Abstract
Heavy metals in wastewater represent a main source of environmental contamination for the ecosystem and aquatic system. Herein, the in situ polymerization method was used to prepare a novel nanocomposite polyaniline/muscovite (PANI/Msc) using ammonium persulphate as an oxidizing agent and HCl as a catalyst. The combination of muscovite with polyaniline (PANI) creates a hybrid material that leverages the strengths of both components. PANI/Msc nanocomposite was characterized using XRD, TEM, FT-IR, SEM, and surface area analyzer. Crystalline size of the prepared nanocomposite found in the range 15.6 – 45 nm , while its surface area was 208.6 m2/g. The resulting nanocomposite was used for Cd2+ and Pb2+ adsorption from their solution. Up to 75.6 and 72.6% of Cd2+ and Pb2+, respectively, were removed in the optimized conditions of metal concentration 75 ppm, pH 6 and 7 for Pb2+ and Cd2+, adsorbent dose 0.1 g, 25 °C solution temperature, and 60 min contact time. Kinetic and adsorption studies clearly demonstrated that the results of the adsorption process followed the pseudo-second order (qe 32.8 and 33.1 mg g−1) and Langmuir models (Q0 36.1 and 61 mg g−1) for Cd2+ and Pb2+, respectively. The thermodynamic indicated favorable, spontaneous and exothermic process. Electrostatic interaction and ion exchange mechanisms were responsible for the adsorption of Cd2+ and Pb2+ by PANI/Msc as demonstrated by FTIR spectroscopy and pH studies. This study ended with the cost- effective preparation of PANI/Msc nanocomposite that offers a promising solution for the removal of heavy metals from contaminated water source. In addition, the capability study regarding Pb2+ and Cd2+ion adsorption over the PANI/Msc nanocomposite clearly revealed that our method is suitable for large- scale application.
Similar content being viewed by others
Introduction
Wastewater containing heavy metal pollutants, when discharged to surface water, causes water pollution and human health. As a result, it is now a global challenge to have access to clean water for drinking and irrigation. It is challenging to remove and recover metal ions from aqueous effluent because of their diverse physical and chemical properties, making it difficult to completely remove and recover them. Pollutants containing heavy metals are introduced into water systems by industrial, mining, and other sources1.
There have been a number of technologies reported for the removal of heavy metals from contaminated water, including complexation, precipitation, membrane filtration, flotation, oxidation, solvent extraction, adsorption, photocatalysis, ion exchange electrodialysis, reverse osmosis, electrochemical and electrocatalysis methods2,3,4,5,6,7. However, the adsorption method is thought to be one of the most effective, practical, and easy to apply. It is considered cost-effective technology for treating wastewater8,9,10.
Different adsorbents have produced effective treatment of wastewater, which include zeolites11, activated carbons12, natural clay minerals4, sludge materials, agricultural waste, biochar, polymeric resins, and industrial waste10.
Recent advancements and methodologies of pollutant removal have been studied. Advanced conditioning techniques, such as those explored by Deng, et al.13, demonstrate enhanced solid–liquid separation efficiencies, which may inform the design of adsorption systems. Recent studies by Wang, et al.14 on the removal of organic and inorganic contaminants underscore the potential of hybrid adsorption-oxidation systems in water treatment. Li, et al.15 highlighted innovative oxidative coupling strategies, providing insights into improving adsorption efficiency for pollutant removal. The simultaneous removal of SO₂ and NO as reported by Li, et al.16 exemplifies the integration of multiple functionalities in environmental remediation technologies. Stress-mediated modifications, as studied by Xie, et al.17, show promise in enhancing the surface reactivity of adsorptive materials. Ali Alshehri and Pugazhendhi18 emphasized the role of biochar and composite materials in achieving efficient contaminant removal, applicable to the current study’s system. Green recycling processes, like those explored by Yu, et al.19, provide a sustainable perspective on material recovery and reuse, potentially aligning with the regenerative aspects of adsorbents.
Because of their affordability and practical uses as adsorbent materials, clay minerals are widely employed. However, the utilization of natural clay minerals is restricted due to their small surface area and the absence of standardized regeneration and recovery processes in aqueous systems.
Contrarily, polymers have demonstrated the ability to overcome clay minerals’ limitations20,21. Clay-polymer nanocomposites (CPNs) have received much interest from researchers to be used as adsorbents22. Due to their diverse uses and significant benefits in a variety of industries, including water purification, CPNs synthesis is growing in popularity. A compound called polymer nanocomposite (PCN) has many more desirable properties than its component polymers23.
Because of its low cost of raw materials, high electrical conductivity, easy synthesis, environmental stability, unique conductivity properties, high stability, redox properties, relatively high porosity, and high surface area, polyaniline PANI has attracted a lot of research interest among conductive polymers24.
Utilising simple synthesis techniques, innovative nanocomposite adsorbents made of polyaniline and another adsorbent with superior adsorption characteristics, strong regeneration ability, and selectivity have been created. These nanoparticles are frequently employed in water treatment; because of their electrical, mechanical, improved conductive, and adsorbing properties25,26.
Solvent intercalation, melt compounding, and in situ, polymerization are techniques used to create clay-polymer nanocomposites. In situ polymerization and solvent intercalation both enable polymer chains to penetrate silicate clays’ galleries27.
By using in-situ polymerization, Kalotra and Mehta22 prepared nanocomposites (polyaniline/clay), and used them to remove the acid green dye from wastewater. Liu, et al.28 prepared polyaniline/vermiculite nanocomposite via in situ polymerization of aniline. Vijayakumar, et al.29 synthesized polyaniline/smectite nanocomposite by heating aniline and ammonium persulfate in the presence of clay at 0–5 °C for 12 h. By utilizing ammonium persulfate as an oxidant in a solid process, Bekri-Abbes and Srasra30 produced the polyaniline (PAn) and polyaniline/montmorillonite (Mt) nanocomposite.
In situ, polymerization was used by Kalotra and Mehta22 to develop nanocomposites of polyaniline and polyaniline/montmorillonite clay. Tang, et al.31 prepared nanocomposites (polyaniline/vermiculite) by in situ polymerization. Piri, et al.32 prepared polyaniline/clay and used it in water treatment.
Muscovite is a member of the mica family of clay minerals. Natural 2M1 Muscovite (Silicate mineral with a 2:1 phyllosilicate ratio) has the chemical formula KAl2(Si3AlO10)(OH)2 with layered silicate mineral created when biotite and iron-bearing phlogopite micas weather together. The mica group minerals consist of two tetrahedral SiO4 sheets and an octahedral Al(O/OH)6sheet33.
