Abstract
Peripheral nerve injury (PNI)-induced neuropathic pain (NP) is a severe disease with high prevalence in clinics. Gene reprogramming and tissue remodeling in the dorsal root ganglia (DRG) and spinal cord (SC) drive the development and maintenance of neuropathic pain (NP). However, our understanding of the NP-associated spatial molecular processing landscape of SC and the non-synaptic interactions between DRG neurons and SC cells remains limited. We here integrate spatial transcriptomics (ST) with single-nucleus RNA-sequencing (snRNA-seq) and bulk RNA-sequencing (bulk RNA-seq) to characterize regional pathological heterogeneity of the SC under NP conditions. First, the SC of NP mice manifests unique spatial atlases of genes, cell populations, cell-cell cross-talks, signaling pathways, and transcriptional regulatory networks compared to sham mice. We further report that injured DRG sensory neurons and the corresponding ventral horn of the SC show similar expression patterns after PNI. In addition, for the first time, we systematically exhibit “cross-talk omics” between the DRG neurons and SC dorsal horn neurons and glial cells, indicating an altered communication profile under NP conditions. Together, our findings decode the spatial and cellular heterogeneity of molecular pathological mechanisms underlying NP, providing a foundation for designing therapeutic targets for this disorder.
Similar content being viewed by others
Introduction
Neuropathic pain (NP) is a debilitating condition affecting hundreds of millions worldwide1. Despite decades of research, the underlying mechanisms of NP remain poorly understood, making it challenging to develop effective treatments. Unlike nociceptive pain, the etiology of NP may be attributed to nerve injury- or lesion-caused structural and functional plastic maladaptive changes in the sensory nervous system based on the alternations of gene expression profiles2,3. However, we know little about it. New ways are needed to holistically understand pathological changes in pain-modulating tissues.
The dorsal horn of the spinal cord (SCDH) is an important integral center of pain sensation, responsible for processing and relaying pain information from the periphery and transmitting it to the brain through a complex circuitry composed of spinal projection neurons, inhibitory interneurons, excitatory interneurons, and peripheral and descending afferents4,5,6. Targeting SC neural activity can relieve NP7. Various regulators, including microglia and astrocytes, also influence and access this circuitry, forming an intricate network, all cells of which have region-specific characteristics3,8. SC is divided into several layers based on anatomy and electrophysiology6. Each layer receives particular primary afferent nerve fibers from different sensory neuron types and coordinates distinct somatic sensory information. For instance, DRG prokineticin-expressing neurons project to a class of excitatory interneurons in the dorsal side of the lamina II inner (IIi) layer, mediating pleasant touch processing via activating the prokineticin receptor 29. Following peripheral nerve injury or lesions, the spinal circuitry and its regulatory network undergo reorganization, which underlies the generation and maintenance of central sensitization2,3,8. For example, after PNI, the central terminals of DRG-injured sensory neurons release CSF1 in SCDH, inducing microgliosis and pain10. Hence, exploring the SC pathology of NP spatially at the single-cell level is imperative.
The distinct combinations and levels of gene expression in a cell determine its molecular composition, signaling capacity, and other defining features11,12. Moreover, the location of the cells with distinct gene expression patterns within tissue defines tissue phenotype11. Thus, mapping spatial gene expression in the spinal cord (SC) at the single-cell level under sham and NP conditions will provide valuable insights into macro- and micro-pathological mechanisms of NP. Cutting-edge sequencing technologies, including single-nucleus RNA-sequencing (snRNA-seq), spatial transcriptomics sequencing (ST), make these insights possible11. The combination of these sequencing approaches is revolutionizing the way cellular heterogeneity and function analysis at the tissue level13,14.
In this work, we carried out ST, snRNA-seq, bulk RNA-seq experiments, and molecular and immunological assays to explore the molecular processing landscape in the SC following PNI. We presented a pipeline that accurately revealed the changes in gene expression, cell populations, intercellular communication in the SC, and molecular cross-talks between DRG and SC under NP conditions. These findings shed light on the molecular pathological mechanisms underlying NP and may provide theoretical bases for designing therapeutic targets for this disorder.
Results
ST and snRNA-seq reveal substantially different gene expression maps in murine SC under sham and NP conditions
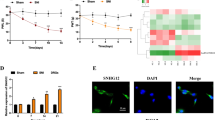
To obtain a comprehensive gene expression atlas that delineated the spatial distribution and cellular specificity of gene expression within the mouse SC under sham and NP conditions, we performed a combined analysis of ST and snRNA-seq (Fig. 1a). Employing the Seurat R package, we identified 8 principal ST-spot clusters in ST data, with each spot representing a fixed-coordinate sampling point on the tissue section. Specifically, the cluster SC_R8 was distinctly observed in SC sections from mice 10 days after L4 spinal nerve ligation (SNL), exhibiting regional specificity (Fig. 1b, c). The identified ST-spot clusters corresponded to distinct neuroanatomical regions of the SC. SC_R7 was distributed in the superficial layers of the SCDH, while SC_R3 ST-spots were located in the deep layers. SC_R1 and SC_R4 were mainly distributed in the white matter of the SC and the dorsal and ventral spinal roots, respectively (Fig. 1b, c). The heatmap and cluster dendrogram (Fig. 1c, d) demonstrated higher similarity and closer distribution within the gray matter for SC_R2, SC_R3, and SC_R7. The top 5 enriched genes in these clusters were illustrated through a heatmap compared to the remaining clusters (Fig. 1e). Our further examination revealed that different cellular subpopulations represented the cluster-specific marker genes. For example, the oligodendrocyte marker gene Plp1 was also a marker gene for SC_R1. Markers of SC excitatory interneurons Sst and Tac2 were also top enriched genes for SC_R7 (Fig. 1e), suggesting a preferential distribution of these excitatory neurons in the superficial layers of the SCDH. Using the Loupe locally distinguishing algorithm, we discovered that Ifi27l2a, Siglech, Ly86, Fcgr1, and Bst2 were highly expressed within SC_R7 ST-spots after SNL, in contrast to sham. Similarly, within the central spinal canal, Zp3, Oals2, Adgb, Zfp523, and Slc41a1 were significantly higher post-SNL versus sham (Fig. 1f, g). In fact, this analytical approach facilitated the comparison of gene expression across any corresponding SC regions between sham and SNL groups.
a Schematic figure illustrating a flow chart of ST combined with snRNA-seq to map mice SC gene expression landscape in the sham and NP states 10 days after surgeries. Created with BioRender. b Hematoxylin-eosin-stained coronal sections of SCs from sham and SNL mice subject to ST analysis (two biological repeats). c Overview of the ST-spot clusters within spinal cord samples, with the left schematic illustrating the anatomical context of the spinal cord and the relative positions of the clusters. The upper right panel displays the spatial distribution across four samples, while the lower right panel provides a UMAP visualization of the clusters. d Heatmap showing each spatial cluster’s correlation according to their gene expression files. e Heatmap shows the top 5 enriched genes in each spacial cluster. f ST showing the top 5 upregulated and downregulated genes in the ipsi DH of the SNL mice compared with the sham mice. g ST showing top 5 upregulated and downregulated genes in the ependyma’s region in the SNL mice compared with the sham mice. h t-SNE plots showing identified cell types within SCDH from mice 10 days after sham and SNI, colored according to identified clusters. The dot plot shows the expression of established marker genes to annotate snRNA-seq-identified subpopulations of the DH. i The top 5 enriched genes in each snRNA-seq identified subpopulation. j, k t-SNE plots and heatmaps showing the top five upregulated and downregulated genes in microglia (j) or excitatory neuron (k) in the SNI mice compared with the sham mice. l t-SNE plots of snRNA-seq and Spatial plot showing the spatial and cellular expression pattern of Slc5a7 in the SC; m Immunofluorescence staining for CHT1 coded by Slc5a7 on SC sections 10 days after surgeries. Scale bar = 200 μm. n t-SNE plots of snRNA-seq and Spatial plot showing the spatial and cellular expression pattern Ly86. o RNA Scope analyses showed Ly86 expression in SC. Scale bar = 200 μm. p Timecourse of Ly86 mRNA expression in SC 10 days after SNL. Values are shown as mean (bar) ± SD (error bar). ****P < 0.0001, N = 5–7, one-way ANOVA followed by Bonferronis test.
