Abstract
Frailty has become a growing global health concern and is associated with social determinants of health (SDoH). However, the relative importance and cumulative contribution of multidomain SDoH to frailty, and whether these relationships differ across countries, require further investigations. We included participants aged ≥45 years from the USA (N = 5792), England (N = 3773), and China (N = 5016). SDoH (n = 121 for the USA, n = 125 for England, and n = 94 for China) were selected across seven domains. Frailty was assessed by the frailty index (FI). We developed Extreme Gradient Boosting to predict frailty at the 4-year follow-up and used SHapley Additive exPlanations to quantify variable-wise and domain-wise contributions of SDoH, and to explore nonlinear relationships between SDoH and frailty. Our models explained 0.242 (95% confidence interval [CI]: 0.203–0.281), 0.258 (95% CI: 0.190–0.324), and 0.172 (95% CI: 0.126–0.215) of the variance in FI among all participants from the USA, England, and China. Health behaviors and social connections or stressors were the most important domains in the USA and England, while material circumstances contributed largely in China. We observed several common important SDoH predictors across countries, such as body mass index and sleep duration, whereas their nonlinear relationship with frailty showed differences, and other country-specific risk factors were also identified. Our findings reveal the priorities of SDoH domains for addressing aging disparities and promoting healthy aging, especially region-specific risk factors for tailored public health prevention strategies.
Similar content being viewed by others
Introduction
Frailty is a syndrome characterized by an age-related functional decline across multiple physiological systems and an increased vulnerability to stressors, and it has been associated with an increased risk of falls, disability, long-term care, and mortality (Clegg et al. 2013; Hoogendijk et al. 2019). With an increasingly aging population, frailty places substantial burdens on individual well-being and healthcare systems (Hoogendijk et al. 2019). Therefore, identifying determinants of frailty is essential for developing public health interventions to prevent and delay this condition, thus ultimately promoting healthy aging (Hoogendijk et al. 2019).
Frailty is influenced by economic, social, environmental, and psychological factors across multiple domains (Feng et al. 2017; Buta et al. 2024; Hoogendijk et al. 2019; Qin et al. 2023), namely, social determinants of health (SDoH), defined as the conditions where we live, work, play, and age (World Health Organization, 2010). Several hypotheses have been proposed, including the fundamental causes theory, the risk environment theory, the syndemic theory, the ecosocial theory, and life-course approaches, to understand the relationship between SDoH and late-life health (Thimm-Kaiser et al. 2023). Bringing these theoretical perspectives together, the SDoH framework of the World Health Organization (WHO) provides a unifying structure by categorizing the SDoH into structural and intermediary determinants (World Health Organization, 2010). This framework emphasizes that structural determinants defined by income, education, occupation, social class, gender, and race/ethnicity, operate through a set of intermediary determinants, including material circumstances, psychosocial circumstances, behavioral and biological factors, and health systems, to shape health outcomes.
There have been much efforts to investigate frailty-related SDoH under this framework, whereas SDoH factors within and between domains are often studied in isolation from each other with a priori hypotheses (Feng et al. 2017; Qin et al. 2023; Puterman et al. 2020). Although several recent studies have examined the association between various factors and frailty, they all used traditional linear regression models that assumed no correlation between independent variables and linear exposure-outcome associations, which may oversimplify the model (Hoogendijk et al. 2018; X. Chen et al. 2022; Niederstrasser et al. 2019; Tan et al. 2022; Cao et al. 2022; Jiang et al. 2023). In contrast, machine learning approaches can capture nonlinear and interaction effects in a data-driven way, especially when dealing with large and high-dimensional datasets. Due to the potential interactions between a series of SDoH factors, machine learning provides a tool to obtain an understanding of the relative importance and cumulative contribution of multidomain SDoH to frailty. Such evidence can inform which SDoH domains can best be targeted for the interventions to address later-life health inequalities.
Even though approximately one-fifth of middle-aged and older populations in the Americas, Europe, and Asia are frail (O’Caoimh et al. 2021), the effect of SDoH on frailty may vary due to the disparities in sociocultural and policy contexts in these regions, especially between Western and Eastern countries (Ailshire and Carr, 2021). Specifically, the USA features a diverse cultural landscape, a predominantly market-based healthcare system, and notable disparities in income and access to services (Ridic et al. 2012). In contrast, England is characterized by a universal healthcare system and robust social welfare policies (Mitonga and Shilunga, 2020). Meanwhile, China represents a rapidly aging society undergoing dramatic urbanization and economic transformation. It is marked by unique cultural norms, such as collectivism and filial piety, along with a near-universal health insurance coverage (Lancet, 2022). However, only limited studies compare the association between risk factors and frailty across countries, and they all focus on specific SDoH domains (Hoogendijk et al. 2018; X. Chen et al. 2022). Therefore, assessing the relative importance of various factors for frailty in both Western and Eastern countries is important to inform universal and region-specific priorities of public policies. Furthermore, this understanding aligns with the recommendation by the WHO – ageing research needs to be better coordinated across countries to discover the most cost-effective approaches to maintain older people’s health and wellbeing (World Health Organization, 2011; Lu et al. 2021).
To bridge these gaps, this study aimed to investigate the relationship between multidomain SDoH and frailty from a longitudinal and cross-national perspective. Using a wide range of the SDoH across three population-based cohort studies in the USA, England, and China, we employed the tree-based machine learning approach Extreme Gradient Boosting (XGBoost) to predict frailty over the follow-up. We also utilized SHapley Additive exPlanations (SHAP) to quantify the variable-wise and domain-wise contributions of SDoH to frailty and investigate the nonlinear associations of SDoH with frailty. The findings could help develop regionally tailored strategies for frailty prevention and intervention, thereby promoting healthy aging and addressing age-related health inequalities within and across countries.
