Abstract
Existing prognostic tools for non-traumatic intracerebral hemorrhage (NCH) mainly focus on neurological injury severity but overlook systemic factors that cause secondary deterioration. This study aimed to evaluate the Advanced Lung Cancer Inflammation Index (ALI), a biomarker combining nutritional parameters (BMI, albumin) and inflammatory parameters (neutrophil-to-lymphocyte ratio, NLR), as a new prognostic indicator for NCH. It aimed to fill the gap in quantifying the synergy between inflammation and malnutrition in neurocritical care.In this retrospective cohort study, 1502 critically ill NCH patients from the MIMIC-IV database were analyzed. ALI was calculated as (BMI × Albumin)/NLR, and patients were divided into groups based on the quartiles of the log-transformed ALI. The outcomes measured were in-hospital, 90-day, and 1-year mortality. Multivariable logistic regression, ROC curve analysis, restricted cubic spline models, and subgroup analyses were used to assess the prognostic value of ALI.The results showed that lower ALI values could independently predict mortality at all time points. After comprehensive adjustment, a 1-unit increase in the log-transformed ALI was associated with a 26% reduction in in-hospital mortality (OR = 0.74, 95% CI: 0.64–0.86, P < 0.001). The fourth quartile (with the highest ALI) had 56% lower mortality compared to the first quartile (OR = 0.44, P = 0.001). ALI had a strong ability to distinguish in-hospital all-cause mortality (AUC = 0.853, 95% CI: 0.826–0.880, P < 0.001). A threshold effect was found (log-transformed ALI = 4.0), below which ALI was inversely related to mortality (P < 0.001), and above which the relationship leveled off. Subgroup analyses confirmed the consistency of these findings across different age groups, comorbidities, and organ dysfunctions (P for interaction > 0.05). In conclusion, ALI is a reliable prognosticator for NCH, reflecting the interaction between systemic inflammation and malnutrition. Its simplicity and potential for threshold-based stratification may help in personalized interventions. Further prospective validation of ALI-guided protocols is needed.
Similar content being viewed by others
Introduction
Non-traumatic intracerebral hemorrhage (NCH), encompassing intracerebral, subarachnoid, subdural, and epidural hemorrhages, represents the second most common stroke subtype, characterized by rapid clinical deterioration and unacceptably high mortality [(20% in-hospital, rising further for ICU(intensive care unit) patients]1,2,3. Current prognostic tools primarily focus on initial neurological injury, while systemic drivers of secondary deterioration remain underrecognized4.
Emerging evidence highlights a self-perpetuating cycle between neuroinflammation and systemic catabolism as a key determinant of poor outcomes. Brain-derived damage-associated molecular patterns immunocytes activate peripheral neutrophils, triggering cytokine storms that suppress appetite and induce muscle wasting5,6. These processes are exacerbated by concurrent complications such as dysphagia and gastrointestinal dysfunction, which impair both nutrient intake and absorption, leading to hypoalbuminemia, negative nitrogen balance, and progressive malnutrition7. This dual burden of sustained inflammation (marked by elevated C-reactive protein and transthyretin dysregulation) and malnutrition disrupts homeostasis, accelerating multi-organ failure across acute and long-term recovery phases8,9.
The Advanced Lung Cancer Inflammation Index [ALI = body mass index (BMI) × Albumin/ the neutrophil-to-lymphocyte ratio (NLR)] quantifies inflammation-nutrition interplay through components directly mapping NCH pathophysiology: BMI/Albumin reflect catabolic states, NLR indicates inflammation10. Although ALI has shown prognostic value in cancer, ischemic stroke, and metabolic disorders, its utility in NCH remains unexplored despite strong mechanistic alignment with the disease’s neuroimmune-metabolic cascade11,12,13.
Using the Medical Information Mart for Intensive Care (MIMIC-IV, version 3.1) database, this study addresses two critical questions: does ALI measured within 24 h of ICU admission independently predict mortality at in-hospital, 90-day, and 365-day timepoints after adjusting for key confounders and does ALI’s predictive performance support its hypothesized role as a composite marker of systemic inflammation-nutrition imbalance in NCH?