We sequenced and analyzed 14,873 and 15,100 cell nuclei of SCDH from the mice 10 days after sham and spared nerve injury (SNI) groups, respectively, to augment the ST analysis. Nine major subtypes of clusters were annotated using previously reported marker genes15: astrocyte, excitatory neuron (Exc.), inhibitory neuron (Inh.), a mixed population of inhibitory/excitatory neuron (Inh./Exc. neuron), microglia, oligodendrocyte precursor cells (OPC), meningeal cells (Men.), oligodendrocyte (Oligo) and vascular cells (Fig. 1h). Certain markers, such as Slc17a6 encoding the vesicular glutamate transporter 2 (VGLUT2) for excitatory neurons and Slc6a5 encoding the glycine transporter 2 (GlyT2) for inhibitory neurons15, were used to categorize these cell types (Fig. 1h and Supplementary Fig. 1a–d). Lmx1b, Tlx3, and Mafa served as markers for SC excitatory neurons, while Gad1, Gad2, Pax2, Lhx1, Lhx5, and Gbx1 were indicative of inhibitory neurons16. The specific expression of these markers in their respective SC neuron types, identified through our snRNA-seq data, suggested the accuracy and high quality of our dataset (Fig. 1h and Supplementary Fig. 1a–d). Sst and Pvalb (PV), markers for brain inhibitory neurons, and Nos1, for excitatory neurons, failed to distinguish these types in the SC, with Sst notably expressed in excitatory neurons there (Supplementary Fig. 1c, d). Notably, most SC neuron clusters could be easily sorted into excitatory or inhibitory neuronal subpopulations. However, a particular group of neurons in the SC had similar gene expression profiles, making it difficult to separate them using resolution values from 0.4 to 1.5 (Supplementary Fig. 1e). We labeled it the Inh./Exc—neuron cluster (Supplementary Fig. 1f). Furthermore, we have highlighted the top 5 enriched genes of each cluster through a heatmap in comparison to the remaining clusters. These genes might serve as markers for distinct cell types in the SC (Fig. 1i). Similar to ST analysis, this snRNA-seq data permitted the identification differentially expressed genes (DEGs) within discrete cell populations or subpopulations between the sham and SNI groups (Supplementary Data 1). After SNI, the top five upregulated genes were identified as Cmss1, Apoe, B2m, Ctss, and C1qb in microglia and as Htr2c, Cdh12, snhg11, Arglu1, and Cmss1 in SNI excitatory neurons as compared to those in the sham group (Fig. 1j, k).
Using the t-SNE plot of snRNA-seq and the spatial map of ST, we showed the expression profiles of any genes of interest in specific cell types and spatial locations under sham and NP conditions. For example, Slc5a7 was expressed in the spinal cord ventral horn (SCVH) neurons, and Ly86 was upregulated in SCDH and SCVH microglia on the ipsilateral side after PNI, confirmed by immunofluorescence staining, RNA Scope and qPCR (Fig. 1l–p). Additionally, neuropeptides (Bdnf, Cbln1, Cck, Grp, Npy), G protein-coupled receptors (Calcrl, Ccr5, Drd2, Npr1, Prokr2), and ion channels (Calna1e, Gabra1, Gria1, Grin2b, Hcn1) implicated in pain modulation exhibit distinct spatial and cellular subpopulation distribution patterns (Supplementary Fig. 2). Neuropeptides Bdnf, Cbln1, and Grp were primarily localized in excitatory neurons, Npy in inhibitory neurons, and Cck in both. Spatially, Grp is concentrated in the superficial SCDH layer, while Cck-expressing cells are in the deep layer (Supplementary Fig. 2). These peptides likely contribute distinct roles across neuron types and engage in the complex spatial circuits involved in sensory processing.
Heterogeneous responses of microglia and astrocytes to PNI
To investigate the response of distinct glial cell subtypes to PNI, we conducted a comprehensive analysis by integrating ST data with snRNA-seq data. Based on snRNA seq, we identified 9 microglia and 10 astrocyte subclusters. After SNI, a significant increase was seen in a subpopulation of microglia (cluster 3) characterized by the expression of Apoe. In contrast, a marked decrease was observed in a subpopulation (cluster 5) of microglia expressing Mrc1 (Cd206), an M2 marker (Fig. 2a–c). The spatial map revealed that Apoe expression primarily increased in the ipsilateral SCDH region, whereas Mrc1 expression predominantly occurred in the superficial layers of the SCDH (Fig. 2d), indicating the distribution patterns of two microglia subpopulations. The spatial distribution of Mrc1 mRNA is consistent with the location of MRC1+ spinal macrophages as in the previous report17. Similar changes were seen in astrocyte subpopulations after SNI. A decrease in the number of Vav3-expressing astrocytes occurred in cluster 0. In contrast, the number of astrocytes in cluster 7 with high A2m expression was significantly increased (Fig. 2e–g). The spatial expression of astrocyte subpopulation marker genes, including A2m, Nnat, Rnf213, and Vav3, may reflect SC astrocyte heterogeneity and localization information (Fig. 2h). Together, these results indicate that PNI induced a widespread response with subtypes of microglia and astrocytes, which may play distinct roles in central pain sensitization.
a Heatmap shows the top 5 enriched genes in each microglia subcluster. b t-SNE plots depicting the identified microglia subtypes across sham and SNI samples are shown, with colors corresponding to the identified subclusters. In the SNI group, microglia account for 7.57% of the total cells, compared to 5.44% in the sham group. c Histograms displaying the percentage of each microglia subcluster in Sham and SNI groups. After SNI, there was an increase in clusters 1, 2, 3, 4, and 7, while a decrease was observed in clusters 0, 5, 6, and 8, suggesting a significant shift in microglia subpopulations following SNI. d Spatial expression of Classical microglia marker Aif1 and selected subcluster marker genes identified by snRNA-seq, illustrating the heterogeneity of microglia. e The top 5 enriched genes in each astrocyte subcluster. f Astrocyte subtypes in sham and SNI samples are shown in t-SNE plots with corresponding subcluster colors. In the SNI group, astrocytes account for 2.49% of the total cells, compared to 3.61% in the sham group. g SNI caused a shift in astrocyte subpopulations, with an increase in clusters 1, 3, 4, 7, and 9 and a decrease in clusters 0, 2, and 6. h The spatial expression pattern of Gfap and subcluster markers show astrocyte diversity.
Potential spatial distribution of glial and neuronal cell subpopulations in the SC
To investigate the spatial topography of the cell subset, CellTrek analysis was applied to directly map single cells back to their spatial coordinates in spinal sections based on our snRNA-seq and ST data (Fig. 3a). The cells obtained from snRNA-seq were mainly located back to SCDH on ST-spot map (Fig. 3b), consistent with our expectations as they were obtained from the dorsal part of the SC. The SColoc module of CellTrek summarized the colocalization patterns among different cell types and revealed that, under NP conditions, there were significant changes in the colocalization patterns among different types of cells in the SC. For example, a high colocalization between microglia and vascular cells was observed in sham mice. However, after PNI, this colocalization disappeared (Fig. 3c). CellTrek analysis accurately located different types of cells back to SC anatomical locations, in which they are highly enriched (Fig. 3d, e). Any gene-specifically expressed cells could be located in their spatial area in the SC through the above framework. Spinal PKCγ positive neurons that are essential in pain sensation and modulation are primarily located in the dorsal horn’s lamina II6. Prkcg (PKCγ coding gene)-positive cells were successfully located in the vicinity of lamina II by CellTrek (Fig. 3f–i). Additionally, this method could accurately reveal the differential distribution of the same type of cells under sham and NP conditions. As shown in Fig. 3j, k, after charting microglia alone to their spatial locations by CellTrek, we demonstrated the phenomenon of microglial proliferation after PNI.
a “CellTrek” imputed near single-cell level information by integrating ST data with snRNA-seq information. b “CellTrek” was used to map single cells onto the spatial coordinates of tissue sections of SC. c The spatial colocalization of cell types in the SC is displayed, where the edge width represents the probability of colocalization. d The spatial location of each identified cell type separately in the snRNA-seq dataset. The translucent blue rectangles indicate SCVH, representing results derived from model-based analysis. e The spacial and cellular expression of marker genes (Plp1, Dcn, Cldn5, Vcan, Trem2, Aldh1l1) displayed by spacial and snRNA-seq t-SNE plot. f Immunofluorescence displays the expression of PKCγ in normal SC. Scale bar: 200 μm (top left), 100 μm (others). g The distribution of Prkcg positive cells (Count > 2) in SC was visualized through the t-SNE diagram. h The spatial location of Prkcg positive cells in SC mapped by CellTrek. i The spatial expression of Prkcg mRNA. j Microglia deconvolution back alone to SC. k The immunofluorescence displays the expression of IBA-1 in SC 10 days after sham and SNL. Scale bar = 200 μm.
