inc. Joshua Sackman is president of AppliedVR, Inc. Drs. Oldstone and Sparks are former employees of AppliedVR, Inc who were employed during execution of the study. Dr. Bonakdar, Dr. Linde-Zwirble, and Kelsey Ffrench are consultants/contractors for AppliedVR, Inc. Dr. Darnall is compensated to serve as chief science advisor for AppliedVR, Inc. Dr. Darnall has authored or coauthored five pain treatment books for patients and clinicians and receives royalties for four. Dr. Darnall is the principal investigator for pain research grants and awards from the National Institutes of Health (NIH) and the Patient-Centered Research Outcomes Research Institute (nonspecific to the current work). Dr. Darnall is a co-investigator on two NIH research grants investigating virtual reality analgesia; neither of these grants is specific to the current work. Dr. Darnall serves on the Board of Directors for the Institute for Brain Potential and on the Medical Advisory Board of the Facial Pain Association Dr. Darnall is a current scientific member of the NIH Interagency Pain Research Coordinating Committee, former member of the Centers for Disease Control and Prevention (CDC) Opioid Workgroup (2020–2021), and the Pain Advisory Group of the American Psychological Association.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Maddox, T., Oldstone, L., Linde-Zwirble, W. et al. Differential treatment response to virtual reality in high-impact chronic pain: secondary analysis of a randomized trial. Sci Rep 15, 14430 (2025). https://doi.org/10.1038/s41598-025-98716-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98716-3