Methods
Study population
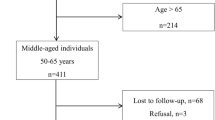
This study used data from three nationally representative longitudinal studies: the US Health and Retirement Study (HRS), the English Longitudinal Study of Ageing (ELSA), and the China Health and Retirement Longitudinal Study (CHARLS), as they provide comprehensive SDoH variables and represent three distinct cultural, socioeconomic, and policy environments. These studies were designed using similar survey protocols, enabling cross-regional comparisons. Additional details for each study have been previously described (Sonnega et al. 2014; Steptoe et al. 2013; Zhao et al. 2014). We restricted our samples to participants without frailty at baseline, defined as having a frailty index below 0.2 (Searle et al. 2008), to mitigate the influence of pre-existing frailty and minimize the possibility of reverse causation. Participants who did not meet the minimal inclusion age (≥51 years in HRS, ≥50 years in ELSA, and ≥45 years in CHARLS), were lost to the 4-year follow-up, or had missing information on the SDoH were excluded (Fig. 1a and Supplementary Methods). All studies received ethical approval from relevant local research ethics committees, and participants were recruited after providing written informed consent.
a Study population included from the HRS (USA), ELSA (England), and CHARLS (China). b Social determinants of health (SDoH) selected from seven domains. c Frailty index evaluated as the accumulation of age-related health deficits. d Extreme Gradient Boosting model as the machine learning approach. e Shapley Additive exPlanations method to analyze the SDoH associated with frailty from domain- and variable-wise importance, as well as their nonlinear relationships. HRS = Health and Retirement Study, ELSA = English Longitudinal Study of Ageing, CHARLS = China Health and Retirement Longitudinal Study.
Measures
Social determinants of health
We selected SDoH variables (n = 121 for HRS, n = 125 for ELSA, and n = 94 for CHARLS) across seven domains (Fig. 1b): adverse childhood experiences (ACEs), socioeconomic status (SES), material circumstances, social connections, social stressors, health behaviors, and healthcare systems (Supplementary Methods and Supplementary Tables 1–3). The selection of these variables was based on a careful review of the literature, with a focus on systematic reviews (Braveman et al. 2011; Braveman and Gottlieb, 2014; Powell-Wiley et al. 2022; Bhutta et al. 2023), the WHO’s report on SDoH (World Health Organization, 2010), and the availability of data from HRS, ELSA, and CHARLS. These selected SDoH variables are highly relevant for frailty prevention as they capture a range of risk factors that influence healthy aging across the life-course (Huang et al. 2024).
According to WHO’s SDoH framework (World Health Organization, 2010), SES refers to an individual’s position in a society and can be measured by education, occupation, income, and social class; material circumstances are defined as physical-environment-related determinants, such as housing, food, and neighborhood environments; health behaviors include lifestyle factors such as smoking, diet, alcohol consumption, and physical activity; healthcare systems comprise the accessibility and quality of medical services, as well as the coverage of healthcare insurance. Social connections and social stressors are derived from psychosocial circumstances of the WHO’s SDoH framework. Specifically, social connections have components pertaining to structure (e.g., social networks and living situation), function (e.g., social support), and quality (e.g., loneliness and relationship quality) (Holt-Lunstad, 2022), while social stressors refer to difficult or challenging circumstances arising from social position and experiences that are expected to be stressful (e.g., financial strain and life events) (Klopack et al. 2022). Additionally, ACEs are potentially traumatic events that occur in childhood, which have been increasingly recognized as SDoH (Bhutta et al. 2023). Categorical variables were dummy coded. We did not normalize numeric variables for further model interpretation. The missing rate of predictors ranged from 0 to 36% across all cohorts (Supplementary Tables 7–9). We imputed missing data using the MissForest method, a non-parametric random-forest-based algorithm for mixed-type data, with 100 trees grown in each forest and five iterations (Stekhoven and Bühlmann, 2012).
Frailty index
The frailty index (FI) was evaluated as the accumulation of age-related health deficits at baseline and the 4-year follow-up. Following the previously described standard procedure (Searle et al. 2008), we constructed the FI based on 45, 52, and 47 items for participants from HRS, ELSA, and CHARLS, respectively (Supplementary Tables 4–6). The deficits included chronic diseases, disability, mobility restrictions, self-rated health, sensory impairments, depressive symptoms, and cognition (Fig. 1c). This multidimensional approach captures a broad range of physical and psychological impairments, providing a comprehensive measure of biological aging and overall health status. Notably, the FI has demonstrated strong predictive validity for adverse health outcomes, such as mortality and hospitalization, across diverse populations (Clegg et al. 2013; Hoogendijk et al. 2019). The total number of deficits was divided by the number of items measured to produce an FI between 0 and 1, where a higher value indicated a higher degree of frailty. Most FI items had a missing rate of less than 5%, and over 70 and 55% of participants had complete data on FI items at baseline and follow-up (Supplementary Tables 4–6). To maximize the sample size and reduce the selection bias of the study population, missing data on FI items were imputed using the MissForest method (Pridham et al. 2022).
Statistical analyses
Descriptive statistics
Characteristics of the total population are presented using mean (standard deviation [SD]) for continuous variables or numbers (percentages) for categorical variables. Spearman correlations between the SDoH variables were less than 0.85 in all cohorts (Supplementary Fig. 1).
Model development and evaluation
We employed the XGBoost model to identify SDoH associated with frailty at the 4-year follow-up (Fig. 1d). XGBoost is an ensemble machine learning algorithm that combines multiple decision trees to generate accurate predictions, and to capture non-linear and interaction effects between predictors (T. Chen and Guestrin, 2016). Additionally, based on our experiments, XGBoost performed better compared with several commonly used machine learning algorithms, including Lasso, elastic net, support vector machines, and random forest (Supplementary Table 10). The dataset was randomly divided into training (80%) and testing (20%) sets. We used the mean squared error as the loss function for our regression task. The hyperparameters, including number of trees, maximum depth, learning rate, gamma, minimum child weight, number of features per tree, percentage of subsample per tree, and early stopping rounds, were chosen by the grid search approach and 10-fold cross-validation (Supplementary Table 11). Model performance on the training and testing sets was evaluated based on root mean squared error (RMSE), mean absolute error (MAE) and coefficient of determination (\({R}^{2}\)). The 95% confidence intervals (CIs) of performance metrics were estimated by bootstrapping the testing set 1000 times.
Model interpretation
Since the XGBoost is a blackbox model, we further employed the SHAP method, a post-hoc explanation algorithm for machine learning models, to understand and interpret the relationships between SDoH variables and frailty (Fig. 1e). SHAP values, computed by a game theoretical approach, can quantify the contribution of each predictor to the prediction for each participant and explain the final prediction as the sum of the SHAP value of each predictor (Lundberg et al. 2020). Higher (or lower) SHAP values imply large positive (or negative) contributions to the FI prediction. We used all participants in the training set to explain our prediction models. First, mean absolute SHAP values across all participants were calculated to obtain the global impact of each SDoH predictor (i.e., variable-wise importance), where more important predictors have higher mean absolute SHAP values. Furthermore, given the additivity of SHAP values, we calculated the domain-wise importance by normalizing mean absolute SHAP values and summing them across all SDoH variables within a given domain (Lundberg et al. 2020; Madakkatel et al. 2021). This approach allows us to estimate the cumulative contribution, ranging between 0 and 1, of each SDoH domain to FI prediction. Finally, we used the partial dependence plot to show the marginal effect of a predictor on FI prediction, which can capture nonlinear relationships between an SDoH predictor and frailty.