Given that SCDH neurons play a central role in the modulation of NP, we performed further clustering analysis by the Findclusters function only on the neurons obtained by snRNA-seq and localized them one by one to the SC by CellTrek. Clustering analysis identified 31 distinct major neuron subpopulations, among which 12 were inhibitory neuronal subtypes, 18 were excitatory neuronal subtypes, and one was the Inh./Exc. neuron (Fig. 4a, b). Euclidean-based hierarchical clustering analysis displayed five major groupings: Groups 1, 3, and 5 shared excitatory neurotransmitter status, and group 4 was inhibitory neurotransmitter status (Fig. 4c). These neurons were primarily located in the gray matter of the SCDH (Fig. 4d). To provide a more intuitive display of the spatial distribution of different types of neuronal subtypes, we simultaneously analyzed the expression patterns of marker genes for each subpopulation and deconvolved them back to the SC (Fig. 4e). The following 4 significant findings were obtained: (1) Within the SCDH, a considerable variety in both inhibitory and excitatory neurons was seen, as evidenced by the unique expression of various classical neuronal markers across different neuron subtypes. (2) The double-positive expression of Pydn and Pld5 was in a subtype of excitatory neurons, although previous studies had reported that PDYN was only a marker for spinal inhibitory neurons18. (3) Subgroups of excitatory neurons were found in the superficial, deep, or both layers in SCDH. For instance, Nmur2-positive (2-E), Tac1-positive neurons (3-E) and Tac2/Nmu double-positive neurons (8-E) were distributed in the superficial layer, while Cck-positive (30-E), Meix2-positive (4-E), and Pbx3-positive neurons (10-E) were distributed in the deep layers of SCDH. Nfib/Tcf4 double-positive neurons (12-E) were found in both layers. (4) Inhibitory neurons were mainly found in the deep layer of SCDH. Sorcs3/Gpc5/Rorb-positive (7-I), mt-Co2/mt-Co3/mt-Atp6-positive (29-I) were distributed in deep layers, while Esrrg/Scn9a/Etl4-positive and Calntl6/Cntn5-positive neurons located in both layers. In summary, This precise spatial localization of SCDH neuron subtypes will provide crucial insights for deciphering the microcircuits involved in pain processing.
a t-SNE visualization of the three main neuron clusters in the SC. b The t-SNE visualization shows 31 subclusters of neurons identified by SC snRNA-seq. The labels “E” and “I” followed by the numerical index represent excitatory and inhibitory neuron subclusters, respectively. “E/I” refers to the mixed subcluster of excitatory and inhibitory neurons. c Unrooted dendrogram displaying the relationships in the 31 neuron subclusters based on mean gene expression for each cluster. The dendrogram was constructed using hierarchical clustering analysis. The distance between them reflects the degree of similarity in gene expression patterns. d The mapping of the 31 neuron subclusters to the spatial coordinates of SC tissue sections was achieved using “CellTrek.” e Heatmap displays the expression patterns of marker genes across the 31 identified subclusters of neurons in the snRNA-seq dataset, and Each subcluster is presented individually along with its corresponding spatial location as determined by CellTrek, providing a holistic and intuitive understanding of each subcluster and their spatial distribution in SCDH. The translucent blue rectangles indicate SCVH part, representing data derived from model-based analysis.
Cellular cross-talking coordination reorganized in the PNI SC
Using the ST dataset, we identified the spatial coordinates of cell-cell interactions based on ligand-receptor (L-R) expression, and with snRNA-seq data, we ascertained the specific cells involved in these interactions. By performing stLearn to calculate the spatial co-expression of L-R genes within and between ST-spots in SC, we identified 1118 valid L_R pairs, including 14 homophilic binding and 1104 heterophilic bindings, which were significantly expressed in close proximity within SC (Fig. 5a and Supplementary Data 2). Mcam-Mcam and Ptprk-Ptprk interactions, essential for oligodendrocyte development and myelination, were predominantly observed among oligodendrocytes. In contrast, Cadm3-Cadm3 and Robo2-Robo2 interactions primarily facilitated neuron-neuron cross-talk (Fig. 5a, b). We further identified multiple heterophilic L-R pairs, including Nmu_Nmur2, Tac1_Tacr1, Tac1_Tacr3, Pdyn_Oprk1, Gal_Gpr151, Npy_Npy1r, Npy_Npy2r, Npy_Npy5r, and Penk_Ogfr (Fig. 5a, b). They were highly relevant to transmitting pain signals and were top enriched in SCDH; moreover, it is noteworthy that these L_R pairs were distributed across distinct subpopulations of excitatory neurons or inhibitory neurons and mediated positive- or negative-feedback connections (Supplementary Fig. 3). In the L_R pair Nmu_Nmur2, both Nmu and Nmur2 were distributed within different subpopulations of excitatory neurons (Fig. 5c and Supplementary Fig. 3), suggesting that Nmu and Nmur mediate the communication of excitatory neurons. In contrast, Npy was predominantly located in inhibitory neurons, while its receptor Npy1r was highly expressed in excitatory neurons (Fig. 5d and Supplementary Fig. 3). Thus, it is plausible that Npy_Npy1r mediates the suppression of excitatory neurons by inhibitory neurons in the superficial SCDH. These findings may provide a neural molecular network for the reciprocal communication between and within subtypes of excitatory and inhibitory neurons.
a The top 50 L_R pairs Ranking based on the number of significant ST-spots. b Heatmaps showing the average expression of the ligand and receptor genes of the top 50 L_R pairs across all cell subpopulations and ST-spot clusters. c, d The spatial plots illustrate the significance [(−log10 (p_adjs).] of L_R pairs of Nmu_Nmur2 and Npy_Npy1r across all ST-spots, t-SNE plots, and spacial plots display mRNA expression of both their ligands and receptors across all cell clusters and ST-spot clusters. e The heatmap from SC bulk RNA-seq data shows the deregulated L_R pairs in SC 10 days after SNL that meet two criteria: differential expression of ligand or receptor genes post-SNL and presence in SC identified by SC ST data. The L_R pairs are categorized into three groups based on their expression features: upregulation in both ligand and receptor, upregulation in ligand only, or upregulation in receptor only. The pairs that exhibit upregulation in both the ligand and receptor are listed. The black/white heatmap highlight pairs with high average expression levels in SC. f The spatial plot displays the L_R scores for Cx3cl1_Cx3cr1. g The spatial mRNA expression of Cx3cl1 and Cx3cr1. h The expression of CX3CR1 in the SC 10 days after SNL was assessed using the GFP in Cx3cr1-GFP reporter mice. Scale bar = 200 μm. i The t-SNE plot illustrates the expression of Cx3cl1 and Cx3cr1 across all cells subjected to SC snRNA-seq.
