Abstract
To understand the relationship between type 2 diabetes (T2D) and risk for developing cognitive impairment, this study is the first to examine association between metabolites measured at mid-life and cognitive performance assessed later in life (8–10 years) in a T2D-enriched cohort. The discovery set included metabolomics from European Americans (EAs; n = 137) and African Americans (AAs; n = 134) from the Diabetes Heart Study (DHS) and the African American-DHS (AA-DHS). The cognitive testing battery included measures of executive function, memory, attention, language, and global cognition. Ancestry-specific linear regression analyses were performed and a false discovery rate (FDR)-corrected p-value was used to assess significance. Overall, fewer significant metabolites were associated with cognitive performance in AAs (n = 19) as compared to EAs (n = 118) suggesting racial differences. There was a positive association between sphingomyelins and cognitive performance, consistent with prior reports. Novel findings implicated partially characterized metabolites linked to oxidative breakdown of bilirubin to multiple cognitive domains suggesting further exploration of this class of metabolites towards improving pathophysiologic understanding and early intervention. Cross-ancestry replication identified four metabolites that generalized to both populations. Replication was performed among additional study participants, i.e. 421 EAs and 167 AAs, followed by a formal meta-analysis. Replication bolstered the association of multiple metabolites with cognitive function. Among these, cortisol was associated in AAs suggesting a link between stress and risk for reduced cognitive function. Further work is needed to provide insight into the pathophysiologic mechanisms and highlight metabolites for inclusion in risk stratification models of cognitive performance.
Similar content being viewed by others
Introduction
People with type 2 diabetes (T2D) have a 60% higher risk for all causes of dementia1 with a 39% increased risk of Alzheimer’s disease (AD) and over 2-fold increased risk of vascular dementia2 The presence of T2D in midlife significantly increases risk for dementia; for example, a recent study found that risk for dementia increases by a hazard ratio of 1.24 for every 5-year younger age of onset of T2D before age 703, i.e. the risk of dementia increased by 24% for every 5-year younger age of onset of T2D before the age of 70, and data from the Atherosclerosis Risk in Communities (ARIC) study show this risk may be even greater for African Americans (AAs)4 The earliest symptoms of dementia include memory impairment although additional cognitive domains, e.g. visuospatial and executive, may be affected5 T2D-associated comorbidities such as cardiovascular disease6,7, hypertension8,9, heart failure10,11 and kidney disease12,13,14 also increase risk of cognitive impairment, making T2D an important modifiable risk factor to target for cognitive health15.
The risk for AD and related dementias is approximately twice as high for AAs compared to European Americans (EAs)16 There is emerging research surrounding potential differences in genetic risk factors that may arise from ancestry17,18,19 However, current evidence suggests much of this disparate risk may be due to or strongly interact with health20,21 and social factors22,23,24 arising from historical inequities in access to education and health care. T2D is also more prevalent among AAs25 Given the strong association of T2D with risk for AD and the co-occurrence of both of these conditions, a better understanding of the contributing factors could provide insight into elevated ancestry-specific prevalence rates of dementia in AAs.
Metabolomics provides a powerful approach for exploring disease-specific indicators to understand biochemical pathways and identify predictive biomarkers with clinical applications. Blood-based metabolite profiles are easily accessible and can be used to reflect metabolic changes in the periphery driven by the interaction of genetic, lifestyle, and environmental risk factors to promote disease risk. With the application of untargeted metabolomics analysis, the biomarker search space can be expanded allowing for the identification of novel compounds not previously suspected to participate in the disease process.
This study used cross sectional data from participants in the Diabetes Heart Study (DHS) and African American-Diabetes Heart Study (AA-DHS) to assess relationships between mid-life metabolomic profiles and cognitive performance 8–10 years later. Cognitive function was assessed using a cognitive battery containing global and domain-specific cognitive tests (verbal learning and memory, working memory, processing speed, and executive functioning). Set- and ancestry-specific association analyses were performed modeling metabolites as predictors and cognitive test scores as outcomes while controlling for appropriate covariates. This approach could provide insight into pathophysiologic mechanisms and highlight metabolites for inclusion in risk stratification models.
Methods
Subjects
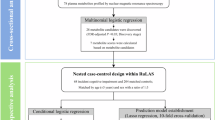
Study participants were recruited to the DHS Family of Studies, i.e., the Diabetes Heart Study (DHS), the African American DHS (AA-DHS), DHS Memory in Diabetes (MIND), and AA-DHS MIND26 (Fig. 1). Briefly, DHS was a genetic and epidemiological study of EA and AA families with multiple cases of T2D. Participants were initially assessed for measures of subclinical CVD. The AA-DHS cohort was used to expand DHS and improve the understanding of ancestry-specific differences in the relationship between T2D and associated chronic illnesses27 Beginning in 2008, the DHS MIND study performed cognitive testing in both EA and AA participants to explore relationships between T2D and cognition.
For all study participants, T2D was diagnosed as having onset after the age of 30 years in the absence of diabetic ketoacidosis with: (1) an active treatment for diabetes (insulin and/or oral hypoglycemic agents), (2) fasting blood glucose ≥ 126 mg/dL or non-fasting blood glucose ≥ 200 mg/dL or (3) hemoglobin (Hb) A1c ≥ 6.5%. Examinations took place in the Wake Forest University School of Medicine (WFUSM) Clinical Research Unit and included collection of medical history, vital signs, and current medications. Educational attainment and diabetes duration were self-reported. Laboratory assays included fasting measurement of serum creatinine, blood urea nitrogen, thyroid-stimulating hormone, vitamin B12 levels, and urine albumin-to-creatinine ratio (LabCorp, Burlington, NC).