Muscovite advantages over other natural adsorbents are low cost as an adsorbent, high availability, and ease of handling34. Rashed, et al.35 used chemically activated Muscovite as an adsorbent of heavy metals.
The interlayer area should ideally be occupied while creating layered silicate nanocomposite materials. So, the ability of layered silicates to exchange charge to cations has led to substantial research and development36.
Rashed, et al.37 synthesize Muscovite nanoparticles using intercalation agents (PA- TTAB-DTAB- DTPA- PN) with its application in the removal of Pb2 and Cd2+ from wastewater. Using K-feldspar, Yuan, et al.38 prepared nano muscovite. Chen, et al.39 prepared exfoliated Muscovite/poly (2, 3-dimethylaniline) nanocomposite. Silica-based nanomaterials have been employed for metal adsorption, because of their extremely adsorptive surface area and pore size.
Preparation of Muscovite nanocomposites was via intercalation with ion exchange between inorganic and organic phases40. Ismail, et al.41 synthesized nanomuscovite using cetyltrimethylammonium bromide and alkaline salt.
Kaolinite/muscovite composite was prepared by activation agents (NaOH and H2SO4) and applied it for Pb2+adsorption42. As a protective coating for mild steel surfaces, Senthilnathan, et al.43 created muscovite polyaniline nanocomposites with superior anti-corrosive properties.
This study aims to prepare polyaniline Muscovite nanocomposite as a new adsorbent for the adsorption of cadmium and lead from liquid waste, focusing on the optimum condition for the maximum adsorption of cadmium and lead from its solution.
The novelty of this work focuses on increasing the surface area and electrostatic properties of nanomuscovite, enabling the optimization of adsorption properties for specific heavy metals, by simply prepared PANI-Muscovite nanocomposite via a combination of polyaniline (PANI) and nanomuscovite which novel and has not been extensively explored.
Material and methods
Sample collection
Bulk Muscovite samples of 10 kg were gathered in Egypt’s southeast desert. Using an agate mortar, the samples were broken up and processed into powder. The powdered muscovite samples were soaked in deionized water for a day, the silica particles from the samples of Muscovite were eliminated, and the solution was then filtered before being dried at 60 °C for 24 h and being sieved to a 63 μm mesh size.
Reagents and chemicals
High analytical—grade chemicals and reagents were used: cadmium and lead standard solutions (1000 ppm) were perched from BDH, UK. Ammonium persulphate (NH4)2S2O8, Alfa Aesar DTAB (1-Dodecyl) trimethyl ammonium bromide (CH3(CH2)11N(CH3)3Br, and aniline (C6H5NH2) were of analytical grade supplied by BHD Chemical Ltd., UK. Aniline was purified before its use as the precursor in the polymerization reaction. Ammonium persulphate was selected as the oxidizing agent for polyaniline synthesis, while DTAB used as an intercalate agent to prepare the nanomuscovite.
Preparation of metal standard solutions
Working standard Pb2+ and Cd2+ solutions were prepared by diluting the stock one (1000 ppm).
Instruments and tools
A flame atomic absorption spectrophotometer Varian GBC 932 AA was used in the quantitative analysis of metal ion concentration.
Characterization techniques
Morphological analysis of polyaniline Muscovite nanocomposite [PANI/Msc] was performed. Power X-ray diffraction patterns (XRD) were performed for the PANI/Msc nanocomposite on a Bruker D advance XRD meter between angle 2θ = 5–60° at 40 kV using X-rays diffraction patterns (Brukeraxs D8, Germany). Scanning electron microscopy (SEM–EDX, JEOL, JSM-5500 LV electron microscopy) technique was used to morphological analyses and characterization of the size and shape of the resulted nanocomposite. Transmission Electron Microscopy (TEM) Investigation was performed using TEM, JEOL-JEM-1230, Tokyo, Japan. The chemical bond characteristic of the PANI/Msc nanocomposite was acquired by Fourier transform infrared (FTIR) spectroscopy (FTIR, JASCO 3600, Tokyo, Japan using KBr pellets in the 400–4000 cm−1 frequency range. Surface area BET techniques was employed to determine the surface area, pore volume and pore size of the prepared adsorbent by Quatachrome Instruments (NOVA 2000 series, UK).
The integration of XRD, SEM–EDX, TEM, FTIR, and surface area BET techniques in this study allowed for a comprehensive understanding of the structural, and chemical properties of the synthesized samples, facilitating a nuanced analysis of their composition and potential applications.
Preparation of polyaniline muscovite nanocomposite [PANI/Msc]
Our previously published research showed that DTAB (1-Dodecyl trimethyl ammonium bromide) was used to prepare the nanomuscovite as published by Rashed, et al.37. The PANI/Msc nanocomposite was prepared with aniline polymerization on the exfoliated nanomuscovite. In a 50 mL solution of 1 M HCl, several ratios of nanomuscovite and aniline were added (Table 1). After being sonicated for one hour, the mixture received 5 g of ammonium persulphate (APS) dissolved in 30 mL of 1 M HCl solution, followed by stirring for 7 h at 25 °C. Following filtration, the precipitates were twice washed with distilled water and vacuum-dried for 24 h at 25 °C. The resulted polyaniline Muscovite nanocomposites were labelled as PANI/Msc1, PANI/Msc2, and PANI/Msc3 (Table 1).
Adsorption properties of polymer muscovite nanocomposite [PANI/Msc]
The adsorbed amount of each metal (qe) and removal percentage (R%) for all conducted experiments was determined by these equations.
where Co and Ce are initial and equilibrium concentrations of metal in (mg L−1), respectively, while V and m are the volume of the aqueous metal solution (L) and dosage of adsorbent (g), respectively.
All the experiments were performed in triplicates, and the average values were used for experimental data evaluation.
pH Effect
The pH level of a solution significantly affects adsorption studies due to its influence on adsorbate solubility and the concentration of metals in adsorbent functional groups. To an aliquot of 25 mL of metal solution concentration (50 ppm Pb, Cd) in an Erlenmeyer flask (50 mL) was added to 0.1 g of PANI/Msc nanocomposite, and the solution mixture was shaken on a shaker at a different pH (2, 4, 5, 6, 7, 8, and 10) for 1 h, and filtered. In the filtrate, lead and cadmium were measured by atomic absorption spectrophotometer.