As the first investigation to evaluate ALI in NCH using a high-resolution ICU registry, our findings may enable practical risk stratification through routine clinical parameters, bypassing the need for specialized biomarker assays.
Materials and methods
Materials
The data used in this study were obtained from the open-source Medical Information Mart for Intensive Care (MIMIC-IV, version 3.1) database. This publicly available, high-quality resource contains de-identified clinical data from patients admitted to the Intensive Care Unit (ICU) at Beth Israel Deaconess Medical Center between 2008 and 2019. The database includes a wide range of patient information, such as demographics, medical records, drug treatments, laboratory test results, and disease classifications based on the International Classification of Diseases (ICD-9 and ICD-10)14,15,16.
Access to the MIMIC-IV database was granted after Peng Zhong completed the National Institutes of Health (NIH) online course and passed the “Protecting Human Research Participants” examination (Certification Number: 14031810). This study was approved by the Institutional Review Boards (IRBs) of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center, and patient privacy was strictly protected.
Study design
This was a retrospective cohort study using the MIMIC-IV database to evaluate the prognostic value of ALI in patients with NCH.
Study site
The study was conducted using data from the Beth Israel Deaconess Medical Center (Boston, USA), an academic medical center with a large volume of critically ill patients.
Study population
Patients included in this study met the following inclusion criteria
-
1.
Adult patients (≥ 18 years old) diagnosed with NCH, identified using ICD-9 and ICD-10 codes.
-
2.
First ICU admission recorded in the database.
-
3.
ICU stay duration > 24 h to ensure sufficient clinical data for analysis.
Patients were excluded if they
-
1.
Had no available ICU records.
-
2.
Lacked key laboratory measurements required to calculate ALI (BMI, albumin, neutrophil count, lymphocyte count).
-
3.
Had > 20% missing data across relevant clinical variables.
A total of 6360 patients were initially identified with an NCH diagnosis, and 1502 adult patients met the final inclusion criteria (Fig. 1).
Primary and secondary endpoints
-
1.
Primary endpoint: All-cause in-hospital mortality.
-
2.
Secondary endpoints: 90-day mortality and 365-day all-cause mortality.
Data collection
Baseline characteristics included demographics (age, sex, BMI), medical history (hypertension, diabetes, dementia, cardiovascular, pulmonary, liver, kidney diseases, and malignancies), vital signs (heart rate, blood pressure, respiratory rate, temperature, and SpO2), disease severity scores, laboratory parameters, and medication use. Disease severity scores included the Glasgow Coma Scale (GCS), assessed at ICU admission to evaluate consciousness (range: 3–15); the Systemic Inflammatory Response Syndrome (SIRS) score, calculated based on temperature, heart rate, respiratory rate, and leukocyte count; the Logistic Organ Dysfunction System (LODS) score17, quantifying dysfunction across six organ systems (neurological, cardiovascular, respiratory, renal, hepatic, and hematologic) using data from the first 24 h of ICU admission; and the Sequential Organ Failure Assessment (SOFA) score, measuring multi-organ dysfunction and calculated daily during the ICU stay. Laboratory tests included blood glucose, coagulation profile, complete blood count, liver/kidney function, electrolytes, albumin, and the neutrophil-to-lymphocyte ratio (NLR). Medication use covered anticoagulants (heparin, warfarin, and DOACs) and NSAIDs (aspirin, ibuprofen). The ALI was calculated within 24 h of ICU admission as ALI = (BMI × Alb)/NLR10. Survival outcomes included in-hospital all-cause mortality (time from ICU admission to death), 90-day mortality (time from hospital discharge to death within 90 days), and 365-day mortality (time from ICU admission to death within 365 days). Patients discharged alive were censored at discharge for in-hospital mortality analysis, while those alive at the end of follow-up or lost to follow-up were censored at the last known date for 90-day and 365-day all-cause mortality analyses. Data were extracted using PostgreSQL software (v15) and Navicat Premium software (v15).