This study was approved by the WFUSM Institutional Review Board and adhered to the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Cognitive testing
Cognitive testing was performed in a quiet room after a midmorning snack. The Rey Auditory Verbal Learning Test (RAVLT) was used to measure learning and memory. The data used for analysis represents the sum of the words recalled correctly over the first five trials (RAVLT Sum of 5 Trials) and recall after a 25-minute delay (RAVLT Delayed Recall)28 Global cognition was assessed using the Modified Mini-Mental State Exam (3MSE)29 The Digit Symbol Coding (DSC) task, representing the number of geometric figures that are accurately decoded to specific numbers in 60 s, assessed processing speed and, to a lesser extent, working memory30 The interference score, which reflects ability to inhibit an overlearned response (an aspect of executive function), was calculated for the modified Stroop test as described in Houx et al.31 Semantic fluency was measured by the number of animals named in 60 s and assesses rapid semantic word retrieval (another aspect of executive function)32.
Metabolomics
Metabolite profiling was performed using plasma samples stored at -80°C from DHS (1998–2000) and AA-DHS (2007–2010). Sample selection was randomized for processing order and balanced by age, sex, BMI, and ancestry. Global untargeted metabolomic profiling was performed by Metabolon Inc. (Durham, North Carolina) on the DiscoveryHD4 panel. Methodology and quality control have been previously described33 Briefly, metabolites were measured using liquid chromatography and high resolution/accurate mass spectrometry. Peaks were annotated by comparison to Metabolon’s library of purified standards or recurrent, uncharacterized entities. In addition to individual named biochemicals, super- and sub-pathways were annotated based on a combination of pathway and chemical structure similarities to serve as a guide for interpretation. Metabolite values were normalized by extraction volume, block corrected for batch and run day, and missing data imputed to the minimum. Study samples were processed in three sets. Discovery samples (Set 1) included 137 EAs and 134 AAs and were processed in 2019. Replication samples (Sets 2 and 3) included 421 EAs and 167 AAs and were processed in 2020 and 2021, respectively (Fig. 1).
Statistical analysis
Set- and ancestry-specific association analyses using linear regression models incorporating generalized estimating equations (GEE) were performed. Models accounted for familial correlation, when necessary, using a sandwich estimator of the variance. Metabolites were inverse normal transformed and modeled as predictors. The residuals of the cognitive test scores were normally distributed, so no transformations were necessary. Cognitive test scores were modeled as the outcomes. All analyses were controlled for age, sex, BMI, HbA1c, educational attainment, statin use, prevalent CVD, smoking status, hypertension status at the MIND exam, and time interval between midlife (plasma collection) and MIND (cognitive assessment) visits. Metabolites not detected in more than 50% of participants were analyzed as both continuous and binary (dichotomized as detectable vs. non-detectable) measures. SAS version 9.4 (SAS Inc., Cary, NC) was used for analysis. In the discovery phase, a false discovery rate (FDR)34 was used to account for multiple comparisons testing, i.e. a PFDR<0.05 was considered statistically significant. For cross-ancestry comparison of significant hits in the discovery phase, a nominally significant threshold of P < 0.05 was considered significant. In the replication phase, additional DHS and AA-DHS participants, recruited and processed identically, were evaluated to assess replication of associated metabolites with cognitive measures. For the replication phase, a Bonferroni-corrected P < 0.05, after accounting for the trait-specific number of metabolites tested, was considered significant. To assess overall evidence of association of significant metabolites identified from the discovery analysis, formal set- and ancestry-specific meta-analyses were calculated using a random effects model implemented in rmeta (R version 4.1.1) and significance was assessed after trait-specific Bonferroni correction (Fig. 1).
Results
Participant demographics for the discovery samples are shown in Table 1. EAs (60.4 ± 9.4 years) were older than AAs (58.5 ± 8.7 years) and included fewer females (47% and 58%, respectively). In addition, AAs had significantly higher average BMI, i.e. p < 0.01 between EAs (31.9 ± 6.7 kg/m2) and AAs (34.5 ± 8.6 kg/m2). Over 85% of participants had hypertension and there was no significant difference between cohorts. The average hemoglobin A1c (HbA1c) approximated 7.0%, indicating generally controlled diabetes and over 40% of EA and 65% of AA participants were on lipid lowering medication. Overall, self-reported educational attainment was high, with over 80% of the total sample reporting a high school education or above.
Association of metabolites with cognition in EAs (Discovery)
In EAs, 1132 metabolites inclusive of amino acid, carbohydrate, cofactors and vitamins, energy, lipid, nucleotide, and peptide super-pathways and partially characterized metabolites were detected and included in analysis. Xenobiotics were excluded. A total of 118 metabolites were associated with cognitive performance 8–10 years later across the five cognitive tests examined (PFDR<0.05; SF1A-D, ST1-6).
Six metabolites, including two annotated and four partially characterized metabolites, were associated with RAVLT Delayed Recall (SF2A, ST2), and included an inverse relationship with retinol (vitamin A; β=-2.10, PFDR=0.020) and positive association with cysteine sulfinic acid (β = 1.00, PFDR=0.028). There were positive associations with four partially characterized metabolites including X-11441 (β = 0.95, PFDR=0.020), X-11442 (β = 0.91, PFDR=0.022), X-15220 (β = 1.69, PFDR=0.037), and X-21448 (β = 0.95, PFDR=0.037).
