Abstract
Preeclampsia (PE) is a common pregnancy disorder characterized by hypertension and proteinuria. Trophoblast behavior severely affect PE progression. Transcription factor Forkhead box protein P2 (FOXP2) was involved in cell migration and invasion, but its role in PE progression remains unknown. Laminin subunit alpha 4 (LAMA4) was predicted as a downstream gene of FOXP2 and related to PE. Thus, we supposed that FOXP2 might regulate PE by regulating LAMA4. We found the decreased FOXP2 expression in patients with PE compared with healthy pregnant women. The rat model of PE was induced by L-NAME oral gavage. FOXP2 overexpression lowered systolic and diastolic blood pressure and restored pathological changes of rats with PE. Trophoblasts under the hypoxia/reoxygenation (H/R) treatment were used to mimic PE in vitro. The results revealed that FOXP2 overexpression inhibited apoptosis but promoted migration, invasion, and angiogenesis of H/R-treated trophoblasts. Dual luciferase and chromatin immunoprecipitation-polymerase chain reaction assays confirmed that FOXP2 transcriptionally upregulated the LAMA4 expression in trophoblasts. LAMA4 knockdown reversed the migration and invasion-promoting role of FOXP2 overexpression in trophoblasts with H/R treatment. Collectively, our findings suggest that the FOXP2/LAMA4 axis regulates PE by suppressing trophoblast apoptosis and promoting its migration, invasion, and angiogenesis.
Similar content being viewed by others
Introduction
Preeclampsia (PE) is a pregnancy disorder characterized by hypertension and proteinuria, occurring after 20 weeks of gestation and involving multi-organ dysfunction1,2,3. It complicates 5 ~ 7% of all pregnancies and leads to over 70,000 maternal and over 500,000 fetal deaths per year worldwide4,5. Termination of pregnancy is the only curative strategy for PE6. However, higher cardiovascular and metabolic disease risks exist in mothers with a medical history and their children7. Therefore, exploring a potential therapeutic target is meaningful to PE treatment. The placenta, which is mainly composed of trophoblasts, is the central organ of the pathogenesis of PE8,9. Trophoblast behavior is closely associated with the development of PE. More frequent trophoblast apoptosis has been observed in the placenta of pregnancies with PE compared to trophoblasts from normal pregnancies10. Inadequate trophoblast migration and invasion were associated with the abnormal placentation11. Trophoblasts also participate in the formation of placental vascular system12. The reduction of placental vascularization is widely acknowledged as a feature of PE13. Based on this, we tried to find the gene that might regulate trophoblast behavior.
To explore a potential target affecting PE progression, we performed mRNA sequencing analysis and found a decreased Forkhead box protein P2 (FOXP2) expression in preeclamptic placentas. FOXP2 is a Forkhead protein family transcription factor and is highly conserved among mammals14,15. It is necessary for the development of speech regions in the embryonic brain16. Previous reports have revealed the roles of FOXP2 in cell apoptosis, migration and invasion, and angiogenesis. FOXP2 knockdown inhibited migration and angiogenesis abilities of microvascular endothelial cells17. The promoting effect of FOXP2 knockdown on cell migration was observed in colon cancer cells18. Importantly, Ke et al. reported that FOXP2 silencing contributed to the trophoblast apoptosis and inflammatory response in high glucose environments19. Nevertheless, how FOXP2 would regulate trophoblasts under the condition of PE has not been elucidated.
Laminin subunit alpha 4 (LAMA4) is a member of the Laminin family that mainly was expressed in the basement membranes20. It was previously reported that the LAMA4 expression was lower in preeclamptic placentas than in healthy controls21,22. Several studies revealed that LAMA4 was related to trophoblast behavior. Increased LAMA4 expression was related to better migration ability of trophoblasts21. Consistently, LAMA4 silencing resulted in a significant decrease in the migration and invasion abilities of human trophoblasts22. In addition, downregulated LAMA4 expression promoted the development of PE by inhibiting the expression of vascular factors23. By UCSC and JASPAR database prediction, we identified potential bind sites of FOXP2 in the promoter region of LAMA4. Therefore, we supposed that FOXP2 might regulate trophoblast behavior by regulating LAMA4 expression.
In the present study, the in vivo and in vitro models of PE were constructed to explore whether and how FOXP2 affects PE, aiming to provide a possible therapeutic option for the treatment of PE.
Results
Decreased FOXP2 expression was related to PE
To explore a potential therapeutic target affecting the development of PE, placental samples of patients with PE and healthy pregnant women were collected for mRNA sequencing and bioinformatics analysis. PCA plots showed marked differences in the gene signatures between the control group and the PE group (Fig. 1A). The volcano plot showed the overall expression pattern of DEGs in placental tissues from patients with PE compared with the normal control (Fig. 1B). The clustered heat map also revealed the expression pattern of DEGs (Fig. 1C). TOP10 enriched biological process (BP), cellular component (CC), and molecular function (MF) terms of DEGs were identified by gene ontology (GO) enrichment analysis. We found that DEGs belonging to FOXP family were enriched in anatomical structure development, multicellular organism development, system development, cell adhesion, cell migration, protein binding (Fig. 1D). Therefore, we put our attention on DEGs of the FOXP family. A heat map showed the expression profiles of these FOXP family DEGs. We found that the prominently downregulated FOXP2 expression in placental tissues of PE patients compared with the normal control (Fig. 1E). The box plot also depicted the decreased FOXP2 expression level in placental tissues of patients with PE compared with the normal control (Fig. 1F). Downregulated FOXP2 mRNA and protein levels also were determined by qRT-PCR and western blot assays (Fig. 1G, H). To increase the reliability of the FOXP2 profile in PE, we collected another batch of placental tissues from patients with PE and healthy pregnant women and performed western blot assay to detect the FOXP2 protein expression. The results confirmed the reduced FOXP2 protein expression in preeclamptic placenta compared with healthy controls (Supplementary Data 1-Fig. S1). All these results indicated that decreased FOXP2 expression might be related to the development of PE.
A The 2D and 3D PCA plots for DEGs from the normal control and PE samples. B Volcano plot of DEGs. C Heat map analysis for DEGs. D GO function analysis of DEGs, including Top 10 of BP, Top10 of CC, and Top 10 of MF. E Heat map analysis for expression of genes belonging to FOXP2 family. F The expression value of FOXP2. G qRT-PCR analysis for the mRNA level of FOXP2. H Western blot analysis for the protein level of FOXP2. Data: mean ± SD. N = 6. *p value < 0.05, **p value < 0.01.