Sensitivity analyses
We performed sensitivity analyses to explore the robustness of our results. First, due to the steeper increase after 65 years old (Raymond et al. 2020) and sex differences in FI (Kane and Howlett, 2021), we developed prediction models by age and sex. Then, we used Explainable Boosting Machine (EBM) models to repeat our primary analyses with the same training and testing sets and using default parameters (Supplementary Methods) (Nori et al. 2019). The EBM is an explainable machine learning method that is derived from the generalized additive model and determines the shape function of each feature using bagging and gradient boosting with round-robin cycles (Nori et al. 2019). Finally, we performed environment-wide association studies (EWAS) to investigate the relationship between SDoH factors and frailty (Supplementary Methods). Similar to genome-wide association studies (GWAS), EWAS is an analytic approach that systematically tests all available factors while reducing the chance of spurious findings by internal validation and false discovery control (Patel et al. 2010).
EBM was performed using Python Version 3.8, and other statistical analyses were conducted using R software Version 4.1.1. A two-sided P < 0.05 was considered statistically significant.
Results
Study population
Baseline characteristics of participants in HRS (N = 5792, 44.0% of males), ELSA (N = 3773, 45.9% of males), and CHARLS (N = 5016, 54.3% of males) are presented in Supplementary Tables 7–9. The mean (SD) age of participants was 65.2 (9.2) years in HRS, 65.9 (8.5) years in ELSA, and 58.1 (9.2) years in CHARLS. At the 4-year follow-up, the mean (SD) FI was 0.14 (0.09) in HRS, 0.11 (0.08) in ELSA, and 0.15 (0.08) in CHARLS (Fig. 2a–c and Supplementary Table 12).
Domain-wise importance
Figure 3 presents the domain-wise importance of seven SDoH domains and demographics along with the explained variance (i.e., \({R}^{2}\)). Our models explained 0.242 (95% CI: 0.203–0.281), 0.258 (95% CI: 0.190–0.324), and 0.172 (95% CI: 0.126–0.215) of the variance in FI among all participants from HRS, ELSA, and CHARLS, while the explained variance was between 0.090–0.319 in subgroups. Social connections (22.3%), social stressors (20.3%), and health behaviors (18.3%) had the largest contribution to frailty in HRS, wherein health behaviors (30.5%) and social connections (20.4%) were also the most important SDoH in ELSA. In CHARLS, SDoH domains with importance scores of ≥10% included material circumstances (16.6%), ACEs (15.5%), health behaviors (15.2%), and social stressors (10.6%). Age- and sex-stratified analyses showed similar patterns of domain-wise importance.
Domain-wise importance of SDoH among the overall population and by subgroups, in the USA (a), England (b), and China (c). Domain-wise importance was calculated by normalizing mean absolute SHAP values and summing them across all SDoH variables within a given domain. The importance of scores ≥10% is presented. The \(R^2\) are reported for each model. SDoH social determinants of health, SHAP SHapley Additive exPlanation, HRS = Health and Retirement Study, ELSA = English Longitudinal Study of Ageing, CHARLS = China Health and Retirement Longitudinal Study.
Variable-wise importance
Figure 4 and Supplementary Fig. 2 summarize the top 20 most important variables in each prediction model. Among all participants in HRS, the most relevant SDoH factors included ongoing health problems, body mass index (BMI), sleep problems, using a computer or email, and government health insurance. In ELSA, the strongest SDoH predictors came from health behaviors, including sleep problems, BMI, vigorous/moderate physical activities, and sleep duration. In CHARLS, access to the shower/bath facility, BMI, sleep problems, out-of-pocket doctor visit expenditure, and father’s farming occupation were the most important SDoH.
Variable-wise importance of SDoH in the overall population, in the USA (a), England (b), and China (c). The top 20 most important SDoH ranked by mean absolute SHAP values are shown for clarity. Each point represents the SHAP value of a specific predictor for each individual. Indigo and cheese color indicate low and high values of the feature, respectively. A higher SHAP value on the x-axis indicated a higher degree of frailty for a given individual. The \({R}^{2}\), MAE, and RMSE are reported for each model. SDoH social determinants of health, HRS = Health and Retirement Study, ELSA = English Longitudinal Study of Ageing, CHARLS = China Health and Retirement Longitudinal Study, MAE = mean absolute error, RMSE = root mean squared error.
We found several strong SDoH predictors shared in three cohorts, including BMI, sleep problems, loneliness, and household wealth. Moreover, similar important SDoH variables were observed across two cohorts, such as physical activities for HRS and ELSA, sleep duration for ELSA and CHARLS, as well as out-of-pocket medical expenditure for HRS and CHARLS. Although most of these factors were also identified in different subgroups, we observed some variations in important SDoH between subpopulations (Supplementary Fig. 2). For instance, using the computer more frequently was associated with frailty among adults aged ≥65 years but not among those aged <65 years in HRS.
Associations between SDoH and frailty
Most SDoH variables were associated with frailty, aligning with the expected direction (Fig. 4). As an example, HRS participants who reported more severe ongoing health problems had a higher SHAP value, representing a higher degree of frailty. Moreover, higher household wealth, higher household income, and lower out-of-pocket expenditure were associated with a lower FI. However, the relationship between several predictors and frailty was opposite to our initial hypothesis and showed differences across cohorts (Supplementary Fig. 3). We observed that, for example, having government health insurance increased the frailty risk in HRS but not in CHARLS. Current smoking was associated with an increased FI in HRS and ELSA but not in CHARLS. Similarly, people with higher drinking frequency or more weekly drinks had a lower FI in ELSA and CHARLS, but a higher FI in HRS.