Adsorbent doses
Adsorbent dosage is known to have a great impact on the overall adsorption process. In order to study the effects of different PANI/Msc doses on the adsorption efficiency of Pb and Cd ions, different doses of PANI/Msc (0.05, 0.1, 0.2, 0.3, 0.5, and 1.0 g) were immersed with 50 mL metal ions (50 ppm Cd2+, Pb2+) with constant pH 6 and 7 for Cd2+and Pb2+, respectively, and stirring for two hours. In the filtrate, lead and cadmium were measured by atomic absorption spectrophotometer.
Metal concentration
The effect of contact time between the adsorbent and the adsorbate on the effectiveness of the adsorption was investigated. 50 mL of various Cd2+ and Pb2+ concentrations (10, 20, 30, 50, 75, and 100 ppm) were mixed with 0.1 g of PANI/Msc and left to adjust at 25ºC for 2 h. In the filtrate, lead and cadmium were measured by atomic absorption spectrophotometer.
Contact time
Equilibrium time as important parameters show the need for time for metal removal in adsorption processes. 0.1 g of PANI/Msc was mixed with 50 mL (50 ppm Cd2+, Pb2+) with a suitable pH and solution temperature 25ºC for interval 30, 60, 120 and 180 min. At predetermined time intervals, in the filtrate, lead and cadmium were measured by atomic absorption spectrophotometer.
Solution temperature
The effect of adsorption temperature was investigated at different solution temperatures of 25, 35, 45 and 55ºC. 0.1 g of PANI/Msc was stirred with 50 ml metal ion (50 ppm Cd2+, Pb2+) with stabilization of all aforementioned parameters. The solution was filtered, and the metal ions concentration Cd2+and Pb2+ in the filtrate were measured by atomic absorption spectrophotometer.
Results and discussion
A comparative study of the PANI/Msc nanocomposites for removal of Pb2+ and Cd2+
Adsorption of Cd2+ and Pb2+ on the various prepared polymer muscovite nanocomposites (PANI/Msc1, PANI/Msc2, and PANI/Msc3) was investigated at the best adsorption conditions in order to compare the prepared nanocomposites. High levels of cadmium and lead were removed by PANI/Msc1 (75.6% and 72.6% for Cd2+ and Pb2+, respectively) more than the others, indicating that the optimal way for preparing polyaniline is to use a 1:1 ratio of polyaniline to nanomuscovite (Table 2). So, the nanocomposites (PANI/Msc1) will be used for further experiments.
Nanocomposite [PANI/Msc] characterization
XRD
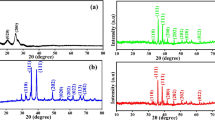
The insertion of PANI chains into the nanomuscovite interlayer space was examined using XRD analysis of the nanocomposite [PANI/Msc1] using the 2Ө range of 0° to 60°. Figure 1 shows the XRD patterns of nanomuscovite and the PANI/Msc1 nanocomposite. This pattern shows a very small peak, approximately at 2θ = 28.8, for amorphous polyaniline due to its lower weight percent in the sample, and this illustrates the formation of the exfoliated state of PANI/Msc nanocomposite (Gholami et al., 2017). In this state, polyaniline chains increase the d-spacing of nanocomposite so that there is not a considerable interaction between silicate layers. Crystalline size was calculated using the Scherer equation and found in the range 15.6–45 nm.
SEM
SEM technique was used to morphological analyses and characterization of the size and shape of the resulted PANI/Msc1 nanocomposite. The granular texture and plate-like morphology of the Msc1 nanomuscovite particles were visible in the SEM images of the Msc1 nanocomposite (Fig. 2a). It is evident from Fig. 2b that the polymerization mostly took place between the Msc1 nanocomposite layers. Also, it confirmed that after modification of Msc1 nanomuscovite by PANI, the flaky Msc1 nanomuscovite structure coated by PANI and many individual platelets are seen in SEM images.This outcome demonstrates that PANI easily inserted itself between the Msc1 nanocomposite layers, expanded, and forced the layers apart, forming intercalated layered silicate PANI particles.
FTIR
An FTIR spectrum of PANI/Msc1 nanocomposite and nanomuscovite is shown in Fig. 3. The nanomuscovite and PANI/Msc1 nanocomposite spectra show the peak at ~ 1016 cm−1, representing Si–O bond stretching. Bands at 2922 and 2855 cm−1 represented CH2 symmetric and asymmetric stretching vibration modes of alkyl chains.
The 440 cm−1 peak results from the Mg-O bending bonds. The Al-O bending bond bands may be detected at 918 cm−1. The hydration -OH vibration and HOH groups between the octahedral and tetrahedral sheets are attributed to the peaks at 1635 and 3298 cm−144.
The 793 cm−1band is associated with the bending vibrations of OH39. The stretching vibrations of the CH3COO- group, which occur symmetrically and asymmetrically, are responsible for the bands at 1542 cm−1 and 1403 cm−1, respectively. The band at 1395–1440 cm−1marks the emergence of interaction between the deformation vibration mode of the O–H group and the C-O vibration45. In the spectrum of the [PANI/Msc1] nanocomposite, the secondary amine is represented by a peak at 3,260 cm−1, and the benzenoid and quinoid rings of polyaniline are represented by peaks at 1,512 cm−1 and 1,592 cm−1, respectively.
Surface area
Nanomuscovite shows a surface area of 195.15 m2/g, while that for PANI/Msc1 nanocomposite was 208.626 m2/g (Table 3). These findings show that the PANI/Msc1 nanocomposite has a larger surface area than nanomuscovite. From Table 3, the pore size of nanomuscovite was 13.82 nm, and that for PANI/Msc nanocomposite was 13.74 nm. Due to the reduction in particle size, the pore size of nanocomposite was less than that of [PANI/Msc1] nanocomposite.
Optimum conditions for Cd2+ and Pb2+ ions adsorption on polyaniline muscovite nanocomposite [PANI/Msc1]
pH Effect
Solution pH has a significant impact on the adsorbent’s surface functional groups and metal ion ionization degree as well as its water solubility. The effect of solution pH on the adsorption processes of Cd2+ and Pb2+ by the prepared PANI/Msc1 nanocomposite was studied with pH range (2, 4, 5, 6, 7, 8, and 10) is shown in Fig. 4. High metal removal of Pb2+ and Cd2+ was found at pH 7.0 (72.6%) and pH 6.0 (75.6%) for Cd2+ and Pb2+, respectively. Increased Pb2+ and Cd2+adsorption occurs at pH levels greater than 4 up to 7 due to more negatively charged the adsorbent and electrostatic repulsion reduction46. However, at pH levels over 7, Pb2+ and Cd2+adsorption rises to 100%; this may be because Pb and Cd hydroxide are poorly soluble in the solution and form an imperceptible precipitate47.