FOXP2 overexpression alleviated the development of PE
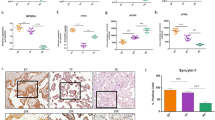
Next, we sought to explore the effects of FOXP2 on PE progression. The rat model of PE was constructed by oral gavage of L-NAME. The FOXP2-overexpressing adenovirus vectorGFP was injected into PE-like rats to determine whether FOXP2 overexpression could regulate the development of PE. We found elevated urine protein content, SBP, and DBP in rats with PE compared with the control, suggesting the successful construction of the PE rat model. However, FOXP2 overexpression significantly lowered these indicators in rats with PE (Fig. 2A, B). IHC staining was used to locate the FOXP2 expression in placental tissues. Representative images showed that L-NAME administration caused the decrease in the FOXP2 expression, but FOXP2 overexpression increased the FOXP2 expression in placental tissues of PE-like rats (Fig. 2C). LAMA4 was predicted as a downstream target gene of FOXP2. Therefore, we detected the mRNA and protein level of LAMA4 by qRT-PCR and western blot assays. Similar to the FOXP2 expression in each group, the LAMA4 expression was reduced in rats with PE, but it was elevated by FOXP2 overexpression (Fig. 2D, E), which demonstrated that FOXP2 might positively regulate the LAMA4 expression. Then, adenovirus infection efficiency was verified by visualization of GFP fluorescence signal in placental tissues of rats. The marked GFP expression in placental tissues after Ad-EVGFP and Ad-FOXP2GFP injection confirmed a high infection efficiency (Fig. 2F). Representative appearances of placenta, fetus, and uterus among groups were recorded. We found the shrunken fetus and the decreased number of fetuses in PE-like rats, which was reversed after FOXP2 overexpression (Fig. 2G). Besides, the weights of placenta and fetuses were reduced in rats with PE, but FOXP2 overexpression restored the weight loss (Fig. 2H). Furthermore, images of H&E staining showed that rats with PE exhibited obvious pathological changes in placental tissues: the reduction in the decidua and junctional zone. These pathological changes were restored by FOXP2 overexpression (Fig. 2I). Collectively, these results showed that FOXP2 overexpression alleviated L-NAME-induced PE.
A Urine protein content in urine samples of rats at GD0 and GD19. B SBP and DBP of rats at GD0 and GD19. C IHC analysis for FOXP2 expression. Scale bar = 200 μm (100 ×) or 50 μm (400 ×). D qRT-PCR analysis for the mRNA level of LAMA4. E Western blot analysis for the protein levels of FOXP2 and LAMA4. F Visualization of FOXP2 expression in placental tissues by a microscope. Scale bar = 100 μm. G Placenta, fetus, and uterus appearances of rats. H Weights of placenta and fetus of rats. I Images of H&E-stained placental tissues of rats. Scale bar = 500 μm. Data: mean ± SD. N = 6. *p value < 0.05, **p value < 0.01.
FOXP2 expression was lowered in trophoblasts subjected to H/R treatment
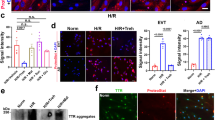
Trophoblast behavior is strongly linked to PE. To dissect the molecular mechanism underlying the protective role of FOXP2 overexpression in PE progression, we isolated primary trophoblasts from human villous tissues. Trophoblasts could fuse into the fully differentiated multinucleated syncytiotrophoblast24. Therefore, we use the characteristics of trophoblasts to identify isolated cells. After 24 h, 72 h, and 96 h of culture of isolated cells at passage 2, immunofluorescence staining was performed to locate the expression of CK-7, a trophoblast marker protein25. Representative images showed an intensive CK-7 expression in isolated cells and the cell-cell fusion after 72 h and 96 h of culture (Fig. 3A). In addition, the secretion of hCG would be increased in multinucleated syncytiotrophoblast26. By performing ELISA, we also found the significantly growing hCG production in the supernatant of isolated cells after 96 h of culture (Fig. 3B). Then, isolated cells after 72 h of culture with multinucleated morphology were used for further identification. Immunofluorescence staining located the CK-7 and FOXP2 expression in isolated cells after 72 h of culture (Fig. 3C). However, no expression of Vimentin, a marker of fibroblasts, was detected in isolated cells (Fig. 3C), indicating that isolated cells were not contaminated by was fibroblasts27. We also performed the western blot assay to detect the CK-7 and Vimentin protein expression in cells. Consistent with immunofluorescence staining, western blot analysis confirmed the obvious expression of CK-7 and invisible expression of Vimentin in isolated cells after 72 h of culture (Fig. 3D). All these results suggested that isolated cells are trophoblasts. Subsequently, primary trophoblasts were subjected to the H/R treatment to construct the in vitro model of PE. The mRNA and protein levels of FOXP2 were markedly decreased in trophoblasts with H/R treatment (Fig. 3E, F). Similarly, analysis of immunofluorescence staining also verified the decreased expression of FOXP2 in trophoblasts under the H/R condition (Fig. 3D). These results demonstrated that the FOXP2 expression was reduced in the in vitro model of PE.
A Images of immunofluorescence staining for CK-7 of isolated cells after 24 h, 72 h, and 96 h of culture. Scale bar = 50 μm. B The secretion of hCG in the supernatant of isolated cell culture medium after 24 h and 96 h of culture. C Images of immunofluorescence staining for CK-7, FOXP2, and Vimentin in isolated cells after 72 h of culture. Scale bar = 50 μm. D Western blot analysis for the protein level of CK-7 and Vimentin in isolated cells after 72 h of culture. E qRT-PCR analysis for the mRNA level of FOXP2 in primary trophoblasts. F Western blot analysis for the protein level of FOXP2 in primary trophoblasts. G Images of immunofluorescence staining for FOXP2 in primary trophoblasts. Scale bar = 100 μm. Data: mean ± SD. N = 3. *p value < 0.05, **p value < 0.01.
FOXP2 overexpression inhibited trophoblast apoptosis induced by H/R treatment
Trophoblast apoptosis is considered a main cause of PE28. To explore whether trophoblast apoptosis was involved in the role of FOXP2 in PE, trophoblasts were infected with the adenovirus vectorGFP overexpressing FOXP2 or the empty adenovirus vectorGFP. Fluorescence images revealed a higher efficiency of infection, as demonstrated by the obvious GFP expression in trophoblasts infected with Ad-EVGFP and Ad-FOXP2GFP (Supplementary Data 1-Fig. S2). Western blot assay determined the increased FOXP2 protein level in the Ad-FOXP2GFP group compared with Ad-EVGFP group, indicating the effective infection of Ad-FOXP2GFP (Fig. 4A). MTT assay indicated that H/R treatment caused the decreased cell viability of trophoblasts, which was enhanced by FOXP2 overexpression (Fig. 4B). Following that, we found that H/R treatment reduced the levels of FOXP2 and anti-apoptotic proteins (Bcl-2 and BCL-xL), in trophoblasts, while increased the protein level of cleaved caspase-3. However, FOXP2 overexpression exhibited the opposite trend (Fig. 4C). These results showed that FOXP2 overexpression inhibited H/R treatment-induced trophoblast apoptosis.
