Abstract
Chronic heart failure (CHF) is closely associated with inflammation and mitochondrial dysfunction in cardiomyocytes. This study attempts to investigate the effects of microRNA-21-3p (miR-21-3p) on macrophage polarization and mitophagy in CHF. Here we found miR-21-3p was upregulated in CHF and negatively correlated with carnitine palmitoyl transferase 1A (CPT1A). L-palmitoyl carnitine (L-PC) exacerbated isoproterenol (ISO)-induced myocardial structural disruption and fibrosis in rats, which was exacerbated by miR-21-3p. Mechanistically, miR-21-3p accelerated M1 macrophage polarization. Both miR-21-3p inhibitor and CPT1A overexpression suppressed mitophagy. The inhibition of CPT1A on mitophagy was reversed by miR-21-3p. MiR-21-3p targeted CPT1A mRNA and co-localized with CPT1A protein in cardiomyocytes. In the co-culture system of M1 macrophages and H9c2 cells, miR-21-3p mimics in H9c2 cells promoted M1 polarization, whereas miR-21-3p inhibitor reduced M1 phenotype. M1 macrophages exacerbated H9c2 cell damage. These findings support the potential therapeutic targeting of miR-21-3p to regulate inflammation and mitophagy by inducing CPT1A in CHF.
Similar content being viewed by others
Introduction
Chronic heart failure (CHF) represents the terminal phase and the most common cause of mortality in the majority of cardiovascular diseases1. It is characterized by myocardial fibrosis and impaired systolic and diastolic function2,3. The majority of the energy required to maintain normal and sustained cardiac contraction is derived from adenosine triphosphate (ATP), which is primarily generated by mitochondrial fatty acid oxidation-phosphorylation4. Fatty acids taken up by cardiacmyocytes are converted to long-chain acylcarnitine and transported to the mitochondria, where they are used to generate ATP5. L-palmitoyl carnitine (L-PC) is an endogenous fatty acid metabolite that is catalyzed by palmitoyl-coenzyme A and L-carnitine. In FeCl3-induced mice, L-PC has been demonstrated to facilitate fibrinolysis while inhibiting arterial thrombotic events6. In vitro, supplementation of L-PC alleviates mitochondrial dysfunction and meiotic defects in oocytes derived from mice fed a high-fat diet7. Metabolome analysis revealed elevated levels of palmitic acid and L-carnitine in patients with congenital heart disease-associated pulmonary arterial hypertension8. Studies have shown that long-term administration of L-carnitine helps protect against cardiomyocyte contractile dysfunction during aging9. Elevated plasma levels of L-PC predict poor prognosis in patients with heart failure (HF)10,11, which support the hypothesis that L-PC involves in the pathogenesis of CHF. Carnitine palmitoyl transferase 1A (CPT1A) is a pivotal enzyme that regulates fatty acid oxidation, and its activity is diminished in failing hearts12,13. Nevertheless, the precise role and regulatory mechanism of L-PC and CPT1A in CHF remain to be elucidated.
HF is associated with both local and systemic activation of inflammation14. The status of macrophages, a critical component of the immune system, influences the development of cardiovascular and other inflammatory diseases15. The heart contains a large number of resident macrophages, which are one of the earliest cell types to respond to cardiac stimulation. Due to their phenotypic plasticity, macrophages play a role in both inflammation and resolution during cardiac injury16. In extreme cases, macrophages differentiate into pro-inflammatory (M1) or pro-repair and pro-resolution (M2) phenotypes17. Extensive research has reported that an increased proportion of the M2 macrophage population contributes to the prevention of CHF18,19. However, it is still unclear whether L-PC mediates the development of CHF by affecting the proportion of macrophages in the heart.
Autophagy is defined as the complex molecular pathway that delivers cellular components to the lysosomal system for degradation and recycling, thereby maintaining cellular and organismal homeostasis20,21. Mitophagy is a form of autophagy that is specialized for the degradation and elimination of damaged or unwanted mitochondria. It is essential for ensuring mitochondrial function and cardiac homeostasis22. The dual role of mitophagy in myocardial injury has been proven23. On the one hand, augmented mitophagy activity is regarded as a defensive mechanism of injured myocardial cells and cardiac dysfunction24,25. However, excessive activation of mitophagy causes excessive self-consumption of essential myocardial cell components, resulting in cardiac toxicity26. Research has shown that Qi-Li-Qiang-Xin inhibits cardiomyocyte apoptosis and excessive autophagy to alleviate isoproterenol (ISO)-induced HF-like pathological changes, including myocardial cell necrosis and fibrosis27. However, it remains to be established whether L-PC and CPT1A influence mitophagy in cardiomyocytes to regulate CHF.
The majority of microRNAs (miRNAs) are conserved in mammals and regulate the expression of target mRNAs28. MiR-21 has been put forth as a potential predictive biomarker for HF patients29. Several studies have reported dysregulation of miR-21 in diabetes-associated HF with preserved ejection fraction (HFpEF)30,31. Reduction of extracellular vesicles carrying miR-21a-5p derived from bone marrow-fibroblast progenitor cells has been found to inhibit pressure overload-induced cardiac fibrosis and treat HF32. In addition, miR-21-3p exhibits a strong correlation with HF-related biomarkers33. However, its role in the pathological processes underlying L-PC-related CHF remains unclear. To address this, an ISO-induced HF-like rat model and an ISO-induced H9c2 cell model were established and treated with L-PC. The involvement of miR-21-3p in myocardial fibrosis, macrophage activation, and excessive mitophagy in CHF-like models and its correlation with CPT1A were investigated.
Results
L-PC exacerbates cardiomyocyte fibrosis and autophagy
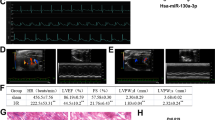
To elucidate the role of L-PC in CHF, L-PC was injected into ISO-induced CHF-like rats. Echocardiography demonstrated that rats treated with L-PC exhibited a further decline in EF and FS, accompanied by an increase in LVIDs compared to the ISO group (Fig. 1A, B). ISO administration resulted in elevated serum CK-MB levels, which were further augmented by L-PC (Fig. 1C). Compared with the ISO group, L-PC further increased the serum levels of LPS, IL-5, IL-8, and TNF-α (Fig. 1D). ISO-induced control rats exhibited structural disarray, increased inflammatory infiltration, and fibrosis in cardiac tissue, and L-PC exacerbated these pathological changes (Fig. 1E, F). Green fluorescence of LC3 was observed in cardiac tissue of rats treated with ISO and L-PC (Figs. 1G and S1A). Compared with the ISO group, L-PC was observed to elevate LC3II/I expression and reduce p62 expression (Fig. 1H). In addition, ISO resulted in a reduction in CPT1A expression in control rats, with L-PC additionally contributing to this decline (Fig. 1I). Taken together, these results indicated that L-PC exacerbated ISO-induced cardiac contractile dysfunction, fibrosis, excessive autophagy, and decreased CPT1A expression.
A Representative echocardiographic images. B EF, FS, and LVIDs. C Changes in serum CK-MB levels by ELISA. D Changes in serum LPS, IL-5, IL-8, and TNF-α levels by ELISA. E Representative images of hematoxylin-eosin-stained cardiac tissue. Scale bar, 100 μm (top) and 25 μm (bottom). F Representative images of cardiac tissue stained with Masson trichrome stain. Scale bar, 100 μm (top) and 25 μm (bottom). G IF staining analysis of LC3 (green) and DAPI (blue) fluorescence in cardiac tissue. Scale bar, 25 μm. H Western blotting analysis of LC3II/I and p62 in cardiac tissue. I Evaluation of CPT1A expression in cardiac tissue after L-PC treatment by qRT-PCR and western blotting. *P < 0.05 vs. control. &P < 0.05 vs. ISO. All experiments consisted of 6 biological replicates, each representing the average of 3 technical replicates.