Statistical analysis
In this study, the Shapiro–Wilk normality test was used to assess the distribution of continuous variables. If a variable followed a normal distribution, it was presented as mean ± standard deviation (SD), and group comparisons were performed using analysis of variance (ANOVA). If the normality assumption was violated, variables were expressed as median (interquartile range, IQR), and group comparisons were conducted using the Kruskal–Wallis H test. As shown in supplementary Fig. 1, the ALI data did not follow a normal distribution; however, after logarithmic transformation, it exhibited normality. The transformed ALI values were then categorized into quartiles based on the IQR: Quartile 1 (− 1.24 to 3.26), Quartile 2 (3.26 to 4.33), Quartile 3 (4.33 to 5.25), and Quartile 4 (5.25 to 13.83).
For categorical variables, data were presented as frequencies and percentages, and associations between groups were analyzed using the chi-square test. If sample sizes were small or expected frequencies were low, the Fisher’s exact test was applied.
To investigate the independent risk factors for in-hospital mortality, 90-day mortality, and 1-year mortality, multiple logistic regression (MLR) analysis was performed with three models18:
-
1.
Model I: Unadjusted analysis.
-
2.
Model II: Adjusted for age, gender, race, vital signs, and comorbidities.
-
3.
Model III: Further adjusted for baseline variables with significant differences, including
race, vital signs (blood pressure, respiratory rate), comorbidities (hypertension, heart diseases, chronic kidney disease, chronic liver disease), severity scores (SOFA, LODS), laboratory markers (INR, WBC, RBC, HB, ALT, AST, BUN, Sodium), medication use (NSAIDs, Anticoagulants), length of ICU stay, and length of hospital stay (for non-in-hospital death analysis).
To assess the predictive ability of ALI and other variables, Receiver Operating Characteristic (ROC) curves were plotted, and the area under the curve (AUC) was calculated to evaluate the discriminatory power of ALI9.
To explore the relationship between ALI and short-term mortality, Kaplan–Meier survival curves were generated and compared using the log-rank test. A Cox proportional hazards regression model was used to examine potential non-linear associations between ALI and endpoints. Restricted cubic splines (RCS) and linear spline regression were employed to characterize the relationship between ALI and patient outcomes11.
Furthermore, subgroup analyses were conducted using stratified Cox proportional hazards models, where continuous variables were categorized based on clinical cut-off points or medians, followed by interaction tests to examine effect modifications.
All statistical analyses were conducted using STATA version 18 and R version 4.4.1. A two-tailed P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 1502 patients were included (age range: 58–79 years; 58% male). Based on the quartiles of log-transformed ALI values, patients were divided into four groups: Group T1 (− 1.24 ≤ log-transformed ALI < 3.26, N = 375), Group T2 (3.26 ≤ log-transformed ALI < 4.33, N = 376), Group T3 (4.33 ≤ log-transformed ALI < 5.25, N = 377), and Group T4 (5.25 ≤ log-transformed ALI < 13.83, N = 374). The baseline characteristics of these patients are presented in Table 1.
Patients in the T1 group (lowest log-transformed ALI) exhibited worse clinical conditions. They showed lower blood pressure, red blood cell counts, hemoglobin levels, and lower rates of NSAID and anticoagulant use, along with a lower prevalence of hypertension. In contrast, T1 patients had higher incidences of cardiac, liver and renal diseases, elevated SOFA and LODS scores, and increased white blood cell, AST, and BUN levels, resulting in longer ICU and hospital stays. No significant differences were observed among the groups for gender, age, heart rate, body temperature, SpO2, histories of diabetes, peripheral vascular disease, dementia, or in GCS, SIRS scores, and certain laboratory markers (e.g., coagulation parameters, Glucose, Creatinine, electrolytes, platelets) (P > 0.05).
