Abstract
Hypoxia is a common feature of glioblastoma (GBM). Circular RNAs (circRNAs) are identified as regulators in cancers. However, the role of circRNAs in GBM remains elusive. Here, circPLOD2a and circPLOD2b, spliced from the same parental gene PLOD2, are identified as hypoxia-responsive circRNAs. Overexpression of circPLOD2a and b enhance while knockdown inhibit GBM cell aggressiveness. The protein partners and downstream molecules were investigated by RNA-pulldown, mass spectrometry and RNA-seq. Mechanistically, HIF1α induces the expression of circPLOD2a and b, which competitively bind to HuR, causing a degradation of XIRP1 in vitro and in vivo. Clinical data demonstrate circPLOD2a and b are highly expressed in GBM negatively correlated with XIRP1, whose lower expression associates with higher glioma grade and worse prognosis. In conclusion, hypoxia-induced circPLOD2a and b are oncogenic regulators of tumour aggressiveness through attenuating the interaction between HuR and XIRP1 in glioblastoma cells and may be potential therapeutic targets for this disease.
Similar content being viewed by others
Introduction
Glioblastoma multiforme (GBM) is the highest grade of gliomas and one of the most malignant brain tumors in adults. Although great progresses have achieved in diagnose and therapy, the 5-year overall survival rate of GBM is still <35%1. One major difficulty of surgical removal is the propensity of GBM to infiltrate into adjacent normal brain tissues. Therefore, it is crucial to excavate mechanisms underlying the aggressiveness of GBM and explore new therapeutic targets for this disease. Hypoxia is a common microenvironment in solid tumors including GBM, which is caused by excessive cell proliferation and poorly organized tumor vasculature2,3. Hypoxia profoundly affects many signaling pathways to induce cellular adaptations4. HIF1α is considered as the core regulator under hypoxia which activates gene transcription and leads to a more aggressive and metastatic phenotype of cells5,6,7,8. Knock-down of HIF1α impairs cell invasion and migration in GBM cells9. However, previous studies have mainly focused on the coding RNAs regulated by hypoxia and HIF1α. The functions of non-coding RNAs, especially circRNAs under hypoxia has been poorly understood in GBM.
Circular RNA (circRNA) is a type of single-stringed RNA that comes from back-splicing of pre-mRNA with a covalently closed loop structure10,11. The conserved sequences and tumor-specific expression make circRNAs potentially valuable biomarkers for cancer diagnoses. Recent studies indicate that circRNAs are involved in the response to hypoxic microenvironment and the progression of cancers12. CircWSB1 is reported to be up-regulated by HIF1α and promotes breast cancer progression13. Another hypoxia-induced exosome circ133 increases cell invasion and migration by acting on miR-133a/GEF-H1/RhoA axis in colorectal cancer14. Dysregulated circRNAs are implicated in GBM as miRNA sponges, molecular partners of RBPs or coding sequences for short peptides15,16,17. Nevertheless, the biological function of circRNAs in response to hypoxia in GBM remains largely unknown.
Human antigen R (HuR), a member of the embryonic lethal abnormal visual protein (ELAV) family, acts as an RNA binding protein in cell cycle progression18. There are three RNA recognition motifs (RRMs) in HuR that bind to the poly-U elements or AU-rich elements (AREs) in the 3′-untranslated region (3′-UTR) of target genes19. Previous studies have revealed that HuR plays a vital role in tumor oncogenesis, hyperplasia, invasion and migration by regulating the stabilization of a large subset of target mRNAs20. For example, HuR stabilizes the mRNA of PTBP1 and promotes lung metastasis of colorectal cancer21. It was also reported that HuR is widely involved in circRNA-mediated signaling regulation22,23,24.
In this study, we identified two hypoxia-induced circRNAs in GBM, derived from the same parental gene PLOD2 and termed as circPLOD2a and circPLOD2b, which are regulated by HIF1α. CircPLOD2a/b promote the invasion and migration of GBM cells both in vivo and in vitro. We further identified HuR as the protein partner of circPLOD2a/b by RNA-pull down plus mass spectrometry. Mechanistically, circPLOD2a/b suppress the expression of XIRP1 mRNA through competitive binding with HuR. Our findings provide insights into the involvement of circRNAs in hypoxic response, and suggest that these two circRNAs may be potential biomarkers and therapeutic targets for GBM.
Results
CircPLOD2a/b are identified as hypoxia-responsive circRNAs in GBM cells
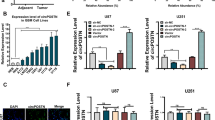
To identify circRNAs which are involved in responses to hypoxia in GBM cells, RNA-seq (GSE245635) was performed in U87 cells cultured under normoxia (21% O2) and hypoxia (1% O2) for 24 h. Data analysis showed that 17 circRNAs were significantly up-regulated under hypoxia with a cut-off criterion of |log2fold-change | > 1.0 and P-value < 0.05 (Fig. 1A, B). Divergent qRT-PCR primers cross the junction sites of these 17 circRNAs were designed in circBase (http://circbase.org/) to testify the RNA-seq data. Nine circRNAs were confirmed to be up-regulated in U87 under hypoxic condition (Fig. 1C). The basal expression levels of these 9 circRNAs in hypoxic U87 cells were showed in Fig. 1D. The existence of these circRNAs were further examined by RT-PCR and agarose-gel electrophoresis in U87, and the results showed that all these 9 circRNAs could be amplified with divergent primers in cDNA samples but not in gDNA samples (Fig. 1E and Supplementary Fig. 8A). Considering both the alteration and abundance of these circRNAs in U87 cells (Fig. 1C–E), hsa_circ_0141963 and hsa_circ_0067682 were chosen as top candidates for further investigation.
A Heatmap shows differentially expressed circRNAs between normoxia and hypoxia in U87 cells on a scale from blue (downregulated, n = 2158) to red (upregulated, n = 2566). B Volcano plot shows differently expressed circRNAs between normoxia and hypoxia in U87 cells, n = 4727. Horizontal dashed line labels P = 0.05. Vertical dashed lines label |log2fold-change | = 1. C Verification of 17 up-regulated circRNAs (P-value < 0.05 and reads counts >10) by qRT-PCR, n = 3. D Basal expression levels of 9 up-regulated circRNAs in U87 cells under hypoxia examined by qRT-PCR, n = 3. E Verification of corresponding circRNAs in U87 cells by RT-PCR using divergent and convergent primers. F Schematic illustration of the generation of circPLOD2a/b. G Junction sites of circPLOD2a/b were validated by Sanger sequencing. H Relative levels of circPLOD2a/b after treated with or without RNase R digesting determined by qRT-PCR in U87 cells, n = 3. I, J Relative expression levels of (I) circPLOD2a and (J) circPLOD2b under normoxia and hypoxia in GBM cell lines determined by qRT-PCR, n = 3. K Cellular localization of circPLOD2b examined by FISH with a junction specific antisense probe (red). Scale bar: 25 μm. L U87 cells were treated for 0 h, 12 h, 24 h, 48 h under 1% O2. Protein level of HIF1α and expression level of circPLOD2a/b were verified by western blot and qRT-PCR respectively, n = 3. M, N Protein level of HIF1α and expression level of circPLOD2a/b in (M) OE-HIF1α and (N) KD-HIF1α U87 cells under hypoxia, n = 3. Relative integrated density normalized to β-actin was marked above each band. Data are shown as mean ± SEM (error bars) and were analyzed using Student’s t-test (M) and ANOVA (C–N). NA, not available.
Referring to the position information in circBase, hsa_circ_0141963 and hsa_circ_0067682 were spliced from the exon 2-3 and exon 2–7 of the same parental gene PLOD2, respectively (Fig. 1F). Thus, we named hsa_circ_0141963 as circPLOD2a and hsa_circ_0067682 as circPLOD2b. The results of sanger sequencing showed continuous signals at the junction sites of both circPLOD2a and circPLOD2b (Fig. 1G). Besides, circPLOD2a/b could be amplified after RNase R digesting, while negative controls, linear PLOD2 and β-actin, showed a significant decrease (Fig. 1H). Furthermore, we examined the expression of circPLOD2a/b under hypoxia in other GBM cell lines except for U87. Both circPLOD2a and circPLOD2b could be significantly induced by hypoxia in all the tested GBM cell lines including DBTRG, LN229, T98G and U251 (Fig. 1I, J). RNA FISH assay was conducted to explore the cellular localization of circRNA2b in U87 cells. A FISH probe mix targeting circPLOD2b junction site were synthesized and incubated with U87 cells. The results showed that circPLOD2b mainly located in cytoplasm, and was significantly induced by hypoxic condition (1% O2, 48 h) (Fig. 1K), which was consistent with the results of qRT-PCR. We were not able to perform FISH on circPLOD2a due to lacking of specific probes.