To analyze changes in the communications between different types of cells in the SCDH under sham and NP conditions, we integrated and analyzed the cellular and spatial characteristics of the cross-talks mediated by differentially expressed ligand and receptor genes detected by bulk RNA-seq (Fig. 5e and Supplementary Fig. 4). The ligand and receptor genes were extracted from enriched L_R pairs identified by stLearn analysis from ST data. To determine the deregulated L_R pairs, we next intersected these genes with the DEGs identified by bulk RNA-seq of the SC from sham and SNL mice 10 days after surgery. After SNL, 27 pairs of both ligands and their corresponding receptors were upregulated. Additionally, there were 95 pairs with only the receptor upregulation and 53 pairs with only the ligand upregulation (Fig. 5e). The heatmap in Supplementary Fig. 4 further detailed the anatomical location, cellular localization, and altered expression of these receptor-ligand pairs in the SC. Cx3cl1_Cx3cr1 signaling promotes pain and spinal inflammation by transmitting information from SCDH neurons to microglia19. Our analysis paradigm confirmed the localization of Cx3cl1 in spinal neurons and its receptor, Cx3cr1, in microglia. Since SNL did not alter the expression of Cx3cl1 in the SC, the enhanced effect of the L_R pair in the SC may be due to an increase in Cx3cr1 expression in microglia (Fig. 5–i). Our analyzed results further verify the previous reports20 and demonstrate the robustness of our analysis paradigm. Collectively, the cell interaction map elucidates the pivotal signaling pathways and cell-to-cell interactions integral to SC pain modulation, laying a robust groundwork for future research validation.
Spatial and cell-type distribution of signaling pathways related to NP
Identifying the localization of signaling pathways within the SC is crucial to understanding their role in pain processing. We analyzed the activity of mouse canonical pathways obtained from MsigDb within each ST-Spot or each cell by AUCell package. AUC values heatmap of different signaling pathways across all SC ST-spots showed that different signaling pathways were active in different regions of the SC (Fig. 6a, Supplementary Data 3). SC_R7 ST-spot clusters exhibited significant enrichment in multiple pain-associated pathways, including TACHYKININ_RECEPTORS_BIND_TACHYKININS, PHASE_1_INACTIVATION_OF_FAST_NA_CHANNELS, ION (Ion Channel and Phorbal Esters Signaling Pathway) and PLCD (Phospholipase C d1 in phospholipid associated cell signaling). Furthermore, the ION and PLCD pathways showed higher activity in the SC of the PNI group (Fig. 6b). snRNA-seq data also demonstrated that different signaling pathways were active among different cell types (Fig. 6c, Supplementary Data 4). Figure 6d showed the top five enriched signaling pathways for different cell types in SCDH and their distribution across different spatial regions. The signaling pathways predominantly enriched in excitatory or inhibitory neurons showed relatively weaker enrichment in other neuronal subtypes (Fig. 6d, Supplementary Data 4). More interesting, we identified several previously unexplored cell type-specific signaling pathways, including the astrocyte ACE_INHIBITOR pathway, excitatory neuron PYRIMIDINE_SALVAGE pathway, inhibitory neuron CA2_ACTIVATED_K_CHANNELS pathway, and microglia ACETAMINOPHEN PATHWAY (Fig. 6d, Supplementary Data 4).
a Heatmap displaying the mean activity of mouse canonical pathways [MsigDb(M2:CP)] in cluster SC_R1 to SC_R8. b Heatmap depicts the average activity of the top 5 pathways in every spatial cluster of SC, while also showcasing their average activity in each cell subpopulation of SC. c Heatmap displaying the mean activity of mouse canonical pathways across all cells subjected to snRNA-seq. d Heatmap showing the mean activity of the top 5 pathways in each cell subpopulation of SC and their average activity in each ST-spot cluster. e Venn plot illustrating the overlapping relationship of each ST-spot cluster’s top 5 regional-specific pathways and the top 5 cell type-specific pathways of each cell subpopulation. f The spatial and t-SNE plots display the AUC score of selected overlapping pathways from e. g Representative deregulated pathways after PNI (SNL/SNI) were analyzed and displayed using spatial and t-SNE plots. h Immunofluorescence assay showing the expression and subcellular localization of C1Q in SC 10 days after sham and SNL, a key component of the upregulated pathway “comp_pathway,” in SC. Scale bar = 200 μm.
Our analysis found 8 unique signaling pathways with specificity to both cell type and anatomical location (Fig. 6e, Supplementary Data 5), identified by intersection analysis of the top five enriched pathways in different spatial ST-spot clusters and cell types. TACHYKININ_RECEPTORS_BIND_TACHYKININS_PATHWAY and the ASSEMBLY_AND_CELL_SURFACE_PRESENTATION OF NMDA_RECEPTORS pathway in SC excitatory neurons (Fig. 6f). The PYRIMIDINE_SALVAGE pathway was found in the ipsi SCVH neurons following PNI (Fig. 6e). In addition, we observed the LEUKOTRIENE_RECEPTORS pathway in OPC of the spinal white and gray matter junction area, the SLRP pathway and CROSSLINKING_OF_COLLAGEN_FIBRILS pathway in dorsal root Schwann cells, and the HISTIDINE_CATABOLISM pathway and INTERLEUKIN_36 pathway in oligodendrocytes of SC white matter tracts (Fig. 6e, f).
By using AUC values, we identified signal pathways with activity differences between sham and PNI SCs and revealed their respective spatial and cellular distributions (Fig. 6g). The COMP pathway displayed increased activity in microglia in the ipsilateral SCVH and SCDH following PNI. The ETS pathway, CTL pathway, and ENDOSOMAL_VACUOLAR pathway exhibited increased activity in PNI microglia and vascular cells in the same spinal regions. In contrast, the BTG2_Pathway showed decreased activity in astrocytes in the ipsilateral spinal SCDH and VH following PNI surgeries (Fig. 6g). Consistent with the distribution pattern of the COMP signaling pathway, our immunofluorescence analysis has shown a similar spatial distribution of C1Q, a pivotal protein within this pathway, in the SC following SNL surgery (Fig. 6h). In conclusion, this analysis delineates the spatial and cellular characteristics of signaling pathways in the SC, offering insights for targeted pain interventions.
Spatial and cell-type-specific gene regulatory networks associated with NP
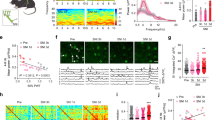
Exploring the spatial distribution characteristics of the transcriptional regulatory network consisting of transcription factors (TFs) and their downstream genes in the SC is vital for understanding NP mechanism. We employed the SCENIC algorithm to explore region-specific regulon networks in the SC, where a regulon refers to the regulatory network consisting of TF and their target genes. Regulon activity was binarized and matched with ST-spot clusters and experimental treatments (sham vs SNL). The distribution of regulons in SC regions exhibited variations between sham and SNL (Fig. 7a), indicating possible changes in gene expression regulation. The heatmap of average AUC scores across different ST-spot clusters for the 32 regulons revealed specific enrichment of certain regulons in each spatial region (Fig. 7b). For instance, Stat3_11g, Jun_74g, and Atf3_extended_62g were actively expressed in the SC_R8 cluster, a distinct subpopulation of ST-spots exclusively present in the SNL mice. Similarly, Hoxb9_29g predominantly displayed activation in the SC_R7 cluster, while Sox10_201g primarily showed the presence in the SC_R1 cluster (Fig. 7b). Spatial maps revealed the distribution of 4 representative regulons, with Hoxb9_29g predominantly present in the spinal SCDH, Sox10_201g primarily located in the spinal white matter, Stat3_11g found to be active in the SCVH, and Egr2_100g enriched in both dorsal and ventral roots (Fig. 7c, upper panel). The t-SNE plot displayed the AUC values of the four aforementioned regulons in the subpopulations of spinal cells. Hoxb9_29g was predominantly expressed in excitatory neurons, Sox10_201g in oligodendrocytes, Stat3_11g among Schwann cells, microglia, astrocytes, and vascular cells, and Egr2_100g in Schwann cells (Fig. 7c, lower panel). The corresponding heatmap provided further insight into the expression pattern of each gene in the representative regulons across the ST-spots, affirming their respective distributions (Fig. 7d). By analyzing the Hoxb9-29 regulon, we found that Hoxb9 targeted Penk promoter region in SCDH neurons. Interestingly, both Hoxb9 and Penk exhibited similar expression patterns in ST-spot maps and snRNA-seq UMAP plots across various cell types (Fig. 7e). Furthermore, luciferase assays have demonstrated that Hoxb9 overexpression significantly increased the activity of the Penk gene promoter, underscoring the potentially crucial role of HOXB9 in regulating Penk expression and supporting the rationality and reliability of our analysis (Fig. 7f). As Penk is a precursor gene for opioid peptides, targeting the HOXB9/PENK axis may represent a potential alternative for activating the SC endogenous analgesia system.