Endpoint analysis
Overall, 18.64% of the patients died during hospitalization. The 90-day and 365-day all-cause mortality rates were 15.14% and 24.39%, respectively. When divided by Log-transformed ALI groups, in-hospital mortality was highest in the T1 group (34.93%) and lowest in the T3 group (10.08%) (P < 0.001). Similarly, the T1 group exhibited the highest 90-day (22.95%) and 1-year (33.61%) mortality rates, while the T4 group showed the lowest 90-day (11.66%) and 1-year (17.18%) mortality (P ≤ 0.001) (Table 2).
Relationship between ALI and endpoint events
Unadjusted (Model 1) and adjusted analyses (Models 2 and 3) revealed a negative correlation between log-transformed ALI and in-hospital mortality. In Model 3, each one-unit increase in log-transformed ALI was associated with a 26% reduction in in-hospital mortality (OR = 0.74; 95% CI: 0.64–0.86; P < 0.001) (Table 3).
When divided into quartiles, patients in Quartile 4 had a 56% lower in-hospital mortality rate compared to Quartile 1 (OR = 0.44; 95% CI: 0.28–0.71; P = 0.001), with trend analyses supporting these findings (P < 0.001). Similar inverse relationships were observed for 90-day and 1-year mortality: in Model 3, each one-unit increase in log-transformed ALI decreased 90-day and 1-year mortality by 19% (OR = 0.81; 95% CI: 0.69–0.94 and OR = 0.81; 95% CI: 0.72–0.91; both P < 0.001) Supplementary Table 1. Quartile analysis using Cox regression demonstrated a graded reduction in 90-day and 365-day all-cause mortality across ascending log-transformed ALI groups (Fig. 2).
Kaplan–Meier survival curves for secondary endpoint events after ischarge across the log—transformed ALI tertiles. (A) Kaplan–Meier survival curves for 90—day all—cause mortality after discharge across the log—transformed ALI tertiles. (B) Kaplan–Meier survival curves for 365—day all—cause mortality after discharge across the log—transformed ALI tertiles. (Note: ALI refers to the advanced lung cancer inflammation index.)
Prediction of endpoints by ALI
In multivariable-adjusted Model III, ALI demonstrated strong predictive performance: In-hospital mortality: AUC = 0.853 (95% CI: 0.822–0.884); 90-day mortality: AUC = 0.791 (95% CI: 0.753–0.829); 1-year mortality: AUC = 0.759 (95% CI: 0.722–0.796) (Fig. 3). For in-hospital mortality, ALI achieved balanced sensitivity (78.2%), accuracy (81.6%) and specificity (82.3%), with slightly lower yet clinically meaningful performance for post-discharge outcomes. (Supplementary Table 2).
Dose–response relationship between ALI and outcome events
A restricted cubic spline model revealed a nonlinear L-shaped association between log-transformed ALI and in-hospital mortality, with a turning point at log-transformed ALI = 4.0. Below this value, higher ALI correlated with reduced mortality risk; above 4.0, the association plateaued (Fig. 4 and supplement Table 3). For 90-day and 1-year mortality, the relationship appeared more linear.
Subgroup analysis
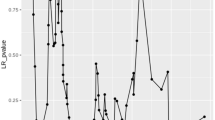
Subgroup analyses using categorical variables (age, gender, hypertension, diabetes, heart disease, liver and renal insufficiency) indicated that the inverse association between log-transformed ALI and mortality (in-hospital, 90-day, and 1-year) remained consistent across these groups (all P for interaction > 0.05, Fig. 5).
Subgroup Analysis of the Relationship between ALI and primary and secondary endpoint events in NCH Patients. (A) Subgroup Analysis of Log-transformed ALI and In-hospital Mortality in NCH. (B) Subgroup Analysis of Log-transformed ALI and 90-day Mortality after Discharge in NCH. (C) Subgroup Analysis of Log-transformed ALI and 365-day Mortality after Discharge in NCH.