Our findings suggest that circPLOD2a and circPLOD2b are hypoxia-induced circRNAs and probably involved in the responses to hypoxia in GBM cells.
CircPLOD2a/b are regulated by HIF1α under hypoxia
HIF1α is a key regulator of hypoxic responses. Previous studies showed that linear PLOD2 was regulated by HIF1α in hypoxic condition to promote tumor invasion and progression25,26,27. To investigate the regulation of HIF1α on circPLOD2a/b, we cultured U87 cells under hypoxic conditions for 12 h, 24 h, and 48 h. The expression of circPLOD2a/b along with HIF1α protein level, were both gradually increased in a time-dependent manner (Fig. 1L and Supplementary Fig. 8B). Then we established stable cell lines with HIF1α overexpression or knockdown in U87 and cultured the cells under hypoxic condition. The overexpression of HIF1α led to elevation of both circPLOD2a and circPLOD2b (Fig. 1M and Supplementary Fig. 8C). In contrast, circPLOD2a/b showed a significant decrease when HIF1α was knocked down (Fig. 1N and Supplementary Fig. 8D).
Linear PLOD2 has been reported to be upregulated in hypoxic cancer cells, including GBM25,28,29. Our data suggest that HIF1α enhances the transcription of the PLOD2 gene. Analysis using the JASPAR database (https://jaspar.elixir.no/) predicted 13 potential HIF1α binding sites within the 0–2000 bp promoter region of PLOD2, with a relative profile score threshold of 80%. Accordingly, dual-luciferase reporter assays demonstrated that transfection with the HIF1α overexpression plasmid significantly elevated the luciferase activity of the PLOD2 promoters (Supplementary Fig. 1C). These data demonstrated that the expression of circPLOD2a/b is notably regulated by HIF1α under hypoxia.
CircPLOD2a/b promote invasion and migration of GBM cells under hypoxia in vitro
To explore the functions of circPLOD2a/b in GBM cells, we inspected the effects of overexpression and knockdown of these two circRNAs on the proliferation, invasion and migration of GBM cells. U87 and LN229 cell lines, which possess relatively high expression levels of circPLOD2a/b under hypoxia (Fig. 1I, J; Supplementary Fig. 1A, B), were employed to establish stable cell lines of circPLOD2a/b knockdown and overexpression. CircPLOD2a/b were knocked down by shRNAs targeting the back-splicing region. The mRNA and protein levels of linear PLOD2 were not affected by the manipulations of circPLOD2a/b in GBM cells (Supplementary Fig. 2). The results of CCK-8 assay showed no significant differences in U87 and LN229 cells when circPLOD2a or circPLOD2b was overexpressed (Fig. 2E, F, M, N), indicating that circPLOD2a/b had no significant influence on the cell viability of GBM cells under hypoxia.
A, B Results of transwell assays in (A) U87 and (B) LN229 OE-circPLOD2a cells under hypoxia. Scale bar: 100 μm. C, D Quantification of transwell assays in (C) U87 and (D) LN229 OE-circPLOD2a cells, n = 3. E, F Results of CCK8 assays on (E) U87 and (F) LN229 OE-circPLOD2a cells under hypoxia, n = 5. G, H Representative pictures and quantification of wound-healing assays in (G) U87 and (H) LN229 OE-circPLOD2a cells under hypoxia. Scale bar: 250 μm, n = 5. I, J Results of transwell assay in (I) U87 and (J) LN229 OE-circPLOD2b cells under hypoxia. Scale bar: 100 μm. K, L Quantification of transwell assays in (K) U87 and (L) LN229 OE-circPLOD2b cells, n = 3. M, N Results of CCK8 assay on (M) U87 and (N) LN229 OE-circPLOD2b cells, n = 5. O, P Representative pictures and quantification of wound-healing assay (O) U87 and (P) LN229 OE-circPLOD2b cells under hypoxia, n = 5. Scale bar: 250 μm. Data are shown as mean ± SEM (error bars) and analyzed using Student’s t-test (C–P) and ANOVA (E–N).
Transwell and wound healing assays were conducted to evaluate the functions of circPLOD2a and circPLOD2b in GBM cell invasion and migration under hypoxia. When circPLOD2a (Fig. 2A–D, G, H) or circPLOD2b (Fig. 2I–L, O, P) was stably overexpressed in U87 and LN229 cells, the cell invasion and migration ability significantly increased under hypoxia. On the other side, when circPLOD2a (Fig. 3A–D) or circPLOD2b (Fig. 3E–H) was knocked down, the cell invasion and migration ability under hypoxia was significantly inhibited.
A, B Results of transwell assays in (A) U87 and (B) LN229 KD-circPLOD2a cells under hypoxia. Scale bar: 100 μm, n = 3. C, D Results of wound-healing assays in (C) U87 and (D) LN229 KD-circPLOD2a cells under hypoxia. Scale bar: 250 μm, n = 5. E, F Results of transwell assays in (E) U87 and (F) LN229 KD-circPLOD2b cells under hypoxia. Scale bar: 100 μm, n = 3. G, H Results of wound-healing assays in (G) U87 and (H) LN229 KD-circPLOD2b cells under hypoxia. Scale bar: 250 μm, n = 5. Data are shown as mean ± SEM (error bars) and analyzed using ANOVA. Scr: Scramble.
As a summary, our results demonstrate that circPLOD2a and circPLOD2b function to promote GBM cell invasion and migration under hypoxia in vitro.
CircPLOD2a/b directly interact with HuR
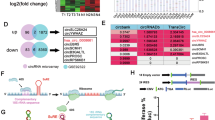
To explore the protein partners of circPLOD2a/b, RNA pull-down assays were performed with biotin-labeled probes targeting the back-splicing site of circPLOD2a (Fig. 4A, B) and circPLOD2b (Fig. 4C, D) in hypoxic U87 cells. Mass spectrometry identified 69 proteins which were pulled down by antisense probe targeting circPLOD2a and 53 proteins by probe targeting circPLOD2b, but not by the scramble probe. Crossing analysis with the established RBP database (http://rbpdb.ccbr.utoronto.ca) and the cancer metastasis-related RBPs revealed by previous studies30,31,32,33 determined five potential interacting partners of circPLOD2a or circPLOD2b (Fig. 4B, D). Further validation of RNA pull-down with circPLOD2a/b probes and corresponding antibodies confirmed the interaction of circPLOD2a/b with HuR (Fig. 4E, F, Supplementary Fig. 9A, B). RIP assay also disclosed the endogenous binding of circPLOD2a/b, but not linear PLOD2 with HuR (Fig. 4G–J and Supplementary Fig. 9C). FISH-IF assay revealed that circPLOD2b and HuR co-localize in the cytoplasm of U87 cells. A stronger binding was observed in U87 cells under hypoxic conditions compared to normoxic conditions (Fig. 4K).
A–D Proteins pulled down by biotin-labeled circPLOD2a (A) or circPLOD2b (C) antisense probes and corresponding scrambled control probes in hypoxic U87 cells were visualized by SDS-PAGE and silver staining. Unique peptides identified by mass spectrometry in (B) circPLOD2a (n = 73) or (D) circPLOD2b (n = 54) compared with corresponding control lanes. E, F Potential proteins interacting with (E) circPLOD2a and (F) circPLOD2b were verified by RNA pull-down assays with corresponding antisense probes in hypoxic U87 cells. Relative integrated density normalized to β-actin was marked above each band. G–J RIP assay confirms the interaction of circPLOD2a/b and linear PLOD2 with HuR. CircPLOD2a/b and linear PLOD2 were detected by RT-PCR (G). The immunoprecipitation products of anti-HuR compared with IgG were treated with or without RNase R. Quantitative enrichment analysis of circPLOD2a (H), circPLOD2b (I) and linear PLOD2 (J) were analyzed by qRT-PCR, n = 3. K Cellular localization of circPLOD2b (red) and HuR (green) were examined by dual RNA-FISH and IF in U87 cells treated with normoxic and hypoxic conditions. Scale bar: 25 μm. L Schematic diagram of wild type and various truncated HuR with Flag-tag. (M) The interaction of circPLOD2a/b with full-length or truncated HuR protein were detected by RIP assay in U87 cells. CircPLOD2a/b were detected by RT-PCR; full-length and truncated HuR proteins were detected by western blot using anti-Flag antibody. N CircPLOD2a and circPLOD2b were immunoprecipitated by anti-Flag beads in U87 cells transfected with plasmids carrying HuR- wildtype (HuR-wt) or truncated forms (HuR-△RRM1/2/3) and detected by qRT-PCR, n = 3. Data are shown as mean ± SEM (error bars) and analyzed using Student’s t-test (H–J) and ANOVA (N).