Nonlinear relationships between SDoH and frailty
Curve shapes indicated the ranges of the predictor value for which the corresponding SHAP values were positive, neutral, or negative (Fig. 5). For example, a sharp increase from zero in SHAP values occurred for a BMI ≥ 30 kg/m2 in HRS and ELSA, whereas positive SHAP values were observed in both BMI ≤ 18.5 kg/m2 and BMI ≥ 25 kg/m2 in CHARLS. Additionally, we found a U-shaped relationship between sleep duration and frailty, with minimal SHAP values observed at 7–8 h in ELSA and 7–9 h in CHARLS. ELSA participants who had <2.5 portions of daily fruit or vegetable consumption had a lower FI. Finally, daily duration of watching television among participants in ELSA was positively associated with frailty, while the threshold was five hours.
Partial dependence plots for body mass index (a), sleep duration (b), fruit and vegetable consumption (c), and watching television (d) among the overall population. Each point represents the SHAP value of a specific predictor for each individual. Predictor values are plotted on the x-axis. A higher SHAP value on the y-axis indicated a higher degree of frailty for a given individual. SHAP = SHapley Additive exPlanation, HRS = Health and Retirement Study, ELSA = English Longitudinal Study of Ageing, CHARLS China Health and Retirement Longitudinal Study.
Sensitivity analyses
When using EBM models to predict frailty, the results of domain-wise importance (Supplementary Fig. 4), variable-wise importance (Supplementary Fig. 5), and relationships between SDoH and frailty (Supplementary Fig. 6) were largely replicated. Additionally, we validated the associations between frailty and most SDoH factors identified by XGBoost models through the EWAS approach (Supplementary Fig. 7).
Discussion
Among middle-aged and older adults from the USA, England, and China, we assessed the impact of individual SDoH factors and SDoH domains on frailty at four years after baseline using machine learning and SHAP approaches. We showed that health behaviors and social connections or stressors were the most important domains in HRS and ELSA, but material circumstances contributed largely to frailty in CHARLS. We observed several common important SDoH predictors across countries, such as BMI and sleep duration, whereas their nonlinear relationship with frailty showed differences. Finally, understudied country-specific risk factors, such as access to the shower/bath facility in CHARLS, were also identified.
Our results showed that health behaviors were the important SDoH domain across the three countries. This is consistent with previous studies that have highlighted the role of lifestyle factors in the development and progression of frailty (Feng et al. 2017; Hoogendijk et al. 2019; Qin et al. 2023). However, the results regarding smoking and alcohol consumption varied between countries. Specifically, we observed that current smoking was associated with frailty in HRS and ELSA but not in CHARLS. A plausible explanation is that participants in CHARLS, especially males, had a much higher proportion of smoking than those in HRS and ELSA (Supplementary Table 9), which may lead to a selection bias or a survivor effect (Kojima et al. 2015). This higher prevalence of smoking in China may be attributed to the later implementation and lower effectiveness of tobacco control measures compared to the USA and England (Chan et al. 2023; World Health Organization, 2023). For example, cigarette package warnings are less noticeable, and the regulation of smoke-free environments is weaker in China. Additionally, cigarettes are often viewed as a form of social currency in China, where offering and sharing cigarettes is a common social practice, making tobacco control more challenging (Rich and Xiao, 2012). Similarly, we found that adults with more drinks per week had a higher FI in HRS, whereas those with a higher drinking frequency had a lower FI in ELSA and CHARLS. This discrepancy may suggest that higher drinking frequency reflects light or social drinking habits, while a greater weekly alcohol intake may indicate heavy drinking or binge drinking, which is more detrimental to health (Probst et al. 2020).
We found that the relationship between BMI and frailty differed between countries, with a J-shaped association in HRS and ELSA, but a U-shaped association in CHARLS. This may reflect the double burden of both underweight and overweight or obesity in Asian low- and middle-income countries (LMICs) (Wells et al. 2020), whereas the USA and England face a persistent obesity epidemic (NCD Risk Factor Collaboration NCD-RisC, 2024). In line with previous findings, we observed a U-shaped relationship between sleep duration and frailty, and found that longer daytime napping could reduce the frailty risk (Pourmotabbed et al. 2020; Zhang et al. 2023). Moreover, our results showed that moderate fruit consumption was associated with a lower FI, consistent with another study using ELSA data (Kojima et al. 2020). Overall, our findings support that the adherence to healthy behaviors plays a role in later-life frailty prevention at the population level, and examining potential nonlinear exposure-outcome associations could provide additional insights for public policies (Fraser et al. 2016).
Psychosocial factors, including social stressors and social connections, contributed substantially to frailty disparities in our study. Through repeated and prolonged activation of neuroendocrine responses, psychosocial stress could accelerate biological aging processes, leading to frailty (Polsky et al. 2022). Our findings further strengthen the evidence showing that stressors, including loneliness, daily discrimination, ongoing health problems, and leaving the job for health conditions were related to increased FI. Both the USA and England have recognized the growing public health challenge posed by loneliness and implemented national-level interventions to address it (The Lancet, 2023). In contrast, while we found that loneliness is also a strong predictor of frailty among Chinese older adults, there are fewer policies or public initiatives specifically targeting loneliness. In China, the cultural emphasis on filial piety and family-centered care traditionally places the responsibility for late-life care on family members rather than the country. However, rapid urbanization and demographic shifts, such as increased migration and smaller household sizes, have weakened traditional family support structures. Older adults in China may experience greater feelings of loneliness if their expectations for family involvement and care are not met (X. Wang, 2024). These findings highlight the urgent need for national policies or community programs to address loneliness in China, thereby mitigating its impact on frailty risk in older populations.
Regarding social connections, we found that using a computer, maintenance or gardening, and watching television were strong predictors of frailty. Social connections may influence frailty through multiple mechanisms, including providing emotional support, reducing stress, encouraging healthy behaviors, and enhancing cognitive stimulation, all of which contribute to better physical and mental function (Holt-Lunstad, 2022). However, we observed that watching television more frequently in HRS or more than5 h per day in ELSA was associated with an increased risk of frailty. This may be because watching television could increase sedentary time and reduce time spent on other social activities beneficial to physical and mental functioning (Fancourt and Steptoe, 2019). These results suggest that social connections could improve healthy aging, aligning with recent studies supporting hobby engagement and internet use as potential global social prescribing strategies (Mak et al. 2023; Luo et al. 2025). However, we also found that some specific activities or activities with improper frequency and duration may attenuate their benefits on health and even increase frailty risk. Thus, further research is needed to investigate the effect of specific types of social connections and their frequency or duration on frailty.