a The binary active heatmap illustrates the activity status of 32 regulons in the SC-spot clusters, as calculated by SECNIC. Black indicates an active status, while white represents an inactive status for the corresponding regulon in that particular ST-spot cluster. b The heatmap illustrates the average AUC score of the 32 regulons in the SC region. The AUC score measures each regulon’s activity level based on its associated geneset. c Spatial AUC scores of selected representative regulons in SC are shown in the upper panel. The lower panel depicts a t-SNE plot displaying the AUC scores of these representative regulons across each cell. d A heatmap displays the expression levels of regulons’ TFs and their target genes across ST-spots, revealing regional-specific distribution patterns. e UMAP and spatial plots depicting the expression patterns of Hoxb9 and Penk. The plots indicate that the two genes exhibit similar patterns. f Left panel (Penk Promoter): the diagram displays the transcription start site (TSS) location and five HOXB9 binding sites. Right Panel (Luciferase Assay): a luciferase assay was performed in HEK-293 cells to evaluate the promoter activity of Penk (−1500 to 0 base pairs) compared to a control vector called pGL3-basic. The effect of HOXB9 overexpression (OE-HOXB9) on Penk promoter activity was compared to a control condition of GFP overexpression (OE-GFP). Values are shown as mean (bar) ± SD (error bar). N = 4, ****P < 0.0001, Student’s t test. g The spatial AUC scores of the Mafb-16g regulon. h The ridge plot (right panel) represents the activity of the Mafb-16g regulon across the sham and SNL ST-spot clusters. i The heatmap illustrates the weighted mean expression of TF Mafb and its target genes from the Mafb-16g regulon across each cell subpopulation identified by snRNA-seq. Microglia exhibit the most prominent expression of these genes.
We identified several differentially expressed regulons by comparing average regulon AUC scores among different ST-spot clusters in sham and SNL groups. Specifically, Irf8_extended_36g and Mafb_16g were found to an increase in SNL SC_R7; Irf9_25g, Stat1_28g, and Irf7_53g showed the increased expression in SC_R1 and SC_R4; and Gata2_extended_10g, Ltf_extended_13g, and Ets2_extended_13g exhibited the elevated expression in SC_R4 (Fig. 7b). Conversely, the expression of Hoxa10_extended_11g, Srebf1_19g, Nr2f2_extended_22g, Eg22_100g, and Maf_77 g was reduced in SC_R4 following SNL (Fig. 7b). These findings offer insights into the differential regulation of regulons in response to SNL and highlight potential targets for further investigation. We then conducted an in-depth analysis and highlighted the key characteristics of the regulon Mafb_16g. The spatial map of AUC regulon showed a significant increase in Mafb_16g expression in both the ipsilateral dorsal and ventral horns of SNL SCs (Fig. 7g). The ridgeline plot indicated a general increase in the range of AUC scores for the Mafb_16g in all SC_clusters, with greater increases observed in SC_R1, SC_R7, and SC_R8 following SNL compared to sham-operated animals (Fig. 7h). Furthermore, the weighted mean expression of genes in Mafb_16g in distinct cell subpopulations based on snRNA-seq data showed that Mafb and the majority of its target genes were mainly expressed in microglial cells of the spinal SCDH, and their expression levels were upregulated after SNI (Fig. 7i). These findings suggest that MAFB may facilitate pain via increasing the expression of downstream genes in spinal microglia.
Similar gene expression patterns in DRG and SCVH after PNI
Proteins synthesized from mRNA transcripts within DRG neurons were transported into the peripheral afferent terminals in the SC. To comprehensively analyze the cross-talking information between DRG neurons and SCDH cells, we examined the changes in gene expression within DRG neurons between sham and SNI mice 10 days after surgeries by bulk-seq (Fig. 8a). The bulk RNA-seq approach identified 183 upregulated and 6 downregulated DEGs in DRG after SNI using the filter criteria of |FoldChange|> 2 and q value < 0.05 (Fig. 8a). Multiple upregulated DEGs in the DRG following SNI overlapped significantly with the genes enriched in SC_R8, a ST-spot cluster that emerged only in the SNL SCVH. Specifically, 55 upregulated DEGs in DRG were enriched in SC_R8, with 29 DEGs exclusively distributed in SC_R8 (Fig. 8b). The violin plot and spatial map displayed the detailed expression patterns of the 29 exclusively distributed genes of SC_R8 (Fig. 8c, d). Immunofluorescence experiments confirmed that ATF3 was upregulated in both the DRG and the ipsilateral SCVH in mice following SNL (Fig. 8e, f).
a Heatmap of DEGs in the DRG after SNI detected by bulk RNA-seq. b The UpSet plot displays the shared genes between DEGs in DRG after SNI and the marker genes that are highly enriched or expressed explicitly in different ST-spot clusters corresponding to different anatomical parts of the SC. c Violin plots illustrating the expression of 29 feature genes in each ST-spot cluster. These genes are uniquely overlapping between the DEGs in DRG and the marker genes specific to the SC_R8 spatial region but not to other clusters. d The spatial expression of the 29 genes as in c in SC connected to the injured peripheral nerve. e, f Immunofluorescence showing the expression of ATF3 in the DRG and SC 10 days after sham and SNL surgeries. Scale bars: 100 μm (e), 200 μm (f).
We also conducted snRNA-seq on DRG taken from mice 10 days post-sham, and SNI surgeries (Fig. 9a). A total of 14,430 and 16,218 cells were successfully sequenced from the sham and SNI groups, respectively. We identified 17 distinct cell clusters in DRG and confirmed their cell types using established marker genes21,22,23. These clusters consisted of fibroblast cells, macrophages, T lymphocytes, satellite glial cells, Schwann cells, vascular cells, and 10 different neuronal subtypes (Fig. 9b, c). The latter included peptidergic nociceptors (PEP1 and PEP2), C-fiber low-threshold mechanoreceptors (cLTMR1), A-fiber LTMR subtypes (NF1, NF2, and NF3), Mrgprd+ non-peptidergic nociceptors (NP), Sst+ pruriceptors (SST), and two subtypes of injured neurons characterized by high Atf3 expression (PNI_N1 and PNI_N2) (Fig. 9c, d). The heatmap highlighted the top five enriched genes for each cluster (Supplementary Fig. 5). These genes had the potential to serve as markers for identifying different cell types in DRG. For example, Nts and Mmp16 could be used as PNI-N1 and PNI-N2 markers, respectively (Fig. 9e and Supplementary Fig. 5). By analyzing the average expression of the above-mentioned 55 overlapping genes across DRG cell clusters, we revealed a distinct preponderance of these genes in two types of injured neuron subtypes, PNI_N1 and PNI_N2 (Fig. 9f). Remarkably, snRNA-seq data served to corroborate our bulk RNA-seq data and further revealed that the average expression of these 55 genes was significantly increased following SNI. The geneset enrichment analysis of GO revealed that the 55 overlapping genes were primarily associated with biological processes such as regulation of the acute inflammatory response, neuropeptide hormone activity, and synapse pruning (Fig. 9g). These genes in injured neurons may be necessary for facilitating intercellular communications within the SCDH, potentially serving as signaling molecules between DRG neurons and spinal cells.
a Schematic representation of the sample preparation process for DRG tissue and workflow for snRNA-seq. Created with BioRender. b Dot plot illustrating the expression of established marker genes used to annotate subpopulations identified through snRNA-seq in DRG. c t-SNE plots displaying identified cell types for sham and PNI (spinal nerve injury) samples, colored based on identified clusters. d t-SNE plots depicting the expression levels of Atf3 in all DRG cells of sham and SNI groups. e t-SNE plots of SNI DRG showing the expression levels of Nts or Mmp16 highly expressing cells. Only cells with expression levels greater than half their respective expression range are displayed. It is observed that Nts are primarily present in the PNI-N1 cluster, while Mmp16 is mainly found in the PNI-N2 cluster, indicating their potential as molecular markers. f Heatmap displaying the weighted mean expression level of 55 shared genes in both the injured DRG and cluster SC_R8. The majority of these genes are distributed within the injured neurons of the DRG, while a small portion is present in macrophages. g Enrichment map of gene ontology (GO) terms belonging to the ‘biological process’ GO category for the 55 overlapping genes. Node size represents the geneset size, and the color scale represents the significance levels. Edges connect highly similar gene sets, which are then grouped into subclusters and annotated using ClueGO.