To probe the structural basis of the interactions between circPLOD2a/b and HuR, we constructed plasmids carrying Flag-tagged wild type HuR (HuR-wt) and its three truncated forms (HuR-△RRM1/2/3) containing different functional domains (Fig. 4L). RIP assay showed that RRM1 and RRM2 domains in HuR are essential for its interaction with circPLOD2a, while all three RRMs domains are involved in binding to circPLOD2b in hypoxic GBM cells (Fig. 4M, N and Supplementary Fig. 9D). The potential binding regions of circPLOD2a/b were predicted using the catRAPID database (http://service.tartaglialab.com/page/catrapid_group). The results indicate that the binding region of circPLOD2a to HuR is likely located between 26–80 nt. For circPLOD2b, multiple binding sites are predicted at 26–80 nt, 268–319 nt, and 418–644 nt (Supplementary Fig. 3A, B).
Taken together, our results suggest that circPLOD2a/b directly interact with HuR, which might explain the regulatory role of circPLOD2a/b in the invasion and migration of GBM cells under hypoxia.
CircPLOD2a/b promote cell invasion and migration by suppressing XIRP1 through interaction with HuR
The human RBP HuR is a conserved mRNA stability regulator34. To identify the downstream molecules of circPLOD2a/b/HuR regulatory axis, we analyzed the differentially expressed genes (DEGs) by performing RNA-seq (GSE245636) in KD-circPLOD2a U87 cells compared with sh-Scramble, and identified 57 up-regulated together with 114 down-regulated genes ( | log2fold-change | ≥ 1 and P < 0.05) (Fig. 5A, B). We examined the expression of 15 up-regulated genes in both U87 KD- and OE-circPLOD2a by qRT-PCR, and identified 4 candidate genes which were up-regulated in KD-circPLOD2a cells and meanwhile down-regulated in OE-circPLOD2a cells (Fig. 5C, D; Supplementary Fig. 4A, B).
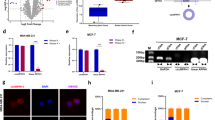
A Heatmap and (B) Volcano plot of DEGs in KD-circPLOD2a U87 cells compared to negative control cells under hypoxia. In total n = 57 up-regulated and n = 114 up-regulated genes were displayed. C, D Expression of 4 up-regulated genes in RNA-seq data in (C) KD-circPLOD2a and (D) OE-circPLOD2a U87 cells examined by qRT-PCR. E Expression of these 4 genes in KD-HuR cells. F, G Expression of XIRP1 in (F) KD-circPLOD2b and (G) OE-circPLOD2b cells. H, I Binding of (H) circPLOD2a and (I) XIRP1 to HuR were detected by RIP assay in U87 OE-circPLOD2a and control cells. J, K Binding of (J) circPLOD2b and (K) XIRP1 to HuR were detected by RIP assay in U87 OE-circPLOD2b and control cells. L, M The expression (L) mRNA and (M) protein level of XIRP1 were rescued by overexpression of HuR in OE-circPLOD2a and OE-circPLOD2b cells. Relative integrated density normalized to β-actin was marked above each band. N OE-HuR U87 and LN229 cells were treated with actinomycin D (5 μg/ml). The relative expression of XIRP1 was detected by qRT-PCR at different time points post actinomycin D treatment. O, P Transwell invasion assays illustrate that knockdown of XIRP1 in (O) KD-circPLOD2a or (P) KD-circPLOD2b U87 cells could rescue the inhibition of cell invasion and migration induced by knockdown of these two circRNAs under hypoxia. scale bar: 100 μm. (Q and R) Wound healing assays illustrate that knockdown of XIRP1 in (Q) KD-circPLOD2a or (R) KD-circPLOD2b U87 cells could rescue the inhibition of cell migration ability under hypoxia. Scale bar: 250 μm. n = 3 replicates unless otherwise noted. Data are shown as mean ± SEM (error bars) and analyzed using ANOVA. Scr: Scramble.
We constructed KD-HuR cell line in U87 (Supplementary Figs. 4C, 13), and further inspected the mRNA expression levels of candidate genes by qRT-PCR. XIRP1 and ANGPTL6 were found to be down-regulated, while GLRA2 was up-regulated in KD-HuR cells (Fig. 5E). Consistent with the regulatory effects of circPLOD2a on XIRP1 expression, XIRP1 was also up-regulated in KD-circPLOD2b cells and down-regulated in OE-circPLOD2b cells (Fig. 5F, G). Previous studies have implied XIRP1 as a potential tumor suppressor35, which is in tune with the promotion of GBM cell invasion and migration by circPLOD2a/b here. Thus, we chose XIRP1 for further investigation.
RIP assay was conducted in OE-circPLOD2a or OE-circPLOD2b U87 cells with anti-HuR antibody (Fig. 5H, J). There was an appreciable enrichment of XIRP1 by anti-HuR antibody pulldown compared with IgG control, indicating that XIRP1 can bind to HuR.
Hypoxia decreased XIRP1 mRNA enrichment in the RIP assay (Supplementary Fig. 4D), likely driven by the hypoxia-induced upregulation of circPLOD2a/b. Overexpression of circPLOD2a (Fig. 5I) or circPLOD2b (Fig. 5J) reduced XIRP1 mRNA enrichment in the RIP assay. Conversely, XIRP1 immunoprecipitation increased following the knockdown of circPLOD2a/b in U87 cells (Supplementary Fig. 4E–H). These findings suggest that circPLOD2a/b may function as protein sponges, binding to HuR to inhibit XIRP1.
To verify that circPLOD2a/b regulate the expression of XIRP1 through HuR, we overexpressed HuR in U87 OE-circPLOD2a or OE-circPLOD2b cells. The results showed that overexpression of HuR could effectively rescue the downregulation of XIRP1 induced by circPLOD2a/b (Fig. 5L, M and Supplementary Fig. 10).
We examined the stability of XIRP1 mRNA in OE-HuR cells followed by actinomycin D treatment, and found that the stability of XIRP1 was enhanced in U87 cells with HuR overexpression, compared with control cells (Fig. 5N). Similarly, knockdown of circPLOD2a and circPLOD2b improved the half-life of XIRP1 mRNA compared to control cells, indicated an inhibited degradation of XIRP1 caused by Actinomycin D treatment in U87 and LN229 under hypoxia. (Supplementary Fig. 5A–D). Therefore, HuR may stabilize XIRP1, which could be disrupted by competitive binding of circPLOD2a/b and consequently lead to the degradation of XIRP1.
The regulatory role of HuR on the translation of XIRP1 was investigated by sucrose gradient ribosome separation assay in U87 KD-HuR and control cells. The knockdown of HuR led to a significant decrease in XIRP1 mRNA within polysome association, indicating HuR enhance the translational efficiency of XIRP1 (Supplementary Fig. 5E–G).
We further explored whether the effect of circPLOD2a/b on GBM cell invasion and migration is dependent on the regulation of XIRP1. Knockdown of XIRP1 could effectively attenuate the inhibition of cell invasion and migration induced by knockdown of circPLOD2a/b in hypoxic U87 cells in transwell assay (Fig. 5O, P). Wound-healing assay further confirmed this hypothesis as knockdown of XIRP1 in KD-circPLOD2a or KD-circPLOD2b U87 cells could rescue the inhibition of cell migration ability under hypoxia (Fig. 5Q, R).
Therefore, we demonstrate that circPLOD2a/b suppress XIRP1 through competitively binding to HuR to interrupt the interaction of HuR and XIRP1 mRNA, thus promoting invasion and migration of GBM cells under hypoxia.