MiR-21-3p mediates ISO-induced M1 macrophage polarization
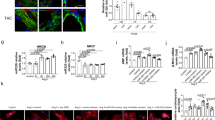
Compared to healthy subjects, an increase in serum levels of miR-21-3p was observed in CHF patients (Fig. 2A). The elevation of miR-21-3p levels in ISO-challenged CHF-like rats was further exacerbated by L-PC in serum and myocardium (Fig. 2B). Furthermore, a negative correlation was observed between miR-21-3p and CPT1A (Fig. 2C). ISO induction in rats resulted in increased CD86 expression (M1 macrophages) and decreased CD206 expression (M2 macrophages) in spleen tissue, which were further enhanced by L-PC (Fig. 2D). In addition, miR-21-3p was detected in peritoneal macrophages (Mφ) isolated from rats. Compared to Mφ, both LPS/IFN-γ and IL-4/IL-13 resulted in an elevation of miR-21-3p levels in M1 or M2 macrophages, with higher levels of miR-21-3p in LPS/IFN-γ-induced M1 macrophages (Fig. 2E). Compared to the M1 group, miR-21-3p inhibitor resulted in a reduction in IL-1β and TNF-α secretion by macrophages, as well as a limitation in CD86 expression. Conversely, miR-21-3p mimics elevated IL-1β and TNF-α levels and enhanced CD86 expression (Fig. 2F, G). The collective data indicated a close association between miR-21-3p and CPT1A, and a role for miR-21-3p in promoting M1 macrophage polarization.
A qRT-PCR detection of miR-21-3p levels in serum of patients with CHF. *P < 0.05 vs. normal. Experiment A consisted of 12 biological replicates, each representing the average of 3 technical replicates. B qRT-PCR detection of miR-21-3p levels in serum and heart of rats. C Pearson correlation analysis of miR-21-3p levels and CPT1A levels in heart. D Flow cytometry analysis of the effect of L-PC on the enrichment of macrophage surface markers CD86 and CD206 in spleen tissues of ISO-induced model. *P < 0.05 vs. control. &P < 0.05 vs. ISO. E qRT-PCR detection of miR-21-3p levels in Mφ, M1 and M2 macrophages. *P < 0.05 vs. Mφ. F qRT-PCR detection of the secretion of cytokines IL-1β and TNF-α in M1 macrophages by miR-21-3p. G Flow cytometry analysis of the effect of miR-21-3p on the surface marker CD86 in M1 macrophages. *P < 0.05 vs. M1. Experiments B to G consisted of 6 biological replicates, each representing the average of 3 technical replicates.
MiR-21-3p promotes excessive mitophagy in rat H9c2 cells via CPT1A
The prediction tool miRmap indicated that miR-21-3p targets CPT1A mRNA, a finding that was subsequently validated through dual-LUC (Fig. 3A, B). The combination of ISO and L-PC increased miR-21-3p levels compared to the untreated cells. Compared to the NC inhibitor group, miR-21-3p levels were reduced by the miR-21-3p inhibitor (Fig. 3C). Furthermore, the combination of ISO and L-PC impaired cell viability, whereas miR-21-3p inhibitor reserved the decrease (Fig. 3D). The combination of ISO and L-PC increased the LC3 II/I ratio and reduced the levels of CPT1A and p62, whereas miR-21-3p inhibitor reversed these trends (Fig. 3E). TEM observation revealed an increase in autophagosome-like structures in H9c2 cells treated with ISO and L-PC. The miR-21-3p inhibitor reduced the number of autophagosomes compared to the NC inhibitor group (Fig. 3F). These data indicated that the miR-21-3p inhibitor mitigated the excessive mitophagy in ISO/L-PC-exposed H9c2 cells.
A Prediction of potential binding sites of miR-21-3p to CPT1A using miRmap. B Validation of miR-21-3p binding to CPT1A by dual-LUC assay. *P < 0.05 vs. CPT1A-WT+NC mimics. C qRT-PCR analysis of miR-21-3p levels in H9c2 cells. D Evaluation of the effect of miR-21-3p on cell viability in H9c2 cells using CCK8 assay. E Analysis of the effects of miR-21-3p on the protein levels of LC3 II, LC3I, p62 and CPT1A in H9c2 cells using Western blotting. F Observation of the effect of miR-21-3p on the number of autophagosome-like structures in H9c2 cells by transmission electron microscopy. Scale bars: 2 μm and 500 nm. *P < 0.05 vs. control. & P < 0.05 vs. NC inhibitor. G qRT-PCR analysis of miR-21-3p and CPT1A mRNA levels in H9c2 cells. H Evaluation of the effect of CPT1A and miR-21-3p on cell viability in H9c2 cells using CCK8 assay. I Western blotting analysis of the effects of CPT1A and miR-21-3p on the protein levels of LC3 II, LC3I, p62, and CPT1A in H9c2 cells. J Observation of the effects of CPT1A and miR-21-3p on the number of autophagosome-like structures in H9c2 cells by transmission electron microscopy. Scale bars: 2 μm and 500 nm. K IF staining showing the subcellular localization of miR-21-3p and CPT1A in H9c2 cells. Scale bar: 25 μm. *P < 0.05 vs. oe-NC. &P < 0.05 vs. oe-CPT1A+NC mimic. All experiments consisted of 6 biological replicates, each representing the average of 3 technical replicates.
The objective of the subsequent experiment was to investigate the potential role of miR-21-3p in regulating excessive mitophagy by targeting CPT1A. Transfection of oe-CPT1A led to increased CPT1A levels in H9c2 cells compared to the oe-NC group, while further transfection of miR-21-3p mimic reversed CPT1A levels (Fig. 3G). Moreover, the transfection of oe-CPT1A enhanced H9c2 cell viability, while the addition of miR-21-3p mimic impaired cell viability (Fig. 3H). Transfection of oe-CPT1A resulted in a reduction in the LC3 II/I ratio and an increase in CPT1A and p62 expression. However, miR-21-3p mimic reversed these effects (Fig. 3I). Additionally, our observations indicated that interference with oe-CPT1A reduced the number of autophagosome-like structures in ISO- and L-PC-exposed H9c2 cells, whereas the number was increased by miR-21-3p mimic (Fig. 3J). Furthermore, H9c2 cells were incubated with fluorescence-labeled miR-21-3p and CPT1A antibodies, and the colocalization of miR-21-3p and CPT1A in H9c2 cells was observed (Figs. 3K and S1B), suggesting that miR-21-3p was taken up into the cells and bound to CPT1A to exert its effects. Taken together, these findings demonstrated that miR-21-3p promoted excessive mitophagy induced by ISO and L-PC in H9c2 cells by downregulating CPT1A expression.