Forty-seven metabolites, including 36 annotated and 11 partially characterized metabolites, were associated with results from the 3MSE (SF2B, ST3). The majority of metabolites represented lipid (n = 17) and amino acid (n = 11) super-pathways. The most significantly associated metabolites with the 3MSE were isoursodeoxycholate sulfate (lipid super-pathway; β=-4.75, PFDR=1.74 × 10− 5) involved in secondary bile acid metabolism and 2-aminobutyrate (amino acid super-pathway; β = 1.79, PFDR=4.96 × 10− 5) involved in glutathione metabolism. Among lipid metabolism pathways, sphingomyelins were positively associated with performance on the 3MSE (β = 1.67-2.00, PFDR=0.021–0.047). Among amino acid metabolism pathways, branched chain amino acid metabolites leucine and N-acetylisoleucine (β = 1.75–1.84, PFDR=0.020–0.033) and phyenylalanine metabolite N-acetylphenylalanine (β = 2.15, PFDR=0.012), historically associated with risk for T2D, were positively associated with performance on the 3MSE. Several partially characterized metabolites were associated with 3MSE performance including X-15666 (β = 2.26, PFDR=0.01) and X-21448 (β = 2.38, PFDR=0.023).
Twenty-eight metabolites, including 19 annotated and nine partially characterized metabolites, were associated with DSC, with the majority representing amino acid (n = 8) and lipid (n = 7) super-pathways (SF2C, ST4). Among these, the most significant metabolites were involved in phenylalanine metabolism with results for N-acetylphenylalanine (β = 3.45, PFDR=1.55 × 10− 4) consistent with associations reported for the 3MSE. In addition, metabolites in tryptophan metabolism were positively associated and included tryptophan (β = 3.26, PFDR=0.0052), indoleacetolycarnitine (β = 2.98, PFDR=0.024) and N-acetyltryptophan (β = 2.68, PFDR=0.025). Notably, two partially characterized metabolites X-15666 and X-21448 were consistently and positively associated with DSC (β = 3.21–4.07, PFDR<0.034, respectively) and 3MSE (β = 2.26–2.38, PFDR<0.023, respectively).
Thirty-seven metabolites, including 34 annotated and three partially characterized metabolites, were associated with semantic fluency (SF2D, ST6). Twenty-eight were ascribed to the lipid super-pathway with a predominance of positively associated sphingomyelin metabolites (n = 11, β = 0.93–1.39, PFDR=2.56 × 10− 5-0.036). Four sphingomyelins associated with semantic fluency were also positively associated with the 3MSE (β = 1.67-2.00, PFDR=0.026–0.047). In addition, four lipids (β = 1.04–1.23, PFDR=2.20 × 10− 4-0.002), two amino acids (kynurenine and 3-(4-hydroxyphenyl) lactate (HPLA), β = 1.25–1.29, PFDR=0.001–0.002), and one partially characterized metabolite (X-24455, β = 1.00, PFDR=0.032) that were positively associated with semantic fluency were also significantly associated with the DSC (β = 2.21–4.20, PFDR=0.0052–0.049). No metabolites were associated with the RAVLT Sum of 5 Trials (ST1) or the Stroop test (ST5) in the EA discovery sample.
Association of metabolites with cognition in AAs (Discovery)
In AAs, among 1132 metabolites, excluding xenobiotics, detected in plasma samples collected at midlife, 19 metabolites were associated across the five cognitive tests administered 8–10 years later (PFDR<0.05; SF2A-B, ST7-12). Fewer significant associations were observed among AAs as compared to 118 metabolites associated with cognition in EA participants. Two metabolites in the lipid super-pathway (SF3A, ST7), cortisol (β=-2.48, PFDR=0.016) and decadienedioic acid (β=-2.68, PFDR=0.029), were inversely associated with performance on the RAVLT Sum of 5 Trials. Three metabolites were associated with results from the 3MSE (SF3B, ST9), i.e. two sphingomyelins (β= -2.6- -2.46PFDR = 0.047 for each) were inversely associated, and one partially characterized metabolite (X-18901, β = 2.38, PFDR=0.047) was positively associated. Fourteen metabolites, including seven annotated and seven partially characterized metabolites were associated with DSC (SF3C, ST10). The most significant associations with the DSC in the AA discovery cohort were glycerate (β = 3.40, PFDR=0.016), a carbohydrate metabolite involved in glycolysis, gluconeogenesis and pyruvate metabolism, a partially characterized metabolite (X-11880; β = 3.24, PFDR=0.016), and chiro-inositol (β = 7.86, PFDR=0.042). Among the cofactors and vitamins super-pathway, two metabolites involved in ascorbate and aldarate metabolism, oxalate (β = 3.32, PFDR=0.045) and threonate (β = 2.77, PFDR=0.048), were positively associated with DSC. There were no significant associations between metabolites and RAVLT Delayed Recall (ST8), Stroop test (ST11) or semantic fluency (ST12).
Cross-ancestry association of metabolites with cognitive performance (Discovery)
A total of 118 metabolites associated with cognitive measures among EAs in the discovery analysis were assessed for cross-ancestry associations among AAs in the discovery analysis (ST13). Of the six metabolites associated with RAVLT Delayed Recall in the EA discovery cohort, four showed a consistent direction of effect in AAs but none reached nominal significance (P < 0.05). Of the 47 metabolites associated with the 3MSE in the EA discovery cohort, 18 were directionally consistent among AAs with one (alpha-CMBHC glucuronide, involved in tocopherol metabolism) obtaining nominal levels of significance. For DSC, 28 metabolites were significantly associated among EAs with 13 observed to have a consistent direction of effect and two achieving nominal significance among AAs (Table 2), the amino acid histidine and a partially characterized metabolite, X-21448. Finally, 37 metabolites were significantly associated with semantic fluency among EAs with 20 observed to have a consistent direction of effect among AAs but none attaining nominal significance.