CircPLOD2a/b promote the progression of GBM xenografts in mice by suppressing XIRP1
We take a close look at the function of circPLOD2a/b-HuR-XIRP1 regulatory axis in GBM progression in vivo using cell line-derived xenograft (CDX) model in mice. Four-week-old BALB/c nude mice were randomly allocated into five groups (control-luc, KD-circPLOD2a-luc, KD-circPLOD2a/KD-XIRP1-luc, KD-circPLOD2b-luc, KD-circPLOD2b/KD-XIRP1-luc). Mice from each group were intracranially transplanted with corresponding genetically modified U87-luc cells (Fig. 6A). Decreased bioluminescence was detected in KD-circPLOD2a and KD-circPLOD2b groups compared with the control group, indicating that knockdown of circPLOD2a/b inhibits the expansion of GBM cells in vivo. This phenotype could be rescued by XIRP1 knockdown as disclosed by the increased bioluminescence in KD-circPLOD2a/KD-XIRP1 and KD-circPLOD2b/KD-XIRP1 groups compared with KD-circPLOD2a and KD-circPLOD2b groups, respectively (Fig. 6A, B). Body weights of tumor-bearing mice from all groups were stable during trial period (Supplementary Fig. 6).
A, B (A) Representative bioluminescence images and (B) tumor growth curve of tumor-bearing mice intracranially transplanted with corresponding genetically modified U87 cells labeled with luciferase (n = 5–6/ per group). C Relative expression of XIRP1 in CDX tumors, n = 3. D Representative images of IHC staining of Vimentin, E-cadherin and N-cadherin in CDX tumors. Scale bar: 100μm. E, F Relative protein level of Vimentin, E-cadherin, N-cadherin, HuR and XIRP1 in different genetically-modified (E) U87 cells in vitro and in (F) CDX tumors in vivo. Relative integrated density normalized to β-actin was marked above each band. Data are shown as mean ± SEM (error bars) and analyzed ANOVA. Scr: Scramble.
Mice were then sacrificed at 52 days post transplantation, and the tumor tissues were collected for further analysis. Epithelial-mesenchymal transition (EMT) is a process that epithelial cells lose their cell-cell adhesion and obtain mesenchymal features, which is critical for tumor metastasis. Thus, we examined the expression of EMT markers in the CDX tumors by western-blot and IHC staining. The results showed that tumors derived from KD-circPLOD2a/b group had higher level of E-cadherin and lower level of Vimentin and N-cadherin (Fig. 6D), suggesting that knockdown of these two circRNAs could inhibit the EMT process of GBM cells in vivo. Consistent with the results of CDX tumor expansion and the in vitro experiments, the effects of KD-circPLOD2a/b on EMT marker expression could be effectively inhibited by knockdown of XIRP1. Besides, knockdown of circPLOD2a/b had no effect on the protein level of HuR (Fig. 6E, F, Supplementary Fig. 11A, B).
Taken together, our results demonstrate that circPLOD2a/b inhibit the expansion of GBM xenografts by suppressing XIRP1 in vivo.
CircPLOD2a/b are highly expressed in GBM tissues and XIRP1 is negatively correlated with glioma grade and prognosis
To clarify the expression and clinical significance of circPLOD2a/b, qRT-PCR was conducted to detect the expression of circPLOD2a/b and XIRP1 in 19 clinical specimens containing grade II-III (n = 10) and IV (n = 9) glioma. The results showed a remarkable enrichment of circPLOD2a/b in GBM compared with lower grade glioma (Fig. 7A, B), and the expression of downstream gene XIRP1 was suppressed in circPLOD2a/b-high group (Fig. 7C, D). These findings suggest that circPLOD2a/b are highly expressed in GBM and negatively correlated with the expression of XIRP1.
A, B Relative expression of (A) circPLOD2a and (B) circPLOD2b were examined by qRT-PCR in clinical samples from patients with grade II-III (n = 10) and grade IV (n = 9) glioma. C Relative expression of XIRP1 in tissues from circPLOD2a-high and -low groups. The GBM tissues were divided into circPLOD2a-high (n = 10) and -low (n = 9) groups according to the expression of circPLOD2a. D Relative expression of XIRP1 in tissues from circPLOD2b-high and -low groups. The GBM tissues were divided into circPLOD2a-high (n = 10) and -low (n = 9) groups according to the expression of circPLOD2b. E Relative expression of XIRP1 in GBM and non-tumor brain tissues were analyzed from Rembrandt database. F Representative immunohistochemistry images of XIRP1 in a tissue microarray containing grade I (n = 3), II (n = 50), III (n = 24) and IV (n = 94) glioma. Scale bar: 100 μm. G, H Expression levels of XIRP1 in tissues from patients with different grade glioma were evaluated by (G) the percentage of cells with strong/moderate/weak intensity of XIRP1 staining and (H) histochemistry score (H-score) of each tissue on this microarray. I Kaplan–Meier survival curves of glioma patients in XIRP1-high and low groups. Glioma patients were categorized into XIRP1-high and -low group by the expression level of XIRP1 based on H-score. J Schematic illustration of HIF1α/circPLOD2a/b/HuR/XIRP1 axis. HIF1α induces the up-regulation of linear PLOD2, circPLOD2a and circPLOD2b under hypoxia. CircPLOD2a/b suppress XIRP1 by competitively binding to HuR and enhance GBM cell aggressiveness. Data are shown as mean ± SEM (error bars) and analyzed using Student’s t-test (A–E), ANOVA (H) and Log-rank test (I).
We further explored the expression and clinical significance of XIRP1. The expression of XIRP1 was lower in GBM than non-tumor tissues according the data from Rembrandt database (Fig. 7E)36. Immunohistochemical analysis of XIRP1 was performed in tissue microarray which contains grade I (n = 3), II (n = 50), III (n = 24) and IV (n = 94) glioma patient tissues. Histochemistry score (H score) was calculated to evaluate the expression level of XIRP1 in each tissue. The results showed that XIRP1 decreased with the increase of glioma grade, and the expression level of XIRP1 was significantly lower in grade IV than grade II (Fig. 7F–H). Survival analysis of IHC data of tissue microarray indicated that patients with low level of XIRP1 had worse overall survival than those with high XIRP1 level (Fig. 7I).
As a summary, circPLOD2a/b are highly expressed in GBM, while its downstream gene XIRP1 was suppressed and low expression of XIRP1 is correlated with higher glioma grade and worse prognosis (Fig. 7J).
Discussion
To gain essential elements such as oxygen and glucose, tumors tend to metastasize under hypoxic microenvironment. A growing number of circRNAs have been implicated in the regulation of cell proliferation, transformation and metastasis of malignant tumors10. Brain tumors such as GBM usually exist in a low-oxygen environment as human brain has lower oxygen concentration than other organs. However, the role of circRNAs responding to hypoxia in brain tumors is still unclear. In order to disclose the circRNAs that are involved in hypoxic responses in GBM, we screened circRNAs induced by hypoxia in U87 cells by RNA sequencing. Here, we identified two hypoxia-induced circRNAs, which were significantly up-regulated by HIF1α and could promote GBM cell invasion and migration under hypoxia both in vitro and in vivo. These two circRNAs, circPLOD2a and circPLOD2b, were derived from the same parental gene PLOD2. Mechanically, circPLOD2a/b directly bind to HuR and inhibit its interaction with XIRP1 mRNA, leading to downregulation of XIRP1. Therefore, our findings have revealed the oncogenic role of hypoxia-induced circPLOD2a/b in GBM cells under hypoxia, and uncovered the underlying mechanisms.
Recent studied revealed that circRNAs have various biological functions, including miRNA sponges37, binding with protein partners16, modulating the expression of parental genes38 and serving as protein-coding sequences for short peptides39. To figure out whether circPLOD2a/b regulate the aggressiveness of GBM through other mechanisms besides competing with XIRP1 mRNA to interact with HuR, we analyzed the sequences of circPLOD2a/b in circRNADb database (http://reprod.njmu.edu.cn/cgi-bin/circrnadb/circRNADb.php)40, which showed that the possibilities of protein coding for these two circRNAs are relatively low (R < 1.6). Besides, circRNAs act as miRNA sponge are widely reported, in which AGO2 is essential for miRNA recruitment41. In our results of pull-down mass spectrometry, AGO2 was not detected, which implies that circPLOD2a/b probably does not act as miRNA sponges.
