Abstract
Ichthyosporea is an underexplored group of unicellular eukaryotes closely related to animals. Thanks to their phylogenetic position, genomic content, and development through a multinucleate coenocyte reminiscent of some animal embryos, the members of Ichthyosporea are being increasingly recognized as pivotal to the study of animal origins. We delve into the existing knowledge of Ichthyosporea, identify existing gaps and discuss their life cycles, genomic insights, development, and potential to be model organisms. We also discuss the underestimated diversity of ichthyosporeans, based on new environmental data analyses. This review will be an essential resource for researchers venturing into the study of ichthyosporeans.
Similar content being viewed by others
Introduction
Ichthyosporea is a clade of unicellular eukaryotes, most of which were isolated from animals as either parasitic or potentially symbiotic organisms. The first species were described in the mid-19th century as agents causing mortality in fish1,2,3,4. This determined the fate of ichthyosporeans to be studied almost exclusively as fish parasites and an economic threat to fisheries for the next several decades. Their destiny changed when detailed phylogenetic analyses identified these once disparate lineages as a monophyletic clade, closely related to animals5,6,7,8. Today, Ichthyosporea comprises at least 30 groups and 50 described species (taken from the Taxonomy database, NCBI)9 and is known as an important lineage that may hold clues to understanding the origin of animals. In addition, their diverse and complex life cycles offer a new framework for expanding our knowledge of eukaryotic cell biology. Before delving into ichthyosporean biology, we will first clarify uncertainties surrounding the group’s nomenclature that originated from the initial difficulties in the taxonomic placement of the many species described before the advent of molecular phylogenetics.
What is in a name?: that which we call an ichthyosporean
In the realm of scientific inquiry, as in Shakespeare’s timeless musings, a name encapsulates identity. The nomenclature of Ichthyosporea has changed over the years and continues to be a basis of confusion. We believe establishing a single, definitive name is pivotal, not just for clarity, but to provide a stable foundation for future studies in the field.
The nomenclature problem arose from the impossibility of assigning taxonomic placement to morphologically diverse organisms discovered decades before taxonomists were aware that this branch in the tree of life existed. Early records of what we now know as Ichthyosporea include species described in the mid 19th century, such as Enterobryus elegans, Amoebidium parasiticum, and Psorospermium. Based on morphological features, they were tentatively placed in different groups of eukaryotes, ranging from trichomycete fungi to algae. The trend of ongoing discoveries of enigmatic species continued throughout the 1900s. It wasn’t until the first ichthyosporean 18S rRNA gene was sequenced, from the “Rosette agent” (later named Sphaerothecum destruens), that their phylogenetic proximity to animals was established7. Additional sequences from Dermocystidium salmonis, Ichthyophonus hoferi, and P. haekeli gave rise to the provisional ‘DRIP’ clade, encompassing the four sequenced organisms at the time, Dermocystidium, Rosette agent, Ichthyophonus and Psorospermium5.
In 1998, Cavalier-Smith renamed DRIP to Ichthyosporea because of their round to ovoid walled spores and life history as parasites of fish (Ichthys in Greek)10. Subsequent studies expanded the DRIPs to include parasites of invertebrates, birds, and mammals. The broader variety of identified host organisms prompted a reevaluation of the nomenclature. The discovery that certain members, for example, Ichthyophonus, harbor chitin synthase genes and lack uniflagellated cells led to the adoption of the name Mesomycetozoa11 and also Class Mesomycetozoae12. This naming came from the intermediary position of the group between animals and fungi and was used to highlight similarities to fungi and the growing number of reclassified species that were symbionts of a broad range of hosts, rather than only fish. Modifications in both the composition and the definition of the group coupled with potential confusion of Mesomycetozoea with Mycetozoa—an unrelated group of eukaryotes—necessitated another modification of nomenclature. Thus, the term originally coined by Cavalier-Smith resurfaced, and Ichthyosporea emerged as an unmistakable term from then onwards among specialists. Therefore, we here embrace Ichthyosporea as the clade’s unequivocal name.
Ichthyosporea is closely related to animals
Phylogenomic analyses clearly show that Ichthyosporea is a member of the clade Holozoa, which also comprises Metazoa (animals) and the three other groups of close unicellular relatives of animals: Choanoflagellatea, Filasterea, and Corallochytrea (also referred to as Pluriformea)13,14 (Fig. 1). The exact phylogenetic relationship of Ichthyosporea among other unicellular Holozoa groups remains a matter of debate15,16. In different phylogenomics analyses, Ichthyosporea branch as either the sister-group to Filozoa (i.e., the clade comprising Metazoa, Choanoflagellatea, and Filasterea)16, the sister group to the rest of Holozoa17,18, or as the sister group to Corallochytrea forming what is known as the Teretosporea clade19,20,21,22. Further taxon sampling across Holozoa is needed to decipher the precise position of Ichthyosporea. Regardless of the specific evolutionary relationship with other Holozoa groups, Ichthyosporea occupies a crucial phylogenetic position for comparative studies as an early-branching lineage.
Ichthyosporea is estimated to have diverged from the lineage leading to animals ~1100 mya according to molecular clock studies23,24. However, these studies only featured three Ichthyosporea species and did not include the closest lineage, Corallochytrea. Given the considerable variability in dating deep branches of the eukaryotic tree—affected by both methodological approaches and the choice of calibrating fossils—more research is needed to accurately date the origin of Ichthyosporea. The preservation of cell content in a wide range of microfossils in Ediacaran age deposits (~600 mya) allowed the description of organisms that could feature potential early-branching holozoans, namely ichthyosporeans25,26,27,28,29,30. However, distinguishing them from other unicellular organisms or early animal embryos remains challenging. Notably, these fossils appeared after the estimated time of the split of Ichthyosporea from the rest of the Holozoa.
Ichthyosporea is divided into two major clades, Ichthyophonida and Dermocystida, a division well supported by molecular phylogenies10,12,19,22 (Fig. 2). Dermocystida is primarily composed of vertebrate parasites and has few known representatives. In contrast, there are many described species of Ichthyophonida, which are associated with various host species12,31. Ichthyphonida comprises Eccrinales, Amoebodiales, Paramoebidales, as well as other groups with fewer representative species. Eccrinales and Amoebidiales were among the first ichthyosporeans discovered and include many described species, but a lack of cultivated members thus far has severely hindered the possibility of further studying them. These two groups were originally classified as trichomycete fungi, which was later found to be polyphyletic, including both fungi and ichthyosporeans32. Currently, the term “protist trichomycete” is still used to refer to Eccrinales and Amoebodiales, which leads to confusion about the phylogenetic position of these groups. To avoid confusion, we recommend referring to them as Ichthyosporea. The exact phylogenetic relationships of the groups within Ichthyophonida remain unclear due to a lack of molecular data. Recent advances in metagenomic and single-cell sequencing techniques may allow for better genomic representation to be achieved.
The tree includes groups and genera described in this review. Species with no clear published morphological description or without full species names are not included. Solid lines represent phylogenetic placements determined through multi-gene analyses19,21,22,97. Dotted lines and polytomies indicate uncertain positions. Groups comprising several species are highlighted in bold.
Life cycles, phenotypic plasticity, and cell types
The different groups of unicellular relatives of animals exhibit various temporary multicellular life stages13,14. Choanoflagellates can form clonal colonies, some of which have the capacity to perform simple coordinated movements33. Filastereans can assemble into aggregates of thousands of cells upon specific molecular signals34,35. Finally, corallochytreans and ichthyosporeans can go through a stage with multinucleated cells, that is known as a coenocyte12,31,36,37.