In our study, material circumstances had a great impact on frailty disparities in CHARLS. Our results showed that in-house shower facilities, internet connection, clean cooking fuel, and geographic region were the most important material factors in CHARLS. These findings are consistent with prior research demonstrating that better housing conditions were associated with healthy aging among older Chinese adults, while those living in coastal regions had a lower frailty prevalence (Gong et al. 2022; Wu et al. 2018). Given the rapid economic development and urbanization process, older Chinese populations, particularly in rural areas, may experience more severe housing poverty than those in the USA and England (Xie and Zhou, 2014). For example, 10% of older adults lack indoor toilets, and only 45% have access to toilets with seats and flushing water rather than conventional squatting toilets, even after a decade of toilet revolution in China (Gong et al. 2022; Cheng et al. 2018). Thus, improving housing conditions and creating age-friendly residential environments, such as shower facilities and toilets with seats and flushing water, could offer an effective solution for preventing frailty or reducing regional health disparities, especially in LMICs like China. Furthermore, recent China’s policies on age-friendly environment underscore the government’s efforts to enhance the living conditions of older adults (National Health Commission of the People’s Republic of China, 2020; Xu et al. 2023). These policies advocate for comprehensive improvements in housing, healthcare services, and community support systems to foster an age-friendly community. Importantly, they also highlight the integration of digital technologies as a future direction for building such environments. Integrating our findings with these policies offers a practical and culturally appropriate pathway to reduce frailty risks.
We demonstrated that several factors related to ACEs, SES, and healthcare systems were strong predictors of frailty. This is consistent with previous findings reporting that childhood adversity has wide-ranging and long-term negative effects on physiological systems, thus increasing the frailty risk (Q. Wang, 2022). Our results also add further evidence to validate the association between SES and frailty (J. Wang and Hulme, 2021). For healthcare systems, we observed that government health insurance was related to a lower risk of frailty in CHARLS but a higher risk in HRS. A possible reason is that the USA provides healthcare coverage for adults aged ≥65 years or with disabilities through Medicare, and China implements a universal health coverage system that entitles all citizens to receive public healthcare. Therefore, having government health insurance may indicate older age or poorer health status, which are risk factors for frailty, in the USA, but reflect higher socioeconomic status or better access to healthcare in China (Cho et al. 2023). Moreover, we found that people with higher out-of-pocket medical expenditures and lower healthcare satisfaction had a higher FI in HRS and CHARLS. Even in England, where there is a public healthcare system, poor access to medical resources due to transportation problems could increase frailty risk. These findings suggest that access to healthcare services is essential to promote optimal human functioning and healthy aging in older populations.
The main strength of our study was that we included three national longitudinal studies, representative of middle-aged and older adults from the USA, England, and China, to evaluate the longitudinal effects of seven-domain SDoH on frailty. Moreover, we combined machine learning models and state-of-the-art explanation methods to identify important SDoH factors for frailty and evaluate the contribution of each SDoH domain to FI prediction. Our cross-national comparisons shed light on the role that SDoH play in shaping healthy aging in later life under specific contexts.
Our findings have important policy implications. Our results support the United Nations’ Decade of Healthy Ageing 2021–2030 initiative by providing empirical evidence to inform international guidelines on healthy aging promotion (United Nations, 2020). Frailty prevention policies targeting modifiable factors, including health behaviors, social connections, and housing conditions, could be adapted globally while accounting for regional differences. Additionally, the identification of country-specific SDoH suggests the need for tailored public health strategies. For example, in LMICs like China, lifestyle modifications such as smoking cessation remain a priority, while in high-income countries, addressing obesity is crucial. Furthermore, machine learning approaches allow us to identify nonlinear relationships that can better inform frailty prevention strategies.
More importantly, as global aging cohort resources continue to expand (Khalatbari-Soltani et al. 2024), our study provides a hypothesis-free discovery framework for identifying potential determinants of healthy aging across countries through machine learning. This approach opens two promising directions for future research. First, a systematic investigation of cross-country discrepancies in key SDoH predictors could uncover possible mechanisms driving health disparities. For instance, our study observed differences in the association between smoking and frailty across countries, which may reflect cultural perceptions of smoking as social currency in China and policy implementation gaps in tobacco control (Rich and Xiao, 2012; Chan et al. 2023). Second, consistent and meaningful SDoH factors can be further validated through epidemiological studies using multi-cohort data. For example, the consistent protective association between internet connection and frailty observed in our study has been independently validated in another multi-cohort study (Li, 2024). These findings also lay the foundation for our work demonstrating the positive relationship between internet use and mental health across 23 countries (Luo et al. 2025). This scalable framework, when incorporated with more global aging cohorts, could inform the development of tailored public health interventions and policies that address population-specific needs while also revealing global patterns in healthy aging.
Several limitations should be noted in our study. First, SDoH variables and FI items were collected by self-reported questionnaires, potentially introducing recall bias and information bias. However, self-reported data allow for the cost-effective collection of a wide range of information, and these measures have been widely used and validated in large-scale epidemiological studies (Sonnega et al. 2014; Steptoe et al. 2013; Zhao et al. 2014). Second, although we selected SDoH based on the same framework, there were still inevitable variations in the included SDoH variables or measures across cohorts, which could reduce the cross-national comparability of our findings. We also recognized that cultural differences may shape the experience and interpretation of SDoH variables. Nonetheless, our results could give insights into universal and country-specific important SDoH for frailty, capturing common dimensions of social and economic life that are comparable across Western and Eastern countries. Third, information on certain SDoH factors, such as dietary patterns, was unavailable in these three cohorts. Future research with more detailed assessments of lifestyle and psychosocial factors could further elucidate their contributions to frailty risk. Fourth, our research does not allow for causal inferences, even though we conducted a longitudinal design and excluded participants with frailty at baseline to minimize the risk of reverse causation. Future research utilizing causal inference methods and interventional studies would be essential to further investigate the causal mechanisms underlying these associations. Fifth, a large proportion of adults were excluded due to missing data on SDoH, which may affect the representativeness of the study population. Larger cohort studies are needed to further verify the results. Sixth, due to the limited accessibility of the CHARLS data, we restricted our analysis to a four-year follow-up across all cohorts to ensure comparability. Future research could extend the follow-up period to capture long-term effects and consider additional health outcomes, such as multimorbidity and functional decline, to provide a more comprehensive understanding of how SDoH influences the aging process. Finally, we only considered one middle-income country (China) and two high-income countries (the USA and England). Validation in diverse populations across low-, middle-, and high-income countries could be further considered using the HRS international families of studies.