MiR-21-3p affects M1 macrophage polarization and autophagy in H9c2 cells
To confirm the role of macrophages in miR-21-3p-mediated autophagy in H9c2 cells, a co-culture system involving H9c2 cells and M1 macrophages was established. The M1+H9c2 (ISO+L-PC+NC) group exhibited increased CD86 expression in macrophages compared to the M1+H9c2 (NC) group. Compared to the M1+H9c2 (ISO+L-PC+NC) group, the M1+H9c2 (ISO+L-PC+miR-21-3p mimics) group exhibited augmented CD86 expression, whereas the M1+H9c2 (ISO+L-PC+miR-21-3p inhibitor) group displayed diminished CD86 expression (Fig. 4A). Furthermore, elevated levels of CK-MB, IL-6, IL-8, and TNF-α were observed in the supernatant of H9c2 cells in the M1+H9c2 (NC) group, and these levels were further increased in the M1+H9c2 (ISO+L-PC+NC) group. Furthermore, interference with miR-21-3p mimics further enhanced this trend, whereas interference with miR-21-3p inhibitor reversed this trend (Fig. 4B, C). Obvious LC3-positive fluorescence was observed in the cells of the M1+H9c2(NC) group. The combined treatment of ISO and L-PC increased the punctate accumulation of LC3 in the cells. This increase was further enhanced by miR-21-3p mimics and inhibited by miR-21-3p inhibitor (Figs. 4D and S1C). Additionally, in comparison to the H9c2 (NC) group, the co-culture system involving H9c2 cells and M1 macrophages exhibited elevated LC3 expression and decreased p62 expression. The M1+H9c2 (ISO+L-PC+NC) group exhibited enhanced LC3 expression and reduced p62 expression compared to the M1+H9c2 (NC) group. The addition of miR-21-3p mimics to the co-culture system further amplified these trends, whereas miR-21-3p inhibitor reversed them (Fig. 4E). These data illustrated that miR-21-3p regulated the secretion of CK-MB and inflammatory cytokines as well as the expression of autophagy-related proteins in the co-culture model.
A Establishment of a co-culture system between LPS/IFN-γ-induced M1 macrophages and H9c2 cells. Examination of the effect of H9c2 cells on the expression of the surface marker CD86 in macrophages by flow cytometry. *P < 0.05 vs. M1+H9c2 (NC). &P < 0.05 vs. M1+H9c2 (ISO+L-PC+NC). B Levels of CK-MB in the supernatant of H9c2 cells after co-culture by ELISA. C Levels of IL-6, IL-8 and TNF-α in the supernatant of H9c2 cells after co-culture by ELISA. D Observation of the punctate accumulation of LC3B in H9c2 cells after co-culture by immunofluorescence. Scale bar: 25 μm. E Western blotting analysis of LC3 II, LC3I and p62 in H9c2 cells after co-culture. *P < 0.05 vs. H9c2 (NC). & P < 0.05 vs. M1+H9c2 (NC). #P < 0.05 vs. M1+H9c2 (ISO+L-PC+NC). All experiments consisted of 6 biological replicates, each representing the average of 3 technical replicates.
MiR-21-3p exacerbates ISO and L-PC-induced cardiac fibrosis and M1 macrophage polarization
The next step was to assess the involvement of miR-21-3p in ISO- and ISO/L-PC-induced CHF animal models. Compared to the control rats, rats in the ISO+NC inhibitor group exhibited structural disarray, increased inflammatory infiltration, and fibrosis in cardiac tissue sections. These pathological changes were exacerbated by L-PC and alleviated by miR-21-3p inhibitor. Compared with the ISO+L-PC+NC inhibitor group, miR-21-3p inhibitor attenuated the inflammatory infiltration and fibrosis in the heart (Fig. 5A, B). Echocardiography demonstrated a decline in EF and FS in the ISO+NC inhibitor group, accompanied by an increase in LVIDs. The ISO+L-PC+NC inhibitor group demonstrated a reduction in EF and FS, while the ISO+miR-21-3p group exhibited an increase in both parameters (Fig. 5C, D). Compared with the control rats, the serum levels of CK-MB, LPS, IL-6, IL-8, and TNF-α were elevated in the ISO+NC inhibitor group. The levels of these indicators were increased by L-PC and reduced by miR-21-3p inhibitor. Compared with the ISO+L-PC+NC inhibitor group, the ISO+L-PC+miR-21-3p inhibitor group showed decreased levels of these indicators (Fig. 5E, F). In addition, the combination of ISO and L-PC increased the expression of CD86 in the spleen tissues. Administration of miR-21-3p inhibitor limited CD86 expression in comparison to the ISO+L-PC+NC inhibitor group (Fig. 5G, H). Furthermore, increased levels of miR-21-3p were detected in serum and cardiac tissues in the ISO+NC inhibitor group compared to the control rats. This trend was enhanced by L-PC and reversed by miR-21-3p inhibitor. The ISO+L-PC+miR-21-3p inhibitor group exhibited a reduction in miR-21-3p levels compared to the ISO+L-PC+NC inhibitor group (Fig. 5I). These findings collectively demonstrated that miR-21-3p exacerbated ISO- and L-PC-induced M1 macrophage polarization and cardiac fibrosis.
A Representative images of hematoxylin-eosin staining of cardiac tissue sections. Scale bar, 100 μm (up) and 25 μm (down). B Representative images of Masson’s trichrome staining of cardiac tissue sections. Scale bar, 100 μm (up) and 25 μm (down). C Representative echocardiographic images. D EF, FS, and LVIDs. E Levels of CK-MB in rat serum by ELISA. F Levels of LPS, IL-6, IL-8, and TNF-α in rat serum by ELISA. G, H Flow cytometry analysis of CD86 expression in rat spleen tissue. I qRT-PCR determined levels of miR-21-3p in rat serum and heart. *P < 0.05 vs. control. &P < 0.05 vs. ISO+NC inhibitor. #P < 0.05 vs. ISO+L-PC+NC inhibitor. All experiments consisted of 6 biological replicates, each representing the average of 3 technical replicates.
MiR-21-3p exacerbates excessive autophagy by ISO and L-PC in the heart
Figure 6A shows the green fluorescence of LC3 in the hearts of rats in the ISO+NC inhibitor group and the ISO+L-PC+NC inhibitor group. miR-21-3p inhibitor caused a decrease in LC3 fluorescence in the heart (Figs. 6A and S1D). The hearts of the rats in the ISO+NC inhibitor group exhibited increased LC3 expression and decreased p62 expression. L-PC further enhanced these trends; in contrast, miR-21-3p inhibitor reversed them. Compared with the ISO+L-PC+NC inhibitor group, miR-21-3p inhibitor reduced LC3 expression and increased p62 expression (Fig. 6B). In addition, CPT1A levels were decreased in the hearts of rats in the ISO+NC inhibitor group. L-PC further decreased CPT1A levels, while miR-21-3p inhibitor reversed this trend. Compared with the ISO+L-PC+NC inhibitor group, miR-21-3p inhibitor upregulated CPT1A expression (Fig. 6C). The above data indicated that in vivo, miR-21-3p exacerbated ISO- and L-PC-induced excessive autophagy in the heart.
A Immunofluorescence images of LC3-labeled cells in the heart. Scale bar, 25 μm. B Western blotting analysis of LC3 II, LC3I and p62 expression in rat heart. C Western blotting analysis of CPT1A expression in rat heart. *P < 0.05 vs. control. &P < 0.05 vs. ISO+NC inhibitor. #P < 0.05 vs. ISO+L-PC+NC inhibitor. All experiments consisted of 6 biological replicates, each representing the average of 3 technical replicates.
Discussion
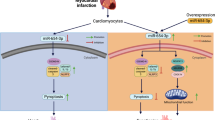
The incidence of HF increases with age, with a prevalence of over 10% in individuals aged 70 years and older34. Today’s society is characterized by an aging population, and despite the introduction of some drugs for the treatment of CHF, the side effects associated with long-term use cannot be ignored35. Therefore, research into the pathological mechanisms of CHF is helpful in finding innovative countermeasures. The present study demonstrates that the fatty acid metabolite L-PC exerts a detrimental effect on cardiomyocyte mitophagy and M1 macrophage polarization. M1 macrophages were observed to promote cardiomyocyte mitophagy. The miR-21-3p inhibitor was found to effectively reverse the cardiomyogenic effects of L-PC via CPT1A (Fig. 7).