A total of 18 metabolites associated with cognitive measures among AAs in the discovery analysis were assessed for cross-ancestry associations among EAs (ST14). Among AAs, two metabolites were associated with performance on the RAVLT Sum of 5 Trials. One of these, decadienedioic acid, was directionally consistent among EAs but did not reach a nominal level of significance (P < 0.05). For 3MSE, three metabolites were assessed for cross-ancestry associations; however, only one partially characterized metabolite (X-18901) was directionally consistent but did not reach nominal significance. For DSC, 14 metabolites were significant among AAs with 10 observed to have a consistent direction of effect and two achieving nominal significance in the EA sample (Table 3), i.e. a partially characterized metabolite X-11880 and the peptide HWESASXX.
Replication of metabolites associated with cognition in EAs
Replication of 118 metabolites significantly associated with measures of cognitive function in EAs was assessed in an additional 421 DHS MIND participants recruited under the same protocol (ST15). Among six metabolites associated with RAVLT Delayed Recall, three were detected in the replication sample and a partially characterized metabolite, X-15220, was significantly associated in the overall meta-analysis (ST16; β = 1.77, P = 2.21 × 10− 5). Forty-two of the 47 metabolites associated with results from the 3MSE were detected in the replication sample (ST17). Nineteen metabolites exhibited a consistent direction of effect with one attaining nominal significance (P < 0.05). A formal meta-analysis revealed a single inversely associated partially characterized metabolite (X-25805) that was nominally significant (β=-1.76, P = 0.00052) even after Bonferroni correction (P < 0.0011) for 42 metabolites.
Of the 28 metabolites associated with DSC performance in the EA discovery sample, 23 were detected in the replication sample, 20 had a consistent direction of effect in the replication sample, and one metabolite (tryptophan, β = 1.85, P = 0.0057) was nominally significant (ST18). In the meta-analysis, eight metabolites were nominally significant with tryptophan remaining significant after applying a Bonferroni correction (P < 0.0022; Table 4).
Among 37 metabolites associated with semantic fluency in the discovery sample, 36 were detected in the replication sample and 26 were directionally consistent (ST19). Among these metabolites, sphingomyelin (d17:1/14:0, d16:1/15:0)* was nominally significant (β = 0.39, P = 0.037) in the replication sample and the meta-analysis (β = 0.59, P = 0.0084) but was not significant after applying a Bonferroni correction (P < 0.0014).
Replication of metabolites associated with cognition in AAs
Replication of the 19 metabolites significantly associated with cognitive function in the AA discovery sample was assessed in an additional 167 AA-DHS MIND participants recruited under the same protocol (ST15). Among the two metabolites associated with RAVLT Sum of 5 Trials in the discovery sample, both were detected in the replication sample, and one was directionally consistent but failed to reach nominal levels of significance (P < 0.05; ST20). However, in the overall meta-analysis, cortisol was significantly inversely associated with scores on the RAVLT Sum of 5 Trials (β=-2.26, P = 5.80 × 10− 5, Table 5). Three metabolites were associated with 3MSE in the AA discovery sample; however, only one metabolite (X-18901) was directionally consistent but did not reach statistical significance in the replication or overall meta-analysis (ST21). All 14 metabolites associated with DSC in the AA discovery sample were detected in the replication sample (ST22). All associations were directionally consistent and three were nominally significant. In the overall meta-analysis, 11 metabolites were nominally significant with eight remaining significant after Bonferroni correction (P < 0.0038). These included glycerate, chiro-inositol, threonate, and five partially characterized metabolites (Table 5).
Discussion
AD and T2D, two diseases common in aging, are associated with midlife risk factors and possess long prodromal periods35,36,37 T2D increases the risk for dementia, including dementia arising from AD, and both T2D and dementia disproportionately affect AAs in the US. Given the increased risk, identifying midlife biomarkers associated with cognitive impairment in people of both AA and EA descent could identify new biomarkers for earlier identification and suggest new pathways for treatment. This report details results toward a more comprehensive identification of predictive biomarkers. We examined a total of 1134 plasma metabolites detected in midlife from samples of EA and AA participants enriched for T2D from DHS and AA-DHS for association with cognitive performance assessed 8–10 years later.
Statistically, the most significant metabolite association with cognitive function measured 8–10 years after metabolomic assessment was identified in the EA discovery sample. Isoursodeoxycholate sulfate, involved in the secondary bile acid metabolism sub-pathway, was significantly associated with results from the 3MSE which measures global cognition. Recent research suggests a role for secondary bile acids as neuromodulators in the brain-gut-microbiota axis which has been implicated in the pathogenesis of AD38.