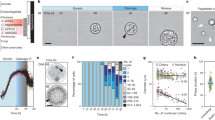
It is worth mentioning that the life cycles of most ichthyosporeans remain cryptic due to their phenotypic plasticity, parasitic nature, and the difficulty in culturing them. The latter is especially relevant for members of the Dermocystida clade, which are mostly generalist parasites of cold-blooded animals (fish and amphibians), except for Rhinosporidium seeberi, a parasite of birds and mammals, including humans38,39. Inside the host, the parasites form cysts containing one or several coenocytes surrounded by a cell wall, often with many small or one large central vacuole. Each coenocyte produces hundreds to thousands of either immobile spores or flagellated mobile zoospores (Fig. 3). It has been suggested that spores drop from the fish gills or amphibian skin into the water to later colonize a new host31,40,41. Interestingly, zoospore formation can be induced in some species by cultivating them in pure water (for example, in S. destruens, Dermocystidium cyprini, and Dermocystidium percae)42,43,44. In D. percae, zoospores can further transition into ameboid cells43. The ability of zoospores to infect a new host was confirmed in D. cyprinid and D. percae suggesting that the zoospores are a free-living, infectious stage in the life cycle of at least some Dermocystida species. Such induced formation of a motile stage indicates that these organisms are capable of complex cellular remodeling in response to environmental factors.
a In Dermocystida species coenocytes produce multiple mononucleated cells (spores), often with vacuoles, and/or zoospores (flagellated cells). A big central vacuole is usually prominent in the coenocytes. In D. percae, nuclei condense and arrange into a mass of reticulated chromatin in growing coenocytes. Coenocytes of D. cyprini form septae that segregate nuclei into groups. b In Ichthyophonida, coenocytes release mononucleated often vacuolated spores or motile amoebas. Some species can form intermediate stages, such as plasmodia (polynucleated ameboid cell) or hyphae. c Eccrinales grow elongated, non-vacuolated coenocytes which shed new cells from one end. Newly released spores can be mono- or polynucleated.
More is known about Ichthyophonida species, as several of them can be cultured in laboratory conditions. However, it is worth noting that culture conditions might not reveal all the diversity of their life stages in nature and several species show remarkable plasticity of forms even under culture conditions. As Dermocystida, Ichthyophonida species also form coenocytes as part of their life cycle. Interestingly, when these multinucleate coenocytes cellularize, the resulting multicellular structure is maintained for some time, blurring the distinction between multicellular and unicellular organisms. How the coenocyte is formed and what other life stages are present in their life cycle varies greatly between species (see Supplementary Data 1). For example, the genera Sphaeroforma includes both free-living and animal-associated species, and their life cycles typically alternate between mononucleated spores and multinucleated cell-walled coenocytes that eventually divide into new mononucleated spores (see Supplementary Data 1). In some Sphaeroforma species, rare plasmodial cells can be spotted but their role and frequency of appearance in nature are unknown45,46.
The mature coenocytes of most ichthyosporean species (both Ichthyophonida and Dermocystida) contain a large central vacuole that pushes the cytoplasm and the nuclei toward the plasma membrane. The function of the vacuole is not known but it could be involved in inducing cellularization along the cell wall. Interestingly, newly formed cells of Sphaeroforma arctica form a tight epithelium-like monolayer along the periphery of the coenocyte before the cells are released. This structure resembles some invertebrate embryos not only morphologically but also because it is formed in a similar way by inward membrane invagination and is also dependent on the actomyosin network47. Interestingly, another similarity to animal embryos is found in the coenocytes of Pirum gemmata37 or S. destruens42, where maturation includes serial cleavage of cytoplasm resembling palintomic division of animal zygotes. A new example of palintomic division in Ichthyosporea was recently described in the dermocystida Chromosphaera perkinsii48. This study also showed that C. perkinsii undergoes asymmetric division with the perpendicular spindle orientation on the two-cell stage, which is reminiscent of the spindle rotation mechanisms involved in cell differentiation in multicellular organisms. A maternal cell generates cells of at least two cell types within the same cell wall - flagellated zoospores and immotile round cells. The cells of the two cell types are arranged in spatial clusters. All these observations place ichthyosporeans in a unique position to address the origin of embryonic development and spatial cell differentiation.
Eccrinales (Ichthyophonida), however, seem to lack large vacuoles, and their coenocytes are long and thin, thalli-like structures that produce spores by basipetal septation, i.e., shedding new cells from the apex to the base of the coenocyte32 (Fig. 3c). This type of sporulation is characteristic of fungi. Interestingly, coenocytes of Eccrinales can produce two types of spores: thin-walled, multinucleated cells that re-infect the same animal and uninucleated cells with a thick cell wall for infecting new hosts32.
It was traditionally thought that an important distinction between Ichthyophonida and Dermocystida was the presence of amoebas, which were thought to be specific to Ichthyophonida (Fig. 4). Notable examples include Creolimax fragrantissima and the genera Amoebidium and Paramoebidium, which release large numbers of actively crawling amoebas from mature coenocytes31,49,50,51. Accordingly, electron microscopy studies have shown the presence of spindle pole bodies in Ichthyophonida31,52, a trait that can be linked to the absence of centrioles and likely the subsequent loss of flagella19,45. In contrast, dermocystid species such as D. percae and C. perkinsii retain both centrioles and flagella44,52. However, there is evidence of amoebas in D. percae and amoeboflagellated cells in C. perkinsii44,48.
The best example of the phenotypic plasticity of Ichthyophonida is, so far, Abeoforma whisleri, which displays several cell morphologies under culture conditions, from amoebas, and budding plasmodia (a multinucleated, wall-less, ameboid-shaped coenocyte) to clover-like tetrads, to coenocytes37. Similarly, the fish parasite I. hoferi can generate spores, plasmodial cells, and multinucleated cysts. Moreover, in response to changes in pH, I. hoferi cells can either grow hypha-like structures or transform into amoebas53.
The existence of meiosis in ichthyosporeans remains unclear and only few studies address ploidy and nuclear division within this group. These studies highlight the diversity of nuclear division mechanisms across different species. For example, Cervinka et al. describes that nuclei in immature coenocyes of D. cyprini are larger (3 um) than in mature coenocytes (1.5 um) suggesting the possibility of endoreplication54. Similarly, a wide range of nuclear sizes has also been observed in various life stages of A. whisleri (unpublished data) and P. gemmata37. The cells of D. cyprini, D. percae, and S. destruens go through nuclear division at least twice. Initially, coenocytes divide to produce spores, which then split into several zoospores when incubated in pure water42,44. Moreover, in D. percae, nuclei de-condense into an amorphous mass of reticular chromatin in growing coenocytes and only re-assemble shortly before division of the coenocyte by plasmotomy (division of a multinucleated cell into new multinucleated cells)44. This array of nuclear behaviors positions ichthyosporeans as valuable models for studying eukaryotic evolution.
Our current knowledge of eukaryotes is based on a handful of model organisms and, therefore, limited. Expanding our cell biological research to include such diverse and exciting organisms like ichthyosporeans, promises to provide new insights into eukaryotic evolutionary processes. In this regard, we have previously hypothesized that the unicellular ancestor of animals had a complex life cycle with different cell types14. The presence of so many distinct cell types (flagellated, ameboid, coenocytic, hyphae, polynucleated cells) within Ichthyosporea supports the idea that cellular diversity is a characteristic shared by all holozoans and likely was inherited from their common unicellular ancestors.
Genomes, genome regulation, gene content
An important step to address the origin of animals is to unravel the gene content of the last unicellular ancestor. The genomes of ichthyosporeans are central to this effort. Sequencing these genomes has posed significant challenges, from difficulties in DNA extraction to technical hurdles in sequencing and assembly. For instance, the genomes of Amoebidium sp., A. whisleri, and P. gemmata have proven particularly challenging to assemble with short-read data due to high repetitiveness19. Long-read sequencing attempts have also faced obstacles, possibly due to metabolite contaminants or unique genomic structures. However, and despite these challenges, the genomes of several ichthyosporean species have been sequenced. In this section, we discuss the most noteworthy aspects of the available genomic and transcriptomic data. We also provide a resource table for all publicly available omics data from ichthyosporeans (Supplementary Data 2).
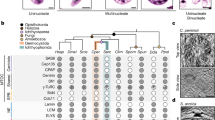
The genomes of ichthyosporeans vary in size and gene content (Fig. 5), but they are the largest among the unicellular relatives of animals, comparable in size to those of early branching animals. For example, the genome sizes of S. arctica, A. whisleri, P. gemmata, A. appalachense, and I. hoferi range between 84.4 and 200 Mb, while the genomes of filastereans and choanoflagellates are estimated to be in the 30–50 Mb range19. Moreover, the current genome assemblies of A. whisleri and P. gemmata are fragmented and likely underestimate their true sizes. Some of the factors that can explain the large genome size are the increased length of intergenic regions (comparable to that in animals), the massive intron-gain event that took place at the root of Ichthyosporea, and an increase of repetitive elements. Indeed, over 400 transposable element superfamilies have been identified in A. whisleri and P. gemmata, 31% of which show sequence similarities with their counterparts in animal genomes19.
Schematic phylogenetic tree of species within Ichthyosporea. The adjacent table summarizes the availability of cultures, genomic data, and genetic tools in each species. Species for which cultures are available are marked19,31,37,45,49. Circles denote available transcriptomes (solid circle) and/or genomes (circle outline)19,47,56,61. Species for which protocols for transfection through electroporation are available are indicated36,64. Dashes denote absence.