The pathogenesis of HF is closely related to fatty acid metabolism36. Abnormal fatty acid metabolism determines the severity and type of HF37. Elevated plasma levels of L-PC, a well-known long-chain acylcarnitine involved in fatty acid oxidation, have been observed in CHF patients10, yet the precise role of L-PC in the pathogenesis of CHF remains uncertain. In this study, L-PC exacerbated the pathological changes in the ISO-induced CHF-like model. Echocardiography revealed increased LVIDs, decreased LVEF and LVFS, which are traditional clinical indicators38. CK-MB, an important marker for the diagnosis of myocardial necrosis, is released from damaged myocardial cells39. The ISO-challenged rats in our study displayed increased LVIDs, decreased LVEF and LVFS, as well as elevated serum levels of CK-MB, consistent with a previous study40. HE and Masson staining showed myocardial structural disruption, increased inflammatory infiltration, and collagen fiber deposition in ISO-exposed rats41. These parameter changes were further enhanced by L-PC, indicating the detrimental effects of L-PC on ISO-stimulated myocardium. Mitochondria are critical for maintaining cellular homeostasis and are key sites of fatty acid metabolism. Long-chain fatty acids enter mitochondria and undergo β-oxidation to generate high levels of ATP, with CPT1A being a key rate-limiting enzyme in this process42. Previous studies have indicated that rhein regulates the prevention of renal fibrosis through the PPARα/CPT1A/L-PC axis43. The CPT1A-specific inhibitor etomoxir was observed to reduce the level of L-PC and fatty acid oxidation in human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs)44. Here, we found that CPT1A expression was upregulated in ISO-stimulated rats, and this trend was further exacerbated by L-PC. These results suggested a close relationship between the development of ISO-induced CHF-like pathology and fatty acid metabolism, with exogenous administration of L-PC exacerbating myocardial injury.
While miR-21 is regarded as a predictive and diagnostic tool for numerous cardiomyopathies, its function remains a subject of contention45. On the one hand, miRNA-21 has been demonstrated to protect human cardiomyocytes AC16 from inflammation, apoptosis, oxidative stress, and metabolic damage induced by high glucose46. miR-21-3p treats transverse aortic constriction and reverses myocardial fibrosis and angiotensin II-induced cardiac hypertrophy by silencing histone deacetylase-847. Analysis of a microarray dataset related to coronary arteries revealed a decrease in miR-21-3p levels in patients48. Conversely, the inhibition of miR-21 has been demonstrated to reverse the survival and viability of living myocardial slices and improve myocardial failure in vitro49. Furthermore, the administration of miR-21-3p antagomir alleviates LPS-induced sepsis-related cardiac dysfunction and prevents mitochondrial structural damage and autophagy50. Bioinformatics analysis indicated that miR-21-3p is upregulated in patients with advanced CHF, myocardial infarction, and coronary artery disease, suggesting its potential as a candidate biomarker for cardiomyopathy33,51. The disparate findings on miR-21 in cardiomyopathy may suggest that its pathological activity differs depending on the cell type and disease context. The present study demonstrated that miR-21-3p levels were elevated in CHF patients and ISO-challenged rats, suggesting that miR-21-3p may represent a potential target for the prevention of the pathological development of HF. MiR-21-3p inhibitor restored decreased rat H9c2 cell viability induced by ISO and L-PC. In rats, miR-21-3p inhibitor attenuated ISO- and L-PC-induced myocardial structural disruption and fibrosis and reduced serum levels of CK-MB. Accumulating studies have demonstrated that miRNAs can target the CPT1A gene to regulate lipid homeostasis and disease development52,53. A previous study demonstrated that miR-21 inhibits PPARα and its downstream target gene CPT1A in human renal cancer cells, thereby mediating lipid metabolism54, suggesting an association between miR-21 and CPT1A in disease development. The present study observed a negative correlation between miR-21-3p and CPT1A, and miR-21-3p inhibitor upregulated CPT1A expression in ISO and L-PC-induced rats. The results of the dual-LUC experiments and double FISH/IF staining confirmed the targeting effect of miR-21-3p on CPT1A. These findings indicated that miR-21-3p inhibitor protected against ISO- and L-PC-induced myocardial injury and increased CPT1A expression.
Autophagy is an intracellular mechanism that can mediate the degradation of protein aggregates, lipid droplets, and injured organelles55. Accumulating evidence supports the significance of mitophagy during cardiac injury56. Excessive mitophagy is considered detrimental and exacerbates fibrosis and myocardial damage. Research has demonstrated that in HF mice challenged with transverse aortic constriction, there is upregulation of LC3 II/I and Beclin 1, along with an increase autophagolysosomes in the hearts, and the administration of autophagy inhibitors help alleviate cardiac fibrosis in HF mice57. Furthermore, disturbances in fatty acid metabolism have been shown to affect mitophagy activity58. ACSS3 reduces fatty acid oxidation by downregulating CPT1A, inhibiting mitophagy, leading to alveolar epithelial cell inactivation and ameliorating pulmonary fibrosis59. L-carnitine induces increased CPT1A activity, promotes mitophagy, and ultimately prevents cardiac microvascular damage in diabetic cardiomyopathy60. These observations suggest that CPT1A may influence mitophagy activity during disease development. In the present study, we observed increased LC3 expression in cardiac tissues after ISO treatment, with stronger LC3 expression observed after combined treatment of ISO and L-PC, but this effect was disrupted by miR-21-3p inhibitor. Moreover, miR-21-3p inhibitor attenuated the expression of autophagy-related proteins and reduced the number of autophagosomes in ISO- and L-PC-induced H9c2 cells. Conversely, miR-21-3p mimic disrupted the protective effect of CPT1A overexpression on excessive mitophagy in H9c2 cells. Collectively, the observations indicated downregulation of CPT1A by miR-21-3p exacerbated excessive myocardial mitophagy.
The maintenance of homeostasis in long-lived cardiac cells relies not only on the classical intracellular autophagy pathway, but also on resident macrophages61,62. Macrophages are responsible for coordinating inflammation and resolution in HF and cardiac repair63. In this study, we found that ISO and L-PC enhanced CD86 expression and suppressed CD206 expression in rat spleen tissues, indicating that ISO and L-PC increased the M1 phenotype of macrophages and decreased the M2 phenotype. In addition, M1 macrophages showed higher levels of miR-21-3p. The promotion of polarization of M1 macrophages by induction of miR-21-3p, while the miR-21-3p inhibitor had an inhibitory effect. Further investigations revealed that H9c2 cells after ISO and L-PC treatment resulted in increased CD86 expression in M1 macrophages, this trend that was further enhanced by induction of miR-21-3p and reversed by restriction of miR-21-3p. Furthermore, co-culture with M1 macrophages resulted in increased secretion of CK-MB, IL-6, IL-8 and TNF-α and upregulated LC3 expression in H9c2 cells. ISO and L-PC treatment promoted the secretion of CK-MB, IL-6, IL-8, TNF-α, and enhanced LC3 expression in the co-culture system involving M1 macrophages and H9c2 cells. This phenomenon was enhanced by induction of miR-21-3p and inhibited by restriction of miR-21-3p. Our results demonstrated M1 macrophage polarization stimulated by miR-21-3p in H9c2 cells. M1 macrophages induced H9c2 cell damage, manifested by increased secretion of CK-MB and inflammatory factors, as well as increased autophagic activity.
This study is not without limitations. The combined use of L-PC was not sufficient to explore its additional effects on cardiac function. The materials used in the study were not human primary cardiomyocytes and human primary macrophages, and the results were not validated in clinical samples or in vivo materials. Further experiments are required to elucidate the precise regulatory mechanisms of miR-21-3p and CPT1A in cardiomyopathy. The influence of fatty acid metabolism on the pathogenesis of cardiomyopathy deserves further investigation. The impact of the interaction between macrophages and cardiomyocytes on the development of cardiomyopathy also requires further examination.