Altered communication profile between DRG and SCDH in NP
PNI-caused changes in the non-synaptic communications between DRG neurons and SCDH cells participate in NP genesis10. However, the details of these changes are still elusive. To comprehensively explore the cross-talking landscape between DRG neurons and SCDH cells, we performed communication analysis using CellChat from snRNA-seq data of DRG neurons and ST-spots from the SCDH region of SC ST data. Our analysis revealed 2800 L-R signal counts transmitting from DRG to SC and 3358 from SC to DRG under sham surgery conditions (Fig. 10a, b, Supplementary Fig. 6a, and Supplementary Data 6). Interestingly, we observed a significant increase in L-R signal counts under PNI conditions. Specifically, 3067 L-R signal counts from DRG to SC and 4012 L-R pairs from SC to DRG were identified (Fig. 10b, Supplementary Fig. 6a, and Supplementary Data 6). The relative information flow plot illustrated that the cumulative communication probability showed the altered patterns of information exchange between the DRG and SC under sham and PNI conditions (Supplementary Fig. 6). For example, communication pathways, referring to the classes of L_R pairs systematically curated by CellChat on functionally related signaling pathways, NPR1 and TWEAK were found exclusively in the sham group. In contrast, NPY and CCK signals are present in the PNI group. UCN and AGT signals are enhanced after PNI (Supplementary Fig. 6b, c). However, the top 5 L_R pairs (including Psap_Gpr37l1, Psap_Gpr37, Nrxn1_Nlgn2, Fgf1_Fgfr3, and Nrxn3_Nlgn2) that were significantly enriched in both the sham and PNI groups were identical, suggesting that they may have a fundamental role for connecting DRG and SC (Supplementary Fig. 7).
a Schematic representation of the complex interactions between primary sensory afferent and various cell types in the spinal dorsal horn, including neurons, microglia, astrocytes, and others. In addition to synaptic transmission, this illustration highlights the intricate network of molecular interactions contributing to pain processing and transmission in the SC. Created with BioRender. b Circle plot representation of an inferred network with the weighted strength of cell interactions from DRG to SC calculated by Cellchat in sham (left) and PNI (right) groups. Node colors indicate cell types, while size corresponds to cell number. Edge thickness reflects interaction strength, with thicker lines indicating stronger interactions. c Injured DRG communicates with SC through the Csf1_Csf1r pathway. The t-SNE plot of DRG cells visualizes the expression of Csf1, and lines indicate potential communication strength with SC ST-spots. A spatial plot on the far right shows Csf1r expression in SC. Additionally, t-SNE and violin plots of SC cells display the expression feature of Csf1r across different cell types. d Similarly, injured DRG communicates with SC via the Tgfb1_Tgfbr1 pathway. The t-SNE plot of DRG cells illustrates the expression of Tgfb1, with lines indicating potential communication strength with SC ST-spots. The far-right spatial plot shows the Tgfbr1 expression in SC. Furthermore, t-SNE and violin plots of SC cells showcase the expression feature of Tgfbr1. e Representative RNA scope imaging showing Tgfb1 mRNA expression on DRG from sham and SNL mice illustrates upregulation in injured DRG neurons (ATF3 positive). Scale bar = 100 μm. f Representative RNA scope imaging demonstrated a specific upregulation of Tgfbr1 mRNA expression in microglia, as indicated by IBA-1 staining, within the ipsilateral SC of mice subjected to SNL, compared to the contralateral side and sham. Scale bar = 200 μm.
As shown in Fig. 10c, we selected a well-known L_R pair Csf1_Csf1r as an example to demonstrate our analysis pipeline, which mediated the DRG-injured-neuron-triggered pro-inflammatory activation of SC microglia10. The t-SNE plot of DRG snRNA-seq data exhibited a notable upregulation in Csf1 expression in the injured DRG subtypes, PNI-N1, and PNI-N2 (Fig. 10c). Following nerve injury, the strength and number of interactions between DRG neuron and SCDH were higher (Fig. 10c). Moreover, ST spatial map, t-SNE and violin plots of snRNA-seq data displayed Csf1r increased in microglia of SCVH and SCDH after PNI (Fig. 10c). These comprehensive analyses provided detailed insights into changes in Csf1_Csf1r communication between injured DRG neuron and spinal microglia. In addition, our analysis paradigm revealed many unreported and meaningful interactions (Fig. 10d). For example, we found that Tgfb1 expression was also increased in injured DRG neurons following SNI. There were increases in both the strength and quantity of Tgfb1_Tgfbr1 communication between DRG and SCDH (Fig. 10d). SnRNA-seq results from the SCDH confirmed an increase in Tgfbr1 expression in microglia and neurons (Fig. 10d). RNAscope confirmed the increase of Tgfb1 in injured DRG neurons and Tgfbr1 in spinal microglia and neurons (Fig. 10e, f). These findings suggest Tgfb1_Tgfbr1 may facilitate functional communication between peripheral afferents, SCDH microglia, and neurons. We investigated the communication between injured DRG neurons (PNI-N1 and PNI-N2) and each ST-spot cluster and identified multiple exciting L-R pairs (Supplementary Fig. 8a–c). For instance, the activity of many L_R pairs, such as Csf1_Csf1r, Ucn_Crhr1, Tac1_Tacr1, and Fgf1_Fgfr3, significantly increased from injured DRG to cells in the area covered by SC_R7 ST-spots after PNI (Supplementary Fig. 8a–c).
Small C-fiber afferents innervate lamina I–II, whereas lamina III-IV are mainly innervated by cutaneous mechanosensory afferents4. CGRP-positive and IB4-positive neurons in DRG project small C-fiber afferents that typically terminate in the superficial laminae II and III of the SCDH. In contrast, NF200-positive neurons project larger myelinated afferents into deeper layers (including laminae III-IV) of the SCDH (Supplementary Fig. 9a). To accurately investigate the connections between CGRP-positive neurons and lamina II and III cells or between NF200-positive neurons and deeper layers in SCDH, we further performed communication analysis between DRG neurons expressing Calca and SC_R7 ST-spots, as well as neurons expressing Nfeh and SC_R3 ST-spots in the SCDH (Supplementary Fig. 9b–d and Supplementary Fig. 10a, b). Our analysis revealed specific and deregulated connections between Calca-positive neurons and lamina II and III cells and between Nfeh-positive neurons and deeper layers cells in the SCDH after PNI (Supplementary Fig. 9c and d and Supplementary Fig. 10a, b). For instance, the counts of the following L_R pairs: Ptn_Sdc4, Fgf1_Fgfr3, Bdnf_Ntrk2, Psap_Gpr37l1, and Enho_Gpr19, were found an increase in the NP conditions, suggesting that they may mediate the interaction between injured Calca-positive DRG neurons and astrocytes (Supplementary Fig. 9c). Likewise, the heightened counts of App_Cd74, Thy1_(Itgam+Itgb2), and Spp1_Itgb5 could potentially facilitate communication between the above two types of DRG neurons and microglia in NP conditions (Supplementary Fig. 9c and d). In addition, there were significantly downregulated signaling pathways (such as Negr1_Negr1, Lama2_Sv2a) mediating the communication between Calca-positive neurons and spinal excitatory neurons or between Nfeh-positive neurons and excitatory/inhibitory neurons, respectively (Supplementary Fig. 9c, d).