Several metabolites were associated with more than one cognitive test in the EA discovery sample. The sphingomyelin pathway was positively associated with 3MSE, DSC, and semantic fluency. Plasma sphingomyelin and other sphingolipids have been shown to increase the risk of cardiovascular disease39 and insulin resistance40 which increases the risk for AD. Cognitive testing association studies have suggested that higher levels of sphingomyelins are associated with less decline over time on the Mini-Mental State Examination (MMSE)41, consistent with the observation herein. Among metabolites with dual associations, N-acetylphenylalanine was positively associated with 3MSE and DSC suggesting a role in global cognitive function and processing speed. Dysregulation of phenylalanine metabolism in the brain, specifically the hippocampus, has been implicated in the pathogenesis of AD42 Similarly, tryptophan was positively associated with DSC and fluency suggesting a role in executive function and processing speed. Tryptophan is an amino acid essential for the production of serotonin and treatment with selective serotonin reuptake inhibitors is associated with improved processing speed43 Tryptophan levels are lower with frailty and correlated with MoCA score,44 lower in people with mild cognitive impairment (MCI)45 and later stages of AD pathology,46 and are implicated in Aβ biochemistry and resultant toxicity47 In the context of T2D, tryptophan levels are observed to be lower in diabetics48 These associations follow the sequela of T2D increasing the risk for cognitive impairment and AD.
Among the novel associations identified in EA discovery samples, the partially characterized metabolite X-21448 was positively associated with RAVLT Delayed Recall, DSC, and 3MSE. It also showed a nominal association with the DSC in the AA discovery cohort. Characterization of metabolite levels using whole-genome sequencing identified association between X-21448 and UGT1A (UDP glucuronosyltransferase family 1 member A complex locus). This association suggests that this and two other metabolites (X-11530 and X-16946) are possible oxidation breakdown products of bilirubin49 Of note, X16946 was also associated with DSC performance in the EA discovery cohort. Bilirubin has been implicated in the pathogenesis of AD through accumulation of Aβ50 Although bilirubin was not associated here, likely due to lack of detection in more than 50% of participants, the increase of bilirubin breakdown products associated with improved cognition across multiple cognitive tests supports this mechanism. In addition to the associations with X-21448 and X-16946 noted above, X-11441 was positively associated with RAVLT Delayed Recall and 3MSE and X-11442 was positively associated with RAVLT Delayed Recall and DSC; both of these metabolites are highly correlated with bilirubin51 Further exploration and identification of partially characterized metabolites could positively influence biomarker discovery towards pathophysiologic understanding and early intervention.
Overall, a greater number of midlife metabolites were significantly associated with later life cognitive function in EAs (n = 118) than AAs (n = 19) in the discovery cohort. This observation is consistent with ethnic disparities impacting T2D and related comorbidities52 As previously suggested53 metabolite abundance, as a result of lifestyle, socioeconomic factors, and psychosocial stressors, may contribute to racial disparities in disease susceptibility, complicating the disentanglement of biological and social effects, and this has clinical implications for assessment, monitoring, and intervention. Among associated metabolites, four displayed generalizability across ancestry. In the EA discovery sample, alpha-CMBHC glucuronide positively associated with 3MSE and histidine and the partially characterized metabolite X-21448 were positively associated with DSC with consistent trait associations in AAs. Alpha-CMBHC glucuronide is a metabolite in tocopherol (Vitamin E) metabolism. Vitamin E is an important antioxidant which protects the central nervous system against oxidative stress and progression of cognitive decline54 Similarly, the antioxidant histidine has been reported to be decreased in AD patients55 In the AA discovery sample, the inflammation associated complement component 3 peptide, HWESASXX, and the partially characterized metabolite X-11880 were positively associated with DSC with consistent significant association in EAs. The exact role of these metabolites in cognitive function remains unclear; however, higher levels of HWESASXX have been positively associated with visceral fat and hypertension56,57, risk factors for AD.
Replication identified multiple metabolites with a consistent direction of effect, bolstering significance in the overall analysis. Among EAs, replication enhanced the association among eight metabolites associated with DSC (tryptophan, indoleacetoylcarnitine, histidine, lysine, sphingomyelin (d18:1/21:0, d17:1/22:0, d16:1/23:0)*, a glutamine conjugate, X-24456, and X-12839), and one each associated with RAVLT Delayed Recall (X-15220), 3MSE (X-25805) and fluency (sphingomyelin (d17:1/14:0, d16:1/15:0)). Among replicated associations, lysine has been identified as an essential amino acid that improves cognitive function58, consistent with the positive association with DSC observed in the EA sample. Notably, lysine was positively correlated among AAs but not significant.
Among AAs, replication enhanced the association among 12 metabolites associated with RAVLT (cortisol) and DSC (glycerate, threonate, chiro-inositol, oxalate (ethanedioate), X-11880, X-24951, X-11372, X-24953, X-21339, X-11308, and X-12812). Interestingly, one of the most significant associations observed overall was between cortisol, a steroid hormone released in response to stress, and RAVLT Sum of 5 Trials which assessed verbal learning. Cortisol was not associated with cognitive test results in EAs. Minorities, including AAs, are hypothesized to be more heavily impacted by chronic stress and resultant high levels of cortisol59 It has also been reported that higher cortisol levels are associated with increased risk of cognitive decline and AD60 Consistent with previous reports61, our results suggest that cortisol may represent a mediator between stress and risk of cognitive decline leading to dementia.
To date, published studies have not evaluated T2D-enriched cohorts to identify metabolomic predictors of cognitive status; however, one other analysis examined associations between midlife metabolomic markers and later life cognition in AAs62 This analysis focused on serum metabolites from Atherosclerosis Risk in Communities (ARIC) Study participants who had baseline and 6-year follow-up scores on the DSC task, a 10-word delayed recall task (similar to the RAVLT) and letter fluency (compared to semantic fluency as used in this study). They observed that change in 6-year cognitive performance on delayed word recall was associated with histidine metabolism (N-acetyl-1-methylhistidine) and on the DSC task was associated with an essential fatty acid (docosapentaenoate (n-6 DPA)) and a partially characterized metabolite (X-12844). Although our findings did not directly replicate findings from the ARIC Study, we did observe associations between 9-hydroxystearate, a fatty acid metabolite and performance on a measure of processing speed (DSC).