Conclusions
By identifying and comparing SDoH factors associated with frailty among middle-aged and older adults in the USA, England, and China, our study emphasizes the need to obtain a comprehensive understanding of the impact of multidomain SDoH on frailty. Our findings reveal the priorities of SDoH domains and factors for addressing aging disparities and promoting healthy aging, especially region-specific risk factors for tailored public health strategies to prevent frailty. More robust and cross-national evidence on the association between SDoH factors and frailty is needed to validate our findings.
Data availability
The data of this study were available on the following websites: the Health and Retirement Study (https://hrs.isr.umich.edu/), the English Longitudinal Study of Ageing (https://www.elsa-project.ac.uk/), the China Health and Retirement Longitudinal Study (http://charls.pku.edu.cn/), and the Gateway to Global Aging (https://g2aging.org/app/home).
Code availability
All code for analyses in this study is available for download from GitHub at https://github.com/YanLuoCityU/social-determinants-frailty.
References
Ailshire J, Carr D (2021) Cross-national comparisons of social and economic contexts of aging. J Gerontology: Ser B 76(Supplement_1):S1–S4. https://doi.org/10.1093/geronb/gbab049
Bhutta ZA, Bhavnani S, Betancourt TS, Tomlinson M, Patel V (2023) Adverse childhood experiences and lifelong health. Nat Med 29(7):1639–1648. https://doi.org/10.1038/s41591-023-02426-0
Braveman P, Egerter S, Williams DR (2011) The social determinants of health: coming of age. Annu Rev Public Health 32(1):381–398. https://doi.org/10.1146/annurev-publhealth-031210-101218
Braveman P, Gottlieb L (2014) The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 129(1_suppl2):19–31. https://doi.org/10.1177/00333549141291s206
Buta B, Twardzik E, Samuel L, Cudjoe T, Teano AL, Langdon J, Thorpe RJ, Walston J, Xue Q-L (2024) Social Determinants of Physical Frailty. In JG Ruiz & O Theou (Eds.), Frailty: A Multidisciplinary Approach to Assessment, Management, and Prevention (pp. 389–401). Springer International Publishing. https://doi.org/10.1007/978-3-031-57361-3_50
Cao X, Ma C, Zheng Z, He L, Hao M, Chen X, Crimmins EM, Gill TM, Levine ME, Liu Z (2022) Contribution of life course circumstances to the acceleration of phenotypic and functional aging: A retrospective study. eClinicalMedicine 51:101548. https://doi.org/10.1016/j.eclinm.2022.101548
Chan KH, Xiao D, Zhou M, Peto R, Chen Z (2023) Tobacco control in China. Lancet Public Health 8(12):e1006–e1015. https://doi.org/10.1016/S2468-2667(23)00242-6
Chen T, Guestrin C (2016) XGBoost: A Scalable Tree Boosting System. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, 785–794. https://doi.org/10.1145/2939672.2939785
Chen X, Yan B, Gill TM (2022) Childhood circumstances and health inequality in old age: comparative evidence from China and the USA. Soc Indic Res 160(2–3):689–716. https://doi.org/10.1007/s11205-020-02436-2
Cheng S, Li Z, Uddin SMN, Mang H-P, Zhou X, Zhang J, Zheng L, Zhang L (2018) Toilet revolution in China. J Environ Manag 216:347–356. https://doi.org/10.1016/j.jenvman.2017.09.043
Cho T-C, Yu X, Gross AL, Zhang YS, Lee J, Langa KM, Kobayashi LC (2023) Negative wealth shocks in later life and subsequent cognitive function in older adults in China, England, Mexico, and the USA, 2012–18: A population-based, cross-nationally harmonised, longitudinal study. Lancet Healthy Longev 4(9):e461–e469. https://doi.org/10.1016/S2666-7568(23)00113-7
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381(9868):752–762. https://doi.org/10.1016/S0140-6736(12)62167-9
Fancourt D, Steptoe A (2019) Television viewing and cognitive decline in older age: Findings from the English Longitudinal Study of Ageing. Sci Rep. 9(1):2851. https://doi.org/10.1038/s41598-019-39354-4
Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, Raat H (2017) Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLOS ONE 12(6):e0178383. https://doi.org/10.1371/journal.pone.0178383
Fraser A, Lawlor DA, Howe LD (2016) Nonlinear exposure-outcome associations and public health policy. JAMA 315(12):1286. https://doi.org/10.1001/jama.2015.18023
Gong J, Wang G, Wang Y, Chen X, Chen Y, Meng Q, Yang P, Yao Y, Zhao Y (2022) Nowcasting and forecasting the care needs of the older population in China: Analysis of data from the China Health and Retirement Longitudinal Study (CHARLS). Lancet Public Health 7(12):e1005–e1013. https://doi.org/10.1016/S2468-2667(22)00203-1
Holt-Lunstad J (2022) Social connection as a public health issue: the evidence and a systemic framework for prioritizing the “Social” in social determinants of health. Annu Rev Public Health 43(1):193–213. https://doi.org/10.1146/annurev-publhealth-052020-110732
Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP (2019) Frailty: Implications for clinical practice and public health. Lancet 394(10206):1365–1375. https://doi.org/10.1016/S0140-6736(19)31786-6
Hoogendijk EO, Rijnhart JJM, Kowal P, Pérez-Zepeda MU, Cesari M, Abizanda P, Flores Ruano T, Schop-Etman A, Huisman M, Dent E (2018) Socioeconomic inequalities in frailty among older adults in six low- and middle-income countries: Results from the WHO Study on global AGEing and adult health (SAGE). Maturitas 115:56–63. https://doi.org/10.1016/j.maturitas.2018.06.011
Huang Z-T, Lai ETC, Luo Y, Woo J (2024) Social determinants of intrinsic capacity: A systematic review of observational studies. Ageing Res Rev 95:102239. https://doi.org/10.1016/j.arr.2024.102239
Jiang R, Noble S, Sui J, Yoo K, Rosenblatt M, Horien C, Qi S, Liang Q, Sun H, Calhoun VD, Scheinost D (2023) Associations of physical frailty with health outcomes and brain structure in 483 033 middle-aged and older adults: A population-based study from the UK Biobank. Lancet Digit Health 5(6):e350–e359. https://doi.org/10.1016/S2589-7500(23)00043-2
Kane AE, Howlett SE (2021) Sex differences in frailty: Comparisons between humans and preclinical models. Mech Ageing Dev 198:111546. https://doi.org/10.1016/j.mad.2021.111546