FOXP2 overexpression promoted migration and invasion of trophoblasts under the H/R condition
Inadequate trophoblast migration and invasion promote the development of PE11. We then verified the effects of FOXP2 overexpression on migration and invasion of trophoblasts by the Transwell assay. Of note, an apoptosis inhibitor Z-VAD-FMK was used to treat trophoblasts under the H/R condition to rule out the effects of apoptosis on migration and invasion. Representative images of Transwell migration and invasion assay showed that H/R treatment decreased trophoblast migration and invasion abilities, but FOXP2 overexpression reversed the effects of H/R treatment on trophoblast migration and invasion (Fig. 5A). The number of migrated and invaded cells also confirmed that FOXP2 overexpression elevated inadequate migration and invasion in H/R-treated trophoblasts (Fig. 5B). MMP-2 and MMP-9 are important regulators of cell migration and invasion. Thus, we next measured the concentrations of MMP-2 and MMP-9 in the supernatant of the culture medium. Similar to the results of Transwell assays, H/R treatment decreased the secretion of MMP-2 and MMP-9 of H/R-treated trophoblasts. However, FOXP2 overexpression promoted the secretion of MMP-2 and MMP-9 of trophoblasts with H/R treatment (Fig. 5C). These results revealed that FOXP2 overexpression promoted migration and invasion of trophoblasts.
FOXP2 overexpression accelerated angiogenesis of trophoblasts under the H/R condition
The reduction in angiogenesis of trophoblasts correlates to the development of PE29. Therefore, we performed the tube formation assay to evaluate whether FOXP2 overexpression would affect the angiogenesis of trophoblasts under the H/R condition. H/R-treated trophoblasts were subjected to Z-VAD-FMK treatment to rule out the effects of apoptosis on angiogenesis. The results suggested that H/R treatment resulted in the reduction in the number of tubes in trophoblasts, which was attenuated by FOXP2 overexpression (Fig. 6A, B). Expression of Flt-1 contributes to the inhibition of angiogenesis30. The qRT-PCR analysis confirmed that H/R treatment upregulated the Flt-1 expression in trophoblasts. FOXP2 overexpression downregulated the mRNA level of Flt-1 in H/R-treated trophoblasts (Fig. 6C), which was consistent with the results of tube formation assay. These results implied that FOXP2 overexpression promoted the angiogenesis of trophoblasts under the H/R condition.
LAMA4 mediated the role of FOXP2 in PE progression
LAMA4 was predicted as one of the target genes of FOXP2. Moreover, through the in vivo experiments, we have found that FOXP2 might have the potential to positively regulate the LAMA4 expression. Therefore, we tried to explore whether FOXP2 could regulate the expression of LAMA4 and further affect the development of PE. Similar to the in vivo results, qRT-PCR and western blot analysis demonstrated that FOXP2 overexpression increased the expression levels of FOXP2 and LAMA4 in trophoblasts exposed to the H/R condition (Fig. 7A, B). In the dual luciferase assay, the pGL-3 basic luciferase reporter contained the LAMA4 promoter form the −1900 to +300 bp. In the promoter region of −1900 ~ + 35 bp, FOXP2 overexpression upregulated the promoter activity of LAMA4. However, FOXP2 overexpression had no effect on the promoter activity of LAMA4 in the promoter region of −1300 ~ + 35 bp or −600 ~ + 35 bp. Therefore, we supposed that there were the FOXP2 binding site in the LAMA4 promoter region of −1900 ~ -1300 bp (Fig. 7C). The direct binding of FOXP2 in the DNA region of LAMA4 was revealed by CHIP-PCR. Compared with the control, H/R treatment decreased the amount of genomic DNA binding to FOXP2, demonstrated by the reduced brightness intensity of the band (Fig. 7D). Next, trophoblasts with LAMA4 knockdown were generated by infection of adenovirus vector carrying shRNA targeting LAMA4. The effective infection was proved by the decreased expression of LAMA4 in the Ad-shLAMA4GFP group compared with the Ad-shNCGFP group (Fig. 7E). Trophoblasts were co-infected with Ad-FOXP2GFP and Ad-shLAMA4GFP for FOXP2 overexpression and LAMA4 knockdown. Afterward, we performed the Transwell assay to assess whether LAMA4 knockdown would affect the protective role of FOXP2 overexpression in the in vitro PE model. We found that the enhanced cell migration and invasion abilities induced by FOXP2 overexpression were weakened by LAMA4 knockdown in trophoblasts exposed to the H/R condition (Fig. 7F, G). These results illustrated that LAMA4 might mediate the effects of FOXP2 expression on PE progression.
A qRT-PCR analysis of the mRNA level of LAMA4. B Western blot analysis of the protein level of LAMA4. C Schematic diagram of LAMA4 promoter-luciferase constructs (left) and luciferase activity was determined by dual luciferase reporter assay in trophoblasts co-transfected with promoter constructs and ovFOXP2/Ev (right). D ChIP-PCR analysis for determining the FOXP2 binding to LAMA4 promoter. E Western blot analysis of the protein level of LAMA4. F Images of migrated and invaded trophoblasts. Scale bar = 100 μm. G The number of migrated and invaded trophoblasts. Data: mean ± SD. N = 3. *p value < 0.05, **p value < 0.01.
Discussion
PE is a common pregnancy complication affecting the healthy status of both the mother and her fetus31. Some methods have been raised to prevent the incidence of PE, such as the administration of aspirin, calcium, or antioxidant vitamins31. However, these therapies are merely applicable to early pregnancy or pregnant women with a deficiency in certain nutrients31. Exploring the treatment strategy remains an urgent need. Placental villus, composed of trophoblasts, is responsible for the oxygen and nutrient exchange of the maternal-fetus32,33. Trophoblast dysfunction primarily mediates the development of PE. Of note, impaired trophoblast invasion is a disadvantage to spiral artery remodeling, contributing to placental ischemia and hypoxia34,35,36. The reduction in placental perfusion leads to maternal hypertension, a typical characteristic of PE37. Due to the important role of trophoblasts in PE progression, the regulation of trophoblast phenotypes is of great significance for PE treatment.
In this study, we identified FOXP2 as a potential target of PE treatment. FOXP2 is a member of FOXP transcription factors, which consist of FOXP1, FOXP2, FOXP3, and FOXP4 and share C2H2 zinc finger and leucine zipper domains38. Previous research involving FOXP2 has mainly focused on speech and language development39. FOXP2 has a high expression level in the brain tissues during fetal development, and its mutation leads to severe speech and language deficits40,41. Recently, a large body of literature has indicated that FOXP2 was associated with cancer progression through regulating cell apoptosis, migration, and invasion. Our findings evidenced the protective effects of FOXP2 overexpression on PE. The underlying mechanism might be associated with the reduction in trophoblast apoptosis while the increase in migration and invasion. The anti-apoptotic effect of FOXP2 overexpression on cardiomyocytes was found previously42. Nishida et al. declared that FOXP2 knockdown promoted colon cancer cell migration18, which was opposite to our results. The different roles of FOXP2 in cell migration might be closely related to cell type and pathological status. Furthermore, impaired angiogenesis also is a disadvantage of the development of PE43. FOXP2 has been previously reported to upregulate the AGGF1 expression, thereby promoting the angiogenesis of glioma-exposed endothelial cells44. Placental-derived soluble Flt-1 could bind to vascular endothelial growth factor and suppress it’s the angiogenesis-promoting effect45. Consistent with the previous study, our results showed that FOXP2 overexpression promoted angiogenesis of trophoblasts by inhibiting the Flt-1 expression. However, the angiogenesis-promoting effect often raise concerns about tumorigenesis. In the current study, FOXP2 overexpression caused the decreased sFlt-1 expression, but there was no significant difference was found between the Control group and the FOXP2 overexpression group. Thus, FOXP2 overexpression was not sufficient to upgrade the pro-angiogenic effect to the tumorigenic effect.