The substantial non-coding regions in ichthyosporean genomes, together with the rich repertoire of splicing factors and genes for nonsense-mediated mRNA decay (NMD), suggests that alternative splicing (AS) plays an important role in genome regulation in this lineage55. Interestingly, in C. fragrantissima AS predominantly occurs through intron retention rather than exon skipping (more typical in animals), although both mechanisms are present56. It remains to be further investigated whether this is true for other ichthyosporeans.
Protein domain expansion at the root of Holozoa is thought to be a key factor in the origin and evolution of animal multicellularity13,57. New genes or new protein domain combinations could have been co-opted to perform novel or modified functions in the first animals13. Accordingly, this trend can be observed in ichthyosporeans as they possess a rich repertoire of genes previously thought to be “animal-specific”. For instance, the genome of C. fragrantissima encodes almost the complete gene set of the integrin adhesome, with these genes upregulated in its ameboid stage56. In S. arctica, genes critical for cell adhesion in animals, such as catenins and cadherins, are upregulated during coenocyte cellularization47. Some “animal-specific” transcription factors (TF) were also shown to be upregulated at different life stages, for example, in the ameboid cells of C. fragrantissima (T-box family TF, Runx, hemophagocytic lymphohistiocytosis TFs)56, and during coenocyte maturation in C. perkinsii (Brachyury, RunX, MYC)48.
The genome data from ichthyosporeans is crucial to unravel the evolutionary history of different gene families. One example is the genes required for nitrate assimilation, encoding a nitrate transporter, and nitrate and nitrite reductases. Nitrate assimilation can prove advantageous for osmotrophs (such as ichthyosporeans and fungi)58. Initially, it was theorized that fungi had acquired the nitrate assimilation genes from Oomycetes, a member of Stramenopiles59. However, a recent study with broader taxon sampling showed that the cluster including these genes was assembled de novo in an ancestor of the Stramenopiles and the entire cluster transferred by horizontal gene transfer (HGT) to basal Opisthokonta60. These genes are maintained in many fungal clades but are absent in holozoans, with a striking exception. In particular, two species of ichthyosporeans, S. arctica and C. fragrantissima, have been found to possess gene clusters encoding homologs of the three proteins, while a gene encoding nitrate reductase was found in C. perkinsii. Additionally, the genome of Sphaeroforma sirkka encodes the three nitrate assimilation genes, where they are potentially clustered (unpublished data). Moreover, it was shown that the gene cluster is transcriptionally regulated in S. arctica and enables the organism to utilize nitrate as its sole nitrogen source. Interestingly, the same study suggests that the nitrate utilization gene cluster of Oomycetes originates from a second HGT event, from an ichthyosporean60. Overall, nitrate assimilation genes are an example of genes that were thought to have been obtained just in fungi but were actually obtained before the divergence between fungi and animals.
Ichthyosporeans reveal complex genome regulation mechanisms, some of which resemble that of animals. For example, besides AS, they also employ nonsense-mediated mRNA decay. The genomes of several Ichthyophonida contain the whole gene set involved in processing microRNAs, including drosha, pasha, dicer, and argonaute55; while no works on Dermocystida have been published yet. Moreover, the functionality of the microRNA machinery in different species of Sphaeroforma was confirmed by showing that the expressed microRNA genes maintain the specific structure that can be recognized by RNAse lll enzymes55. It demonstrates that this transcriptional regulatory mechanism was already present before the onset of animals and was later lost in both Filasterea and Choanoflagellatea55.
Long intergenic non-coding RNAs (lincRNAs) have also been identified in various ichthyosporean species47. Some lincRNAs are conserved within the Ichthyosporea lineage, although there are also species-specific lincRNAs. Interestingly, some lincRNAs are co-expressed with the genes that are upregulated during coenocyte maturation and cellularization. In addition, Suga and Ruiz-Trillo demonstrated the effectiveness of short interfering RNA (siRNA) for gene knockdown in C. fragratissima36. Altogether, these findings suggest that Ichthyosporea is the only lineage of the unicellular relatives of animals that employs different RNA regulatory mechanisms to fine-tune gene expression.
The study of genome regulatory mechanisms in unicellular holozoans has just recently begun and it is showing to be challenging due to the complex evolutionary histories of the genes involved, including lineage-specific retentions and losses. For example, Amoebidium appalachense has retained the ability to perform 5mC methylation by DNA methytransferase 1 (DNMT1). In contrast, other unicellular holozoans have lost DNMT1, retaining only those methyltransferases required for RNA-specific methylation, i.e., DNMT2-DNMT661. The presence of DNMT1 might have allowed the recurrent giant viral invasions in the genome of A. appalachense61. Moreover, it was experimentally shown that 5mC methylation in this species acts as a silencing mechanism of the endogenized viral DNA fragments, strongly suggesting that this strategy is essential in this ichthyosporean to tame the consequences of parasitic sequence integration.
Another important level of genome regulation is post-translational modification (PTM) of histones and the corresponding proteins involved in writing and reading these modifications. This avenue of research is just starting to be paved in a wider holozoan context. To date, only a few representatives from each of the four unicellular clades have been inspected62,63. These early findings reveal a diverse range of histone PTMs, underscoring the need for further investigations in Ichthyosporea to fully understand the evolution of this genomic regulation mechanism.
Emerging model systems among Ichthyosporea: an ongoing effort
A powerful way to unravel the secrets of Ichthyosporea and to gather deeper insights into animal origins is to establish model organisms among this clade. However, this is not an easy task, especially for groups such as the Ichthyosporea where much about their biology remains unknown. In this section, we outline the progress made toward developing model systems within this group.
Several species of Ichthyosporea that can be cultured axenically were tested as potential models. In the case of P. gemmata and S. arctica a thick cell wall was on the way and any attempts to establish transfection failed64. However, successful transient transfection has been achieved in C. fragrantissima and A. whisleri. C. fragrantissima was the first unicellular holozoan established as a model organism a decade ago36. More recently, A. whisleri has been transfected by nucleofection, an electroporation based method64. These advances allowed for the specific visualization of the plasma membrane, nucleus, and cytoskeleton of the cell when labeled with marker proteins coupled with fluorescent proteins. This approach was especially useful for understanding the cell division and development of C. fragrantissima, and ongoing studies are underway for A. whisleri. For example, the details on the synchronous division of nuclei in C. fragrantissima coenocytes sparked the hypothesis that the cellularization of a coenocyte could be one of the possible mechanisms by which initial multicellularity in animals evolved (see also ref. 14).
In those species in which transfection has not yet been established, other methods have been used to gain further biological insights. For example, the use of fluorescent dyes combined with specific pharmacological inhibitors in S. arctica allowed the detection of similarities between animal epithelia and the arrangement of the newly formed cells in S. arctica before they are released from the common cell wall47. In addition, a method to manipulate the size of the S. arctica coenocyte by changing culture conditions was established65. It helped to show that the nuclear-cytoplasmic ratio is the trigger for the onset of cellularization in this organism, which is also a key regulator for the maternal to zygotic transition in animal embryos. In addition, ultrastructural expansion microscopy was recently established in several ichthyosporean species and used to demonstrate that C. perkinsii has open mitosis while some representatives of Ichthyophonida have closed mitosis52.
A good example of the importance of having genetic tools in these taxa is the analysis of the regulatory mechanism of Src tyrosine kinase in C. fragrantissima which is, so far, the first functional analysis in Ichthyosporea of a conserved gene involved in multicellulararity in Metazoa66. Src triggers various cellular activities including proliferation, migration, and differentiation. The Csk tyrosine kinase suppresses Src activity by phosphorylating its catalytic domain. Interestingly, C. fragrantissima possesses Src (CfrSrc), but not Csk. Over-expression of CfrSrc in C. fragrantissima negatively impacted cell growth and impeded the coenocyte maturation stage and release of new cells. Further studies using co-overexpression of CfrSrc and the tyrosine phosphatase 3 (CfrPTP-3), rescued the growth defect caused by CfrSrc overexpression. This suggests that a pre-existing PTP was co-opted to take the place of Csk for negative regulation of Src kinase66.
Finally, it is also possible to perform gene expression knock down through RNAi (RNA interference) and Morpholinos in C. fragrantissima36. The successful gene silencing through siRNA indicated the functionality of the C. fragrantissima dicer gene as in Metazoa (see above also section on gene regulation and ref. 55). Overall, the establishment of genetic tools in ichthyosporeans marks a significant stride in evolutionary biology, enabling us to unravel the complexities of these enigmatic organisms and their developmental processes, as well as to interrogate the potential homology of cellular processes between animals and ichthyosporeans.