Strengths of the current study include untargeted metabolomic profiling of a T2D-enriched cohort with multi-domain cognitive assessment. Inclusion of two ancestral populations further facilitated generalizability of the findings. We detected novel metabolites associated with domains of cognition and characterized the underlying pathways. However, this study has limitations. The current metabolite datasets represent untargeted metabolomic profiling using LCMS, which captures only a proportion of the metabolome. This coverage could be enhanced with complementary technologies, e.g. nuclear magnetic resonance (NMR) or gas chromatography mass spectrometry. In addition, the results described herein were based on cross-sectional data collection precluding establishment of a causal relationship and the overall sample size may have negatively affected our ability to observe more modest effects and assess cross-ancestry replication. Finally, genetic contributions to disease risk, e.g. the apolipoprotein E gene (APOE), were not included in the statistical analysis.
In conclusion, results from this study implicated metabolites, e.g. tryptophan and histidine, and pathways, e.g. sphingomyelin, for association with cognitive function and were consistent with the published literature. It also highlighted novel metabolites, e.g. N-acetylphenylalanine and X21448 involved in bilirubin breakdown, for further investigation. Taken together, these observations could provide insight into the pathophysiologic mechanisms that predispose to cognitive decline in the setting of T2D and allow for earlier identification of individuals at higher risk for dementia for earlier intervention. This is particularly important during midlife when the disease trajectory could be augmented with regular evaluation of metabolites associated with declining cognitive function. The use of ancestry stratification has provided evidence to support generalizability of metabolite associations but also insight into the metabolomic differences between ancestry groups. Our study and previous reports have established association among partially characterized metabolites that represent an unexplored pathway to reveal novel biological insight and putative biomarkers. Future studies, bolstered by larger sample sizes, may further strengthen the evidence of association; however, it will also be interesting to explore if the associations are unique to the metabolic dysregulation present in T2D or broadly generalizable.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Hendrie, H. C. et al. Changes of glucose levels precede dementia in African-Americans with diabetes but not in Caucasians. Alzheimer’s Dement. 14, 1572–1579. https://doi.org/10.1016/j.jalz.2018.03.008 (2018).
Lu, F. P., Lin, K. P. & Kuo, H. K. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 4, e4144. https://doi.org/10.1371/journal.pone.0004144 (2009).
Amidei, C. B. et al. Association between age at diabetes onset and subsequent risk of dementia. Jama-J Am. Med. Assoc. 325, 1640–1649. https://doi.org/10.1001/jama.2021.4001 (2021).
Mayeda, E. R. et al. Type 2 diabetes and cognitive decline over 14 years in Middle-Aged African Americans and Whites: the ARIC brain MRI study. Neuroepidemiology 43, 220–227. https://doi.org/10.1159/000366506 (2014).
Barnes, J. et al. Alzheimer’s disease first symptoms are age dependent: evidence from the NACC dataset. Alzheimers Dement. 11, 1349–1357. https://doi.org/10.1016/j.jalz.2014.12.007 (2015).
Deckers, K. et al. Coronary heart disease and risk for cognitive impairment or dementia: systematic review and meta-analysis. PLoS One. 12, e0184244. https://doi.org/10.1371/journal.pone.0184244 (2017).
Eggermont, L. H. et al. Cardiac disease and cognitive impairment: a systematic review. Heart 98, 1334–1340. https://doi.org/10.1136/heartjnl-2012-301682 (2012).
Gottesman, R. F. et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 71, 1218–1227. https://doi.org/10.1001/jamaneurol.2014.1646 (2014).
Group, S. M. I. S. R. et al. Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 321, 553–561. https://doi.org/10.1001/jama.2018.21442 (2019).
Gure, T. R. et al. Prevalence of cognitive impairment in older adults with heart failure. J. Am. Geriatr. Soc. 60, 1724–1729. https://doi.org/10.1111/j.1532-5415.2012.04097.x (2012).
Pastva, A. M. et al. Physical function, and quality of life in older patients with acute decompensated heart failure. J. Card Fail. 27, 286–294. https://doi.org/10.1016/j.cardfail.2020.09.007 (2021). Cognition.
Zammit, A. R., Katz, M. J., Bitzer, M. & Lipton, R. B. Cognitive impairment and dementia in older adults with chronic kidney disease: A review. Alzheimer Dis. Assoc. Disord. 30, 357–366. https://doi.org/10.1097/WAD.0000000000000178 (2016).
Freedman, B. I. et al. Associations of early kidney disease with brain magnetic resonance imaging and cognitive function in African Americans with type 2 diabetes mellitus. Am. J. Kidney Dis. 70, 627–637. https://doi.org/10.1053/j.ajkd.2017.05.006 (2017).
Palmer, N. D. et al. Kidney disease and cognitive function: African American-Diabetes Heart Study MIND. Am. J. Nephrol. 40, 200–207. https://doi.org/10.1159/000367669 (2014).
Ott, A. et al. Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 53, 1937–1942. https://doi.org/10.1212/wnl.53.9.1937 (1999).
Kornblith, E. et al. Association of race and ethnicity with incidence of dementia among older adults. Jama-J Am. Med. Assoc. 327, 1488–1495. https://doi.org/10.1001/jama.2022.3550 (2022).