Khalatbari-Soltani S, Si Y, Dominguez M, Scott T, Blyth FM (2024) Worldwide cohort studies to support healthy ageing research: Data availabilities and gaps. Ageing Res Rev 96:102277. https://doi.org/10.1016/j.arr.2024.102277
Klopack ET, Crimmins EM, Cole SW, Seeman TE, Carroll JE (2022) Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the US Health and Retirement Study. Proc Natl Acad Sci 119(25):e2202780119. https://doi.org/10.1073/pnas.2202780119
Kojima G, Iliffe S, Jivraj S, Walters K (2020) Fruit and vegetable consumption and incident prefrailty and frailty in community-dwelling older people: the English Longitudinal Study of ageing. Nutrients 12(12):3882. https://doi.org/10.3390/nu12123882
Kojima G, Iliffe S, Walters K (2015) Smoking as a predictor of frailty: A systematic review. BMC Geriatr 15:131. https://doi.org/10.1186/s12877-015-0134-9
Li L (2024) Internet use and frailty in middle-aged and older adults: Findings from developed and developing countries. Glob Health 20(1):53. https://doi.org/10.1186/s12992-024-01056-6
Lu W, Pikhart H, Sacker A (2021) Comparing socio-economic inequalities in healthy ageing in the United States of America, England, China and Japan: Evidence from four longitudinal studies of ageing. Ageing Soc 41(7):1495–1520. https://doi.org/10.1017/S0144686X19001740
Lundberg SM, Erion G, Chen H, DeGrave A, Prutkin JM, Nair B, Katz R, Himmelfarb J, Bansal N, Lee S-I (2020) From local explanations to global understanding with explainable AI for trees. Nat Mach Intell 2(1):56–67. https://doi.org/10.1038/s42256-019-0138-9
Luo Y, Yip PSF, Zhang Q (2025) Positive association between Internet use and mental health among adults aged ≥50 years in 23 countries. Nat Hum Behav 9(1):90–100. https://doi.org/10.1038/s41562-024-02048-7
Madakkatel I, Zhou A, McDonnell MD, Hyppönen E (2021) Combining machine learning and conventional statistical approaches for risk factor discovery in a large cohort study. Sci Rep. 11(1):22997. https://doi.org/10.1038/s41598-021-02476-9
Mak HW, Noguchi T, Bone JK, Wels J, Gao Q, Kondo K, Saito T, Fancourt D (2023) Hobby engagement and mental wellbeing among people aged 65 years and older in 16 countries. Nat Med. https://doi.org/10.1038/s41591-023-02506-1
Mitonga HK, Shilunga APK (2020) International Health Care Systems: Models, Components, and Goals. In Haring, R, Kickbusch I, Ganten D, Moeti M (Eds.), Handbook of Global Health (pp. 1–20). Springer International Publishing. https://doi.org/10.1007/978-3-030-05325-3_60-1
National Health Commission of the People’s Republic of China. (2020) Notice on Building a National Model of Age-Friendly Communities. https://www.gov.cn/zhengce/zhengceku/2020-12/14/content_5569385.htm
NCD Risk Factor Collaboration (NCD-RisC) (2024) Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403(10431):1027–1050. https://doi.org/10.1016/S0140-6736(23)02750-2
Niederstrasser NG, Rogers NT, Bandelow S (2019) Determinants of frailty development and progression using a multidimensional frailty index: Evidence from the English Longitudinal Study of Ageing. PLOS ONE 14(10):e0223799. https://doi.org/10.1371/journal.pone.0223799
Nori H, Jenkins S, Koch P, Caruana R (2019) InterpretML: A Unified Framework for Machine Learning Interpretability. https://doi.org/10.48550/ARXIV.1909.09223
O’Caoimh R, Sezgin D, O’Donovan MR, Molloy DW, Clegg A, Rockwood K, Liew A (2021) Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 50(1):96–104. https://doi.org/10.1093/ageing/afaa219
Patel CJ, Bhattacharya J, Butte AJ (2010) An Environment-Wide Association Study (EWAS) on Type 2 Diabetes Mellitus. PLoS ONE 5(5):e10746. https://doi.org/10.1371/journal.pone.0010746
Polsky LR, Rentscher KE, Carroll JE (2022) Stress-induced biological aging: A review and guide for research priorities. Brain Behav Immun 104:97–109. https://doi.org/10.1016/j.bbi.2022.05.016
Pourmotabbed A, Boozari B, Babaei A, Asbaghi O, Campbell MS, Mohammadi H, Hadi A, Moradi S (2020) Sleep and frailty risk: A systematic review and meta-analysis. Sleep Breath 24(3):1187–1197. https://doi.org/10.1007/s11325-020-02061-w
Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, Pita MA, Potharaju KA, Tamura K, Wallen GR (2022) Social determinants of cardiovascular disease. Circ Res 130(5):782–799. https://doi.org/10.1161/circresaha.121.319811
Pridham G, Rockwood K, Rutenberg A (2022) Strategies for handling missing data that improve Frailty Index estimation and predictive power: Lessons from the NHANES dataset. GeroScience 44(2):897–923. https://doi.org/10.1007/s11357-021-00489-w
Probst C, Kilian C, Sanchez S, Lange S, Rehm J (2020) The role of alcohol use and drinking patterns in socioeconomic inequalities in mortality: A systematic review. Lancet Public Health 5(6):e324–e332. https://doi.org/10.1016/S2468-2667(20)30052-9
Puterman E, Weiss J, Hives BA, Gemmill A, Karasek D, Mendes WB, Rehkopf DH (2020) Predicting mortality from 57 economic, behavioral, social, and psychological factors. Proc Natl Acad Sci 117(28):16273–16282. https://doi.org/10.1073/pnas.1918455117
Qin Y, Hao X, Lv M, Zhao X, Wu S, Li K (2023) A global perspective on risk factors for frailty in community-dwelling older adults: A systematic review and meta-analysis. Arch Gerontol Geriatr 105:104844. https://doi.org/10.1016/j.archger.2022.104844
Raymond E, Reynolds CA, Dahl Aslan AK, Finkel D, Ericsson M, Hägg S, Pedersen NL, Jylhävä J (2020) Drivers of frailty from adulthood into old age: results from a 27-year longitudinal population-based study in Sweden. J Gerontology: Ser A 75(10):1943–1950. https://doi.org/10.1093/gerona/glaa106
Rich ZC, Xiao S (2012) Tobacco as a social currency: Cigarette gifting and sharing in China. Nicotine Tob Res 14(3):258–263. https://doi.org/10.1093/ntr/ntr156
Ridic G, Gleason S, Ridic O (2012) Comparisons of Health Care Systems in the United States, Germany and Canada. Mater Socio-Med 24(2):112–120. https://doi.org/10.5455/msm.2012.24.112-120