As a transcription factor, FOXP2 could affect the development of diseases via transcriptionally regulating the downstream target genes. In the present study, we determined that LAMA4 might mediate the effects of FOXP2 overexpression on PE progression. Previous studies showed that LAMA4 expression was decreased in preeclamptic placentas, and LAMA4 silencing inhibited trophoblast migration and invasion22,23. Similarly, we verified the decreased LAMA4 expression in both the in vivo and in vitro models of PE. Although previous report and our study suggested that a decreased LAMA4 expression was associated with the development of PE, whether the inhibition of LAMA4 is sufficient to induce PE remains unknown. Tissue-specific LAMA4 knockout animals would help to determine whether the inhibition of LAMA4 has the potential to induce the pathological changes of PE. Furthermore, LAMA4 RNA interference suppressed migration and tube formation of human umbilical vein endothelial cells, which might play an important role in the association between oxidative stress and PE46. FOXP2 and LAMA4 are associated with oxidative stress or inflammation19,46, which are regarded as vital contributors to PE47. These prior studies indicated that the FOXP2/LAMA4 axis might affect PE progression by regulating other pathological processes. In addition to LAMA4, previous research has demonstrated that FOXP2 could regulate other proteins that associated with cell apoptosis, migration, or invasion. Devanna et al. found that FOXP2 upregulated the expression of retinoic acid receptor β (RARβ)48, which could promote the expression of apoptosis-related genes in cholangiocarcinoma cells and inhibit the invasion of pancreatic cancer cells49,50. Moreover, FOXP2 was able to suppress the Sushi repeat containing protein X-linked 2 (SRPX2) promoter and its transcript51. A previous study showed that SRPX2 overexpression promoted the migration of gastric cancer cells52. However, the effects of RARβ and SRPX2 on trophoblasts have not yet been investigated. Therefore, our study cannot rule out the possibility that FOXP2 might affect trophoblasts by regulating other downstream targets.
Although our study provides an insight into PE treatment, there are several limitations to note here. At present, no drugs have been developed to target FOXP2 activation, which highlights the potential difficulties in the clinical translation of our findings. Furthermore, hemolysis elevated liver enzymes and low platelets (HELLP) syndrome is a complication of PE53. It occurs more frequently in patients with PE, but can also occur alone54. In this study, we focused on the progression of PE and potential treatment strategies. Therefore, whether patients with PE had HELLP symptom was not been carefully identified. Our results only indicated that FOXP2 overexpression could alleviate the development of PE, but cannot determine whether there is a similar effect on HELLP symptom.
This work determined the protective effect of FOXP2 overexpression on the development of PE. In detail, FOXP2 overexpression transcriptionally upregulated LAMA4 expression and thereby inhibited apoptosis but promoted migration and invasion, and angiogenesis of trophoblasts. However, we only pointed out that LAMA4 might mediate the effects of FOXP2 on PE progression by regulating trophoblasts, which failed to rule out that FOXP2 affects PE by other pathways. Thus, further experimental verification is needed to clarify whether the role of FOXP2 in PE progression was related to other pathways or other downstream targets. Some complications, such as HELLP syndrome, could be considered in clinical samples to explore PE progression more fully. What’s more, exploring strategies for activating FOXP2 is key to increasing the clinical translational value of this study in the future.
Collectively, we found a decreased FOXP2 expression in the placenta tissues of patients with PE. FOXP2 overexpression protected against PE by inhibiting apoptosis and promoting migration, invasion, and angiogenesis of trophoblasts. The effects of FOXP2 overexpression on trophoblasts might be mediated by LAMA4.
Ethics approval and consent to participate
The study protocols were approved by Medical Ethics Committee of Shengjing Hospital of China Medical University. All procedures involving human patients were in accordance with the Declaration of Helsinki. All participants have written the informed consent. All ethical regulations relevant to human research participants were followed. All procedures involving animals were in accordance with the Guide for the Care and Use of Laboratory Animals. We have complied with all relevant ethical regulations for animal use.
Materials and methods
Database source
UCSC database (https://genome.ucsc.edu/) was used to obtain the promoter sequence of LAMA4. JASPAR database (https://jaspar.elixir.no/) was used to predict the binding sites of FOXP2 in the promoter region of LAMA4.
Placental tissue collection
Placental samples were collected from patients with PE (n = 6) and from normal pregnant women (n = 6) for mRNA sequencing (Lc-bio Technologies, Hangzhou, China). The screening criteria for differentially expressed genes (DEGs) were |Log2Fold change (FC)| > 1 and p value < 0.05. The clinical data of patients with PE and normal pregnant women are shown in Table 1.
To verify the FOXP2 expression pattern in the PE condition, another batch of placental tissues were collected from patients with PE (n = 8) and normal pregnant women (n = 9) and subjected to western blot analysis. The clinical data of patients with PE and normal pregnancy are shown in Supplementary Data 1 (Table S1).
The study protocols were approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University and were in accordance with the Declaration of Helsinki. All participants have written the informed consent.
Animal model of PE
Sprague-Dawley (SD) rats were purchased from Liaoning Changsheng (Benxi, China). Female SD rats (8–10 weeks) in an estrous phase were mated with weight-matched male SD rats overnight. The vaginal plug was monitored the next morning to determine the status of pregnancy, and the day of vaginal plug formation was determined as the gestational day (GD) 0. Pregnant rats were randomly divided into four groups, including the Control group, the L-NAME group, the L-NAME + Ad-EVGFP group, and the L-NAME + Ad-FOXP2GFP group. For rats in the L-NAME group, L-NAME (Cat#S22013, Yuanye Bio-Technology, Shanghai, China) was given to rats at a dose of 60 mg/kg/d by oral gavage from GD10 to GD19 to induce pathological features of PE55. Accordingly, rats in the Control group were orally administrated with normal saline to use as the healthy control of PE-like rats. For rats in the L-NAME + Ad-FOXP2GFP group, L-NAME treatment (60 mg/kg/d) was orally given to rats from GD10 to GD19, and CMV-driven FOXP2-overexpressing recombinant adenovirus vectorsGFP (Ad-FOXP2GFP, 5 × 108 PFU) were injected into the tail vein of rats at GD10 to explore whether FOXP2 would modulate PE progression56. Rats in the L-NAME + Ad-EVGFP group were orally gavaged with L-NAME (60 mg/kg/d) from GD10 to GD19 and injected with the empty vectorGFP (5 × 108 PFU) via the tail vein to use as the negative control of rats in the L-NAME + Ad-FOXP2GFP group56. All rats were subjected to euthanasia at GD20, and placental tissues were collected for subsequent analysis. The study protocols were approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University and were in accordance with the Guide for the Care and Use of Laboratory Animals.