The proliferation and metastasis are hallmarks of cancer progression, which are modulated by various signaling pathways such as PI3K/AKT/mTOR and TGF-beta42,43. CircRNAs participate in the regulation of tumor cell metastasis. It was reported that circRHOBTB3 and circEXOC6B repress tumor cell metastasis by decreasing epithelial-mesenchymal transition (EMT) in colorectal and prostate cancer respectively21,44. Under hypoxic microenvironment, tumor cells tend to restrain the cell proliferation and develop more aggressive phenotype45. In this process, HIF1α plays an important role to enhance EMT, leading to cell migration and invasion46. Consistent with the above theory of hypoxia inducing metastasis, circPLOD2a/b promote cell invasion and migration while had no significant influence on the proliferation of GBM cells under hypoxia.
In this study, we observed that the circular isoforms of PLOD2 (circPLOD2a and circPLOD2b) were significantly upregulated following HIF1α overexpression and correspondingly downregulated upon HIF1α knockdown in U87 cells. Additionally, our luciferase reporter assay confirmed that HIF1α significantly enhances PLOD2 promoter activity, providing strong evidence that HIF1α transcriptionally regulates the expression of circPLOD2a/b under hypoxic conditions. However, the exact mechanism linking HIF1α-mediated transcription of PLOD2 and its circular isoform generation, such as whether the circularization occurs spontaneously or concurrently with transcription, remains to be fully elucidated. Investigating the alternative splicing or back-splicing events associated with circPLOD2 formation following HIF1α expression modulation is one of the key directions for future studies.
It was reported that PLOD2, the parental gene of circPLOD2a/b, promotes GBM cell proliferation and invasion under hypoxia by regulating downstream mRNA such as MT1-MMP, CD44, CD99, and MMP226. Previous studies reported that the expression of parental genes could be modulated by corresponding circRNAs in some cases. For example, CircSEP3 binds to its cognate DNA locus and pause the transcriptional of SEP3 by forming R-loops47. Nuclei-localized circRNA ci-ankrd52 suppresses host gene ANKRD52 through RNase H138. Thus, it is possible that circPLOD2a/b could also promote cell metastasis by regulating its parental gene PLOD2. However, both the mRNA and protein levels of PLOD2 were not altered when circPLOD2a/b was knocked down or overexpressed (Supplementary Figs. 2, 12), which indicates that circPLOD2a/b may not modulate the expression of its parental gene. Besides, we found that overexpression of circPLOD2a or circPLOD2b have no effect on the expression levels of each other (Supplementary Fig. 7). Considering the fact that circPLOD2a and circPLOD2b directly bind to the same RNA binding protein HuR, the potential synergistic effects of circPLOD2a/b could be further explored. Taken together, our model is that both the linear transcript of PLOD248, and its circular forms, circPLOD2a/b are up-regulated by HIF1α in GBM cells under hypoxic microenvironment to promote invasion and migration, while their expression are not affected by each other.
HuR, a vital RNA binding protein in tumor progression and metastasis, has post-transcriptional influence on target RNAs49. It was reported that HuR promotes tumor invasion by interacting with the 3’ UTR and stabilizing coding mRNA such as snail24 and MMP950. Here, we identified HuR as the protein partner of circPLOD2a/b in the responses to hypoxia, but its expression is not regulated by circPLOD2a/b (Fig. 6E, F). Our results showed that HuR could positively regulate the expression of XIRP1 through directly binding with the mRNA of this gene. Therefore, HuR may stabilize XIRP1 mRNA, which is disrupted by competitive binding by circPLOD2a/b and consequently lead to the degradation of XIRP1 mRNA. Previous studies have shown that HuR plays an oncogenic role under hypoxic conditions by regulating gene expression at the post-transcriptional level51. However, as a multi-target regulator, HuR is involved in the post-transcriptional regulation of various cancer-associated genes, with some promoting GBM aggressiveness52,53 and others inhibiting its progression54,55,56,57. Given the complex cellular functions of HuR, targeting the circPLOD2a/b–HuR–XIRP1 axis for GBM treatment should focus on inhibiting circPLOD2a/b expression or disrupting the interaction between XIRP1 and HuR, rather than directly downregulating HuR, to minimize potential side effects from other downstream targets.
To summarize, our study identifies two hypoxia-response circRNAs, circPLOD2a and circPLOD2b, in GBM cell lines, which could be induced by HIF1α and directly interact with HuR to inhibit the mRNA level of XIRP1, thus promoting the aggressiveness of GBM cells both in vitro and in vivo. As a result, our study provides therapeutic targets for GBM.
Methods
Cell culture
Human GBM cell lines (DBTRG, LN229, T98G, U251 and U87) and HEK 293 T were purchased from American Type Culture Collection (ATCC). All cells were maintained in a humidified incubator at 37 °C with 5% CO2 and cultured in high glucose DMEM medium, supplemented with 10% fetal bovine serum, 100 mg/mL streptomycin and 100 U/mL penicillin. For the induction of hypoxia, cells were cultured in a tri-gas chamber (GeneScience E500, USA) with 1% O2, 94% N2 and 5% CO2 for 24 h if not indicated.
RNA isolation, RNA sequencing, RNase R treatment and qRT-PCR
Total RNA was extracted using TRIZOL reagent (Solarbio, China) and quantified by Nanodrop2000 spectrophotometer (Thermo Fisher Scientific, USA). RNA-seq was conducted using Illumina Hiseq by Novogene Co., Ltd (China). For RNase R treatment, 1ug of total RNA was treated with 0.05uL RNase R (Lucigen Middlesex, UK) for 10 min at 37 °C. For qRT-PCR, the total RNA was synthesized into cDNA with PrimeScript RT Reagent Kit (Takara, China) in accordance with the manufacturer’s protocols. The cDNA was amplified with 2x M5 Ultra SYBR Mixture (Mei5bio, China) on a Bio-Rad CFX96 system (Bio-Rad, USA). The expression of circRNAs and mRNAs were analyzed by 2–ΔΔCT, and normalized against β-actin. The primers were purchased from GeneCreate (China), and the sequences are detailed in Supplementary Table 1.
Plasmids and cell transfection
For overexpression of circRNAs (OE-circPLOD2a/b), linear sequences of circPLOD2a/b were synthesized by GeneCreate (Wuhan, China) and subcloned into pLCDH-ciR vector. For HIF1α overexpression (OE-HIF1α), HIF1α coding sequence was obtained from HA-HIF1α-pcDNA3, which was purchased from Addgene (plasmid # 18949), and subcloned into pLenti-IRES-puro. 3xFlag-tagged HuR protein was synthesized by GeneCreate (Wuhan, China) and subcloned into pLVX-IRES-neo vector. 3xFlag-tagged HuR RRM domain-truncated plasmids were amplified by overlapping PCR from pLVX-3xFlag-HuR-neo plasmid and cloned into pLVX-IRES-neo vector.
Short-harpin RNA (shRNA) was used to knockdown gene expression. For knockdown of circRNA, shRNA targeting sites were designed at circinteractome (https://circinteractome.nia.nih.gov/). For knockdown of HIF1α, HuR and XIRP1, shRNAs were designed at sigma Aldrich analyses (https://www.sigmaaldrich.cn/CN/zh/semi-configurators/shrna?activeLink=product Search). Corresponding oligonucleotides targeting circPLOD2a/b and HIF1α were annealed and ligated into pGreen-puro vector, while shRNAs targeting HuR and XIRP1 were cloned into pLKO.1-neo vector. Empty vector and scrambled sequence were used as negative controls for overexpression and shRNAs respectively.
To establish luciferase-labeled cells, a plasmid expressing luciferase and the BSD gene was transfected into U87 cells, followed by selection with Blasticidin S HCl.
All the primers were purchased from GeneCreate (China), and the sequences are detailed in Supplementary Table 1 and Supplementary Table 2. The expression plasmids, packaging plasmid containing gag-pol and envelope plasmids containing VSVG were co-transfected into HEK293T cells by using Lipofectamine 3000 (Invitrogen, USA) following the manufacturer’s instructions. Lentivirus were collected after 72 h and used for transfection in U87 or LN229 cells. Puromycin or neomycin (G418) was used to select the stable cell lines according to the antibiotic resistance genes carried by the plasmids.
Western blotting
GBM cells were collected and lysed in RIPA lysis buffer (Beyotime, China). The protein concentrations were determined using a BCA Protein Assay Kit (Beyotime, China). Proteins were separated by 8–12% SDS-PAGE and then transferred to PVDF membranes (Millipore, Schwalbach, Germany). The membrane was blocked with PBST buffer containing 5% skim milk and incubated with the corresponding primary antibodies at 4 °C overnight. Then, the membrane was washed and hybridized with an HRP-conjugated secondary antibody at room temperature for 2 h. The signals were detected using ChemiDoc XRS+ (Bio-Rad, USA).