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K (2008) A standard procedure for creating a frailty index. BMC Geriatr 8(1):24. https://doi.org/10.1186/1471-2318-8-24
Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR (2014) Cohort Profile: The Health and Retirement Study (HRS). Int J Epidemiol 43(2):576–585. https://doi.org/10.1093/ije/dyu067
Stekhoven DJ, Bühlmann P (2012) MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 28(1):112–118. https://doi.org/10.1093/bioinformatics/btr597
Steptoe A, Breeze E, Banks J, Nazroo J (2013) Cohort profile: The English Longitudinal Study of Ageing. Int J Epidemiol 42(6):1640–1648. https://doi.org/10.1093/ije/dys168
Tan V, Chen C, Merchant RA (2022) Association of social determinants of health with frailty, cognitive impairment, and self-rated health among older adults. PLOS ONE 17(11):e0277290. https://doi.org/10.1371/journal.pone.0277290
The Lancet (2022) Population ageing in China: Crisis or opportunity? Lancet 400(10366):1821. https://doi.org/10.1016/S0140-6736(22)02410-2
The Lancet (2023) Loneliness as a health issue. Lancet 402(10396):79. https://doi.org/10.1016/S0140-6736(23)01411-3
Thimm-Kaiser M, Benzekri A, Guilamo-Ramos V (2023) Conceptualizing the mechanisms of social determinants of health: a heuristic framework to inform future directions for mitigation. Milbank Q 101(2):486–526. https://doi.org/10.1111/1468-0009.12642
United Nations (2020) Decade of Healthy Ageing: Plan of Action
Wang J, Hulme C (2021) Frailty and socioeconomic status: a systematic review. J Public Health Res 10(3):jphr.2021.2036. https://doi.org/10.4081/jphr.2021.2036
Wang Q (2022) Association of adverse childhood experiences with frailty index level and trajectory in China. JAMA Netw Open 5(8):e2225315. https://doi.org/10.1001/jamanetworkopen.2022.25315
Wang X (2024) Toward a national profile of loneliness in old-age China: Prevalence and lonely life expectancy. J Gerontol Ser B Psychol Sci Soc Sci 80(1):gbae187. https://doi.org/10.1093/geronb/gbae187
Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, Demaio A (2020) The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 395(10217):75–88. https://doi.org/10.1016/S0140-6736(19)32472-9
World Health Organization (2010) A conceptual framework for action on the social determinants of health. https://www.who.int/publications/i/item/9789241500852
World Health Organization (2011) Global Health and Aging. National Institute on Aging, National Institutes of Health. https://www.nia.nih.gov/sites/default/files/2017-06/global_health_aging.pdf
World Health Organization (2023) WHO report on the global tobacco epidemic, 2023: Protect people from tobacco smoke. https://www.who.int/publications/i/item/9789240077164
Wu C, Smit E, Xue Q-L, Odden MC (2018) Prevalence and correlates of frailty among community-dwelling Chinese older adults: The China health and retirement longitudinal study. J Gerontology: Ser A 73(1):102–108. https://doi.org/10.1093/gerona/glx098
Xie Y, Zhou X (2014) Income inequality in today’s China. Proc Natl Acad Sci 111(19):6928–6933. https://doi.org/10.1073/pnas.1403158111
Xu W, Zhu J, Xi W, Cui J (2023) Creating age-friendly environments in a smart society in China: A policy review. J Aging Soc Policy, 1–20. https://doi.org/10.1080/08959420.2023.2284058
Zhang Y, Zhou L, Ge M, Lin X, Dong B (2023) Association between daytime nap duration and risks of frailty: Findings from the China Health and Retirement Longitudinal Study. Front Public Health 10:1098609. https://doi.org/10.3389/fpubh.2022.1098609
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G (2014) Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol 43(1):61–68. https://doi.org/10.1093/ije/dys203
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 71972164 and 72401210) and China Postdoctoral Science Foundation Funded Project (grant no. 2024M752282). We thank the HRS, ELSA, and CHARLS research and field teams for collecting the data. We also appreciate the Gateway to Global Aging team providing harmonized datasets and codebooks for the HRS, ELSA, and CHARLS.
Author information
Authors and Affiliations
Contributions
YL contributed to conceptualization, data curation, methodology, statistical analyses, visualization, writing the original draft, and reviewing and editing the manuscript. MG contributed to the methodology and reviewed and edited the manuscript. QZ contributed to conceptualization, funding acquisition, and reviewing and editing the manuscript. YL and QZ verified the data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The US Health and Retirement Study (HRS) and the China Health and Retirement Longitudinal Study (CHARLS) received ethical approval from the Health Sciences/Behavioral Sciences Institutional Review Board at the University of Michigan (Ann Arbor, MI, USA; HUM00061128) and the Biomedical Ethics Review Committee of Peking University (Beijing, China; IRB00001052–11015), respectively. The English Longitudinal Study of Ageing (ELSA) received ethical approvals for each wave of data collection as follows: Wave 3 was approved by the London Multi-Center Research Ethics Committee on 27 October 2005 (05/MRE02/63); Wave 4 by the National Hospital for Neurology and Neurosurgery & Institute of Neurology Joint Research Ethics Committee on 12 October 2007 (07/H0716/48); Wave 5 by the Berkshire Research Ethics Committee on 21 December 2009 (09/H0505/124); Wave 6 by the NRES Committee South Central – Berkshire on 28 November 2012 (11/SC/0374); and Wave 7 by the same committee on 28 November 2013 (13/SC/0532).
Informed consent
Informed consent was not required for this secondary data analysis study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, Y., Guo, M. & Zhang, Q. Cross-national analysis of social determinants of frailty among middle-aged and older adults: a machine learning study in the USA, England, and China. Humanit Soc Sci Commun 12, 738 (2025). https://doi.org/10.1057/s41599-025-05088-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1057/s41599-025-05088-0