Measurement of urine protein content
Urine samples of rats were collected within 24 h at GD0 and GD19. Urine protein content was detected using the commercially available kit (Cat#C035-2, Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions provided by the manufacturer.
Measurement of blood pressure
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) of rats were measured using a Blood Pressure Analysis System (ALC-NIBP, Shanghai, China) at GD0 and GD19.
Cell isolation and culture
Human primary trophoblasts were isolated from the placental villous tissues of healthy term placenta57. Briefly, placental villous tissues were rinsed several times with sterile 0.9% NaCl solution and cut into small pieces (2 cm × 2 cm). Then, tissues were transferred to a cell strainer and rinsed with sterile 0.9% NaCl solution until the rinse appeared clear. Tissues were collected and digested with 0.2 mg/mL DNase (Cat#BS137, Biosharp, Hefei, China) and 0.25% trypsin (Cat#T4799, Sigma, Saint Louis, MI, USA) at 37 °C with shaking for 15 min. After being filtered through a mesh screen, cell suspension was obtained and neutralized by fetal bovine serum (FBS). Centrifugation was performed on collected cells, which were re-suspended in Iscove’s modified dulbecco medium (IMDM) (Cat#G4640, Servicebio, Wuhan, China) supplemented with 10% FBS. Cell suspension was digested again and passed through 100 μm filter. Cell pellets were re-suspended in Hank’s solution and purified by Percoll gradient. Cells between 30% and 50% of Percoll were collected and washed with Hank’s solution. IMDM supplemented with 10% FBS was used to suspend cells, which were cultured in an incubator at 37°C under the condition of 8% oxygen. For the construction of the in vitro model of PE, trophoblasts were exposed to the hypoxia environment (0.5% O2) for 4 h followed by the normoxia condition (8% O2) for 18 h58, referred to as hypoxia/reoxygenation (H/R)58. To rule out the effects of apoptosis on migration, invasion, and angiogenesis of trophoblasts with FOXP2 overexpression, a caspase inhibitor z-VAD-FMK (25 µM) was used to treat trophoblasts under the H/R condition59.
Cell infection
For FOXP2 overexpression, trophoblasts were infected with the CMV-driven adenovirus vectorGFP overexpressing FOXP2 or the empty adenovirus vectorGFP. For FOXP2 knockdown, trophoblasts were infected with the CMV-driven adenovirus vectorGFP encoding a shRNA targeting LAMA4 (AGAACTGTGCAGTGTGCAACT) or the adenovirus vectorGFP encoding a non-targeting shRNA (TTCTCCGAACGTGTCACGT). To achieve overexpression of FOXP2 and knockdown of LAMA4, trophoblasts were co-infected with the adenovirus vectorGFP overexpressing FOXP2 and the adenovirus vectorGFP encoding a shRNA targeting LAMA4.
Western blot analysis
The total protein was extracted using the radio immunoprecipitation assay (RIPA) buffer (Cat#R0010, Solarbio, Beijing, China) supplemented with phenylmethanesulfonyl fluoride (PMSF) (Cat#P0100, Solarbio, Beijing, China). Then, these total protein samples were quantified using a bicinchoninic acid assay (BCA) Protein Assay Kit (Cat#PC0020, Solarbio, Beijing, China) on a microplate reader (BIOTEK, Winooski, VT, USA). After being separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred onto polyvinylidene fluoride (PVDF) membranes. Subsequently, membranes were blocked with skim milk and incubated with primary (4 °C, overnight) and secondary antibodies (37 °C, 1 h). Afterward, blots were developed with electrochemiluminescence (ECL) reagent and imaged by a Chemiluminescence Image Analysis System (Tanon, Shanghai, China). Primary antibodies: antibodies against FOXP2 (1:500, Cat#A5677), B-cell lymphoma 2 (Bcl-2) (1:500, Cat#A0208), B-cell lymphoma-extra large (Bcl-xl) (1:1000, Cat#A19703) (Abclonal, Wuhan, China), LAMA4 antibody (1:1000, Cat#ab242198, Abcam, Cambridge, UK), cleaved caspase-3 antibody (1:1000, Cat#9664, CST, MA, USA), antibodies against Cytokeratin 7 (CK7) (1:1000, Cat#R24065) and Vimentin (1:500, Cat#R22775) (Zenbio, Chengdu, China). The secondary antibody used for the above primary antibodies was goat anti-rabbit Immunoglobulin G (IgG)-horseradish peroxidase (HRP) (1:3000, Cat#SE134, Solarbio, Beijing, China). GAPDH antibody (1:20000, Cat#60004-1-Ig, Proteintech, Wuhan, China) was used as an internal reference, and its corresponding secondary antibody was goat anti-mouse IgG-HRP (1:3000, Cat#SE131, Solarbio, Beijing, China). The original blot images are provided in Supplementary Data 2.
Quantitative real-time (qRT)-polymerase chain reaction (PCR) analysis
The total RNA was isolated using TRIpure (Cat#RP1001, BioTeke, Beijing, China) and reversely transcribed to cDNA by BeyoRT™ Q M-MLV (Cat#D7160L, Beyotime, Shanghai, China). After that, qRT-PCR was performed using primers (Generalbiol, Chuzhou, China), 2×Taq PCR MasterMix (Cat#PC1150, Solarbio, Beijing, China), and SYBR Green (Cat#SY1020, Solarbio, Beijing, China) on Exicycler 96 (BIONEER, Daejeon, South Korea). The 2−ΔΔ CT method was used to calculate relative mRNA levels for animal and cell samples, while the 2−Δ CT method was used for clinical samples. Primer sequences were as follows: homo FOXP2 (Forward: CTGGAAAGCAAGCGAAAGAG; Reverse: GAATGGAGATGAGTCCCTGA), homo LAMA4 (Forward: TGGGACCTGACTGATGAC; Reverse: CTTTCTTAGGGCGTATTG), homo fms-like tyrosine kinase (Flt)-1 (Forward: TGGGACAGTAGAAAGGG; Reverse: AAGGGAGTGGTAGCAGTA), rat LAMA4 (Forward: TTACAGCCAAATGGGTTA; Reverse: GACGACGAAGTGGGACA).