Piri, et al.32studied the removal of Pb(II) on polyaniline-modified clay nanocomposite and discovered that Pb was most effectively removed at a pH of 5.0. Gapusan and Balela48 reported the maximum removal of Pb ions from polluted water using polyaniline-kapok fibre nanocomposite at pH 4–6, which agreed with our results.
Mahmoud and Fekry49 studied the removal of divalent cadmium and lead on nanopolyaniline (NPANI) and cross-linked nanopolyaniline (CrossNPANI)/silica nanocomposites and found that the maximum capacity was at pH 6., which agreed with our results. Ahmad, et al.50 found that the highest amount of Cd (II) adsorbed from aqueous solutions on chitosan-grafted polyaniline-OMMT nanocomposite was at pH value 5.
Effect of adsorbent dose
The removal of Cd2+ and Pb2+ ions versus the adsorbent dosage of PANI/Msc1 nanocomposite (0.05–0.5 g) is demonstrated in Fig. 5. The results show that the metal’s removal increases with increasing dose of adsorbent from 0.05 g to 0.1 g. The increasing metal adsorption on the adsorbent was related to increasing the active sites in the adsorbent because of the adsorbed equilibrium status between the solid and solution phase51. According to Hua52, the high adsorbent dose and the split in the concentration gradient between the adsorbent dose in the solution and on the adsorbent surface may result in shielding of binding sites due to screening impact on the impermeable outer layer. Rafiei, et al.53 reported that the maximum Pb removal efficiency (99.6%) on poly(acrylic acid)/bentonite nanocomposite was at 7.5 g L−1. Yadav, et al.54 studied the adsorption of Pb ions on clay-CNT nanocomposite in aqueous media achieved with an adsorbent dose (1.4 g L−1).
Effect of initial metals concentration
Figure. 6 represents the relationship between the adsorption efficiency of Cd2+ and Pb2+ on PANI/Msc1 and the metal initial concentration. The results reveal that the removal percentage of Cd2+ and Pb2+ increased gradually with the metal concentration and reached the maximum adsorption (95.5% and 97% for Cd2+ and Pb2+, respectively) at 75 ppm. So, Cd2+ and Pb2+concentration (75 ppm) was used for further experiments. The amount of metal adsorbed increased with increasing the metal concentration as a result of the rising driving force of metal ions onto the adsorbent active sites12.
Lead adsorption on clay-CNT nanocomposite in aqueous media was reported by Yadav et al. (2019), who ended that maximum Pb adsorption was at an initial lead concentration of 15 mg L−1. Piri, et al.32 studied the removal of Pb(II) ions on polyaniline-modified clay nanocomposite and found that the optimum metal adsorption was at 200 mg L−1 initial Pb(II) concentration.
Effect of contact time
The effect of the contact time on the removal of Cd2+ and Pb2+ by PANI/Msc nanocomposite adsorbent is illustrated in Fig. 7. The removal efficiency of Cd2+ and Pb2+ ions by the PANI/muscovite nanocomposite increases as the contact time increase and reach the maximum metal adsorption at 60 min (87.2% and 89% of Cd2+ and Pb2+ ions, respectively). Rapid Cd2+ and Pb2+adsorption on PANI/Msc1 nanocomposite may be due to the abundance of active sites on the adsorbent. The removal process is slowed after 60 min due to the progressive decline in the number of active sites, which causes the remaining unoccupied sites to be difficult to access due to repulsive forces between the metal ions on the solid surface and the bulk phase55.
Karimi, et al.56 reported that the optimum time for the maximum adsorption of Pb (II) on polythiophene/Al2O3 and its modified poly(vinyl alcohol) was 40 min. Pb(II) ion adsorption was investigated by Msaadi, et al.57 on a polymer/clay nanocomposite, and it was discovered that equilibrium was reached in 15 min. Rafiei, et al.53 reported that Pb ion removal on poly(acrylic acid)/bentonite nanocomposite was maximum at 30 min. Yadav, et al.54 studied Pb ions adsorption on clay-CNT nanocomposite was achieved within 120 min.
Effect of temperature
The results (Fig. 8) of solution temperature on the adsorption of the metals showed that the percentage adsorption efficiency of Cd2+ and Pb2+was high at 25 °C (97.8% and 98.8% for Cd and Pb, respectively); after that, it diminishes as a result of the force of the van der Waals bonds breaking down, resulting in a reduction of active sites58.
Adsorption isotherms
The adsorption isotherms parameters (Langmuir, Freundlich, Temkin, and Dubinin-Raduskevich (D-R) isotherms) of Cd2+ and Pb2+ on PANI/Msc1 nanocomposite are presented in Table 4.
For adsorption isotherm and kinetic models to evaluate the goodness of the estimates, we used the following established criteria:- R2 > 0.9: Indicates a good fit of the model to the experimental data.
Freundlich isotherm
To determine how heterogeneous surface energy is consumed during adsorption, the Freundlich isotherm is applied. The practical Freundlich formula is:
The adsorption intensity and capacity are represented by the constants n and Kf, respectively. From the equation, linear relationship’s slope and intercept between log qe and log Ce can be utilized to calculate Kf and n. The information is displayed in Table 4. As a result, 1/n values less than 1 imply normal adsorption with a significant contact between the adsorbent and the metal, whereas cooperative adsorption is indicated by 1/n values more than 1.
The results show that the values for Cd2+ Kf and 1/n were 0.363 and 2.1, while those for Pb2+ were 0.264 and 2.3, respectively. The results reveal that 1/n value was less than 1, which indicates that the adsorption of Cd2+ and Pb2+ onto PANI/Msc1 nanocomposite was favorable. The R2 values were 0.805 for Cd2+ and 0.773 for Pb2+ lower than those with Langmuir, which indicated that this isotherm did not fit this adsorption.
Langmuir isotherm
The saturated monolayer adsorption on the uniform surface was characterized using the Langmuir isotherm model at constant energy. The equation may quantify the Langmuir equation.:
Ce, the metals’ equilibrium concentration (ppm), Qo for capability of monolayer adsorption (mg/g), qe for the adsorbent’s metal equilibrium capacity (mg/g), and b for the coefficient of binding energy in Langmuir’s model (L/mg). From the equation, the slope and intercept of Ce/qe vs. Ce may be used for computations b and Qo. The information is displayed in Table 4.