Discussion
In this retrospective cohort study, we demonstrated that the Advanced Lung Cancer Inflammation Index (ALI) is a robust prognostic marker in critically ill patients with non-traumatic intracerebral hemorrhage (NCH). Our results indicate that lower ALI values—reflecting the dual burden of malnutrition (lower BMI and albumin) and heightened systemic inflammation (higher NLR)—are strongly associated with increased mortality at in-hospital, 90-day, and 1-year timepoints. These findings are consistent with previous investigations that identified nutritional depletion and systemic inflammation as key drivers of adverse outcomes in various critical illnesses, including ischemic stroke and cancer11,12,13.
Strengths and limitations relative to prior studies
Although NLR, albumin, and BMI have individually been linked to stroke mortality5,19,20; their isolated use fails to capture the synergistic effects of inflammation and malnutrition—a gap addressed by our composite ALI index in NCH. This approach aligns with emerging trends in critical care prognostication, where integrated biomarkers (e.g., the Controlling Nutritional Status score in sepsis) outperform single parameters, yet contrasts with stroke models still reliant on neurological scales alone21.While traditional prognostic tools (e.g., GCS, SOFA, LODS) mainly capture the severity of neurological injury22,23,24, our data suggest that ALI provides additional prognostic value by incorporating systemic factors. This observation supports recent evidence that emphasizes the interplay between neuroinflammation and catabolic processes in secondary brain injury23,25,26,27. Similar to reports in ischemic stroke and other inflammatory conditions, our findings reinforce that a lower ALI, driven by both chronic inflammatory states and acute injury, is predictive of worse outcomes11,12,13.
Inflammation-nutrition synergy: mechanisms and implications
The L-shaped ALI-mortality association mirrors thresholds observed in heart failure, , suggesting a universal pathophysiological ‘point of no return’ where inflammation-nutrition imbalance triggers irreversible organ failure28. While this concept is well-established in cardiology < sup > 40,41 < /sup > , our study extends it to neurocritical care by demonstrating that BBB disruption—previously attributed solely to neuroinflammation—is equally driven by systemic hypoalbuminemia and NLR elevation29,30,31. However, our finding that ALI’s post-discharge predictive capacity diminishes (AUC 0.76 at 1-year vs. 0.85 in-hospital) contrasts with ischemic stroke studies12 possibly reflecting NCH’s distinct secondary injury cascades.
The malnutrition-inflammation cycle we describe parallels reports in cirrhotic encephalopathy but differs in magnitude: NCH patients exhibited much higher hypoalbuminemia rates than general ICU populations, likely due to unique neurogenic gut dysfunction23,32,33. While prior works emphasized acute-phase proteins (e.g., CRP), our data highlight NLR-albumin interplay as the dominant driver, consistent with emerging “neuro-immunometabolism” paradigms34.
Clinical translation
ALI’s operational simplicity addresses limitations of current NCH prognostication. Unlike imaging-based scores requiring specialized modalities, ALI uses universally available metrics, similar to its validated role in prehospital trauma triage35. The Intracerebral Hemorrhage Score’s focus on initial injury severity, inherently overlooks dynamic secondary insults like systemic inflammation—a limitation mitigated by ALI. Similar to how SOFA scores refine sepsis prognostication beyond GCS ALI provides a ‘second-layer’ risk stratification, particularly relevant given NCH’s delayed mortality peaks beyond the acute phase36,37,38. Notably, ALI’s 78% sensitivity for in-hospital mortality exceeds the 65% reported for SIRS in NCH, though specificity remains comparable18. This positions ALI as a “rule-out” tool for early targeted interventions, akin to its use in oncology10.
Therapeutic implications
The strong ALI-mortality association supports dual-pathway interventions. The ALI threshold of 4.0 identifies a subgroup benefiting most from dual-pathway interventions, echoing recent guidelines advocating immune-nutrition in neurocritical care39. However, our null effect beyond this threshold challenges the ‘one-size-fits-all’ nutritional support prevalent in ICUs, underscoring the need for ALI-driven precision medicine akin to oncology40. Emerging NLR-modulating strategies—colchicine in heart failure41, canakinumab in atherosclerosis42—warrant exploration in NCH, particularly given our subgroup analyses showing consistent ALI effects across comorbidities.