In conclusion, our findings indicate that elevated levels of miR-21-3p may serve as a potential biomarker for patients diagnosed with CHF. The level of miR-21-3p is elevated in CHF patients, and this increase may facilitate cardiomyocyte mitophagy and M1 macrophage polarization, thereby triggering an excessive inflammatory response and abnormal cardiac contraction and relaxation. Furthermore, we demonstrate the correlation between miR-21-3p, the fatty acid metabolite L-PC, and CPT1A, a pivotal rate-limiting step in fatty acid metabolism, in the pathogenesis of CHF. These findings provide clues for the future investigation of cardiovascular diseases and clinical phenotypes associated with CHF.
Materials and Methods
Clinical subjects
Serum samples were collected from 12 CHF patients (CHF group) and 12 healthy subjects (Normal group) in the Second Affiliated Hospital of Fujian Medical University from September 2021 to November 2021. CHF was diagnosed according to the 2016 European Society of Cardiology (ESC) Guidelines. Healthy people in the same period and excluded CHF were selected as the Normal group. The healthy subjects did not take any medication, while the CHF patients received continuous medical treatment for 28 days prior to sample collection. Subjects with infection, rheumatoid arthritis, renal failure, severe valvular heart disease, intestinal disease, cancer, or autoimmune disease were excluded. None of the participants had any known immune system diseases or were receiving immunomodulatory therapy. Specific information on each subject is presented in Table 1. The study was approved by the Ethics Committee of Second Affiliated Hospital of Fujian Medical University (No. 2020-163). The research was conducted according to the World Medical Association Declaration of Helsinki and the Guidance of the Ministry of Science and Technology (MOST) for the Review and Approval of Human Genetic Resources. All ethical regulations relevant to human research participants were followed. All the information about the study will be fully explained to the subjects by the researchers. All the participants provided informed consent before sampling.
Animal models
Male Sprague-Dawley (SD) rats (250–270 g) were obtained from Hunan SJA Laboratory Animal Co., Ltd (Changsha, China). The rats were acclimated to a specific pathogen-free (SPF) environment for 1 week and then grouped according to the different purposes (Fig. 8). To simulate CHF pathology, the rats were injected abdominally and subcutaneously with 5 mg kg−1 ISO for 7 days27. Left ventricular ejection fraction (LVEF), fractional shortening (FS), and left ventricular internal diameter systolic (LVIDs) were analyzed by echocardiography. LVEF < 55% was defined as CHF.
To investigate the role of L-PC, rats were randomly divided into control, ISO, and ISO+L-PC groups (n = 6). Rats in the ISO and ISO+L-PC groups were injected abdominally and subcutaneously with 5 mg kg−1 ISO for 7 days. Subsequently, rats in the ISO+L-PC group received a daily intraperitoneal injection of 4 μM L-PC for additional 21 days64. The ISO and control groups received injections of the same volume of solvent.
To study the involvement of miR-21-3p, rats were randomly assigned to control, ISO+NC inhibitor, ISO+L-PC+NC inhibitor, ISO+miR-21-3p inhibitor, and ISO+L-PC+miR-21-3p inhibitor groups (n = 6). Except for the control group, all rats were injected abdominally and subcutaneously with 5 mg kg−1 ISO for 7 days. Following ISO intervention, rats in the ISO+NC inhibitor group received an additional intravenous tail injection of 200 μg negative control (NC) inhibitor every 3 days for 21 days. Following ISO intervention, rats in the ISO+L-PC+NC inhibitor group received a daily intraperitoneal injection of 4 μM L-PC and an additional intravenous tail injection of 200 μg NC inhibitor every 3 days for 21 days. Following ISO intervention, rats in the ISO+miR-21-3p inhibitor group received an additional intravenous tail injection of 200 μg miR-21-3p inhibitor65 every 3 days for 21 days. Following ISO intervention, rats in the ISO+L-PC+miR-21-3p inhibitor group received a daily intraperitoneal injection of 4 μM L-PC and an additional intravenous tail injection of 200 μg miR-21-3p inhibitor every 3 days for 21 days. MiR-21-3p inhibitor and its random sequence negative control were purchased from HonorGene (Changsha, China).
Rats were sacrificed by intraperitoneal injection of 200 mg kg−1 sodium pentobarbital, and blood, spleen and heart tissues were collected.
All experimental procedures and animal handling were performed with the approval of the Animal Care and Use Committee of the Second Affiliated Hospital of Fujian Medical University (No. 2020-347), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and studies involving laboratory animals follows the ARRIVE guidelines.
H9c2 cell culture and treatment
Rat cardiomyocyte line H9c2 (AW-CNR083, Abiowell, Changsha, China) was cultured in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were grown in a 5% CO2 incubator at 37 °C. H9c2 cells passed mycoplasma detection and STR testing.
H9c2 cells were divided into control, ISO+L-PC, NC inhibitor, and miR-21-3p inhibitor groups. In the ISO+L-PC group, H9c2 cells were exposed to 10 μM L-PC11 and 80 µM ISO27 for 48 h. In the NC inhibitor group, cells were exposed to 10 μM L-PC and 80 µM ISO and simultaneously transfected with NC inhibitor for 48 h. In the miR-21-3p inhibitor group, cells were exposed to 10 μM L-PC and 80 µM ISO and simultaneously transfected with miR-21-3p inhibitor for 48 h.
H9c2 cells were divided into oe-NC, oe-CPT1A, oe-CPT1A+NC mimic, and oe-CPT1A+miR-21-3p mimic groups. In the oe-NC group, cells were exposed to 10 μM L-PC and 80 μM ISO and simultaneously transfected with oe-NC. In the oe-CPT1A group, cells were exposed to 10 μM L-PC and 80 µM ISO and simultaneously transfected with CPT1A overexpression vectors (oe-CPT1A). In the oe-CPT1A+NC mimic group, cells were exposed to 10 μM L-PC and 80 µM ISO and simultaneously transfected with oe-CPT1A and NC mimic. In the oe-CPT1A+miR-21-3p mimic group, cells were exposed to 10 μM L-PC and 80 µM ISO and simultaneously transfected with oe-CPT1A and miR-21-3p mimic.
All vectors were purchased from HonorGene (Changsha, China), including miR-21-3p mimics, miR-21-3p inhibitor, and their negative control (NC) with random sequences (as NC mimics or NC inhibitor), as well as CPT1A overexpression vectors (oe-CPT1A) and its negative control (oe-NC). All transfection procedures were performed according to the instructions for Lipofectamine 2000 Reagent (11668019, Invitrogen, Carlsbad, CA, USA).
Macrophage polarization
After the rats were euthanized, peritoneal cells were immediately isolated by abdominal lavage. The isolated peritoneal cells were cultured in 1640 medium containing 10% FBS, and the medium was replaced after 2 h to remove suspended cells. The cells adhering to the walls and bottom of the culture dish were peritoneal macrophages (Mφ). Mφ macrophages (1 × 106 cells per well) were seeded in 12-well plates. For M1 polarization, Mφ macrophages were stimulated with lipopolysaccharides (LPS; 1 µg mL−1) and interferon-γ (IFN-γ; 20 ng mL−1) for 4 h. For M2 polarization, Mφ macrophages were stimulated with interleukin-4 (IL-4; 20 ng mL−1) and IL-13 (20 ng mL−1) for 4 h66.
Co-culture system of H9c2 cells and macrophages
A co-culture system using 0.4 μm Transwell inserts (#3450, Corning Inc., Corning, NY, USA)67 was established to investigate whether miR-21-3p mediated functional changes induced by co-culture of H9c2 cells and M1 macrophages. M1 macrophages induced by LPS and IFN-γ (1 × 106 cells per well) were seeded in the 6-well plate, while various treated H9c2 cells (5 × 105 cells per well) were seeded in the transwell inserts and co-cultured for 48 h. In another co-culture system, the positions of M1 macrophages and H9c2 cells were reversed. Cells in the lower chamber were considered the focus of the study and used for subsequent analyses.