The uncultured majority: ichthyosporean diversity and environmental distribution
Our present knowledge of Ichthyosporea is based primarily on animal-associated species obtained in culture (Fig. 5), and from observations of members long known as parasites that have remained recalcitrant to cultivation12,38,67,68. In the case of cultured Ichthyosporea, host effects along the parasite-mutualist spectrum remain unclear, and whether host-association is obligate, facultative, or even opportunistic. Indeed, some isolates may simply have been obtained from hosts by chance and in actuality originate from the surrounding environment. Furthermore, free-living ichthyosporean species have now been isolated, S. sirkka and Sphaeroforma napiecek from the Sub-Arctic Bering Sea46 and C. perkinsii from shallow marine sediments in Hawai’i19, highlighting the potential for an unknown and uncultivated diversity of free-living Ichthyosporea. Cultivation-independent 18S rRNA gene metabarcoding studies, and several metagenomic and metatranscriptomic investigations, show that our current cultured representation of Ichthyosporea is biased, presenting an incomplete view of diversity across the group. To take full advantage of the clues Ichthyosporea has to offer concerning animal origins, further efforts to uncover the uncultivated diversity of Ichthyosporea are crucial. Here, we compile published cultivation-independent studies on Ichthyosporea environmental diversity (Supplementary Data 3). In addition, we collected 18S rRNA gene sequences from various sources to gain an up-to-date picture of Ichthyosporea diversity and distribution.
Ichthyosporean species have been detected using 18S rRNA gene metabarcoding in diverse multicellular hosts including invasive fish populations69, mosquito larvae70, starfish71, and carnivorous pitcher plants72. More importantly, several studies have uncovered a large uncultivated diversity of Ichthyosporea in a wide variety of environmental samples73,74,75, and new clades composed solely of uncultivated Ichthyosporea lineages discovered, such as Marine Ichthyosporean 1 (MAIP1) and Freshwater Ichthyosporean 1 (FRESHIP1)73. Further studies have also detected Ichthyosporea in a wide variety of geographically distinct marine-associated environments76,77,78,79. In particular, undescribed sequences have been found in low-oxygen marine environments including anoxic marine water, soil, and marine sediment samples80,81,82,83,84. Ichthyosporea has also been found at low relative abundances in globally distributed freshwater samples85,86,87,88,89,90,91. Unexpectedly, this includes the detection of typically marine-associated genera at higher relative abundances92, showcasing the large unexplored diversity of Ichthyosporea in non-marine environments. Ichthyosporea has also been found to be ubiquitous in diverse soil samples93, and present in marine ikaite tufa column formations94, and in coastal salt marshes95. Overall, the 26 cultivation-independent surveys of eukaryotic diversity outlined above (Supplementary Data 3) demonstrate that Ichthyosporea is ubiquitously distributed across a wide variety of environments including host-associated, freshwater, marine water, and sediment, and soil.
To have a better understanding of the environmental diversity of Ichthyosporea, we here compiled data from the EukBank database96 to compare the broad environmental distribution of well-known unicellular Holozoa lineages. The results show that Ichthyosporea is ubiquitous, with particularly high relative abundance among unicellular Holozoa in sediments and soil (Fig. 6). Ichthyophonida is the dominant lineage in marine sediments and soil, and also has higher relative abundances in sediment and freshwater. Dermocystida, on the other hand, has low relative abundances across environments, although slightly higher in sediment samples (Fig. 6). Interestingly, and despite being known for parasites, Dermocystida has low relative abundance in organism-derived samples, while Ichthyophonida are relatively abundant in marine organisms.
a Relative abundance and environmental distribution of well-characterized unicellular Holozoa lineages across samples assigned to different overarching environment categories in EukBank96. Specifically, the categories land_freshwater (freshwater collected from a terrestrial environment), land_organism (organism(s) collected from a terrestrial environment), land_sediment (sediment collected from a terrestrial environment), land_soil (soil collected from a terrestrial environment), land_water (water collected from saline or other terrestrial environments), marine_ice (ice collected from a marine environment), marine_organism (organism(s) collected from a marine environment), marine_sediment (sediment collected from a marine environment), and marine_water (water collected from a marine environment). b Number of reads assigned to the ichthyosporean lineages Ichthyophonida and Dermocystida across environment categories. Bars are colored according to the Figure legend and the number at the top of each column in panel a indicates the total number of samples from the given environment. See Supplementary Data 4.
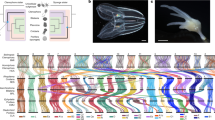
With the aim of updating our understanding of Ichthyosporea diversity we collected 315 unique ichthyosporean 18S rRNA genes from a variety of sources and inferred a maximum likelihood (ML) phylogeny with other Opisthokonta as an outgroup (Fig. 7, Supplementary Data 4, 5, and 6). Consistent with prior studies19,21,22,73,97, we recover Ichthyosporea (ultrafast bootstrap support (ufboot): 98, non-parametric bootstrap support (BP): 36) and the two major divisions, Dermocystida (ufboot: 100, BP: 99) and Ichthyophonida (ufboot: 100, BP: 83) (Fig. 7). When excluding short sequences and clustering at species-level (97% identify), we obtained 11 Dermocystida and 105 Ichthyophonida species representatives (Supplementary Data 6), consistent with the higher number of described Ichthyophonida species. We also inferred a ML phylogeny with species representatives and Ichthyosporea amplicon sequence variants (ASVs) from EukBank96, allowing us to investigate environmental distribution at a finer scale (Fig. 8).
Maximum-likelihood phylogeny of 315 unique Ichthyosporea 18S rRNA genes (and 108 outgroup sequences) collected from various data sources (see Methods and Supplementary Data 4) inferred using the GTR + FO + R7 model of evolution (alignment length: 1800 nt). Supported and newly described clades are collapsed and those newly identified are indicated in pink. Sequences within each clade were assigned to environment categories according to EukBank96 criteria as outlined in the Fig. 6 legend, and are indicated by circles according to the legend (land_water was excluded). A star next to a clade indicates that it includes a cultured representative. Support from ultrafast bootstraps (ufboot) and non-parametric bootstraps (BP) is indicated by circles according to the legend. Branches with less than 90% ufboot support are collapsed. See Supplementary Data 5 and 6 for alignments and uncollapsed trees and for a maximum-likelihood phylogeny including only long (≥1000 nt) 18S rRNA genes from species representatives (97% sequence identity).
Maximum-likelihood phylogeny of the 324 EukBank96 amplicon sequence variants (ASVs) classified as Ichthyosporea (in black) in the context of the 116 Ichthyosporea species representatives clustered at 97% sequence identity (in green) inferred using the GTR + FO + R7 model of evolution (alignment length: 1728 nt). Read counts, normalized per environment category, for each ASV are indicated by bars colored according to the legend (See Supplementary Data 4 for ASV information and the Fig. 6 legend for a description of each environment category). Support from ultrafast bootstraps (ufboot) and non-parametric bootstraps (BP) is indicated by circles according to the legend. Branches with less than 90% ufboot support are collapsed. The outgroup and sequence IDs can be found in the labeled phylogeny in Supplementary Data 6, and alignment and tree files in Supplementary Data 5.
Dermocystida has low diversity on an 18S rRNA gene level and forms three consistent clades: a Chromosphaera clade (ufboot: 98, BP: 43), a Sphaerothecum clade (ufboot: 100, BP: 100), and a clade composed of the remaining genera, Amphibiocystidium, Dermocystidium, Dermotheca, Rhinosporidium, and Valentines (ufboot: 100, BP: 77) (Fig. 7 and Supplementary Data 6). In the later clade, there are no clear distinctions between genera, which have long been known for parasites with most sequences retrieved from mammals, fish, or amphibians (Supplementary Data 4). However, affiliated ASVs have low read counts in freshwater samples, which may represent a promising environment for the retrieval of isolates or single-cell genomes (Fig. 8). The Chromosphaera clade includes the free-living C. perkinsii and sequences from diverse environmental samples, and is associated with ASVs that have the highest read counts in marine sediment, and include a sub-clade found in freshwater sediment.