β-actin was used as a protein internal control due to its independence from hypoxia and HuR regulation. The primary antibodies used for western blotting were anti-β-actin antibody (66009-1-Ig, Proteintech, 1:2000), anti-HIF1α antibody (ab179483, Abcam, 1:1000), anti-PLOD2 antibody (66342-1-Ig, Proteintech, 1:2000), anti-ALDA antibody (11217-1-AP, Proteintech, 1:2000), anti-BIP antibody (11587-1-AP, Proteintech, 1:2000), anti-DDX5 antibody(67025-1-Ig, Proteintech, 1:2000), anti-HuR antibody (11910-1-AP, Proteintech, 1:2000), anti-IGF2BP3 antibody (14642-1-AP, Proteintech, 1:2000), anti-E-cadherin antibody (20874-1-AP, Proteintech, 1:1000), anti-N-cadherin antibody (22018-1-AP, Proteintech, 1:1000), anti-Vimentin antibody (10366-1-AP, Proteintech, 1:1000), anti-Flag antibody (20543-1-AP, Proteintech, 1:2000), anti-XIRP1 antibody(PA5-48605, Invitrogen, 1:1000), HRP Goat anti-Rabbit antibody (AS014, Abclonal, 1:5000), and HRP Goat anti-Mouse antibody (SA00001-1, Proteintech, 1:5000).
Cell viability analysis
Cell viability was detected by Cell Counting Kit 8 assays (GLPBIO, USA). 4 * 103 cells per well were plated into 96-well plate, and incubated in normoxic condition for 8 h. Then the plates were transferred into hypoxic chamber and treated for 24 h or 48 h. Finally, CCK8 reagent was added to cell medium for absorbance detection according to the manufacturer’s guidelines.
Transwell assay
Transwell chambers were used in migration assays and chambers precoated with 10% Matrigel (Corning, USA) were used in invasion assays. For each replicate, 1 * 104 cells were plated in 200 μL of serum-free medium on upper chambers which were inserted into a 24-well plate, and 500 μL of medium containing 10% FBS was added to the bottom chamber. After incubation for 24 h under hypoxia, upper chamber cells were gently removed, and the invaded cells in the lower filters were fixed with 4% polymethanol for 10 min, followed by staining with crystal violet for 10 min. The experiments were performed in triplicate and more than five photos of each replicate were taken by Leica DMi8 microscope (Leica, Germany). Cell counting was conducted using ImageJ (version 1.53q, USA).
Wound-healing assay
For wound-healing assay, cells were seeded in 6-well plate at a concentration of 4 * 105/well. After 24 h, 10 μL pipet tip was used to create a wound scratch. Cells were cultured in 1% O2 hypoxic condition for 24 h with serum-free medium. The migration of cells at the wound site was measure by taking images of the wound site at 0 h and 24 h post the wound scratch. ImageJ (version 1.53q, USA) was used to analyze the percentage of wound closure. The experiments were performed in triplicate and >5 photos of each replicate were taken by Leica DMi8 microscope (Leica, Germany).
Fluorescence in situ hybridization (FISH) and Immunofluorescence staining (IF)
Fluorescence in situ hybridization (FISH) was performed as the manufacturer’s guidelines of Fluorescent in Situ Hybridization Kit (RiboBio, China). Briefly, 2 * 104 of U87 cells were seeded on a cover clip with a diameter of 14 mm in 24-well plate and treated in normoxia or hypoxia condition. 48 h after treatment, cells were fixed by 4% polymethanol and then incubated with biotin-labeled circPLOD2b probe, which was designed and synthesized by RiboBio (Guangzhou, China). Product number for the specific FISH probe is lnc1100936 RiboTM h-hsa_circ_0067682_FISH Probe Mix (Red, 20 T), and the ordering URL is: https://www.ribobio.com/product-and-service/gene-ncrna-mechanism/fish-probes/. Nuclei were stained by DAPI. For dual RNA-FISH and immunofluorescent staining, immunofluorescence staining (IF) was conducted after FISH. Briefly, after performing RNA-FISH, the slides were blocked with 2% BSA and incubated with HuR antibody at a dilution of 1:200 (11910-1-AP, Proteintech), followed by incubation with 488 goat anti-rabbit IgG (H + L) (A-11008, Invitrogen, 1:1000). Images were acquired using Andor Revolution WD spinning disc confocal microscope (Oxford Instruments, England).
RNA pull-down and mass spectrometry
RNA pull-down assay was carried out with Pierce™ Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific, USA) following the manufacturer’s instructions. The biotin-labeled probes targeting the junction site of circPLOD2a and circPLOD2b were synthesized by Genecreate (China) and the sequences are detailed in Supplementary Table 3. Briefly, streptavidin magnetic beads were incubated with circPLOD2a-specific or circPLOD2b-specific probes, and then incubated with U87 cell lysate. The product was separated by SDS PAGE. Silver staining was performed to observe the differences between the proteins pulled down by scrambled control and circPLOD2a/b probes. Each lane of gel was cut and analyzed by mass spectrometry at Genecreate (China). The proteins pulled down were further verified by western blot with anti-ALDA antibody (11217-1-AP, Proteintech, 1:2000), anti-BIP antibody (11587-1-AP, Proteintech, 1:2000), anti-DDX5 antibody (67025-1-Ig, Proteintech, 1:2000), anti-HuR antibody (11910-1-AP, Proteintech, 1:2000) and anti-IGF2BP3 antibody (14642-1-AP, Proteintech, 1:2000).
RNA immunoprecipitation (RIP)
RNA immunoprecipitation assay was performed as previously described58. Briefly, U87 cells were crosslinked with 1% formaldehyde. Cell lysates were collected in RIPA buffer and sonicated using the Bioruptor Plus (Diagenode, Belgium) at 4 °C in high-power mode for 8 cycles (30 s ON, 30 s OFF). The lysates were then centrifuged at 12,000 rpm at 4 °C for 10 min, and the supernatant was diluted with lysis buffer and incubated with antibodies specific for HuR (11910-1-AP, Proteintech, 1:100) or IgG control (30000-0-AP, Proteintech, 1:500) with 100 unites of RNase inhibitor, followed by rotating for 10-14 h at 4 °C. Mix of Dynabeads protein A (10001D, Invitrogen, USA) and Dynabeads protein G (10003D, Invitrogen, USA) were used to immunoprecipitated protein and associate RNA. The RNA-protein-beads complex and input sample were digested by 500 μg/mL protein kinase K at 55 °C for 1 h, followed by retro-crosslinking with 100 mM Tris-HCl (pH = 7.4), 1% SDS and 200 mM NaCl at 70 °C for 10-12 h. The co-precipitated RNAs were extracted by TRIZOL and detected by qRT-PCR. Immunoprecipitated proteins were boiled at 99 °C for 10 min and separated by western blot. For circPLOD2a/b and HuR mapping assays, plasmids carrying Flag-tagged HuR (wt/△RRM1/2/3) were transiently transfected into U87 using Lipofectamine 3000 (Invitrogen, USA). Anti-Flag Magnetic Beads (Beyotime, China) was used for RNA immunoprecipitation. Anti-Flag antibody (20543-1-AP, Proteintech, 1:200) was used to detect the immunoprecipitated protein.
GBM xenograft mouse model
The animal study was performed after receiving approval from the Institutional Animal Care and Use Committee of Hubei University of Medicine (approval number: 2023008).
Four-week-old male BALB/c nude mice were purchased from Beijing Vitalstar Biotechnology Co. Ltd. (China) and housed under the standard conditions at the Center of Experimental Animals of Hubei University of Medicine. Mice were randomly allocated into different groups for luciferase-labeled (-luc) U87 cell injection (control-luc, KD-circPLOD2a-luc, KD-circPLOD2a/KD-XIRP1-luc, KD-circPLOD2b-luc, KD-circPLOD2b/KD-XIRP1-luc). For the intracranial xenografts, mice were anesthetized and placed in a stereotact, and 5 * 105 U87 cells labeled with luciferase were intracranially injected at 2 mm lateral and 1 mm anterior to bregma, 2 mm below the skull into the brain of randomly grouped nude mice. To image the tumor xenografts, the mice were anesthetized using isoflurane and intraperitoneally injected with 150 mg/kg D-luciferin (Beyotime, China) once a week starting at 10 days after the cell injection. Tumor cells labeled by luminescence were imaged using a spectrum in vivo imaging system (IVIS) (PerkinElmer, USA). Fifty-two days post injection, tumor-bearing mice were humanely sacrificed and brains were harvested and fixed with phosphate-buffered formalin for further immunohistochemistry (IHC) staining.