On the contrary, SC cells also acted on the primary afferents from DRG neurons, participating in presynaptic modulation. Pdyn_Oprm1 and Penk_Oprm1 signaling in the endogenous analgesic system that mediates the communication from SCDH neurons to Calca-positive DRG neurons were enhanced after PNI and may act as positive feedback (Supplementary Fig. 10a). Mdk_Ptprz1 facilitated the transmission of information from spinal astrocytes to Nfeh-positive DRG neurons. Its expression was decreased following PNI (Supplementary Fig. 10b). Our analysis in this part provides valuable insights into the interactions between DRG neurons and various cell types within the SC.
Discussion
The structural and signaling networks between primary afferents from DRG neurons and the cells in the SC, including synaptic transmission, L-R systems, and cell-cell junctions, are responsible for encoding and modulating the transmission of different somatosensory information to the central nervous system under physiological or pathophysiological conditions10,24. However, there was no direct high-throughput way to depict the holistic landscape of the static and dynamic mutualisms between DRG and SCDH, especially under NP conditions. This study revealed the intra-SCDH- and inter-DRG/SCDH cellular interactomes based on a combined analysis of multi-transcriptomics data of mouse DRG and SC following sham or PNI surgeries (SNL/SNI). We summarize our findings as the following 4 key points: (1) An integrated snRNA-seq and ST map provided a complete NP-associated picture of the spatial localization, cellular localization, and expression changes of all genes in SC; (2) ST combined with snRNA-seq analysis highlighted the presence of glial and neuronal heterogeneity within SC and outlined the deregulated spatial and cellular distribution of intercellular communication, intracellular signaling, and transcription regulatory networks; (3) DEGs predominantly in injured DRG neurons under PNI conditions overlapped significantly with the genes highly enriched in ipsilateral SCVH, and (4) A comprehensive characterization of the communications between DRG neurons and various SC cells under sham and NP conditions was achieved. These findings may provide support in the direction of future sensory transmission research, especially for pain and peripheral nerve regeneration.
One of the identified ST-spot clusters, SC_R8, was detected explicitly in PNI SC slices in the VH of SC. The SCVH contains motor neurons that send the signals from the SC to muscles, allowing for voluntary movement5. However, recent studies have shown that motor nerve injury contributed to NP genesis5,25,26,27. Our data and the previous studies showed that PNI induced glial cell activation and alterations of gene expression and connectivity in SCVH10,25,26, which may be involved in pain processing. This finding highlights the complexity of pain processing in the SC. Further investigation of SC_R8 and its potential involvement in pain processing could reveal insights into the molecular mechanisms underlying NP.
Revealing the spatial distribution and heterogeneity of different cell subsets is crucial for advancing our understanding of the transmission and modulation of pain and other sensory modalities2,6. Under NP conditions, various subpopulation dynamics of microglia, astrocytes, and neurons have been identified in the SC. Notably, the Apoe-positive microglia subset increased after PNI, while the Mrc1-positive microglia subset decreased. It is well-documented that Apolipoprotein E (Apoe) has been shown to contribute to microglial response in Alzheimer’s disease and microglia functions28. In line with our findings, a prior study also found Apoe to be the most upregulated gene in SC microglia during the chronic phase after peripheral nerve injury in mice, as determined by single-cell RNA sequencing of FACS-sorted microglia29. In addition, we accurately located different types of cells back into the SC spatial structure via CellTrek, providing valuable insights into the heterogeneity and spatial distribution of glial cells and neuron subtypes in the SC. The study revealed subgroups of excitatory neurons, including neurons located simultaneously in either the superficial or deep layer of SCDH or both layers. Inhibitory neuronal subtypes were mainly distributed in the deep layers of SCDH, although some exceptions, such as Gal/Ptprk/Kcnt2 triple-positive neurons, were localized in the superficial layer.
Based on ST and snRNA-seq data under sham and NP conditions, we contextualized SC genes within multiple biological frameworks, including how and where extracellular signals activate intracellular signals to trigger gene regulatory networks. Firstly, we analyzed L_R pairs in the SC and assigned them to cell types based on snRNA-seq data, providing a comprehensive understanding of cell communication. Secondly, our study identified active cell signaling pathways in the SC, showing differences between sham and PNI groups. These findings provide spatial information for targeted intervention and further research. The top enriched signaling pathways in the SCDH superficial layers were related to pain modulation. Specifically, TACHYKININ_RECEPTORS_BIND_TACHYKININ pathway responded to noxious and pruritic stimuli30,31. Inhibition of spinal protein kinase C, a main component of ION_PATHWAY, alleviates pain31,32; Administering PLC inhibitor U73122 intraperitoneally, suppressing PLCD_PATHWAY, increased the pain threshold33. Lastly, our findings also revealed transcriptional regulatory networks exhibited spatial and cell-type-specific features. Specifically, we found that HOXB9 affects Penk expression in SCDH neurons. Tusda’s laboratory has shown that MFAB plays a crucial role in activating SC microglia34. Our analysis also revealed that the Mafb regulon is specifically active in microglia located in the SCDH and SCVH on the ipsilateral side of the nerve injury.
A very impressive finding in this study was that after PNI, DEGs in DRG, most of which are upregulated genes, overlapped significantly with highly expressed genes in the SCVH, in which motor neurons are located. Most overlapping DEGs were located in the injured DRG neurons (PNI-N1 and PNI-N2). This phenomenon may be attributed to the damaged nerves containing both sensory and motor nerves in the mouse models of NP, including SNL, SNI, and CCI. Similar gene expression patterns occur in DRG sensory neurons and SC motor neurons, including Fgf335, Fst36, Gal37, Sox1138, Atf321, and Sprr1a39, suggesting conservative repair signals and regulatory mechanisms. Notably, many of these genes have been proven to be closely related to pain modulation, such as Gpr15140, Anxa1041, Csf110, Nts42, Gal43,44, Gadd45a45, and Npy46. We believe that these genes may not only promote nerve tissue repair and axonal regeneration but also participate in the generation and maintenance of NP. A Previous study suggested that damaging sensory nerves alone cannot cause NP, but damaging motor nerves can25. Although this view is still controversial and remains to be further confirmed, damaged motor neurons are likely implicated in NP genesis.
The connections between the peripheral afferents and SC neurons/glial cells include synaptic transmission and non-synaptic intercellular communications. These specific connections are responsible for coding various sensory modalities, such as sensations of touch, temperature, proprioception, and nociception, from the skin, muscles, and visceral organs to the SC9. DRG Prok2 and spinal Prokr2 neurons were connected by the PROK2_PROKR2 L_R pair, responsible for pleasant touch sensation9. Similarly, DRG Tac1- and spinal Tac1r-neurons were involved in pain sensation through the Substance P_NK1R pair47. DRG Grp- and Npbb-neurons were connected with spinal Grpr- and Npr1-neurons, respectively, contributing to the transmission of itch48. By analyzing the gene expression profile of DRG neurons, we gained insight into the afferently distributed and released proteins, including ion channels, receptors, neuropeptides, and cytokines. Thus, we conducted communication analysis using CellChat between DRG neurons identified by snRNA-seq and ST-spots within the SCDH region and mapped out the interactome. We discovered several traditional communication pathways that facilitated the transmission of pain and itch, including Tac1_Tacr149, Nppb_Npr150, and Calca_Calcrl51. Our analysis provided the advantage of identifying the specific DRG neurons and SC cells involved in particular intercellular communication and the spatial location in SCDH. More importantly, we identified various previously undiscovered communication pathways which provided new perspectives and understanding for research in sensory neuroscience.