Within Ichthyophonida, Amoebidinium, Eccrinales, Ichthyophonus, Parataeniella, and several uncultivated groups form a supported clade AEI (ufboot: 100, BP: 72), and affiliated with FRESHIP1, a previously identified uncultivated freshwater group (Fig. 7)73. Most sequences in the AEI clade were derived from animals, with the exception of the uncultivated groups (Supplementary Data 4). FRESHIP3 (described in ref. 97) and FRESHIP4 (identified here) sequences were instead obtained from freshwater and freshwater sediment. In addition, we newly identified the “Deep Ichthyophonus” clade composed of sequences from deep aphotic marine water samples (between 800 and 3300 meters below sea level)98. Deep Ichthyophonus is the only AEI group with a substantial number of affiliated ASVs and is detected in marine water (Fig. 8).
Creolimax, Sphaeroforma, Anurofeca, and MAIPH1 (described in ref. 97) also form a supported clade CSA within Ichthyophonida (ufboot: 97, BP: 30; ufboot: 100, BP: 75 with species representatives) (Fig. 7 and Supplementary Data 6). Anurofeca has particularly high diversity, while the one described species Anurofeca richardsi is a frog larvae pathogen found in digestive tracts99, most other sequences were obtained from diverse non-marine rather than host-associated samples (Supplementary Data 4). Many ASVs affiliate with Anurofeca with high read counts in non-marine organisms, soil, and freshwater sediment (Fig. 8). In contrast, sequences from diverse marine habitats form the Creolimax and Sphaeroforma clade, with affiliated ASVs primarily detected across marine samples, with one Sphaeroforma-related ASV having particularly high read counts.
Finally, we recovered the CABEPI clade97, including Caullerya, Pirum, Abeoforma, and uncultivated lineages (ufboot: 100, BP: 68; BP:100 with species representatives) (Fig. 7 and Supplementary Data 6). MAIP1 was recently identified as paraphyletic and reclassified into MACABEPI1, MACABEPI2, and MAIPh1 (part of CSA)73,97, which we recovered alongside additional uncultured CABEPI groups (Fig. 7). In particular, we were able to newly identify FRECABEPI1 from freshwater samples98, which is on a long branch together with ASVs detected in marine sediment (Fig. 8). ASVs affiliated with MACABEPI2, Pirum, and Pirum-related CABEPI suggest the groups are exclusively marine and are particularly abundant in marine water and sediment (Fig. 8). MACABEPI1 ASVs were not detected in organism-derived samples, with subclades exclusive to marine and freshwater samples (Fig. 8). MACABEPI1 is thus an intriguing target for isolating putative free-living Ichthyosporea, such as from anoxic sediment samples from which a number of sequences were obtained (Supplementary Data 4)82,100. More generally, there are sequences found across CAPEBI that were retrieved from anoxic or low-oxygen environments, and several from uncultivated groups were obtained from marine sediment foraminifera, a group of rhizarian protists, suggesting associations with other unicellular eukaryotes101 (Supplementary Data 4). FRESHIP2, a previously identified group73, is composed of sequences from freshwater environments and is supported as sister to CABEPI in some phylogenies (Fig. 8 and Supplementary Data 6). The remaining orphan Ichthyophonida groups Psorospermium and SOILIP2, identified here from soil-derived sequences, have no clear affiliations.
All together, our analysis of Ichthyosporea 18S rRNA gene diversity suggests that around 95% of species-level diversity remains uncultivated, showcasing the potential insights to be gained from exploring abundant undescribed lineages. Furthermore, our results suggest anoxic, sediment, deep marine, freshwater, and soil environments as ideal targets for future sampling and cultivation-independent efforts to capture these groups.
Conclusions
The study of animal origins underwent a significant change in the last two decades when a new research approach based on comparative genomics was established. Choanoflagellates were the first to re-emerge as models to study the origin of animals, and later filastereans, ichthyosporeans, and corallochytreans. Together they have broadened our understanding of how the transition from a unicellular eukaryote to multicellularity in animals could have occurred. It is now becoming clearer that the inclusion of ichthyosporeans in this paradigm strengthens the hypothesis of a unicellular ancestor of animals being more complex than previously thought, both on the genomic and developmental levels. In particular, this clade contributes to the evolutionary reconstruction of this ancestor by representing diverse cell types and life stages that require complex genomic and transcriptional regulation. Even more exciting is the potential of ichthyosporeans to elucidate the possible origin of embryonic development due to the resemblance of their coenocytic division to embryo cleavage. Of course, we should be critical in the interpretation of the data obtained and these results must be further validated. First, because ichthyosporeans split from the animal ancestor ~1100 mya and have independently evolved since then. Second, because there is a lot of unknown diversity among Ichthyosporea and among unicellular holozoans. With the advancing development of molecular and genetic tools and the ongoing search for new species, we believe that ichthyosporeans will bring us new and exciting discoveries in the field of animal origins and eukaryotic evolution.
Methods
Environmental distribution of unicellular Holozoa
The environmental distribution of unicellular Holozoa was assessed using the EukBank v. 1 18S rRNA gene V4 dataset96. The number of reads clustered in non-metazoan Holozoa ASVs was summed according to taxonomic assignments at the levels of “taxogroup1” (Choanoflagellatea, Filasterea (and Tunicaraptor), and Pluriformea (Corallochytrea)) and “taxogroup2” (Ichthyophonida, Dermocystida, Acanthoecida and Craspedida), and the relative abundance of each group calculated across environments based on the category “envplot” (Fig. 6 and Supplementary Data 4).
Ichthyosporea 18S rRNA gene sequences from publically available metagenomes
Reference unicellular Holozoa sequences were used as a query to search against publicly available NCBI WGS metagenomic assemblies with “metagenomes” taxids (both ecological and organismal) in the Taxonomy database9. Specifically, the “taxid2wgs.pl” WGS tools script from NCBI was used to retrieve WGS accessions corresponding to the different metagenome taxids. Then the “blastn_vdb” tool from BLAST+ v. 2.13.0102 was used to search the reference sequences against each WGS metagenome project accession with a cut-off of 90% “perc_identity” and 50% “qcov_hsp_perc”. Metagenomic contigs including hits were then retrieved using “efetch” from EDirect v. 15.1103 and the 18 S rRNA gene region extracted using Barrnap v. 0.9104 with the “--kingdom euk” flag. Initial taxonomic classification of sequences was performed using Crest4 v. 4.3.6105. With broad sampling of eukaryotic diversity from EukProt v. 3106 and the reference unicellular Holozoa sequences an initial 18 S rRNA gene phylogeny was then inferred and used to identify metagenome-derived 18S rRNA genes that consistently clustered with Ichthyosporea, which were included in further analyses.
Opisthokonta 18S rRNA gene phylogenies
Opisthokonta sequences were collected from EukProt v. 3106, EukRibo v. 1.0107, and select sequences from NCBI108, and ref. 97. In addition, 18S rRNA gene sequences classified as Ichthyosporea were retrieved from a recent eukaryotic long-read metabarcoding study98 and the metagenome search outlined above. These Opisthokonta outgroup and Ichthyosporea sequences were compiled and unique sequences retained using cd-hit 4.8.1109 at 100% identity. Finally, additional Ichthyosporea sequences were retrieved by searching reference ichthyosporean sequences against NCBI’s108 “nt” database with a cut-off of 90% “perc_identity” and 50% “qcov_hsp_perc”. Sequence redundancy was reduced using cd-hit 4.8.1109 at 99% identity and an initial phylogeny inferred and sequences consistently clustering with Ichthyosporea retained. Across all longer sequences, the region corresponding to the 18S rRNA gene was extracted using Barrnap v. 0.9104 with the “--kingdom euk” flag.
An 18S rRNA gene phylogeny with the resulting 423 Opisthokonta outgroup and Ichthyosporea sequences was then inferred (Fig. 7, Supplementary Data 4, Supplementary Data 5 and 6). Sequences were aligned with MAFFT v. 7.407 “einsi”110 and poorly aligned regions trimmed using trimAl v. 1.4.1111 with the “-gt 0.2” option resulting in 1800 retained positions. A ML phylogeny was then inferred using IQ-TREE v. 2.2.2.2112 with model selection using ModelFinder113 from among GTR models with the options “-m MFP”, “ -mset GTR”, “-mfreq, F, FO”, and, “-mrate ALL”, and with 1000 ufboot114 and 1000 SH-like approximate likelihood ratio test (SH-aLRT) replicates115. An additional ML phylogeny was then inferred using IQ-TREE v. 2.2.2.2112 with the selected model (GTR + FO + R7) and 100 BP.