Solvent-treated H9c2 cells transfected with NC mimic and NC inhibitor were referred to as the H9c2 (NC) group. M1 macrophages were co-cultured with solvent-treated H9c2 cells transfected with NC mimic and NC inhibitor, which was termed the M1+H9c2 (NC) group. M1 macrophages were co-cultured with H9c2 cells treated with 10 μM L-PC and 80 μM ISO and transfected with NC mimic and NC inhibitor, which was designated as M1+H9c2 (ISO+L-PC+NC) group. M1 macrophages were co-cultured with H9c2 cells treated with 10 μM L-PC and 80 µM ISO and transfected with miR-21-3p mimic, which was designated as M1+H9c2 (ISO+L-PC+miR-21-3p mimic) group. M1 macrophages were co-cultured with H9c2 cells treated with 10 μM L-PC and 80 µM ISO and transfected with miR-21-3p inhibitor, which was designated as M1+H9c2 (ISO+L-PC+miR-21-3p inhibitor) group.
Cell counting kit-8 (CCK8)
Cell viability was assessed using the CCK8 assay. Briefly, 5 × 103 cells (100 μL per well) were seeded in 96-well plates and treated. Each well was supplemented with 100 μL containing 10% CCK-8 reagent (NU679, Dojindo, Tokyo, Japan) and incubated for an additional 4 h in a 37 °C, 5% CO2 incubator. The optical density (OD) at 450 nm was measured using a microplate reader (MB-530, HEALES, Shenzhen, China).
Enzyme-linked immunosorbent assay (ELISA)
Serum levels of creatine kinase-myocardial band (CK-MB), LPS, IL-6, IL-8, and TNF-α were determined according to the corresponding instructions. Serum samples were obtained from blood after centrifugation at 1000 × g for 15 min. The kits used included the Rat CK-MB Kit (CSB-E14403r, CUSABIO Co., Ltd, Wuhan, China), Rat LPS Kit (CSB-E14247r, CUSABIO Co., Ltd), Rat IL-6 Kit (CSB-E04640r, CUSABIO Co., Ltd), Rat IL-8 Kit (ml002885, Mlbio, Shanghai, China), and Rat TNF-α Kit (CSB-E11987r, CUSABIO Co., Ltd).
Flow cytometry
CD86 and CD206 expression in spleen tissue and peritoneal macrophages were detected by flow cytometry. Spleen tissue was minced, filtered, and centrifuged to obtain a cell pellet. Red blood cell lysis buffer was added to lyse red blood cells in the single-cell suspension of spleen tissue, followed by cell resuspension in culture medium. For CD206 detection, Intracellular Fixation Buffer was added to the spleen cell suspension and macrophage suspension to fix the cells for 30 min. Permeabilization buffer was then added for membrane permeabilization. The spleen cell suspension and macrophage suspension were supplemented with CD206 antibody (PA5-114370, Invitrogen) for 30 min and then resuspended in 0.5% bovine serum albumin-phosphate-buffered saline (BSA-PBS). For CD86 detection, the spleen cell suspension and macrophage suspension were supplemented with CD86 antibody (12-0860-83, eBioscience, San Diego, CA, USA) for 30 min and then resuspended in culture medium. The resuspended cells were analyzed by flow cytometer (A00-1-1102, Beckman Coulter, Fullerton, CA, USA) and CyExpert 2.5.0.77 software (Beckman Coulter). All gating images of flow cytometry are shown in Supplementary Fig. 2.
Tissue staining
Hematoxylin-eosin (HE) and Masson staining were used to detect pathological changes in cardiac tissue. Rat heart tissue was fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, and sectioned at 4–5 μm. After deparaffinization and hydration, sections were stained with hematoxylin-eosin (AWI0020a, Abiowell) or Masson’s trichrome stain (AWI0253a, Abiowell). The sections were then mounted with neutral resin and observed under a microscope (BA410T, Motic, Xiamen, China).
Immunofluorescence (IF)
IF staining was performed to assess light chain-3 (LC3) expression in H9c2 cells and the heart. For H9c2 cells, cells were fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, and blocked with 5% BSA for 60 min. For heart tissue, the tissue sections were placed in xylene and graded ethanol. Then, the sections were immersed in ethylenediaminetetraacetic acid (EDTA) buffer (pH 9.0) and heated to boiling for antigen retrieval. After cooling at room temperature, the sections were treated with NaBH4 solution and Sudan Black solution. Subsequently, the tissue was blocked with 10% serum containing 5% BSA for 60 min. After the cells and tissues were blocked, they were incubated overnight at 4 °C with LC3 antibody (1:50, 18725-1-AP, Proteintech, Rosemont, IL, USA) and then incubated for 90 min with Goat Anti-Rabbit IgG (H+L) (1:200, SA00013-2, Proteintech). The cell nuclei were stained with DAPI. Finally, multiple fields were randomly captured using a fluorescence microscope (CX41-72C02, Olympus, Tokyo, Japan), and the fluorescence intensity of LC3 was quantified.
Transmission electron microscopy (TEM)
To assess the amount of autophagosomes and mitochondrial structure of H9c2 cells, TEM was performed. Briefly, harvested cells were fixed with 2.5% glutaraldehyde and 1% osmium tetroxide, followed by dehydration in graded ethanol and propylene oxide. Then, the cells were infiltrated with propylene oxide and epoxy resin. The pure epoxy embedding block was cut into ultrathin sections (60 nm) and stained with 4% uranyl acetate and 0.5% lead citrate. Finally, the specimens were observed, and images captured under TEM.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from serum, tissues, and H9c2 cells using Trizol (15596026, Thermo Scientific, Portsmouth, NH, USA), and cDNA was synthesized using the cDNA synthesis kit (CW2569, CWBIO, Taizhou, China). Amplification was performed on a QuantStudio 1 Real-Time PCR system (Thermo Scientific) using the UltraSYBR Mixture reagent kit (CW2601, CWBIO). The amplification program was set to 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s, and finally melting curve analysis (60–95 °C). The primer sequences for targets were designed using Primer5 (PREMIER Biosoft, Vancouver, Canada) (Table 2). The relative expression of the target genes was calculated using the 2−ΔΔCt method, with GAPDH as the gene reference, U6 as the hsa-miR-21-3p reference, and 5S as the rno-miR-21-3p reference. All melting curves of targets in qRT-PCR are shown in supplementary data 2.
Western blotting
Total protein was extracted from heart tissue and H9c2 cells using radioimmunoprecipitation assay (RIPA) buffer (AWB0136, Abiowell), and the protein concentration was then determined using the bicinchoninic acid assay (BCA) kit (AWB0104, Abiowell). Total proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked with phosphate buffered saline with Tween-20 (PBST) containing 5% skimmed milk to remove nonspecific binding and then incubated with LC3 (1:2000, 14600-1-AP, Proteintech), sequestosome 1 (p62; 1:4000, 18420-1-AP, Proteintech), CPT1A (1:10,000, 15184-1-AP, Proteintech), and GAPDH (1:5000, 10494-1-AP, Proteintech) overnight at 4 °C, followed by incubation with HRP goat anti-rabbit IgG (1:6000, SA00001-2, Proteintech). Finally, the membranes were exposed to ECL Plus Luminol (Abiowell, AWB0005) and imaged using a gel imaging system (ChemiScope6100, CLiNX, Shanghai, China). All original immunoblot images are shown in Supplementary Fig. 3.