The correlation coefficients (R2) of Cd2+ and Pb2+ on PANI/Msc1 nanocomposite were determined from the data in Table 4 to be 0.999 and 0.98, respectively, which suggested that the adsorption mechanism is of a monolayer.
Furthermore, it was discovered that Cd2+ and Pb2+ each had a maximum monolayer adsorption capacity (Qo) of 61 and 36.1 mg g−1, respectively. The sorption of Cd2+ and Pb2+ ions on PANI/Msc1 nanocomposite is favorable according to the Langmuir isotherm, which is also supported by the results bL < 1 for the concentration examined. Since the Langmuir equation presumes surface homogeneity, the finding that the Langmuir model best matches the experimental results may be related to the uniform distribution of active sites on the adsorbent surfaces.
The maximum adsorption capacity of PANI/Msc1 nanocomposite for Cd2+ and Pb2+ ions was compared with values of different adsorbents reported in the literature, as presented in Table 5
Temkin isotherm
Temkin isotherm model predicts that the binding energies of adsorbents on a surface will be uniformly distributed throughout the population.
The linear form of the Temkin equation is given by the following:
The sorption heat is denoted by the Temkin constant b. The constants B, associated with the heat of sorption (J/mol), and K, associated with Temkin equilibrium binding constant, can be calculated from a plot of qe vs. ln Ce. From the Temkin plot, the Cd2+ and Pb2+ adsorption constant values were KT 1.84 and 1.12 L g−1, R2 = 0.94 and 0.94, and B = 12.44 and 14.1 J mol−1, respectively.
The isothermal Dubinin-Radushkevich model
The Dubinin-Radushkevich isotherm is commonly used to explain the adsorption mechanism with Gaussian energy distribution over a heterogeneous surface. It is employed to distinguish between chemical and physical metal adsorption. The linearized D-R equation:
It may determine the values from the slope and the qm values from the intercept by plotting ln qe vs. E2. Regarding both chemical and physical adsorption, information is provided by the mean adsorption energy (E).
As seen in Table 4, the calculated mean free energy (E) values for Pb2+ and Cd2+ are 0.5 kJ/mol and 0.32 kJ/mol, respectively. The type of Cd2+ and Pb2+ adsorption onto PANI/Msc1 nanocomposite was characterized as physical adsorption because it was found that (E) for Cd2+ and Pb2+ is lower than the range of adsorption reaction 8–16 kJ mol−1.
Compared to the other Isotherm models, the Cd2+ and Pb2+ adsorption on PANI/Msc1 nanocomposite suited Langmuir isotherm best (Table 4).
Adsorption kinetic
The mechanism of the adsorption process has been determined using kinetic models65, which gives significant information to increase adsorption efficiency and the viability of process scaling up.
The sorption kinetics of Cd2+ and Pb2+ on polyaniline muscovite [PANI/Msc1] nanocomposite adsorbent were investigated using the Pseudo-first order, Pseudo-second order, Elovich, and intra-particle diffusion models.
All these adsorption steps, including adsorption, internal particle diffusion, and external film diffusion, are incorporated into these models. Table 6 represents the Cd2+ and Pb2+ adsorption kinetic data on the polyaniline muscovite [PANI/Msc1] nanocomposite.
Pseudo–first order model
The pseudo-first-order kinetic is depicted in the following model:
The pseudo-first order (K1) is the rate constant, and the adsorption capacities at equilibrium and t (in minutes) are qe and qt (in mg/g), respectively.
By plotting log (qe − qt) vs. (t), the kinetic constants for the pseudo-first order adsorption of Cd2+ and Pb2+ on PANI/Msc1 nanocomposite were obtained from the slope and the intercept (Table 6). The results revealed the correlation coefficient (R2) were 0.61 and 0.73 for Cd2+ and Pb2+ onto PANI/Msc1 nanocomposite, respectively, while k1 values were 0.02 and 0.01 min−1.
In this model (The model of pseudo first order) R2 value for Cd2+ and Pb2+ is lower than those in other models, implying that the model is not applicable and that there is not a great deal of agreement between the theoretical and experimental qe values. This suggests that the pseudo-first order model may not be sufficient to understand how Cd2+ and Pb2+ interact with PANI/Msc1 nanocomposite.
Pseudo–second order model
A depiction of the pseudo-second order kinetic model is as follows:
K2, the rate constant (g/mg/min).
By charting t/qt vs. time (t) using the equation, qe, and K2 can be calculated from the intercept and slope. The results show high correlation coefficients (R2) of 0.998 and 0.997 for Cd2+ and Pb2+ ions, respectively (Table 6). Also, the estimated adsorption capacity at equilibrium (qe) were 32.8 and 33.1 mg g−1 for Cd2+ and Pb2+, respectively. The values qe cal were near qe exp values, which indicates that the adsorption of Cd2+ and Pb2+ ions behaved in a pseudo-second-order approach.
Intra-particle diffusion model
An intra-particle diffusion model is frequently used to characterize solid–liquid adsorption’s solute transfer, and it was utilized to identify the adsorption mechanism.
Ki (mg g−1 min−1/2) is the intra-particle diffusion rate constant, while C (mgg−1) indicates the boundary layer effect and thickness.
From the equation, the slope and intercept of the linear plot of qt vs. t1/2 can be used to get the Ki and C (Fig. 9). Intraparticle diffusion constants were calculated and are presented in Table 5 for the sorption of Cd2+ and Pb2+. Kid values were 0.49 and 0.5 mg g−1 min−1/2 for Cd2+ and Pb2+, respectively, while R2 were 0.708 and 0.723, respectively. The C values (31.5 and 31.1 mg g−1).
Figure 9depicts multilinearity forms, and this may involve two or more additional phases. The first demonstrates that the greater sharpness of the line is due to the propagation of divalent ions from the solution to the adsorbent’s exterior surface or to the diffusion of solute ions through boundary strata. Intraparticle diffusion is the rate-limiting process during the progressive adsorption stage66,67,68.
Elovich model
Elovich equation characterizes the activated chemisorption:
α, the initial adsorption, and β, the adsorption coefficient.