Species representatives were then selected by retaining sequences with a minimum length of 1000 nt using the Seqkit v. 2.4.0116 seq “--min-len” option and clustering sequences at 97% identity using cd-hit 4.8.1109 (Supplementary Data 4). The resulting 206 species representative sequences were then aligned as outlined above, and poorly aligned regions trimmed using Gblocks v. 0.91b117 with all less stringent options selected in SeaView v. 4118 resulting in 1482 retained nt positions. ML phylogenies were then inferred as outlined above with the GTR + FO + I + R5 model of evolution selected (Supplementary Data 5 and 6).
Phylogenetic position and environmental distribution of Ichthyosporea ASVs
ASVs classified as Ichthyosporea in EukBank v. 196 were retrieved (Supplementary Data 4) and added to the species representatives alignment using the “--addfragments” option in MAFFT v. 7.407110. The 530 sequences were then trimmed using trimAl v. 1.4.1111 with the “-gt 0.2” option resulting in 1728 retained positions. A ML phylogeny was then inferred as outlined above with 1000 ufboot114 and 1000 SH-aLRT replicates115, with the GTR + FO + R7 model of evolution selected (Fig. 8, Supplementary Data 5 and 6). Read counts for each ASV were summed across all samples categorized into each environment category “envplot” and normalized by rescaling between 0 and 1 for each category (Fig. 8, Supplementary Data 4). The environment categories “land_water” and “marine_ice” were excluded, as fewer than 100 reads were assigned to each category across the Ichthyosporea ASVs.
Data visualization
Phylogenetic trees were visualized using iTOL119 and Figtree v1.4.4120. The bar plots in Fig. 8 were visualized in iTOL119 and the bar plots in Fig. 6 visualized using ggplot2 3.5.0121 implemented in R version 4.3.3 (R Core Team, 2024).
Change history
22 August 2024
In this article the ORCiD for Elena Casacuberta https://orcid.org/0000-0002-8203-8357, Patricia S. Ara https://orcid.org/0000-0002-3508-9688 and Fernando J. Bascon https://orcid.org/0009-0009-4473-4405 were inadvertently omitted. The original article has been corrected.
References
Leidy, J. Enterobrus, a new genus of Confervaceae. Proc. Acad. Nat. Sci. Phila. 4, 225–227 (1849).
Lieberkühn, N. Ueber parasitische Schläuche auf einigen Insectenlarven. Arch. Anat. Physiol. Wiss. Med. 25, 494–495 (1856).
Schenk, A. Ueber parasitische Schläuche auf Crustaceen. Phys. Med. Ges. 8, 252–259 (1858).
Haeckel, E. Ueber die gewebe des flusskrebses. Arch. Anat. Physiol. Wiss. Med. 24, 469–568 (1857).
Ragan, M. A. et al. A novel clade of protistan parasites near the animal-fungal divergence. Proc. Natl Acad. Sci. USA 93, 11907–11912 (1996).
Medina, M. et al. Phylogeny of Opisthokonta and the evolution of multicellularity and complexity in Fungi and Metazoa. Int. J. Astrobiol. 2, 203–211 (2003).
Kerk, D. et al. The rosette agent of chinook salmon (Oncorhynchus tshawytscha) is closely related to choanoflagellates, as determined by the phylogenetic analyses of its small ribosomal subunit RNA. Mar. Biol. 122, 187–192 (1995).
Ruiz-Trillo, I. et al. Capsaspora owczarzaki is an independent opisthokont lineage. Curr. Biol. 14, R946–R947 (2004).
Schoch, C. L. et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database 2020, baaa062 (2020).
Cavalier-Smith, T. A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc. 73, 203–266 (1998).
Herr, R. A., Ajello, L., Taylor, J. W., Arseculeratne, S. N. & Mendoza, L. Phylogenetic analysis of Rhinosporidium seeberi’s 18S small-subunit ribosomal DNA groups this pathogen among members of the protoctistan Mesomycetozoa clade. J. Clin. Microbiol 37, 2750–2754 (1999).
Mendoza, L., Taylor, J. W. & Ajello, L. The class mesomycetozoea: a heterogeneous group of microorganisms at the animal-fungal boundary. Annu Rev. Microbiol 56, 315–344 (2002).
Ros-Rocher, N., Pérez-Posada, A., Leger, M. M. & Ruiz-Trillo, I. The origin of animals: an ancestral reconstruction of the unicellular-to-multicellular transition. Open Biol. 11, 200359 (2021).
Ruiz-Trillo, I., Kin, K. & Casacuberta, E. The origin of metazoan multicellularity: a potential microbial black swan event. Annu. Rev. Microbiol. 77, 499–516 (2023).
Liu, H. et al. A genome-scale Opisthokonta tree of life: toward phylogenomic resolution of ancient divergences. 2023.09.20.556338. Preprint at https://doi.org/10.1101/2023.09.20.556338 (2023).
Tikhonenkov, D. V. et al. New lineage of microbial predators adds complexity to reconstructing the evolutionary origin of animals. Curr. Biol. 30, 4500–4509.e5 (2020).
Hehenberger, E. et al. Novel predators reshape holozoan phylogeny and reveal the presence of a two-component signaling system in the ancestor of animals. Curr. Biol. 27, 2043–2050.e6 (2017).
Tikhonenkov, D. V. et al. Insights into the origin of metazoan multicellularity from predatory unicellular relatives of animals. BMC Biol. 18, 39 (2020).
Grau-Bové, X. et al. Dynamics of genomic innovation in the unicellular ancestry of animals. eLife 6, e26036 (2017).
López-Escardó, D. et al. Reconstruction of protein domain evolution using single-cell amplified genomes of uncultured choanoflagellates sheds light on the origin of animals. Philos. Trans. R. Soc. B Biol. Sci. 374, 20190088 (2019).
Ocaña-Pallarès, E. et al. Divergent genomic trajectories predate the origin of animals and fungi. Nature 609, 747–753 (2022).
Torruella, G. et al. Phylogenomics reveals convergent evolution of lifestyles in close relatives of animals and fungi. Curr. Biol. 25, 2404–2410 (2015).
Parfrey, L. W., Lahr, D. J. G., Knoll, A. H. & Katz, L. A. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl Acad. Sci. USA 108, 13624–13629 (2011).
Eme, L., Sharpe, S. C., Brown, M. W. & Roger, A. J. On the age of eukaryotes: evaluating evidence from fossils and molecular clocks. Cold Spring Harb. Perspect. Biol. 6, a016139 (2014).
Hagadorn, J. W. et al. Cellular and subcellular structure of neoproterozoic animal embryos. Science 314, 291–294 (2006).
Cunningham, J. A., Vargas, K., Yin, Z., Bengtson, S. & Donoghue, P. C. J. The Weng’an Biota (Doushantuo Formation): an Ediacaran window on soft-bodied and multicellular microorganisms. J. Geol. Soc. 174, 793–802 (2017).
Yin, Z. et al. The early Ediacaran Caveasphaera foreshadows the evolutionary origin of animal-like embryology. Curr. Biol. 29, 4307–4314.e2 (2019).
Strother, P. K. et al. A possible billion-year-old holozoan with differentiated multicellularity. Curr. Biol. 31, 2658–2665.e2 (2021).
Yin, Z., Sun, W., Reitner, J. & Zhu, M. New holozoans with cellular resolution from the early Ediacaran Weng’an Biota, SW China. J. Geol. Soc. 179, jgs2021–jgs2061 (2022).
Sun, W., Yin, Z., Liu, P., Zhu, M. & Donoghue, P. Developmental biology of Spiralicellula and the Ediacaran origin of crown metazoans. Proc. Biol. Sci. 291, 20240101 (2024).
Marshall, W. L., Celio, G., McLaughlin, D. J. & Berbee, M. L. Multiple isolations of a culturable, motile Ichthyosporean (Mesomycetozoa, Opisthokonta), Creolimax fragrantissima n. gen., n. sp., from marine invertebrate digestive tracts. Protist 159, 415–433 (2008).
Cafaro, M. J. Eccrinales (Trichomycetes) are not fungi, but a clade of protists at the early divergence of animals and fungi. Mol. Phylogenet. Evol. 35, 21–34 (2005).
Brunet, T. et al. Light-regulated collective contractility in a multicellular choanoflagellate. Science 366, 326–334 (2019).
Ros-Rocher, N. et al. Chemical factors induce aggregative multicellularity in a close unicellular relative of animals. Proc. Natl Acad. Sci. USA 120, e2216668120 (2023).
Sebé-Pedrós, A. et al. Regulated aggregative multicellularity in a close unicellular relative of metazoa. eLife 2, e01287 (2013).