Limitations
However, our study also has limitations. As with previous retrospective analyses, causality cannot be definitively established, and residual confounding cannot be completely ruled out. Additionally, unlike studies that assess dynamic changes in biomarkers, our static measurement of ALI within 24 h of ICU admission does not capture its temporal evolution. Future prospective, multicenter studies are needed to validate our findings and explore whether ALI-guided interventions can improve long-term outcomes in NCH patients.
Conclusion
In summary, our results not only align with but also extend previous observations by demonstrating that ALI—a composite marker of nutritional status and systemic inflammation—has strong prognostic significance in NCH. These findings highlight the importance of addressing both inflammatory and nutritional pathways in the management of these critically ill patients and pave the way for further research into targeted therapies that may alter the disease course.
Data availability
The data supporting the findings of this study can be obtained from the corresponding author.
References
Ferrari, A. J. et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161. https://doi.org/10.1016/s0140-6736(24)00757-8 (2021).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222. https://doi.org/10.1016/s0140-6736(20)30925-9 (2020).
He, Q. et al. Global, regional, and national burden of stroke, 1990–2021: A systematic analysis for global burden of disease 2021. Stroke 55, 2815–2824. https://doi.org/10.1161/strokeaha.124.048033 (2024).
Wang, J., Meng, J. F., Wang, S., Zhao, J. Z. & Cao, Y. Risk stratification of delayed causative aneurysm detection and long-term outcome in angiographically negative spontaneous subarachnoid haemorrhage. Stroke Vasc. Neurol. 9, 595–603. https://doi.org/10.1136/svn-2023-002546 (2024).
Gusdon, A. M. et al. CSF and serum inflammatory response and association with outcomes in spontaneous intracerebral hemorrhage with intraventricular extension: An analysis of the CLEAR-III Trial. J. Neuroinflamm. 18, 179. https://doi.org/10.1186/s12974-021-02224-w (2021).
Correia, J. C. et al. Zfp697 is an RNA-binding protein that regulates skeletal muscle inflammation and remodeling. Proc. Natl. Acad. Sci. USA 121, e2319724121. https://doi.org/10.1073/pnas.2319724121 (2024).
Badjatia, N. et al. Inflammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology 84, 680–687. https://doi.org/10.1212/wnl.0000000000001259 (2015).
Morotti, A. et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke 47, 1473–1478. https://doi.org/10.1161/strokeaha.116.013176 (2016).
Ozgen, E. et al. The relationship between neutrophil/lymphocyte, monocyte/ /lymphocyte, platelet/lymphocyte ratios and clinical outcomes after ninety days in patients who were diagnosed as having acute ischemic stroke in the emergency room and underwent a mechanical thro. Bratisl. Lek. Listy 121, 634–639. https://doi.org/10.4149/bll_2020_102 (2020).
Jafri, S. H., Shi, R. & Mills, G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 13, 158. https://doi.org/10.1186/1471-2407-13-158 (2013).
Chen, Y., Guan, M., Wang, R. & Wang, X. Relationship between advanced lung cancer inflammation index and long-term all-cause, cardiovascular, and cancer mortality among type 2 diabetes mellitus patients: NHANES, 1999–2018. Front. Endocrinol. (Lausanne) 14, 1298345. https://doi.org/10.3389/fendo.2023.1298345 (2023).
Huang, Y., Wang, X., Li, Z. & Yin, X. A novel nutritional inflammation index for predicting mortality in acute ischemic stroke patients: Insights into advanced lung cancer inflammation index from the medical information mart for intensive care-IV database. Front. Nutr. 11, 1408372. https://doi.org/10.3389/fnut.2024.1408372 (2024).
Zhang, Y. et al. The advanced lung cancer inflammation index predicts long-term outcomes in patients with hypertension: National health and nutrition examination study, 1999–2014. Front. Nutr. 9, 989914. https://doi.org/10.3389/fnut.2022.989914 (2022).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Goldberger, A. L. et al. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 101, E215-220. https://doi.org/10.1161/01.cir.101.23.e215 (2000).