Deters, K. D. et al. L. TOMM40-APOE haplotypes are associated with cognitive decline in non-demented Blacks. Alzheimers Dement. 17, 1287–1296. https://doi.org/10.1002/alz.12295 (2021).
Kunkle, B. W. et al. Novel alzheimer disease risk loci and pathways in African American individuals using the African genome resources panel: A Meta-analysis. JAMA Neurol. 78, 102–113. https://doi.org/10.1001/jamaneurol.2020.3536 (2021).
Sarnowski, C. et al. Meta-analysis of genome-wide association studies identifies ancestry-specific associations underlying Circulating total Tau levels. Commun. Biol. 5, 336. https://doi.org/10.1038/s42003-022-03287-y (2022).
Kumar, V. V. et al. Baseline results: the association between cardiovascular risk and preclinical Alzheimer’s disease pathology (ASCEND) study. J. Alzheimers Dis. 75, 109–117. https://doi.org/10.3233/JAD-191103 (2020).
Xiong, C. et al. Complex interactions underlie Racial disparity in the risk of developing Alzheimer’s disease dementia. Alzheimers Dement. 16, 589–597. https://doi.org/10.1002/alz.12060 (2020).
Meeker, K. L. et al. Socioeconomic status mediates Racial differences seen using the AT(N) framework. Ann. Neurol. 89, 254–265. https://doi.org/10.1002/ana.25948 (2021).
Vonk, J. M. J. et al. Education moderates the relation between APOE varepsilon4 and memory in nondemented Non-Hispanic black older adults. J. Alzheimers Dis. 72, 495–506. https://doi.org/10.3233/JAD-190415 (2019).
Weuve, J. et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of alzheimer disease dementia. Epidemiology 29, 151–159. https://doi.org/10.1097/EDE.0000000000000747 (2018).
Control, C. f. D. Summary Health Statistics: National Health Interview Survey: 2018. Centers for Disease Control and Prevention http://www.cdc.gov/nchs/nhis/shs/tables.htm (2021).
Bowden, D. W. et al. Review of the Diabetes Heart Study (DHS) family of studies: a comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. Rev. Diabet. Stud. 7, 188–201. https://doi.org/10.1900/RDS.2010.7.188 (2010).
Divers, J. et al. Genome-wide association study of coronary artery calcified atherosclerotic plaque in African Americans with type 2 diabetes. BMC Genet. 18, 105. https://doi.org/10.1186/s12863-017-0572-9 (2017).
Lezak, M. D., Howieson, D. B., Loring, D. W., Hannay, H. J. & Fischer, J. S. Neuropsychological assessment, 4th Edition (Oxford University Press, 2004).
Teng, E. L. & Chui, H. C. The modified Mini-Mental state (3MS) examination. J. Clin. Psychiatry. 48, 314–318 (1987).
Wechsler, D. Manual for the Wechlser Adult Intelligence Scale-Revised (Psychological Corporation, 1981).
Houx, P. J., Jolles, J. & Vreeling, F. W. Stroop interference: aging effects assessed with the Stroop color-word test. Exp. Aging Res. 19, 209–224. https://doi.org/10.1080/03610739308253934 (1993).
Benton, A. L., Hamsher, K. & Sivan, A. B. Multilingual Aphasia Examination (AJA Associates, 1994).
Chevli, P. A. et al. Plasma metabolomic profiling in subclinical atherosclerosis: the Diabetes Heart Study. Cardiovasc. Diabetol. 20, 231. https://doi.org/10.1186/s12933-021-01419-y (2021).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Royal Stat. Soc. Ser. B: Stat. Methodol. 57, 289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x (1995).
Li, G. et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology 69, 631–639. https://doi.org/10.1212/01.wnl.0000267428.62582.aa (2007).
Price, J. L. et al. Neuropathology of nondemented aging: presumptive evidence for preclinical alzheimer disease. Neurobiol. Aging. 30, 1026–1036. https://doi.org/10.1016/j.neurobiolaging.2009.04.002 (2009).
Li, G. et al. Cognitive trajectory changes over 20 years before dementia diagnosis: A large cohort study. J. Am. Geriatr. Soc. 65, 2627–2633. https://doi.org/10.1111/jgs.15077 (2017).
Mulak, A. Bile acids as key modulators of the Brain-Gut-Microbiota Axis in Alzheimer’s disease. J. Alzheimers Dis. 84, 461–477. https://doi.org/10.3233/JAD-210608 (2021).
Ichi, I. et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids 41, 859–863. https://doi.org/10.1007/s11745-006-5041-6 (2006).
Summers, S. A. Sphingolipids and insulin resistance: the five Ws. Curr. Opin. Lipidol. 21, 128–135. https://doi.org/10.1097/MOL.0b013e3283373b66 (2010).
Mielke, M. M. et al. Plasma sphingomyelins are associated with cognitive progression in Alzheimer’s disease. J. Alzheimers Dis. 27, 259–269. https://doi.org/10.3233/jad-2011-110405 (2011).
Liu, P. et al. Phenylalanine metabolism is dysregulated in human Hippocampus with Alzheimer’s disease related pathological changes. J. Alzheimers Dis. 83, 609–622. https://doi.org/10.3233/JAD-210461 (2021).
Gudayol-Ferre, E., Duarte-Rosas, P., Pero-Cebollero, M. & Guardia-Olmos, J. The effect of second-generation antidepressant treatment on the attention and mental processing speed of patients with major depressive disorder: A meta-analysis study with structural equation models. Psychiatry Res. 314, 114662. https://doi.org/10.1016/j.psychres.2022.114662 (2022).