The constants α and β were obtained from the equation by plotting qe vs. ln(t) and represented in Table 6. The α values were 7.1 and 6.8 mg min−1 for Cd2+ and Pb2+, respectively, while β values were 0.46 and 0.46 for Cd2+ and Pb2+, respectively. A plot of qt versus ln t shows that the adsorption of Cd2+ and Pb2+ onto PANI/Msc nanocomposite did not obey the Elovich equation due to small R2 values.
According to the Elovich model, the rate-controlling step (RCS) is the chemical reaction between Cd2+ and Pb2+and the surface of the adsorbent and the removal procedure is carried out by multiple-layer adsorption69.
Thermodynamics of adsorption
Several indicators for particle applications are entropy (ΔS°), free energy (ΔG°), and enthalpy (ΔH°). In this study, the thermodynamics of Cd2+ and Pb2+ adsorption on the prepared adsorbent were evaluated in light of various temperatures (298, 313, and 333 K).
For the purpose of calculating the thermodynamic parameters, the following equation was utilized70.
R, and T are constants related to gas (8.314 J/mol K) and temperature (K), respectively.
The equation below determines the free energy of specific adsorption ΔGo (KJ mol−1).
Table 7 lists the estimated thermodynamic parameters.
The results reveal that G° values for Cd2+ and Pb2+ were negative at all temperatures, which points to a physical adsorption mechanism for the adsorption of Cd2+ and Pb2 on PANI/Msc1 nanocomposite. In order to indicate a favourable and spontaneous absorption of Cd2+ and Pb2+, the free energies ΔG° of the PANI/Msc1 nanocomposite were calculated in negative signs. ΔH° parameter shows negative values, which indicate that adsorption is exothermic, and the low value of ΔH° indicates that this adsorption system might function primarily via physical adsorption (Draman et al. 2014). When Cd2+ and Pb2+ were adsorbed on the active sites of the adsorbent surface, the negative ΔS° indicated enhanced randomness at the solid-solution interface.
Adsorption mechanism of Cd2+ and Pb2+ on PANI/Msc1 nanocomposite
The adsorption mechanism of Cd2+ and Pb2+on PANI/Msc1 nanocomposite involves several steps. Firstly, the nano Muscovite (Msc1) serves as a growth template, allowing the Polyaniline (PANI) to form a nanocomposite with enhanced surface area and reactivity71. Generally, the adsorption of heavy metal ions onto PANI/Msc1 nanocomposite occurs through electrostatic interactions and ion exchange mechanisms between the heavy metal ions and the adsorbent. The PANI component plays a crucial role in the adsorption process, as it provides amino and imino functional groups that can chelate with Cd2+ and Pb2+ ions. It is very clear where nitrogen atoms exist in amine compounds due to the presence of electron in SP3 orbital of nitrogen can makes coordinate bond with positive charge of analytes (Cd2+, Pb2+). This chelation process is facilitated by the electrostatic attraction between the positively charged Pb and Cd ions and the negatively charged PANI chains32.
Conclusion
A novel form of polyaniline/muscovite nanocomposite (PANI/Msc1) was synthesized from nanomuscovite by polymerization of aniline on the surface of nanomuscovite. The adsorption of Cd2+ and Pb2+ onto the PANI/Msc1 nanocomposite was found to be more favourable at pH 6 and 7 for Pb2+ and Cd2+, respectively, with an adsorbent dose 0.1 g, metal concentration 75 ppm, solution temperature 25 °C, and contact time 60 min. Adsorption isotherm and kinetics show that the adsorption of Pb2+ and Cd2+ agrees with the Langmuir isotherm model and follows the pseudo-second order kinetic model. Furthermore, thermodynamic studies reveal that adsorption processes are both endothermic and spontaneous.
The prepared novel nanocomposite can be used successfully for the treatment of polluted water from heavy metal pollution. The synthesis process of the PANI/Msc1 nanocomposite reduces the overall cost of the adsorbent material, and can be scaled up for industrial production, making it a viable option for large-scale wastewater treatment applications.
The preparation of PANI/Msc1 nanocomposite offers significant advantages as it greatly enhances its surface and morphological properties, thereby improving its capacity to adsorb heavy metals. Also, the incorporation of nanomuscovite into the PANI matrix enhances the mechanical strength and improves thermal stability. The use of naturally abundant muscovite reduces the overall cost of the nanocomposite.
Our future research will focus on the application of PANI-Muscovite nanocomposite for the removal of other pollutants, such as dyes, pesticides, or pharmaceuticals, from water and soil.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Chai, W. S. et al. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 296, 126589 (2021).
Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 141, 227–239 (2017).
Gunatilake, S. K. Methods of removing heavy metals from industrial wastewater. Methods 1, 14 (2015).
Han, H. et al. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 369, 780–796 (2019).
Burakov, A. E. et al. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 148, 702–712 (2018).
Yu, F. et al. Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 260, 127650 (2020).
Ajiboye, T. O., Oyewo, O. A. & Onwudiwe, D. C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 262, 128379 (2021).
Rashed, M. N. & Palanisamy, P. N. Introductory chapter: Adsorption and ion exchange properties of zeolites for treatment of polluted water. Zeolites Appl. https://doi.org/10.5772/intechopen.77190 (2018).
Gomaa, H. et al. Mesoscopic engineering materials for visual detection and selective removal of copper ions from drinking and waste water sources. J. Hazard. Mater. 406, 124314 (2021).
Singh, N. B., Nagpal, G. & Agrawal, S. Water purification by using adsorbents: A review. Environ. Technol. Innov. 11, 187–240 (2018).
Hong, M. et al. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 359, 363–372 (2019).
Wang, B., Lan, J., Bo, C., Gong, B. & Ou, J. Adsorption of heavy metal onto biomass-derived activated carbon. RSC Adv. 13, 4275–4302 (2023).
Deng, J. et al. Enhanced sludge solid-liquid separation based on Fe2+/periodate conditioning coupled with polyoxometalates: Cell destruction and protein adsorption. J. Environ. Manage. 373, 123552 (2025).
Wang, Y. et al. Efficient removal of dibutyl phthalate from aqueous solutions: Recent advances in adsorption and oxidation approaches. React. Chem. Eng. https://doi.org/10.1039/D4RE00055B (2024).
Li, B. et al. O3 oxidation excited by yellow phosphorus emulsion coupling with red mud absorption for denitration. J. Hazard. Mater. 403, 123971 (2021).
Li, B. et al. Simultaneous removal of SO2 and NO using a novel method with red mud as absorbent combined with O3 oxidation. J. Hazard. Mater. 392, 122270 (2020).