We have complied with all relevant ethical regulations for animal use.
Immunohistochemistry staining
Mice were sacrificed at 52 days post injection and immediately perfused with normal saline and 4% paraformaldehyde solution continuously. The brains were dissected, postfixed for 24 h at 4 °C, and sectioned with a thickness of 10 μm using Leica cryostat microtome (Leica CM1860, Germany). For immunohistochemistry, the slices were blocked in 2% BSA and then incubated with anti-E-cadherin antibody (20874-1-AP, Proteintech, 1:100), anti-N-cadherin antibody (22018-1-AP, Proteintech, 1:100) and anti-Vimentin antibody (10366-1-AP, Proteintech, 1:100). HRP Goat anti-Rabbit antibody (AS014, Abclonal, 1:100) was used as the secondary antibody. DAB Horseradish Peroxidase Color Development Kit (Beyotime, China) was used as chromogenic reagent. Nuclei were stained by hematoxylin. More than five photos of each group were taken by Leica DMi8 microscope (Leica, Germany).
Human tissue samples
Tumor tissues from patients with grade II-III (n = 10) and IV (n = 9) glioma used for qRT-PCR were collected from Taihe Hospital, Hubei, China. Informed consents were obtained from glioma patients for sample collection, and the study was approved by the Ethics Committee of Shiyan Taihe Hospital (approval number: 2023KS11). All tissues were preserved in liquid nitrogen immediately after the surgery at Taihe Hospital, Hubei, China. Patient information is shown in Supplementary Table 4.
A tissue microarray (TMA) containing grade I (n = 3), II (n = 50), III (n = 24) and IV (n = 94) glioma tissues was purchased from Zhuoli Biotechnology (ZL-BraGsur1801, Zhuoli Biotechnology Co., Shanghai, China). The patient information with tumor grade and survival time is shown in Supplementary Tables 5, 6 respectively.
IHC staining of tissue microarray was performed to evaluate the expression level of XIRP1 in these patients. Generally, TMA was incubated with anti-XIRP1 antibody (PA5-48605, Invitrogen, 1:10) for immunohistochemistry staining. By using the Arrayimager software module from Visiopharm (Hoersholm, Denmark), individual digital images of each core was automatically extracted from the full slide images of the TMA. Intensity of staining (weak/ moderate/ strong) was measured to evaluated the expression of XIRP1 in each cell of these tissues. Histochemistry score (H-score) was calculated to represent the expression level of XIRP1 for each tissue in microarray. [H-Score = ∑ (pi×i) = (percentage of weak intensity×1) + (percentage of moderate intensity×2) + (percentage of strong intensity × 3)].
All ethical regulations relevant to human research participants were followed.
Dual-luciferase reporter assay
Human PLOD2 promoter from NC_000003.12:c146163185-146161185 were amplified from 293 T gDNA and subcloned into pGL3-basic to construct luciferase reporter vectors. Cells expressing HIF1α were co-transfected with pGL3-basic empty vector or pGL3-basic-PLOD2 reporter constructs and renilla luciferase plasmids. The luciferase activity of the reporters was detected after 48 h post transfection with Dual-Lumi II Luciferase Assay Kit (Beyotime, China) and normalized to renilla luciferase activity.
Sucrose gradient ribosome separation assay
Ribosomes from U87 KD-HuR and control cells were separated and analyzed following previously established protocols59,60. Briefly, a sucrose gradient solution was prepared in ultracentrifuge tubes with layers at concentrations of 10%, 20%, 30%, 40%, and 50%, containing 100 mM NaCl, 20 mM Tris-HCl (pH 7.5), and 5 mM MgCl2. Cells were incubated with 100 μg/mL cycloheximide (GC17198, GLPBIO, USA) for 10 min and washed by pre-cold PBS also contain 100 μg/mL cycloheximide. Cell lysates were prepared in 450 μL of PEB buffer (20 mM Tris-HCl (pH 7.5), 100 mM KCl, 5 mM MgCl2, 0.5% Nonidet P-40, 100 μg/mL cycloheximide, protease inhibitors, and 100 units of RNase inhibitor). 120 μg of RNA was determined by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and layered on top of the sucrose gradient solution. The mixture was centrifuged for 120 min at 190,000 g and 4 °C in a SW41Ti swinging-bucket rotor using a Beckman ultracentrifuge, with maximum acceleration and brake applied. Polysome profiling was analyzed using a piston gradient fractionator (153-002, BioComp, Canda). Ten polysome fractions were sequentially collected from the top to the bottom of the gradient. Total RNA was extracted using TRIzol, and qRT-PCR was performed to measure the expression of HuR and XIRP1 in each fraction. β-actin served as the internal control.
Statistics and reproducibility
Data are shown as the mean ± SEM (error bars) of at least three independent experiments. Statistical analyses were performed with GraphPad Prism 9.0 (GraphPad Prism, USA). Student’s t-test or analysis of variance (ANOVA) were applied in the comparison of differences between groups. Survival curves were plotted by Kaplan–Meier method and assessed by log-rank test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA-seq data were deposited in GEO accession GSE245635, GSE245636 and GSE226538. Other data that support the findings of this study are available from the corresponding author upon reasonable request. The source data behind the graphs in the paper can be found in Supplementary Data 1. The uncropped images of western blots can be found in Supplementary Information. Newly generated plasmids production and their reference sequences have been deposited in Addgene (pLKO.1-neo-shHuR, ID: 232044; pLCDH-circPLOD2a, ID: 232045; pLCDH-circPLOD2b, ID: 232046; pGreen-puro-circPLOD2a-sh1#, ID: 232047; pGreen-puro-circPLOD2a-sh2#, ID: 232048; pGreen-puro-circPLOD2b-sh1#, ID: 232049; pGreen-puro-circPLOD2b-sh2#, ID: 232050; pLKO.1-neo-shXIRP1, ID: 232051; pGreen-puro-HIF1α-sh1#, ID: 232052; pGL3-Basic-PLOD2, ID: 232053; pGreen-puro-HIF1α-sh2#, ID: 232054).
References
Lapointe, S., Perry, A. & Butowski, N. A. Primary brain tumours in adults. Lancet 392, 432–446 (2018).
Ortiz-Prado, E., Dunn, J. F., Vasconez, J., Castillo, D. & Viscor, G. Partial pressure of oxygen in the human body: a general review. Am. J. Blood Res. 9, 1–14 (2019).
Muz, B., de la Puente, P., Azab, F. & Azab, A. K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 3, 83–92 (2015).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1 alpha., and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Peng, G. et al. The HIF1 alpha-PDGFD-PDGFR alpha axis controls glioblastoma growth at normoxia/mild-hypoxia and confers sensitivity to targeted therapy by echinomycin. J. Exp. Clin. Cancer Res. 40, 16 (2021).
Joseph, J. V. et al. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1 alpha-ZEB1 axis. Cancer Lett. 359, 107–116 (2015).
Goldberg, M. A., Dunning, S. P. & Bunn, H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242, 1412–1415 (1988).
Ma, Z. W. et al. Targeting hypoxia-inducible factor-1-mediated metastasis for cancer therapy. Antioxid Redox Signal 34, 1484–1497 (2021).
Mendez, O. et al. Knock down of HIF-1 alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol. Cancer 9, 10 (2010).
Liu, C. X. & Chen, L. L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2016–2034 (2022).
Kristensen, L. S. et al. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691 (2019).
Meng, S. J. et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol. Cancer 16, 8 (2017).
Yang, R. et al. Hypoxia-induced circWSB1 promotes breast cancer progression through destabilizing p53 by interacting with USP10. Mol. Cancer 21, 88 (2022).
Yang, H. O. et al. Hypoxia induced exosomal circRNA promotes metastasis of colorectal cancer via targeting GEF-H1/RhoA axis. Theranostics 10, 8211–8226 (2020).
Wang, R. et al. CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res. 78, 4812–4825 (2018).
Du, W. W. et al. Identifying and characterizing circRNA-protein interaction. Theranostics 7, 4183–4191 (2017).