Dual-luciferase (Dual-LUC) assay
The binding of CPT1A and miR-21-3p was predicted using miRmap and then verified by Dual-LUC assay. Luciferase reporter plasmids psiCHECK-2-rno-CPT1A and psiCHECK-2-rno-CPT1A-Mut were obtained and then co-transfected with rno-miR-21-3p mimics or NC mimics into 293T cells (HG-NC071, HonorGene). After transfection, the detection was performed according to the instructions of the Dual-Luciferase® Reporter Assay System (E1910, Promega, Madison, WI, USA). The ratio of firefly luciferase/renilla luciferase was used as the relative luciferase activity.
Statistics and reproducibility
Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad, La Jolla, CA, USA). Data are presented as mean ± standard deviation. All data were in accordance with the normal distribution according to the Shapiro-Wilk test. Unpaired t test was used to analyze the difference between two groups. Multiple group comparison was analyzed using one-way analysis of variance (ANOVA) and post hoc Tukey’s test. Pearson’s correlation coefficient was used to detect association between CPT1A and miR-21-3p. Human sample experiments included 12 biological replicates. Animal and cell experiments consisted of 6 biological replicates. Each biological replicate represented the average of 3 technical replicates. P < 0.05 was considered statistically significant.
Data availability
All data discovered and analyzed during this study are included in this paper and its supplementary files. The source data for the graphs presented in the main figure can be found in Supplementary Data 1(Collection of raw data). Melting curves of targets in qRT-PCR can be found in Supplementary Data 2. The gating images of flow cytometry can be found in Supplementary Fig. 2. The original immunoblot images can be found in Supplementary Fig. 3.
References
Kaspar, M. et al. Underestimated prevalence of heart failure in hospital inpatients: a comparison of ICD codes and discharge letter information. Clin. Res. Cardiol. 107, 778–787 (2018).
Zhang, Y. et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br. J. Anaesth. 121, 595–604 (2018).
Frangogiannis, N. G. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 65, 70–99 (2019).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128, 1487–1513 (2021).
Fillmore, N., Mori, J. & Lopaschuk, G. D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 171, 2080–2090 (2014).
Yang, J. et al. L-Palmitoylcarnitine potentiates plasmin and tPA to inhibit thrombosis. Nat. Prod. Bioprospect. 13, 48 (2023).
Ge, J. et al. L-Palmitoylcarnitine supplementation improves oocyte quality and embryo development derived from obese mice. Fundam. Res. https://doi.org/10.1016/j.fmre.2024.05.013 (2024).
Chen, C. et al. Metabolomics reveals metabolite changes of patients with pulmonary arterial hypertension in China. J. Cell. Mol. Med. 24, 2484–2496 (2020).
Gökçe, Y., Danisman, B., Akcay, G., Derin, N. & Yaraş, N. L-Carnitine improves mechanical responses of cardiomyocytes and restores Ca(2+) homeostasis during aging. Histochem. Cell Biol. 160, 341–347 (2023).
Ueland, T. et al. Disturbed carnitine regulation in chronic heart failure — Increased plasma levels of palmitoyl-carnitine are associated with poor prognosis. Int. J. Cardiol. 167, 1892–1899 (2013).
Roussel, J. et al. Palmitoyl-carnitine increases RyR2 oxidation and sarcoplasmic reticulum Ca2+ leak in cardiomyocytes: role of adenine nucleotide translocase. Biochim. Biophys. Acta Mol. Basis Dis. 1852, 749–758 (2015).
Shao, M. et al. β-elemene blocks lipid-induced inflammatory pathways via PPARβ activation in heart failure. Eur. J. Pharmacol. 910, https://doi.org/10.1016/j.ejphar.2021.174450 (2021).
Tang, B., Zhang, J.-G., Tan, H.-Y. & Wei, X.-Q. Astragaloside IV inhibits ventricular remodeling and improves fatty acid utilization in rats with chronic heart failure. Biosci. Rep. 38, https://doi.org/10.1042/bsr20171036 (2018).
Hanna, A. & Frangogiannis, N. G. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 34, 849–863 (2020).
Li, C. X. & Yue, L. The multifaceted nature of macrophages in cardiovascular disease. Biomedicines 12, https://doi.org/10.3390/biomedicines12061317 (2024).
Rurik, J. G., Aghajanian, H. & Epstein, J. A. Immune cells and immunotherapy for cardiac injury and repair. Circ. Res. 128, 1766–1779 (2021).
Stevens, T. W. et al. Dirty jobs: macrophages at the heart of cardiovascular disease. Biomedicines 10, https://doi.org/10.3390/biomedicines10071579 (2022).
Dong, X. et al. Nuanxinkang protects against ischemia/reperfusion-induced heart failure through regulating IKKβ/IκBα/NF-κB-mediated macrophage polarization. Phytomedicine 101, 154093 (2022).
Wang, Z. et al. CCL24/CCR3 axis plays a central role in angiotensin II–induced heart failure by stimulating M2 macrophage polarization and fibroblast activation. Cell Biol. Toxicol. 39, 1413–1431 (2022).
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, https://doi.org/10.15252/embj.2021108863 (2021).
Li, W. et al. Selective autophagy of intracellular organelles: recent research advances. Theranostics 11, 222–256 (2021).
Zheng, H. et al. Mitophagy in diabetic cardiomyopathy: roles and mechanisms. Front. Cell Dev. Biol. 9, 750382 (2021).
Li, A. et al. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 13, https://doi.org/10.1038/s41419-022-04906-6 (2022).
Titus, A. S., Sung, E. A., Zablocki, D. & Sadoshima, J. Mitophagy for cardioprotection. Basic Res. Cardiol. 118, 42 (2023).
Yu, H. et al. α-Ketoglutarate improves cardiac insufficiency through NAD(+)-SIRT1 signaling-mediated mitophagy and ferroptosis in pressure overload-induced mice. Mol. Med. 30, 15 (2024).
Lu, L. et al. Follistatin-like protein 1 attenuates doxorubicin-induced cardiomyopathy by inhibiting MsrB2-mediated mitophagy. Mol. Cell Biochem. https://doi.org/10.1007/s11010-024-04955-9 (2024).
Fan, C. et al. Qi-Li-Qiang-Xin alleviates isoproterenol-induced myocardial injury by inhibiting excessive autophagy via activating AKT/mTOR pathway. Front. Pharmacol. 10, https://doi.org/10.3389/fphar.2019.01329 (2019).
Das, K. & Rao, L. V. M. The role of microRNAs in inflammation. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms232415479 (2022).
Marketou, M. et al. Peripheral blood MicroRNA-21 as a predictive biomarker for heart failure with preserved ejection fraction in old hypertensives. Am. J. Hypertens. 37, 298–305 (2024).
Huang, J. P. et al. Exosomal microRNAs miR-30d-5p and miR-126a-5p are associated with heart failure with preserved ejection fraction in STZ-induced Type 1 diabetic rats. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms23147514 (2022).
Mone, P. et al. Empagliflozin improves the MicroRNA signature of endothelial dysfunction in patients with heart failure with preserved ejection fraction and diabetes. J. Pharmacol. Exp. Ther. 384, 116–122 (2023).
Ranjan, P. et al. Bone marrow-fibroblast progenitor cell-derived small extracellular vesicles promote cardiac fibrosis via miR-21-5p and integrin subunit αV signalling. J. Extracell. Biol. 3, e152 (2024).
Galluzzo, A. et al. Identification of novel circulating microRNAs in advanced heart failure by next‐generation sequencing. ESC Heart Fail. 8, 2907–2919 (2021).
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. J. Heart Fail. 24, 4–131 (2022).
Xing, Z. et al. Aconitum carmichaelii Debx. attenuates heart failure through inhibiting inflammation and abnormal vascular remodeling. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms24065838 (2023).