Johnson, A., Bulgarelli, L., Pollard, T., Gow, B., Moody, B., Horng, S., Celi, L. A. & Markl, R. (2024). MIMIC-IV (version 3.1). PhysioNet. 10.13026/kpb9-mt58.
Brummel, N. E. et al. Systemic inflammation and delirium during critical illness. Intensive Care Med. 50, 687–696. https://doi.org/10.1007/s00134-024-07388-6 (2024).
Hagen, M. et al. Systemic inflammatory response syndrome and long-term outcome after intracerebral hemorrhage. Neurol. Neuroimmunol. Neuroinflamm. 6, e588. https://doi.org/10.1212/nxi.0000000000000588 (2019).
Zhang, D. et al. An analysis of neutrophil-to-lymphocyte ratios and monocyte-to-lymphocyte ratios with six-month prognosis after cerebral contusions. Front. Immunol. 15, 1336862. https://doi.org/10.3389/fimmu.2024.1336862 (2024).
Ma, Y. et al. Risk factor analysis and creation of an externally-validated prediction model for perioperative stroke following non-cardiac surgery: A multi-center retrospective and modeling study. PLoS Med. 22, e1004539. https://doi.org/10.1371/journal.pmed.1004539 (2025).
Toscano, A. et al. Unlocking the predictive power of nutritional scores in septic patients. Nutrients https://doi.org/10.3390/nu17030545 (2025).
Vreeburg, R. J. G. et al. Validation of the GCS-pupil scale in traumatic brain injury: Incremental prognostic value of pupillary reactivity with GCS in the prospective observational cohorts CENTER-TBI and TRACK-TBI. J. Neurotrauma https://doi.org/10.1089/neu.2024.0458 (2024).
Shestopalov, A. E., Yakovleva, A. V., Yadgarov, M. Y., Sergeev, I. V. & Kuzovlev, A. N. Prevalence and impact of malnutrition risk on outcomes in critically ill patients with traumatic brain injury and stroke: A retrospective cohort study using electronic health records. Nutrients https://doi.org/10.3390/nu16152396 (2024).
Aytuluk, H. G., Basaran, S., Dogan, N. O. & Demir, N. Comparison of conventional intensive care scoring systems and prognostic scores specific for intracerebral hemorrhage in predicting one-year mortality. Neurocrit. Care 34, 92–101. https://doi.org/10.1007/s12028-020-00987-3 (2021).
Nicholson, J. P., Wolmarans, M. R. & Park, G. R. The role of albumin in critical illness. Br. J. Anaesth. 85, 599–610. https://doi.org/10.1093/bja/85.4.599 (2000).
Limaye, K., Yang, J. D. & Hinduja, A. Role of admission serum albumin levels in patients with intracerebral hemorrhage. Acta Neurol. Belg. 116, 27–30. https://doi.org/10.1007/s13760-015-0504-2 (2016).
Malakouti, A. et al. Nutrition support and deficiencies in children with severe traumatic brain injury. Pediatr. Crit. Care Med. 13, e18-24. https://doi.org/10.1097/PCC.0b013e31820aba1f (2012).
Sun, X. et al. Advanced lung cancer inflammation index is associated with mortality in critically ill patients with heart failure. ESC Heart Fail. 12, 508–516. https://doi.org/10.1002/ehf2.15098 (2025).
Jeppesen, R., Orlovska-Waast, S., Sørensen, N. V., Christensen, R. H. B. & Benros, M. E. Cerebrospinal fluid and blood biomarkers of neuroinflammation and blood-brain barrier in psychotic disorders and individually matched healthy controls. Schizophr. Bull. 48, 1206–1216. https://doi.org/10.1093/schbul/sbac098 (2022).
Cao, Y. & Yao, X. Acute albumin administration as therapy for intracerebral hemorrhage: A literature review. Heliyon 10, e23946. https://doi.org/10.1016/j.heliyon.2023.e23946 (2024).