Suga, H. & Ruiz-Trillo, I. Development of ichthyosporeans sheds light on the origin of metazoan multicellularity. Dev. Biol. 377, 284–292 (2013).
Marshall, W. L. & Berbee, M. L. Facing unknowns: living cultures (Pirum gemmata gen. nov., sp. nov., and Abeoforma whisleri, gen. nov., sp. nov.) from invertebrate digestive tracts represent an undescribed clade within the unicellular opisthokont lineage ichthyosporea (Mesomycetozoea). Protist 162, 33–57 (2011).
Gozlan, R. E. et al. Current ecological understanding of fungal-like pathogens of fish: what lies beneath? Front. Microbiol. 5, 62 (2014).
Vilela, R. & Mendoza, L. The taxonomy and phylogenetics of the human and animal pathogen Rhinosporidium seeberi: a critical review. Rev. Iberoam. de. Micología 29, 185–199 (2012).
Olson, R., Dungan, C. & Holt, R. Water-born transmission of Dermocystidium salmonis in the laboratory. Dis. Aquat. Org. 12, 41–48 (1992).
Fagotti, A. et al. Developmental stages of Amphibiocystidium sp., a parasite from the Italian stream frog (Rana italica). Zoology 141, 125813 (2020).
Arkush, K. D., Mendoza, L., Adkison, M. A. & Hedrick, R. P. Observations on the life stages of Sphaerothecum destruens n. g., n. sp., a mesomycetozoean fish pathogen formerly referred to as the rosette agent [correction]. J. Eukaryot. Microbiol 50, 430–438 (2003).
Lotman, K., Pekkarinen, M. & Kasesalu, J. Morphological observations on the life cycle of Dermocystidium cyprini Červinka and Lom, 1974, parasitic in carps (Cyprinus carpio). Acta Protozool. 39, 125–134 (2000).
Pekkarinen, M. & Lotman, K. Occurrence and life cycles of Dermocystidium species (Mesomycetozoa) in the perch (Perca fluviatilis) and ruff (Gymnocephalus cernuus) (Pisces: Perciformes) in Finland and Estonia. J. Nat. Hist. 37, 1155–1172 (2003).
Marshall, W. L. & Berbee, M. L. Comparative morphology and genealogical delimitation of cryptic species of sympatric isolates of Sphaeroforma (Ichthyosporea, Opisthokonta). Protist 164, 287–311 (2013).
Hassett, B. T., López, J. A. & Gradinger, R. Two new species of marine saprotrophic sphaeroformids in the Mesomycetozoea isolated from the sub-Arctic Bering Sea. Protist 166, 310–322 (2015).
Dudin, O. et al. A unicellular relative of animals generates a layer of polarized cells by actomyosin-dependent cellularization. eLife 8, e49801 (2019).
Olivetta, M., Bhickta, C., Chiaruttini, N., Burns, J. & Dudin, O. A multicellular developmental program in a close animal relative. bioRxiv https://doi.org/10.1101/2024.03.25.586530 (2024).
Whisler, H. C. Pure culture of the Trichomycete, Amoebidium parasiticum. Nature 186, 732–733 (1960).
Whisler, H. C. Developmental control ofAmoebidium parasiticum. Dev. Biol. 17, 562–570 (1968).
White, M. M., Siri, A. & Lichtwardt, R. W. Trichomycete insect symbionts in Great Smoky Mountains National Park and vicinity. Mycologia 98, 333–352 (2006).
Shah, H. et al. Life-cycle-coupled evolution of mitosis in close relatives of animals. Nature 630, 116–122 (2024).
Okamoto, N. et al. Life history and morphology of ichthyophonus hoferi in vitro. Fish. Pathol. 20, 273–285 (1985).
Červinka, S., Vítovec, J., Lom, J., Hoška, J. & Kubů, F. Dermocystidiosis–a gill disease of the carp due to Dermocystidium cyprini n.sp. J. Fish. Biol. 6, 689–699 (1974).
Bråte, J. et al. Unicellular origin of the animal microRNA machinery. Curr. Biol. 28, 3288–3295.e5 (2018).
de Mendoza, A., Suga, H., Permanyer, J., Irimia, M. & Ruiz-Trillo, I. Complex transcriptional regulation and independent evolution of fungal-like traits in a relative of animals. eLife 4, e08904 (2015).
Suga, H. et al. The Capsaspora genome reveals a complex unicellular prehistory of animals. Nat. Commun. 4, 2325 (2013).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of earth’s nitrogen cycle. Science 330, 192–196 (2010).
Slot, J. C. & Hibbett, D. S. Horizontal transfer of a nitrate assimilation gene cluster and ecological transitions in fungi: a phylogenetic study. PLoS ONE 2, e1097 (2007).
Ocaña-Pallarès, E., Najle, S. R., Scazzocchio, C. & Ruiz-Trillo, I. Reticulate evolution in eukaryotes: origin and evolution of the nitrate assimilation pathway. PLOS Genet. 15, e1007986 (2019).
Sarre, L. A. et al. DNA methylation enables recurrent endogenization of giant viruses in an animal relative. Science Advances 10, eado6406 (2024).
Sebé-Pedrós, A. et al. The dynamic regulatory genome of capsaspora and the origin of animal multicellularity. Cell 165, 1224–1237 (2016).
Grau-Bové, X. et al. A phylogenetic and proteomic reconstruction of eukaryotic chromatin evolution. Nat. Ecol. Evol. 6, 1007–1023 (2022).
Faktorová, D. et al. Genetic tool development in marine protists: emerging model organisms for experimental cell biology. Nat. Methods 17, 481–494 (2020).
Olivetta, M. & Dudin, O. The nuclear-to-cytoplasmic ratio drives cellularization in the close animal relative Sphaeroforma arctica. Curr. Biol. 33, 1567–1605 (2023).
Suga, H. & Miller, W. T. Src signaling in a low-complexity unicellular kinome. Sci. Rep. 8, 5362 (2018).
Rowley, J. J. L. et al. Impacts of mesomycetozoean parasites on amphibian and freshwater fish populations. Fungal Biol. Rev. 27, 100–111 (2013).
Glockling, S. L., Marshall, W. L. & Gleason, F. H. Phylogenetic interpretations and ecological potentials of the Mesomycetozoea (Ichthyosporea. Fungal Ecol. 6, 237–247 (2013).
Spikmans, F. et al. Impact of the invasive alien topmouth gudgeon (Pseudorasbora parva) and its associated parasite Sphaerothecum destruens on native fish species. Biol. Invasions 22, 587–601 (2020).
Cuesta, E. B. et al. Comprehensive ecological and geographic characterization of eukaryotic and prokaryotic microbiomes in African Anopheles. Front. Microbiol. 12, 635772 (2021).
Hewson, I. & Sewell, M. A. Surveillance of densoviruses and mesomycetozoans inhabiting grossly normal tissues of three Aotearoa New Zealand asteroid species. PLoS One 16, e0241026 (2021).
Grothjan, J. J. & Young, E. B. Diverse microbial communities hosted by the model carnivorous pitcher plant Sarracenia purpurea: analysis of both bacterial and eukaryotic composition across distinct host plant populations. PeerJ 7, e6392 (2019).
del Campo, J. & Ruiz-Trillo, I. Environmental survey meta-analysis reveals hidden diversity among unicellular opisthokonts. Mol. Biol. Evol. 30, 802–805 (2013).
de Vargas, C. et al. Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean. Science 348, 1261605 (2015).
Arroyo, A. S., Lannes, R., Bapteste, E. & Ruiz-Trillo, I. Gene Similarity Networks Unveil a Potential Novel Unicellular Group Closely Related to Animals from the Tara Oceans Expedition. Genome Biol. Evol. 12, 1664–1678 (2020).
Hu, Y. O. O., Karlson, B., Charvet, S. & Andersson, A. F. Diversity of pico- to mesoplankton along the 2000 km salinity gradient of the Baltic Sea. Front. Microbiol. 7, 679 (2016).
Savin, M. C., Martin, J. L., LeGresley, M., Giewat, M. & Rooney-Varga, J. Plankton diversity in the Bay of Fundy as measured by morphological and molecular methods. Micro. Ecol. 48, 51–65 (2004).
Cleary, A. & Durbin, E. Unexpected prevalence of parasite 18S rDNA sequences in winter among Antarctic marine protists. J. Plankton Res. 38, fbw005 (2016).