Xia, X. et al. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol. Cancer 18, 16 (2019).
Srikantan, S. Gorospe M. HuR function in disease. Front. Biosci. 17, 189–205 (2012).
Doller, A. et al. Posttranslational modification of the AU-rich element binding protein HuR by protein kinase C delta elicits angiotensin II-induced stabilization and nuclear export of clooxygenase 2 mRNA. Mol. Cell Biol. 28, 2608–2625 (2008).
Brennan, C. M. Steitz JA. HuR and mRNA stability. Cell. Mol. Life Sci. 58, 266–277 (2001).
Chen, J. et al. Circular RNA circRHOBTB3 represses metastasis by regulating the HuR-mediated mRNA stability of PTBP1 in colorectal cancer. Theranostics 11, 7507–7526 (2021).
Zhu, T. et al. Oncogenic circTICRR suppresses autophagy via binding to HuR protein and stabilizing GLUD1 mRNA in cervical cancer. Cell Death Dis. 13, 479 (2022).
Song, X. et al. CircEIF3H-IGF2BP2-HuR scaffold complex promotes TNBC progression via stabilizing HSPD1/RBM8A/G3BP1 mRNA. Cell Death Discov. 8, 261 (2022).
Abdelmohsen, K. et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 14, 361–369 (2017).
Okumura, Y. et al. Hypoxia-induced PLOD2 is a key regulator in epithelial-mesenchymal transition and chemoresistance in Biliary tract cancer. Ann. Surg. Oncol. 25, 3728–3737 (2018).
Kresse, N. et al. PLOD2 is a prognostic marker in glioblastoma that modulates the immune microenvironment and tumor progression. Int J Mol Sci 23, 6037 (2022).
Du, H., Pang, M., Hou, X., Yuan, S. & Sun, L. PLOD2 in cancer research. Biomed. Pharmacother. 90, 670–676 (2017).
Noda, T. et al. PLOD2 induced under hypoxia is a novel prognostic factor for hepatocellular carcinoma after curative resection. Ann. Surg. Oncol. 19, S121–S121 (2012).
Lal, A. et al. Transcriptional response to hypoxia in human tumors. JNCI J. Natl Cancer Institute 93, 1337–1343 (2001).
Sun, J. et al. A novel lncRNA ARST represses glioma progression by inhibiting ALDOA-mediated actin cytoskeleton integrity. J. Exp. Clin. Cancer Res. 40, 20 (2021).
Li, X. et al. Aldolase A enhances intrahepatic cholangiocarcinoma proliferation and invasion through promoting glycolysis. Int. J. Biol. Sci. 17, 1782–1794 (2021).
Wang, R. et al. P68 RNA helicase promotes invasion of glioma cells through negatively regulating DUSP5. Cancer Sci. 110, 107–117 (2019).
Nabors, L. L. B. et al. Tumor necrosis factor a induces angiogenic factor up-regulation in malignant glioma cells: a role for RNA stabilization and HuR. Cancer Res. 63, 4181–4187 (2003).
Lebedeva, S. et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell 43, 340–352 (2011).
Lee, D. J. et al. Multiple tumor-suppressor genes on chromosome 3p contribute to head and neck squamous cell carcinoma tumorigenesis. Cancer Biol. Ther. 10, 689–693 (2010).
Madhavan, S. et al. Rembrandt: helping personalized medicine become a reality through integrative translational research. Mol Cancer Res. 7, 157–167 (2009).
Hansen, T. B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013).
Li, X. et al. Linking circular intronic RNA degradation and function in transcription by RNase H1. Sci China Life Sci. 64, 1795–1809 (2021).
Legnini, I. et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37 (2017).
Chen, X. P. et al. circRNADb: A comprehensive database for human circular RNAs with protein-coding annotations. Sci. Rep. 6, 6 (2016).
Arroyo, J. D. et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl Acad. Sci. USA 108, 5003–5008 (2011).
Du, A. S. et al. M6A-mediated upregulation of circMDK promotes tumorigenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. Mol. Cancer 21, 18 (2022).
Huang, Y. H., Hong, W. Q. & Wei, X. W. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 15, 27 (2022).
Zhang, C. et al. The short inverted repeats-induced circEXOC6B inhibits prostate cancer metastasis by enhancing the binding of RBMS1 and HuR. Mol. Ther. 31, 1705–1721 (2023).
Vaupel, P. & Mayer, A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 26, 225–239 (2007).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Conn, V. M. et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants 3, 5 (2017).
Dong, S. M. et al. Histology-based expression profiling yields novel prognostic markers in human glioblastoma. J. Neuropathol. Exp. Neurol. 64, 948–955 (2005).
Majumder, M., Chakraborty, P., Mohan, S., Mehrotra, S. & Palanisamy, V. HuR as a molecular target for cancer therapeutics and immune-related disorders. Adv. Drug Deliv. Rev. 188, 114442 (2022).
Yang, L. Q. et al. Muscone derivative ZM-32 inhibits breast tumor angiogenesis by suppressing HuR-mediated VEGF and MMP9 expression. Biomed. Pharmacother. 136, 11 (2021).
Guha, A. et al. The versatile role of HuR in glioblastoma and its potential as a therapeutic target for a multi-pronged attack. Adv. Drug Deliv. Rev. 181, 13 (2022).
Akool, E. S. et al. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol. Cell Biol. 23, 4901–4916 (2003).
Wang, J. P. et al. Deletion of the RNA regulator HuR in tumor-associated microglia and macrophages stimulates anti-tumor immunity and attenuates glioma growth. Glia 67, 2424–2439 (2019).
Gaur, A. B., Holbeck, S. L., Colburn, N. H. & Israel, M. A. Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol. 13, 580–590 (2011).
Wigington, C. P. et al. Post-transcriptional regulation of programmed cell death 4 (PDCD4) mRNA by the RNA-binding proteins human antigen R (HuR) and T-cell intracellular antigen 1 (TIA1). J. Biol. Chem. 290, 3468–3487 (2015).
Casolaro, V. et al. Posttranscriptional regulation of IL-13 in T cells: role of the RNA-binding protein HuR. J. Allergy Clin. Immunol. 121, 853–859 (2008).
Thaci, B. et al. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro Oncol. 16, 1304–1312 (2014).
Di Ruscio, A. et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 503, 371–376 (2013).
Panda, A. C., Martindale, J. L. & Gorospe, M. Polysome fractionation to analyze mRNA distribution profiles. Bio. Protoc. 7, e2126 (2017).
Zeng, X. et al. CircHIPK2 contributes cell growth in intestinal epithelial of colitis and colorectal cancer through promoting TAZ translation. Adv. Sci. (Weinh) 11, 2401588 (2024).
Acknowledgements
This work was supported by Key Project of Research and Development of Hubei Province (2022BCE049), Natural Science Foundation of Hubei Province (2023AFB1037, 2017CFA064), Fundamental Research Funds for the Central Universities (2662021SYQD001), National Natural Science Foundation of China (82103674).
Author information
Authors and Affiliations
Contributions
Aixin Yu: Writing original draft, Validation, Methodology, Conception, Investigation. Yiqi Wang: Methodology, Investigation. Chao Duan: Methodology, Investigation. Jun Qin: Conception. Miaomiao Liao: Investigation. Haiyan Zhu: Investigation. Junrong Lei: Investigation. Jun Liu: Investigation. Zehao Yang: Investigation. Li Yu: Investigation. Hui Gui: Investigation. Jinxin Xin: Investigation. Weiwei Tao: Conception, Funding acquisition. Wendai Bao: Conception, Writing—review and editing, Supervision, Funding acquisition. Zhiqiang Dong: Conception, Writing—review and editing, Supervision, Funding acquisition. All authors: Final approval of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The animal study was performed after receiving approval from the Institutional Animal Care and Use Committee of Hubei University of Medicine (approval number: 2023008). Informed consents were obtained from GBM patients for sample collection, and the study was approved by the Ethics Committee of Shiyan Taihe Hospital (approval number: 2023KS11).
Peer review
Peer review information
Communications Biology thanks Vivek Sharma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Kaliya Georgieva. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, A., Wang, Y., Duan, C. et al. Hypoxia-induced circPLOD2a/b promotes the aggressiveness of glioblastoma by suppressing XIRP1 through binding to HuR. Commun Biol 8, 71 (2025). https://doi.org/10.1038/s42003-025-07503-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07503-3