Histology and immunohistochemistry
The paraffin-embedded placental tissues were cut into slices with 5-μm thickness. Histological analysis was performed using hematoxylin and eosin (H&E) staining. In detail, dewaxed and rehydrated sections were stained with hematoxylin (Cat#H8070, Solarbio, Beijing, China) and counterstained with eosin (Cat#A600190, Sangon, Shanghai, China). After being dehydrated, cleared, and mounted, sections were observed and captured using a microscope (DP73, OLYMPUS, Tokyo, Japan). For immunohistochemistry analysis, rehydrated sections were subjected to antigen retrieval and blocked with 3% hydrogen peroxide (Cat#10011218, Sinopharm Chemical Reagent, Shanghai, China). Afterward, sections were blocked with 1% bovine serum albumin (BSA) solution (Cat#A602440-0050, Sangon, Shanghai, China), incubated with primary (4 °C, overnight) and secondary antibody (37 °C, 1 h). After being developed with 3,3-Diaminobenzidine (DAB) reagent (Cat#DAB-1031, Maixin, Fuzhou, China) and counterstained with hematoxylin, sections were subjected to dehydration and clearance. Finally, sections were mounted and imaged using a microscope (DP73, OLYMPUS, Tokyo, Japan). FOXP2 antibody (1:100, Cat#20529-1-AP, Proteintech, Wuhan, China) and goat anti-rabbit IgG-HRP (1:500, Cat#31460, ThermoFisher, Pittsburgh, USA) were used for immunohistochemistry analysis.
Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay
Trophoblasts were seeded into 96-well plates at a density of 5 × 103 per well. After being infected with the adenovirus vectorGFP expressing FOXP2 or the empty adenoviral vectorGFP, cells were placed under the H/R condition and cultured in an incubator with 5% CO2 at 37 °C. The 50 μL of MTT (Cat#KGA311, Keygenbio, Nanjing, China) solution was added to each well to incubate cells for another 4 h. Then the cell supernatant was removed, and 150 μL of dimethyl sulfoxide (DMSO) (Cat#KGT5131, Keygenbio, Nanjing, China) was used to dissolve the purple crystals formed in cells. The optical density (OD) value at 490 nm was measured using a microplate reader (800TS, BIOTEK, Winooski, USA).
Transwell assays
Transwell assays were performed to determine cell migration and invasion abilities. Cell suspension was prepared with a serum-free medium. For cell migration assay, Transwell chambers (Cat#14341, LABSELECT, Hefei, China) were placed in 24-well plates, and 200 μL of cell suspension containing 5 × 103 cells were added to the upper chamber. For cell invasion assay, Transwell chambers coated with Matrigel (Cat#356234, Corning, NY, USA) were placed in 24-well plates, and 200 μL of cell suspension containing 5 × 104 cells were added to the upper chamber. A culture medium containing 10% FBS was loaded into the lower chamber in both migration and invasion assay. Then 24-well plates were placed in a 37 °C incubator with 5% CO2. Trophoblasts were fixed with 4% paraformaldehyde (Cat#C104188, Aladdin, Shanghai, China) for 20 min at room temperature and stained with 0.5% crystal violet dye (Cat#0528, Amresco, Solon, USA) for 5 min. The lower side of the membrane of Transwell chambers was observed using a microscope. The number of migrated or invaded cells was recorded by calculating the average in five views.
Enzyme-linked immunosorbent assay (ELISA)
The secretion of human chorionic gonadotropin (hCG), matrix metallopeptidase 2 (MMP-2), and matrix metallopeptidase 9 (MMP-9) in the supernatant of the cell culture medium was detected by corresponding ELISA kits. The hCG ELISA Kit (Cat#EK1299), Human MMP-2 ELISA Kit (Cat#EK1M02), and Human MMP-9 ELISA Kit (Cat#EK1M09) were purchased from Liankebio (Hangzhou, China).
Tube formation assay
The cell suspension containing 1 × 104 cells was added to per well of the 96-well plates coated with Matrigel. Subsequently, the plates were placed in an incubator with 5% CO2 at 37 °C for 4 h. After incubation, trophoblasts were observed under a microscope. The number of tubes was counted to assess the tube formation ability. Specifically, complete rings in five random fields of view were respectively counted and calculated as an average, which was used as the number of tubes in one well.
Chromatin immunoprecipitation (CHIP)-PCR assay
CHIP assay was performed using the ChIP Assay Kit (Cat#P2078, Beyotime, Shanghai, China). Briefly, trophoblasts were treated with formaldehyde to cross-link proteins to the genomic DNA and mixed with Glycine Solution. Then cells were washed with PBS supplemented with PMSF and collected into centrifuge tubes, followed by centrifugation to precipitate cells. After that, sodium dodecyl sulfate (SDS) Lysis Buffer supplemented with PMSF was used to lyse cells, and genomic DNA was sheared by sonicate. NaCl solution was added to cell lysates to remove cross-links between protein and genomic DNA. The supernatant of cell lysates was collected and then diluted with ChIP Dilution Buffer supplemented with PMSF. The part of diluted solution was used as Input. Other diluted solution was mixed with Protein A + G Agarose/Salmon Sperm DNA. The mixture was centrifuged to obtain the supernatant, which was treated with the antibody for target protein or IgG, followed by centrifugation. The sediments were washed with Complex Wash Buffer. After that, DNA precipitation from the washed samples was amplified using PCR, and the PCR products were detected by agarose gel electrophoresis and analyzed by a gel imaging analyzer (WD-9413B, Liuyi Biotechnology, Beijing, China). The DNA marker used for CHIP-PCR was purchased from Biosharp (Hefei, China). The original blot images are provided in Supplementary Data 2.
Dual luciferase assay
The LAMA4 promoter was inserted into the pGL3-basic vector (Cat#BR014, Hunan Fenghui Biotechnology, Changsha, China) to generate the pGL3-LAMA4 promoter. Trophoblasts were co-transfected with pGL3-LAMA4 promoter and FOXP2 overexpression/empty vector (Cat#G118443, YouBio, Changsha, China) using Lipo3000. The pRL-TK (Cat#BR018, Hunan Fenghui Biotechnology, Changsha, China) was used as the control. Luciferase activity was determined by Luciferase Assay Kit (Cat#KGAF040, Keygenbio, Nanjing, China).
Immunofluorescence
Cell climbing sheets were fixed with 4% paraformaldehyde (Cat#80096618, Sinopharm Chemical Reagent, Shanghai, China) for 15 min, incubated with 0.1% tritonX-100 (Cat#ST795, Beyotime, Shanghai, China) for 30 min, and blocked with 1% BSA solution (Cat#A602440-0050, Sangon, Shanghai, China) for 15 min, followed by incubation with primary antibodies and corresponding secondary antibodies. Subsequently, DAPI (Cat#D106471, Aladdin, Shanghai, China) was used to counterstain the nucleus of cells. The sheets were mounted with an anti-fluorescence quencher (Cat#S2100, Solarbio, Beijing, China). Primary antibodies: CK-7 (1:100, Cat#R24065, Zenbio, Chengdu, China), Vimentin (1:100, Cat#R22775, Zenbio, Chengdu, China), FOXP2 antibody (1:100, Cat#20529-1-AP, Proteintech, Wuhan, China). Secondary antibodies: Anti-rabbit IgG (1:500, Cat#4413, CST, MA, USA) and Goat-anti rabbit IgG-Cy3 (1:200, Cat#ab6939, Abcam, Cambridge, UK).