Pagenkopp Lohan, K. M., Fleischer, R. C., Carney, K. J., Holzer, K. K. & Ruiz, G. M. Amplicon-based pyrosequencing reveals high diversity of protistan parasites in ships’ ballast water: implications for biogeography and infectious diseases. Microb. Ecol. 71, 530–542 (2016).
Stoeck, T. et al. Massively parallel tag sequencing reveals the complexity of anaerobic marine protistan communities. BMC Biol. 7, 72 (2009).
Takishita, K., Miyake, H., Kawato, M. & Maruyama, T. Genetic diversity of microbial eukaryotes in anoxic sediment around fumaroles on a submarine caldera floor based on the small-subunit rDNA phylogeny. Extremophiles 9, 185–196 (2005).
Takishita, K. et al. Genetic diversity of microbial eukaryotes in anoxic sediment of the saline meromictic lake Namako-ike (Japan): on the detection of anaerobic or anoxic-tolerant lineages of eukaryotes. Protist 158, 51–64 (2007).
Takishita, K., Yubuki, N., Kakizoe, N., Inagaki, Y. & Maruyama, T. Diversity of microbial eukaryotes in sediment at a deep-sea methane cold seep: surveys of ribosomal DNA libraries from raw sediment samples & two enrichment cultures. Extremophiles life Extrem. Cond. 11, 563–576 (2007).
del Campo, J. et al. Diversity and distribution of unicellular opisthokonts along the European coast analysed using high-throughput sequencing. Environ. Microbiol. 17, 3195–3207 (2015).
Metz, S. et al. Diversity of photosynthetic picoeukaryotes in eutrophic shallow lakes as assessed by combining flow cytometry cell-sorting and high throughput sequencing. FEMS Microbiol Ecol. 95, fiz038 (2019).
Chen, M., Chen, F., Yu, Y., Ji, J. & Kong, F. Genetic diversity of eukaryotic microorganisms in Lake Taihu, a large shallow subtropical lake in China. Micro. Ecol. 56, 572–583 (2008).
Khomich, M., Kauserud, H., Logares, R., Rasconi, S. & Andersen, T. Planktonic protistan communities in lakes along a large-scale environmental gradient. FEMS Microbiol. Ecol. 93, fiw231 (2017).
Machado, K. B. et al. Diversity patterns of planktonic microeukaryote communities in tropical floodplain lakes based on 18S rDNA gene sequences. J. Plankton Res. 41, 241–256 (2019).
Arroyo, A. S., López-Escardó, D., Kim, E., Ruiz-Trillo, I. & Najle, S. R. Novel diversity of deeply branching holomycota and unicellular holozoans revealed by metabarcoding in Middle Paraná River, Argentina. Front. Ecol. Evol. 6, 99 (2018).
Debroas, D. et al. Overview of freshwater microbial eukaryotes diversity: a first analysis of publicly available metabarcoding data. FEMS Microbiol. Ecol. 93, fix023 (2017).
Zheng, B.-H. et al. Structural characteristics and driving factors of the planktonic eukaryotic community in the Danjiangkou Reservoir, China. Water 12, 3499 (2020).
Mitsi, K. et al. Taxonomic composition, community structure and molecular novelty of microeukaryotes in a temperate oligomesotrophic lake as revealed by metabarcoding. Sci. Rep. 13, 3119 (2023).
Geisen, S. et al. Metatranscriptomic census of active protists in soils. ISME J. 9, 2178–2190 (2015).
Stougaard, P., Jørgensen, F., Johnsen, M. G. & Hansen, O. C. Microbial diversity in ikaite tufa columns: an alkaline, cold ecological niche in Greenland. Environ. Microbiol 4, 487–493 (2002).
Xiao, R., Guo, Y., Zhang, M., Pan, W. & Wang, J. J. Stronger network connectivity with lower diversity of soil fungal community was presented in coastal marshes after sixteen years of freshwater restoration. Sci. Total Environ. 744, 140623 (2020).
Berney, C. et al. EukBank 18S V4 Dataset [Data Set]. Zenodo https://doi.org/10.5281/zenodo.7804946 (2023).
Lu, Y. et al. Revisiting the phylogenetic position of Caullerya mesnili (Ichthyosporea), a common Daphnia parasite, based on 22 protein-coding genes. Mol. Phylogenet. Evol. 151, 106891 (2020).
Jamy, M. et al. Global patterns and rates of habitat transitions across the eukaryotic tree of life. Nat. Ecol. Evol. 6, 1458–1470 (2022).
Baker, G. C., Beebee, T. J. C. & Ragan, M. A. Prototheca richardsi, a pathogen of anuran larvae, is related to a clade of protistan parasites near the animal-fungal divergence. Microbiology 145, 1777–1784 (1999).
Dharamshi, J. E. et al. Marine sediments illuminate chlamydiae diversity and evolution. Curr. Biol. 30, 1032–1048.e7 (2020).
Woehle, C. et al. Denitrification in foraminifera has an ancient origin and is complemented by associated bacteria. Proc. Natl Acad. Sci. USA 119, e2200198119 (2022).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinform. 10, 421 (2009).
Kans, J. Entrez Direct: E-utilities on the Unix Command Line. in Entrez Programming Utilities Help [Internet] (National Center for Biotechnology Information (US), 2024).
Seemann, T. barrnap 0.8: rapid ribosomal RNA prediction.
Lanzén, A. et al. CREST—classification resources for environmental sequence tags. PLoS ONE 7, e49334 (2012).
Richter, D. J. et al. EukProt: a database of genome-scale predicted proteins across the diversity of eukaryotes. Peer Community J. 2, e56 (2022).
Berney, C., Henry, N., Mahé, F., Richter, D. & de Vargas, C. EukRibo: a manually curated eukaryotic 18S rDNA reference database to facilitate identification of new diversity. bioRxiv https://doi.org/10.1101/2022.11.03.515105 (2022).
Sayers, E. W. et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 50, D20–D26 (2022).
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Shen, W., Sipos, B. & Zhao, L. SeqKit2: a Swiss army knife for sequence and alignment processing. iMeta https://doi.org/10.1002/imt2.191 (2024).
Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552 (2000).
Gouy, M., Guindon, S. & Gascuel, O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224 (2010).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242–W245 (2016).
Wickham, H. Ggplot2 (Springer International Publishing, 2016). https://doi.org/10.1007/978-3-319-24277-4.
Acknowledgements
This work has been supported by grant PID2020-120609GB-I00 funded by MICIU/ AEI /10.13039/501100011033/ and by “ERDF A way of making Europe”. Also, funded by the European Union (ERC, MISSINGRELATIVES, 101097659). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them. We also acknowledge support to Departament de Recerca i Universitats de la Generalitat de Catalunya (exp. 2021 SGR 00751). P.S.A. is supported by grant PRE2018-085438 funded by MICIU/AEI /10.13039/501100011033 and by FSE Investing in your future. F.J.B is supported by grant FPU21/04058 funded by the Ministry of Science, Innovation and Universities, Spanish Government. J.E.D is supported by a grant from the Swedish Research Council (VR grant 2022-06250) and computational resources from the National Academic Infrastructure for Supercomputing in Sweden (NAISS) at UPPMAX with the project numbers NAISS 2023/22-491 and NAISS 2023/23-512. We acknowledge many researchers who have contributed to the establishment of cultures, sequences of genomes and/or genetic tools in Ichthyosporea, including Javier del Campo, Núria Sánchez-Pons, Helena Parra, Matija Harcet, Omaya Dudin, Andrej Ondracka, Sebastián Najle, Cristina Aresté, Guifré Torruella, Alex de Mendoza, Xavier Grau-Bové.
Author information
Authors and Affiliations
Contributions
Writing—original draft preparation: V.S., J.E.D., P.S.A., M.A., F.J.B., H.S., W.M., C.S., E.C., I.R.T.; writing—reviewand editing: V.S., J.E.D., P.S.A., M.A., F.J.B., H.S., W.M., C.S., E.C., I.R.T.; 18S rRNA gene analyses—J.E.D.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Natalia Mallo and other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Manuel Breuer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shabardina, V., Dharamshi, J.E., Ara, P.S. et al. Ichthyosporea: a window into the origin of animals. Commun Biol 7, 915 (2024). https://doi.org/10.1038/s42003-024-06608-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-06608-5
This article is cited by
-
A smartphone analogy to explore the origin of animals
The EMBO Journal (2026)
-
Genetic switch between unicellularity and multicellularity in marine yeasts
Nature (2026)
-
The evolutionary foundations of transcriptional regulation in animals
Nature Reviews Genetics (2025)