Xie, Y. et al. Stress-mediated copper-molybdenum alloy enables boosted hydrogen evolution activity. Acta Mater. 286, 120706 (2025).
Ali Alshehri, M. & Pugazhendhi, A. Biochar for wastewater treatment: Addressing contaminants and enhancing sustainability: Challenges and solutions. J. Hazard. Mater. Adv. 16, 100504. https://doi.org/10.1016/j.hazadv.2024.100504 (2024).
Yu, Y. et al. Green recycling of end-of-life photovoltaic modules via Deep-Eutectic solvents. Chem. Eng. J. 499, 155933 (2024).
Unuabonah, E. I., Ugwuja, C. G., Omorogie, M. O., Adewuyi, A. & Oladoja, N. A. Clays for efficient disinfection of bacteria in water. Appl. Clay Sci. 151, 211–223 (2018).
Mukhopadhyay, R. et al. Clay–polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 383, 121125 (2020).
Kalotra, S. & Mehta, R. Synthesis of polyaniline/clay nanocomposites by in situ polymerization and its application for the removal of Acid Green 25 dye from wastewater. Polym. Bull. 78, 2439–2463 (2021).
Nigam, V. & Lal, G. Review on recent trends in polymer layered clay nanocomposites. Indian Natn. Sci. Acad. Proc. 74, 87–96 (2008).
Benhebal, H., Chaib, M., Leonard, A., Crine, M. & Lambert, S. D. Preparation of polyaniline-modified local clay and study of its sorption capacity. J. Nanostruct. Chem. 4, 1–6 (2014).
Doyo, A. N., Kumar, R. & Barakat, M. A. Facile synthesis of the polyaniline@ waste cellulosic nanocomposite for the efficient decontamination of copper (II) and phenol from wastewater. Nanomaterials 13, 1014 (2023).
Hajjaoui, H., Soufi, A., Boumya, W., Abdennouri, M. & Barka, N. Polyaniline/nanomaterial composites for the removal of heavy metals by adsorption: A review. J. Compos. Sci. 5, 233 (2021).
Abedi, S. & Abdouss, M. A review of clay-supported Ziegler-Natta catalysts for production of polyolefin/clay nanocomposites through in situ polymerization. Appl. Catal. A 475, 386–409 (2014).
Liu, D., Du, X. & Meng, Y. Facile synthesis of exfoliated polyaniline/vermiculite nanocomposites. Mater. Lett. 60, 1847–1850 (2006).
Vijayakumar, B., Anjana, K. O. & Rao, G. R. 1 edn 012112 (IOP Publishing).
Bekri-Abbes, I. & Srasra, E. Electrical and dielectric properties of polyaniline and polyaniline/montmorillonite nanocomposite prepared by solid reaction using spectroscopy impedance. J. Nanomater. 16, 428–428 (2015).
Tang, Z., Liu, P., Guo, J. & Su, Z. Preparation of polyaniline/vermiculite clay nanocomposites by in situ chemical oxidative grafting polymerization. Polym. Int. 58, 552–556 (2009).
Piri, S. et al. Potential of polyaniline modified clay nanocomposite as a selective decontamination adsorbent for Pb (II) ions from contaminated waters; Kinetics and thermodynamic study. J. Environ. Health Sci. Eng. 14, 1–10 (2016).
Mu’azu, N. D., Bukhari, A. & Munef, K. Effect of montmorillonite content in natural Saudi Arabian clay on its adsorptive performance for single aqueous uptake of Cu (II) and Ni (II). J. King Saud University-Sci. 32, 412–422 (2020).
Katsou, E., Malamis, S., Tzanoudaki, M., Haralambous, K. J. & Loizidou, M. Regeneration of natural zeolite polluted by lead and zinc in wastewater treatment systems. J. Hazard. Mater. 189, 773–786 (2011).
Rashed, M. N., Arfien, A. A. & El-Dowy, F. A. Adsorption of heavy metals on chemically modified muscovite. Aswan University J. Environ. Stud. 1, 183–203 (2020).
Bao, T. et al. Catalytic degradation of P-chlorophenol by muscovite-supported nano zero valent iron composite: Synthesis, characterization, and mechanism studies. Appl. Clay Sci. 195, 105735 (2020).
Rashed, M. N., Arifien, A. E. & El-Dowy, F. A. Preparation and characterization of nanomuscovite by intercalation method for adsorption of heavy metals from polluted water. Environ. Geochem. Health 5, 1–18 (2023).
Yuan, J. et al. Green synthesis of nano-muscovite and niter from feldspar through accelerated geomimicking process. Appl. Clay Sci. 165, 71–76 (2018).
Chen, S., Chen, F., Di, Y., Han, S. & Zhu, X. Preparation and characterisation of exfoliated muscovite/poly (2, 3-dimethylaniline) nanocomposites with an enhanced anticorrosive performance. Micro Nano Lett. 15, 509–513 (2020).
Yu, X. The preparation and characterization of cetyltrimethylammonium intercalated muscovite. Microporous Mesoporous Mater. 98, 70–79 (2007).
Ismail, N. H. C., Bakhtiar, N. S. A. A. & Akil, H. M. Effects of cetyltrimethylammonium bromide (CTAB) on the structural characteristic of non-expandable muscovite. Mater. Chem. Phys. 196, 324–332 (2017).
Tetteh, S., Ofori, A., Quashie, A., Jääskeläinen, S. & Suvanto, S. Modification of kaolinite/muscovite clay for the removal of Pb (II) ions from aqueous media. Phys. Sci. Rev. https://doi.org/10.1515/9783110783643-005 (2022).
Senthilnathan, A., Dissanayake, S., Rathuwadu, N., Mantilaka, P. G. & Rajapakse, R. M. G. Preparation of Muscovite Nanocomposites of Polyaniline with Superior Anti-Corrosive Property as the Protective Coating on Mild Steel Surfaces. Conference: 36th Annual Technical Sessions of Geological Society of Sri LankaAt: Colombo, Sri Lanka (2020).
Shabani-Nooshabadi, M., Ghoreishi, S. M., Jafari, Y. & Kashanizadeh, N. Electrodeposition of polyaniline-montmorrilonite nanocomposite coatings on 316L stainless steel for corrosion prevention. J. Polym. Res. 21, 1–10 (2014).
Ding, S. L., Xu, B. H., Liu, Q. F. & Sun, Y. Z. Preparation of nano-kaolinite and mechanism. Adv. Mater. Res. 204, 1217–1220 (2011).