Statistical analysis
All data were presented in the form of mean ± standard deviation (SD). Statistical analysis was performed using Graphpad Prism 9. An unpaired t-test or Mann-Whitney test was used to analyze differences between two groups. One-way ANOVA with Tukey’s post hoc analysis or Two-way ANOVA was used for analyzing differences among three or more than three groups. Differences were considered significant if the p value was less than 0.05.
Data availability
The mRNA sequencing data have been deposited in the Gene Expression Omnibus database (Accession number: GSE279757). Numerical source data for graphs and charts can be found in Supplementary Data 3.
References
Rana, S., Lemoine, E., Granger, J. P. & Karumanchi, S. A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 124, 1094–1112 (2019).
Chappell, L. C., Cluver, C. A., Kingdom, J. & Tong, S. Pre-eclampsia. Lancet 398, 341–354 (2021).
Phipps, E. A., Thadhani, R., Benzing, T. & Karumanchi, S. A. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 15, 275–289 (2019).
Si, S. et al. The Association of Vitamin D and Its Pathway Genes’ Polymorphisms with Hypertensive Disorders of Pregnancy: A Prospective Cohort Study. Nutrients 14, https://doi.org/10.3390/nu14112355 (2022).
Sutan, R., Aminuddin, N. A. & Mahdy, Z. A. Prevalence, maternal characteristics, and birth outcomes of preeclampsia: A cross-sectional study in a single tertiary healthcare center in greater Kuala Lumpur Malaysia. Front Public Health 10, 973271 (2022).
Hahn, L. et al. Gal-2 Increases H3K4me(3) and H3K9ac in Trophoblasts and Preeclampsia. Biomolecules 12, https://doi.org/10.3390/biom12050707 (2022).
Wang, Y., Li, B. & Zhao, Y. Inflammation in Preeclampsia: Genetic Biomarkers, Mechanisms, and Therapeutic Strategies. Front. Immunol. 13, 883404 (2022).
Lackner, H. K. et al. Disturbed Cardiorespiratory Adaptation in Preeclampsia: Return to Normal Stress Regulation Shortly after Delivery? International journal of molecular sciences 20, https://doi.org/10.3390/ijms20133149 (2019).
Hada, M. et al. Highly rigid H3.1/H3.2-H3K9me3 domains set a barrier for cell fate reprogramming in trophoblast stem cells. Genes Dev. 36, 84–102 (2022).
Mlyczyńska, E. et al. Anti-Apoptotic Effect of Apelin in Human Placenta: Studies on BeWo Cells and Villous Explants from Third-Trimester Human Pregnancy. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22052760 (2021).
van Zuylen, W. J., Ford, C. E., Wong, D. D. & Rawlinson, W. D. Human Cytomegalovirus Modulates Expression of Noncanonical Wnt Receptor ROR2 To Alter Trophoblast Migration. J. Virol. 90, 1108–1115 (2016).
Zhang, D. et al. poFUT1 promotes uterine angiogenesis and vascular remodeling via enhancing the O-fucosylation on uPA. Cell death Dis. 10, 775 (2019).
Kulandavelu, S. et al. S-Nitrosoglutathione Reductase Deficiency Causes Aberrant Placental S-Nitrosylation and Preeclampsia. J. Am. Heart Assoc. 11, e024008 (2022).
Meng, W. et al. A genome-wide association study finds genetic variants associated with neck or shoulder pain in UK Biobank. Hum. Mol. Genet. 29, 1396–1404 (2020).
Hickey, S. L., Berto, S. & Konopka, G. Chromatin Decondensation by FOXP2 Promotes Human Neuron Maturation and Expression of Neurodevelopmental Disease Genes. Cell Rep. 27, 1699–1711.e1699 (2019).
Auvinen, P. et al. Chromatin modifier developmental pluripotency associated factor 4 (DPPA4) is a candidate gene for alcohol-induced developmental disorders. BMC Med. 20, 495 (2022).
Liu, Y. et al. Vaccarin Regulates Diabetic Chronic Wound Healing through FOXP2/AGGF1 Pathways. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21061966 (2020).
Nishida, K., Kuwano, Y. & Rokutan, K. The MicroRNA-23b/27b/24 Cluster Facilitates Colon Cancer Cell Migration by Targeting FOXP2. Cancers 12, https://doi.org/10.3390/cancers12010174 (2020).
Ke, W. et al. miR-134-5p promotes inflammation and apoptosis of trophoblast cells via regulating FOXP2 transcription in gestational diabetes mellitus. Bioengineered 13, 319–330 (2022).
Yang, Z. S. et al. Regulation and Function of Laminin A5 during Mouse and Human Decidualization. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms23010199 (2021).
Zhao, N. et al. Genome-Wide Identification of Laminin Family Related to Follicular Pseudoplacenta Development in Black Rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms231810523 (2022).
Shan, N. et al. Laminin α4 (LAMA4) expression promotes trophoblast cell invasion, migration, and angiogenesis, and is lowered in preeclamptic placentas. Placenta 36, 809–820 (2015).
Ji, Y. et al. Role of LAMA4 Gene in Regulating Extravillous Trophoblasts in Pathogenesis of Preeclampsia. Med. Sci. Monit. : Int. Med. J. Exp. Clin. Res. 25, 9630–9636 (2019).
Nedder, M. et al. Uptake of Cerium Dioxide Nanoparticles and Impact on Viability, Differentiation and Functions of Primary Trophoblast Cells from Human Placenta. Nanomaterials (Basel, Switzerland) 10, https://doi.org/10.3390/nano10071309 (2020).
Park, J. Y. et al. A microphysiological model of human trophoblast invasion during implantation. Nat. Commun. 13, 1252 (2022).
Bucher, M., Kadam, L., Ahuna, K. & Myatt, L. Differences in Glycolysis and Mitochondrial Respiration between Cytotrophoblast and Syncytiotrophoblast In-Vitro: Evidence for Sexual Dimorphism. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms221910875 (2021).
Tilburgs, T. et al. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc. Natl. Acad. Sci. USA 112, 7219–7224 (2015).
Li, Y. et al. Autophagy Activation by Hypoxia Regulates Angiogenesis and Apoptosis in Oxidized Low-Density Lipoprotein-Induced Preeclampsia. Front. Mol. Biosci. 8, 709751 (2021).
Murrieta-Coxca, J. M. et al. Role of IL-36 Cytokines in the Regulation of Angiogenesis Potential of Trophoblast Cells. Int. J Mol. Sci. 22, https://doi.org/10.3390/ijms22010285 (2020).
Zhou, C. C. et al. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ. Res. 100, 88–95 (2007).
Burton, G. J., Redman, C. W., Roberts, J. M. & Moffett, A. Pre-eclampsia: pathophysiology and clinical implications. BMJ (Clin. Res. ed.) 366, l2381 (2019).