Kameda, M., Teruya, T., Yanagida, M. & Kondoh, H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc. Natl. Acad. Sci. U S A. 117, 9483–9489. https://doi.org/10.1073/pnas.1920795117 (2020).
Kaddurah-Daouk, R. et al. Alterations in metabolic pathways and networks in Alzheimer’s disease. Transl Psychiatry. 3, e244. https://doi.org/10.1038/tp.2013.18 (2013).
Whiley, L. et al. Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimers Res. Ther. 13, 20. https://doi.org/10.1186/s13195-020-00741-z (2021).
Howell, E. H. & Cameron, S. J. Neprilysin Inhibition: A brief review of past Pharmacological strategies for heart failure treatment and future directions. Cardiol. J. 23, 591–598. https://doi.org/10.5603/CJ.a2016.0063 (2016).
Morris, J. K., Piccolo, B. D., Shankar, K., Thyfault, J. P. & Adams, S. H. The serum metabolomics signature of type 2 diabetes is obscured in Alzheimer’s disease. Am. J. Physiol. Endocrinol. Metab. 314, E584–E596. https://doi.org/10.1152/ajpendo.00377.2017 (2018).
Long, T. et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 49, 568–578. https://doi.org/10.1038/ng.3809 (2017).
Hong, L., Huang, H. C. & Jiang, Z. F. Relationship between amyloid-beta and the ubiquitin-proteasome system in Alzheimer’s disease. Neurol. Res. 36, 276–282. https://doi.org/10.1179/1743132813Y.0000000288 (2014).
Krumsiek, J. et al. Mining the unknown: a systems approach to metabolite identification combining genetic and metabolic information. PLoS Genet. 8, e1003005. https://doi.org/10.1371/journal.pgen.1003005 (2012).
Vasishta, S., Ganesh, K., Umakanth, S. & Joshi, M. B. Ethnic disparities attributed to the manifestation in and response to type 2 diabetes: insights from metabolomics. Metabolomics 18, 45. https://doi.org/10.1007/s11306-022-01905-8 (2022).
Butler, F. M. et al. Plasma metabolomics profiles in black and white participants of the adventist health Study-2 cohort. BMC Med. 21, 408. https://doi.org/10.1186/s12916-023-03101-4 (2023).
Alghadir, A. H., Gabr, S. A., Anwer, S. & Li, H. Associations between vitamin E, oxidative stress markers, total homocysteine levels, and physical activity or cognitive capacity in older adults. Sci. Rep. 11, 12867. https://doi.org/10.1038/s41598-021-92076-4 (2021).
Gonzalez-Dominguez, R., Garcia-Barrera, T. & Gomez-Ariza, J. L. Metabolite profiling for the identification of altered metabolic pathways in Alzheimer’s disease. J. Pharm. Biomed. Anal. 107, 75–81. https://doi.org/10.1016/j.jpba.2014.10.010 (2015).
Menni, C. et al. Metabolomic profiling to dissect the role of visceral fat in cardiometabolic health. Obes. (Silver Spring). 24, 1380–1388. https://doi.org/10.1002/oby.21488 (2016).
Altmaier, E. et al. Metabolomics approach reveals effects of antihypertensives and lipid-lowering drugs on the human metabolism. Eur. J. Epidemiol. 29, 325–336. https://doi.org/10.1007/s10654-014-9910-7 (2014).
Suzuki, H. et al. Intake of seven essential amino acids improves cognitive function and psychological and social function in Middle-Aged and older adults: A Double-Blind, randomized, Placebo-Controlled trial. Front. Nutr. 7, 586166. https://doi.org/10.3389/fnut.2020.586166 (2020).
Pearlin, L. I., Schieman, S., Fazio, E. M. & Meersman, S. C. Stress, health, and the life course: some conceptual perspectives. J. Health Soc. Behav. 46, 205–219. https://doi.org/10.1177/002214650504600206 (2005).
Ouanes, S. & Popp, J. High cortisol and the risk of dementia and Alzheimer’s disease: A review of the literature. Front. Aging Neurosci. 11, 43. https://doi.org/10.3389/fnagi.2019.00043 (2019).
Kulshreshtha, A. et al. Association of stress with cognitive function among older black and white US adults. JAMA Netw. Open. 6 https://doi.org/10.1001/jamanetworkopen.2023.1860 (2023).
Bressler, J. et al. Metabolomics and cognition in African American adults in midlife: the atherosclerosis risk in communities study. Transl Psychiatry. 7, e1173 https://doi.org/10.1038/tp.2017.118 (2017).
Funding
The study was supported by National Institutes of Health (NIH) Grant R01 AG058921. DHS and AA-DHS were supported by the NIH Grants R01 HL92301 and DK071891.
Author information
Authors and Affiliations
Contributions
J.P., C.E.H., and N.D.P. wrote the main manuscript, F.H. performed the statistical analysis, C.E.H. and L.A.F. interpreted the cognitive testing results, N.D.P. interpreted the metabolomic data, J.X. managed the study data and created figures, D.W.B., B.I.F., and N.D.P. provided study funding. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Powell, J., Hugenschmidt, C.E., Hsu, FC. et al. Metabolomic signatures of cognitive function in a type 2 Diabetes-Enriched cohort. Sci Rep 15, 14688 (2025). https://doi.org/10.1038/s41598-025-99606-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99606-4