Jokar, M., Mirghaffari, N., Soleimani, M. & Jabbari, M. Preparation and characterization of novel bio ion exchanger from medicinal herb waste (chicory) for the removal of Pb2+ and Cd2+ from aqueous solutions. J. Water Process Eng. 28, 88–99. https://doi.org/10.1016/j.jwpe.2019.01.007 (2019).
Pillai, S. S. et al. Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 98, 352–360. https://doi.org/10.1016/j.ecoenv.2013.09.003 (2013).
Gapusan, R. B. & Balela, M. D. L. Adsorption of anionic methyl orange dye and lead (II) heavy metal ion by polyaniline-kapok fiber nanocomposite. Mater. Chem. Phys. 243, 122682 (2020).
Mahmoud, M. E. & Fekry, N. A. Fabrication of engineered silica-functionalized-polyanilines nanocomposites for water decontamination of cadmium and lead. J. Polym. Environ. 26, 3858–3876 (2018).
Ahmad, R., Hasan, I. & Mittal, A. Adsorption of Cr (VI) and Cd (II) on chitosan grafted polyaniline-OMMT nanocomposite: isotherms, kinetics and thermodynamics studies. Desalin. Water Treat. 58, 144–153 (2017).
Nasrullah, A., Bhat, A. H., Naeem, A., Isa, M. H. & Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 107, 1792–1799 (2018).
Hua, J. Adsorption of low-concentration arsenic from water by co-modified bentonite with manganese oxides and poly (dimethyldiallylammonium chloride). J. Environ. Chem. Eng. 6, 156–168 (2018).
Rafiei, H. R., Shirvani, M. & Ogunseitan, O. A. Removal of lead from aqueous solutions by a poly (acrylic acid)/bentonite nanocomposite. Appl. Water Sci. 6, 331–338 (2016).
Yadav, V. B., Gadi, R. & Kalra, S. Adsorption of lead on clay-CNT nanocomposite in aqueous media by UV-Vis-spectrophotometer: kinetics and thermodynamic studies. Emerg. Mater. 2, 441–451 (2019).
Tan, Y., Chen, M. & Hao, Y. High efficient removal of Pb (II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 191, 104–111 (2012).
Karimi, Z., Khalili, R. & Ali Zazouli, M. Surface modified polythiophene/Al2O3 and polyaniline/Al2O3 nanocomposites using poly (vinyl alcohol) for the removal of heavy metal ions from water: kinetics, thermodynamic and isotherm studies. Water Sci. Technol. 84, 182–199 (2021).
Msaadi, R., Ammar, S., Chehimi, M. M. & Yagci, Y. Diazonium-based ion-imprinted polymer/clay nanocomposite for the selective extraction of lead (II) ions in aqueous media. Eur. Polymer J. 89, 367–380 (2017).
Negm, S. H. et al. Appreciatively efficient sorption achievement to U (VI) from the El Sela Area by ZrO2/Chitosan. Separations 9, 311 (2022).
Zhu, S. et al. Magnetic Nanoscale Zerovalent Iron Assisted Biochar: Interfacial Chemical Behaviors and Heavy Metals Remediation Performance. ACS Sustain. Chem. Eng. 5, 9673–9682 (2017).
Reddy, D.H.K. & Lee, S.-M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids Surfaces A Physicochem. Eng. Asp. 454, 96–103 (2014).
Huda Bonusa Nabila , Endang Tri Wahyuni , Mudasir Mudasir. Simultaneous adsorption of Pb(II) and Cd(II) in the presence of Mg(II) ion using eco-friendly immobilized dithizone on coal bottom ash. South African J. Chem. Eng. 45, 315–327 (2023).
Jiang, M.-Q., Jin, X.-Y., Lu, X.-Q. & Chen, Z.-l. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252(1–3), 33–39 (2010).
Shao, D., Chen, C. & Wang, X. Application of polyaniline and multiwalled carbon nanotube magnetic composites for removal of Pb(II). Chem Eng J. 185–186, 144–150 (2012).
Baranchiyeva, Z., Seilkhanova, G., Rakhym, A., Mastai, Y. & Ussipbekova, Y. Adsorption of Pb(II) and Cd(II) from Aqueous Solutions on Polyvinylpyrrolidone Modified Kyzylsok Natural Clay. Int. J. Biol. Chem. 14(1), 164–171 (2021.).
Eftekhari, S., Habibi-Yangjeh, A. & Sohrabnezhad, S. Application of AlMCM-41 for competitive adsorption of methylene blue and rhodamine B: Thermodynamic and kinetic studies. J. Hazard. Mater. 178, 349–355 (2010).
Bany-Aiesh, H., Banat, R. & Al-Sou’od, K. Kinetics and adsorption isotherm of ibuprofen onto grafted [Beta]-CD/chitosan polymer. Am. J. Appl. Sci. 12, 917 (2015).
Srivastava, P. & Hasan, S. H. Biomass of mucor heimalis for the biosorption of cadmium from aqueous solutions: equilibrium and kinetic studies. BioRes. https://doi.org/10.15376/biores.6.4.3656-3675 (2011).
Dawodu, F. A. & Akpomie, K. G. Kinetic, equilibrium, and thermodynamic studies on the adsorption of cadmium (II) ions using “Aloji Kaolinite” mineral. Pac. J. Sci. Technol 15, 268–276 (2014).
Hosseini-Bandegharaei, A. et al. Sorption efficiency of three novel extractant-impregnated resins containing vesuvin towards Pb (II) ion: effect of nitrate and amine functionalization of resin backbone. Colloids Surf. Physicochem. Eng. Asp. 504, 62–74 (2016).
Ghosal, P. S. & Gupta, A. K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 225, 137–146 (2017).
Anoja Kawsihan, Sandun Dissanayake, Nadeesha Rathuwadu, Prasanga Gayanath Mantilaka & Rajapakse, R. M. G. in 36th Annual Technical Sessions of Geological Society of Sri Lanka (Colombo, Sri Lanka, February 2020).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by F. A. El-Dowy. The first draft of the manuscript was written by M.Nageeb Rashed and A.S.A. Arifien,, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashed, M.N., Arifien, A.S.A. & El-Dowy, F.A. Muscovite based polyaniline nanocomposite as effective adsorbent for removal of Cd2+ and Pb2+ ions from liquid waste. Sci Rep 15, 20234 (2025). https://doi.org/10.1038/s41598-025-99686-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99686-2