Ljubkovic, M. et al. Disturbed fatty acid oxidation, endoplasmic reticulum stress, and apoptosis in left ventricle of patients with type 2 diabetes. Diabetes 68, 1924–1933 (2019).
Li, X. & Bi, X. Integrated control of fatty acid metabolism in heart failure. Metabolites 13, https://doi.org/10.3390/metabo13050615 (2023).
Ahmad, F. et al. Nicotinamide riboside kinase-2 alleviates ischemia-induced heart failure through P38 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 1866, https://doi.org/10.1016/j.bbadis.2019.165609 (2020).
Liu, P. et al. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 139, https://doi.org/10.1016/j.biopha.2021.111552 (2021).
Wan, S. et al. Ginsenoside Rd promotes omentin secretion in adipose through TBK1-AMPK to improve mitochondrial biogenesis via WNT5A/Ca2+ pathways in heart failure. Redox Biol. 60, https://doi.org/10.1016/j.redox.2023.102610 (2023).
Xu, G.-R. et al. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 126, https://doi.org/10.1016/j.biopha.2020.110071 (2020).
Sainero-Alcolado, L., Liaño-Pons, J., Ruiz-Pérez, M. V. & Arsenian-Henriksson, M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Diff. 29, 1304–1317 (2022).
Xiao, Q. et al. An integrated network pharmacology and cell metabolomics approach to reveal the role of rhein, a novel PPARα agonist, against renal fibrosis by activating the PPARα-CPT1A axis. Phytomedicine 102, 154147 (2022).
Li, D. et al. AMPK activator-treated human cardiac spheres enhance maturation and enable pathological modeling. Stem Cell Res. Ther. 14, 322 (2023).
Surina, S. et al. miR-21 in human cardiomyopathies. Front. Cardiovasc. Med. 8, 767064 (2021).
Scisciola, L. et al. The pivotal role of miRNA-21 in myocardial metabolic flexibility in response to short- and long-term high glucose treatment: evidence in human cardiomyocyte cell line. Diabetes Res. Clin. Pract. 191, 110066 (2022).
Yan, M. et al. miR-21-3p regulates cardiac hypertrophic response by targeting histone deacetylase-8. Cardiovasc. Res. 105, 340–352 (2015).
Miao, L. et al. A novel circRNA-miRNA-mRNA network identifies circ-YOD1 as a biomarker for coronary artery disease. Sci. Rep. 9, 18314 (2019).
Abbas, N. et al. Inhibition of miR-21: cardioprotective effects in human failing myocardium ex vivo. Eur. Heart J. 45, 2016–2018 (2024).
Wang, H. et al. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. J. Mol. Cell Cardiol. 94, 43–53 (2016).
Samadishadlou, M. et al. Unlocking the potential of microRNAs: machine learning identifies key biomarkers for myocardial infarction diagnosis. Cardiovasc. Diabetol. 22, 247 (2023).
Yang, Z., Cappello, T. & Wang, L. Emerging role of microRNAs in lipid metabolism. Acta Pharm. Sin. B 5, 145–150 (2015).
Tyagi, A. et al. Exosomal miR-4466 from nicotine-activated neutrophils promotes tumor cell stemness and metabolism in lung cancer metastasis. Oncogene 41, 3079–3092 (2022).
Goujon, M. et al. A double-negative feedback interaction between miR-21 and PPAR-α in clear renal cell carcinoma. Cancers 14, 795 (2022).
Kitada, M. & Koya, D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 17, 647–661 (2021).
Ajoolabady, A., Chiong, M., Lavandero, S., Klionsky, D. J. & Ren, J. Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment. Trends Mol. Med. 28, 836–849 (2022).
Zhang, L. et al. Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. 12, 470 (2021).
Zhang, T., Liu, Q., Gao, W., Sehgal, S. A. & Wu, H. The multifaceted regulation of mitophagy by endogenous metabolites. Autophagy 18, 1216–1239 (2021).
Wang, L. et al. ACSS3 regulates the metabolic homeostasis of epithelial cells and alleviates pulmonary fibrosis. Biochim. Biophysica Acta Mol. Basis Dis. 1870, https://doi.org/10.1016/j.bbadis.2023.166960 (2024).
Li, S. et al. L-carnitine alleviates cardiac microvascular dysfunction in diabetic cardiomyopathy by enhancing PINK1-Parkin-dependent mitophagy through the CPT1a-PHB2-PARL pathways. Acta Physiol. 238, e13975 (2023).
Nicolás-Ávila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e123 (2020).
Jefferies, J. L. & Saffitz, J. E. Autophagy and reverse remodeling. J. Am. College Cardiol. 79, 802–804 (2022).
Halade, G. V. & Lee, D. H. Inflammation and resolution signaling in cardiac repair and heart failure. eBioMedicine 79, https://doi.org/10.1016/j.ebiom.2022.103992 (2022).
Maruyama, K., Hara, A., Hashizume, H., Ushikubi, F. & Abiko, Y. Ranolazine attenuates palmitoyl-L-carnitine-induced mechanical and metabolic derangement in the isolated, perfused rat heart. J. Pharm. Pharmacol. 52, 709–715 (2020).
EW, W., XS, J., CW, R. & ZR, G. miR-487b mitigates chronic heart failure through inhibition of the IL-33/ST2 signaling pathway. Oncotarget 8, 51688–51702 (2017).
Kain, V., Ingle, K. A., Rajasekaran, N. S. & Halade, G. V. Activation of EP4 receptor limits transition of acute to chronic heart failure in lipoxygenase deficient mice. Theranostics 11, 2742–2754 (2021).
Wang, S. et al. NF-κB activator 1 downregulation in macrophages activates STAT3 to promote adenoma-adenocarcinoma transition and immunosuppression in colorectal cancer. BMC Med. 21, https://doi.org/10.1186/s12916-023-02791-0 (2023).
Acknowledgements
This work was supported by Natural Science Foundation of Fujian Province, P.R.C (2023Y9259), and Quanzhou High-level Talent Innovation and Entrepreneurship Project (2023C002YR). The experimental flowchart and the graphical abstract were drawn by Figdraw.
Author information
Authors and Affiliations
Contributions
Yujing Huang, Yalin Huang, and Zhaoling Cai contributions to conceptualization, data curation, investigation, methodology, validation and writing of the original draft. Markus W. Ferrari and Chengyi Li, Tianzhang Zhang, and Guorong Lyu contributed to formal analysis, software and validation. Zhenhua Wang contributed to funding acquisition, project administration, supervision, and review. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Ethics Committee of Second Affiliated Hospital of Fujian Medical University (2020-163). The research was conducted according to the World Medical Association Declaration of Helsinki and the Guidance of the Ministry of Science and Technology (MOST) for the Review and Approval of Human Genetic Resources. All ethical regulations relevant to human research participants were followed. All the information about the study will be fully explained to the subjects by the researchers. All the participants provided informed consent before sampling. All experimental procedures involving animals were approved by the Ethics Committee of Second Affiliated Hospital of Fujian Medical University (2020-347) and carried out according to the guidelines for care and use of laboratory animals and the principles of laboratory animal care and protection.
Peer review
Peer review information
Communications Biology thanks Yasin Gökçe and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Huang, Y., Cai, Z. et al. MiR-21-3p inhibitor exerts myocardial protective effects by altering macrophage polarization state and reducing excessive mitophagy. Commun Biol 7, 1371 (2024). https://doi.org/10.1038/s42003-024-07050-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-07050-3
This article is cited by
-
Circulating miRNAs correlate with clinical evaluation of activity in ANCA-associated glomerulonephritis
European Journal of Medical Research (2025)
-
Identifying key palmitoylation-associated genes in endometriosis through genomic data analysis
BMC Women's Health (2025)