Wu, H. et al. Molecular evidence suggesting the persistence of residual SARS-CoV-2 and immune responses in the placentas of pregnant patients recovered from COVID-19. Cell Prolif. 54, e13091 (2021).
Toothaker, J. M. et al. Immune Cells in the Placental Villi Contribute to Intra-amniotic Inflammation. Front. Immunol. 11, 866 (2020).
Liu, Y. et al. The metabolic role of LncZBTB39-1:2 in the trophoblast mobility of preeclampsia. Genes Dis. 5, 235–244 (2018).
Wang, C. et al. Krüppel-like factor 17 upregulates uterine corin expression and promotes spiral artery remodeling in pregnancy. Proc. Natl. Acad. Sci. USA 117, 19425–19434 (2020).
Lv, L. J. et al. Deep metagenomic characterization of gut microbial community and function in preeclampsia. Front. Cell. Infect. Microbiol. 12, 933523 (2022).
Huang, R. et al. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ. Health : a Glob. Access Sci. source 18, 5 (2019).
Kim, J. H. et al. Molecular networks of FOXP family: dual biologic functions, interplay with other molecules and clinical implications in cancer progression. Mol. cancer 18, 180 (2019).
Neubauer, S., Hublin, J. J. & Gunz, P. The evolution of modern human brain shape. Sci. Adv. 4, eaao5961 (2018).
Kozlenkov, A. et al. Evolution of regulatory signatures in primate cortical neurons at cell-type resolution. Proc. Natl. Acad. Sci. USA 117, 28422–28432 (2020).
Adegbola, A. A. et al. Monoallelic expression of the human FOXP2 speech gene. Proc. Natl. Acad. Sci. USA 112, 6848–6854 (2015).
Peng, H., Luo, Y. & Ying, Y. lncRNA XIST attenuates hypoxia-induced H9c2 cardiomyocyte injury by targeting the miR-122-5p/FOXP2 axis. Mol. Cell Probes 50, 101500 (2020).
Brownfoot, F. C. et al. Sulfasalazine reduces placental secretion of antiangiogenic factors, up-regulates the secretion of placental growth factor and rescues endothelial dysfunction. EBioMedicine 41, 636–648 (2019).
He, Q. et al. circ-SHKBP1 Regulates the Angiogenesis of U87 Glioma-Exposed Endothelial Cells through miR-544a/FOXP1 and miR-379/FOXP2 Pathways. Mol. Ther. Nucleic acids 10, 331–348 (2018).
Huda, S. S. et al. Visceral adipose tissue activated macrophage content and inflammatory adipokine secretion is higher in pre-eclampsia than in healthy pregnancy. Clin. Sci. (Lond., Engl. : 1979) 131, 1529–1540 (2017).
Shan, N. et al. The Role of Laminin α4 in Human Umbilical Vein Endothelial Cells and Pathological Mechanism of Preeclampsia. Reprod. Sci. (Thousand Oaks, Calif.) 22, 969–979 (2015).
Pérez-Roque, L. et al. Pregnancy-Induced High Plasma Levels of Soluble Endoglin in Mice Lead to Preeclampsia Symptoms and Placental Abnormalities. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22010165 (2020).
Devanna, P., Middelbeek, J. & Vernes, S. C. FOXP2 drives neuronal differentiation by interacting with retinoic acid signaling pathways. Front Cell Neurosci. 8, 305 (2014).
Ren, H. Y., Chen, B., Huang, G. L., Liu, Y. & Shen, D. Y. Upregulation of retinoic acid receptor-β reverses drug resistance in cholangiocarcinoma cells by enhancing susceptibility to apoptosis. Mol. Med. Rep. 14, 3602–3608 (2016).
Matellan, C. et al. Retinoic acid receptor β modulates mechanosensing and invasion in pancreatic cancer cells via myosin light chain 2. Oncogenesis 12, 23 (2023).
Roll, P. et al. Molecular networks implicated in speech-related disorders: FOXP2 regulates the SRPX2/uPAR complex. Hum. Mol. Genet. 19, 4848–4860 (2010).
Tanaka, K. et al. SRPX2 is overexpressed in gastric cancer and promotes cellular migration and adhesion. Int J. Cancer 124, 1072–1080 (2009).
Saums, M. K. et al. Combination Antiretroviral Therapy and Hypertensive Disorders of Pregnancy. Obstet. Gynecol. 134, 1205–1214 (2019).
Wallace, K. et al. Hypertension, Anxiety, and Blood-Brain Barrier Permeability Are Increased in Postpartum Severe Preeclampsia/Hemolysis, Elevated Liver Enzymes, and Low Platelet Count Syndrome Rats. Hypertension 72, 946–954 (2018).
Martinez-Fierro, M. L. et al. Fibroblast Growth Factor Type 2 (FGF2) Administration Attenuated the Clinical Manifestations of Preeclampsia in a Murine Model Induced by L-NAME. Front. Pharmacol. 12, 663044 (2021).
Li, Q. et al. Int6/eIF3e Silencing Promotes Placenta Angiogenesis in a Rat Model of Pre-eclampsia. Sci. Rep. 8, 8944 (2018).
Hung, T. H., Chen, S. F., Li, M. J., Yeh, Y. L. & Hsieh, T. T. Differential effects of concomitant use of vitamins C and E on trophoblast apoptosis and autophagy between normoxia and hypoxia-reoxygenation. PloS one 5, e12202 (2010).
Sagrillo-Fagundes, L., Assunção Salustiano, E. M., Ruano, R., Markus, R. P. & Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 65, e12520 (2018).
Lv, Z. et al. Role of GRK2 in Trophoblast Necroptosis and Spiral Artery Remodeling: Implications for Preeclampsia Pathogenesis. Front Cell Dev. Biol. 9, 694261 (2021).
Acknowledgements
This research was funded by the 345 Talent Project of Shengjing Hospital of China Medical University and the Science and Technology Projects of Liaoning Province, China (2021JH2/10300081).
Author information
Authors and Affiliations
Contributions
Sishi Liu: Conceptualization, methodology, investigation, and writing – original draft. Man Gao: Methodology, investigation, and writing – original draft. Xue Zhang: Formal analysis and visualization. Jun Wei: Formal analysis and visualization. Hong Cui: Conceptualization, supervision, and writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to publish
All authors of the manuscript agree to article publication.
Peer review
Peer review information
Communications Biology thanks Chidambra Halari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jesmond Dalli and Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, S., Gao, M., Zhang, X. et al. FOXP2 overexpression upregulates LAMA4 expression and thereby alleviates preeclampsia by regulating trophoblast behavior. Commun Biol 7, 1427 (2024). https://doi.org/10.1038/s42003-024-07149-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07149-7
This article is cited by
-
LAMA4, regulated by the transcription factor SP1 or the ubiquitin ligase NEDD4, mediates HUVEC dysfunction under hypoxia/reoxygenation conditions
Cytotechnology (2026)
-
Unraveling the oxidative stress landscape in diabetic foot ulcers: insights from bulk RNA and single-cell RNA sequencing data
Biology Direct (2025)