Zuo, L. et al. Low HALP (hemoglobin, albumin, lymphocyte, and platelet) score increases the risk of post-stroke cognitive impairment: A multicenter cohort study. Clin. Interv. Aging 19, 81–92. https://doi.org/10.2147/cia.S432885 (2024).
Romeiro, F. G. & Augusti, L. Nutritional assessment in cirrhotic patients with hepatic encephalopathy. World J. Hepatol. 7, 2940–2954. https://doi.org/10.4254/wjh.v7.i30.2940 (2015).
Rhee, H. et al. Worsening or improving hypoalbuminemia during continuous renal replacement therapy is predictive of patient outcome: A single-center retrospective study. J. Intensive Care 10, 25. https://doi.org/10.1186/s40560-022-00620-9 (2022).
Bonifacio, C. et al. The gut-brain axis: Correlation of choroid plexus volume and permeability with inflammatory biomarkers in Crohn’s disease. Neurobiol. Dis. 192, 106416. https://doi.org/10.1016/j.nbd.2024.106416 (2024).
Ferrazzano, P. A. et al. MRI and clinical variables for prediction of outcomes after pediatric severe traumatic brain injury. JAMA Netw. Open 7, e2425765. https://doi.org/10.1001/jamanetworkopen.2024.25765 (2024).
Pezzini, A. et al. Long-term risk of arterial thrombosis after intracerebral hemorrhage: MUCH-Italy. Stroke 55, 634–642. https://doi.org/10.1161/strokeaha.123.044626 (2024).
Yang, Y. et al. Association of nutritional and inflammatory status with all-cause and cardiovascular mortality in adults with sarcopenia: Insights from NHANES. Maturitas 196, 108233. https://doi.org/10.1016/j.maturitas.2025.108233 (2025).
Zhang, X. et al. The association between nutritional-inflammatory status and chronic kidney disease prognosis: A population-based study. Ren. Fail. 47, 2471016. https://doi.org/10.1080/0886022x.2025.2471016 (2025).
Hemphill, J. C. 3rd. et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46, 2032–2060. https://doi.org/10.1161/str.0000000000000069 (2015).
Feinberg, C., Dickerson Mayes, K., Jarvis, R. C. 3rd., Carr, C. & Mannix, R. Nutritional supplement and dietary interventions as a prophylaxis or treatment of sub-concussive repetitive head impact and mild traumatic brain injury: A systematic review. J. Neurotrauma 40, 1557–1566. https://doi.org/10.1089/neu.2022.0498 (2023).
Pascual-Figal, D. et al. Colchicine in acute heart failure: Rationale and design of a randomized double-blind placebo-controlled trial (COLICA). Eur. J. Heart Fail. 26, 1999–2007. https://doi.org/10.1002/ejhf.3300 (2024).
Everett, B. M. et al. Inhibition of interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS trial. J. Am. Coll. Cardiol. 76, 1660–1670. https://doi.org/10.1016/j.jacc.2020.08.011 (2020).
Acknowledgements
We express our sincere gratitude to all members of the MIMIC-IV research group for their steadfast support and collaborative contributions.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
PZ. and YFL. played pivotal roles in the comprehensive planning of the research projects, were responsible for devising the experimental design, and contributed to the writing of the article. PZ., XYC.,and PM. carried out the statistical analysis of the data and offered interpretations of the results. PZ., XYC., PM., and YMX. were engaged in the extraction, acquisition, and collation of the data. PZ. validated the accuracy of the data and examined for any errors in the charts. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The data utilized in this study were sourced from the MIMIC-IV database. The MIMIC-IV project received approval from the Institutional Review Board (IRB) at both the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Since patient information has been anonymized, this research project was deemed exempt from additional ethical review committee approval.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhong, P., Chen, X., Miao, P. et al. Advanced lung cancer inflammation index is associated with mortality in critically ill patients with non-traumatic cerebral hemorrhage. Sci Rep 15, 14972 (2025). https://doi.org/10.1038/s41598-025-98854-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98854-8