PNI leads to altered gene expression profiles in DRG neurons to repair damaged nerves, which also sequentially affects gene expression in SC neurons and glial cells3,8,10. These processes produce maladaptive responses, including a fundamental reshaping of the connection between peripheral afferents and SC cells and the rewiring of the dorsal horn circuit, resulting in the sensitization of peripheral and SC connections52. Our analytical paradigm found significant communication changes between DRG neurons and SCDH cells, particularly between injured DRG neurons and SCDH cells. Our result showed that the L_R pairs Csf1_Csf1r and Tgfb1_Tgfbr1 showed strengthened communications from the injured DRG neurons to spinal microglia after PNI. The CSF1_CSF1R from injured DRG neurons to SC contributing to microglia activation and NP have been well-characterized, suggesting the reliability of our analysis10. Tgfb1_Tgfbr1 also showed strengthened communication from the injured DRG neurons to spinal microglia53. Given that TGF-β signaling plays an important role in promoting the generation of tissue repair macrophage or microglia53,54,55, future work is needed to address the implications of Tgfb1_Tgfbr1 for the generation of spinal pain-resolving microglia56,57. In addition, we also identified enhanced L_R pairs, namely Npy_Gpr83, Ucn_Crhr1, and Fgf1_Fgfr3, which may be closely associated with the modulation of NP. Indeed, it is worth noting that we developed methods to analyze the communication links between a specific type of DRG neuron and cells in the fine laminae of the SC using our data and analytical paradigm, such as the interaction between DRG CGRP neurons and cells in superficial layers of SCDH.
The present study acknowledges the limitation of using different NP models for integrated analysis to reduce the use of laboratory animals. Although slight variations may exist, the SNL and SNI models have demonstrated similar gene expression profiles in both DRG and SC8,21,23,58. Another limitation of the present study is that the snRNA-seq, which analyzes nuclei instead of intact cells, was used to generate sufficient quantities of DRG and SC snRNA-seq data, leading to somewhat reduced coverage due to missing mRNA information from parts of the cytoplasm21,59. Since RNA transcription and splicing occur in the cell nucleus, the nucleus mRNA could reflect the quantity of RNA within cells. Moreover, extensive studies have substantiated the utility of snRNA-seq in analyzing diverse tissues21,60. Despite using a single biological replicate for our snRNA-seq of DRG and SC, pooling tissues from multiple mice provided sufficient sequencing coverage and minimized bias. Moreover, the high consistency of our snRNA-seq data with bulk RNA-seq and ST data further confirms the reliability of our snRNA-seq results. Our research provided valuable information into the molecular pathological alterations in DRG and SC during the NP maintenance phase. However, without analyzing pain progression over time, we could not achieve a comprehensive understanding of the dynamic molecular changes. Future studies should incorporate longitudinal sampling to fully delineate the molecular dynamics of pain.
Conclusion
In summary, our findings provide a comprehensive understanding of the genes, intricate cellular interactions, signaling cascades, and gene regulation networks within the context of cellular and spatial organization in the SC under both sham and NP conditions. We have obtained a comprehensive understanding of the intricate network and communication between DRG and SC at the tissue level, shedding light on their potential involvement in pain regulation. Overall, our work represents a significant step forward in our understanding of the molecular basis of NP and may contribute to developing therapeutic strategies for pain management.
Methods
Animals and pain model generation
Adult (6–8 week old) male ICR mice and C57BL/6 J mice used for ST, bulk RNA-seq or snRNA-seq were purchased from the Experimental Animal Center of Nantong University and Jiangsu Wukong Biotechnology Co. (Nanjing, China). Cx3cr1-GFP mice (005582, B6.129P2(Cg)-Cx3cr1tm1Litt/J, RRID:IMSR_JAX:005582) were obtained from Jackson Lab (Bar Harbor, ME, USA). Simple randomization: Animals are randomly assigned to different experimental groups, ensuring that each animal has an equal chance of being allocated to any group. Randomization of Treatments: The order of treatments was randomized across experimental units to minimize any systematic bias that could arise from a particular sequence. Standardization of Animal/Cage Location: To control for potential location-based biases, efforts were made to standardize the environment across all experimental units. This might include maintaining consistent temperature, humidity, lighting conditions, and cage setups throughout the experiment. The unilateral SNL and SNI models, along with their shams, were created using established methods3,61,62,63. All animals were kept in a 12-h light/dark cycle, Euthanize experimental animals humanely. All experiments conformed to the NIH Guide for Care and Use of Laboratory Animals and the Committee for Research and Ethical Issues of IASP guidelines and were approved by the Animal Care and Use Committee of Nantong University and Zhejiang University (IACUC20220210-2001 and AIRB-2023-0881). We have complied with all relevant ethical regulations for animal use. The work has been reported in accordance with the ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments)64.
10× Visium ST
SC cryosections were sliced to a thickness of 10 μm and attached to GEX arrays. To avoid potential compression on the surgical side, we positioned the lumbar enlargement segment (L4) of the SCs from mice 10 days after the Sham and SNL in horizontally opposite directions, ensuring that they were placed away from each other on the surgical side. ST sequencing, with a sequencing panel area of 0.65 by 0.65, only allows for two SC samples per group (Supplementary Table 1). Then, the sections were incubated at 37 °C, for 1 min with the active surface facing upwards on a Thermocycler Adaptor. Afterward, they were fixed for 30 min using methyl alcohol at −20 °C, and then stained with H&E (Eosin, Dako CS701, Hematoxylin Dako S3309, bluing buffer CS702, CA, USA). At 10X resolution, the brightfield images were scanned with a Leica DMI8 whole-slide scanner (Wetzlar, Germany). Spatial gene expression analysis was performed using the Visum spatial gene expression slide and Reagent Kit (10x Genomics, PN-1000184). A Slide Cassette was utilized to ensure the formation of leakproof wells for the addition of reagents. The Permeabilization enzyme (70 μl) was introduced and incubated at 37 °C, for 30 min. Post incubation and washing by 100 μl of SSC buffer (sodium chloride/sodium citrate buffer), 75 μl of reverse transcription Master Mix was added for the first-strand cDNA Synthesis. The second-strand of cDNA was synthesized in 75 μl of the Second-Strand Mix using an S1000TM Touch Thermal Cycler (Bio-Rad, Hercules, CA, USA) after steps of 0.08 M KOH incubating and 100 μl EB buffer (19086, Qiagen, Hilden, Germany) washing. The Visum spatial Library construction kit (10x Genomics, PN-1000184, Pleasanton, CA, USA) was used to construct Visum spatial libraries. Finally, the libraries were sequenced using an Illumina Novaseq 6000 sequencer (Illumina, San Diego, CA, USA) with a sequencing depth of no less than 100,000 reads per ST-spot, adopting a pair-end 150 bp (PE150) reading strategy (performed by CapitalBio Technology, Beijing).
snRNA-seq
For snRNA-seq, one sample per group was created by pooling ipsilateral spinal dorsal horn (SDH) at the lumbar enlargement segment (L4) or L3-L5 DRGs from three mice 10 days after unilateral SNI or sham surgeries (Supplementary Table 1). To obtain a homogenate, the DRG or SC tissues were ground in pre-cooled lysis buffer. The resulting homogenate was then washed using nuclear wash buffer and filtered through a 70-μM strainer to remove debris. Next, around 1 mL of the filtrate was mixed successively with 1 mL of 33% and 2 mL of 50% iodixanol and centrifuged at 4 °C for 20 minutes at 10,000 × g. After centrifugation, about 3.5 mL of the upper layer was transferred to a new 15 mL tube, washed with nuclear wash buffer for 8 minutes, and then centrifuged at 4 °C, 500 × g for 8 minutes. The resulting supernatant was filtered through a 40-μM strainer and underwent standard processing to remove debris using the Debris Removal Kit (Miltenyi Biotech, Bergisch-Gladbach, Germany). Finally, nuclei were counted by trypan blue staining, and the nucleus suspension was adjusted to a final concentration of 700 ~ 1200 nucleus/µl. Next, the nucleus suspensions were loaded onto a 10X Genomics GemCode Single-cell device (10x Genomics, Pleasanton, CA, USA) to create single-nucleus Gel Bead-In-EMlusion (GEMs). Libraries were produced and sequenced from the cDNAs using Chromium Next GEM Single Cell 3’ Reagent Kits v3.1 (10× Genomics, PN-1000121, Pleasanton, CA). Upon dissolving the Gel Bead within a GEM, primers that contain (i) an Illumina® R1 sequence (sequencing primer for read 1), (ii) a 16 nt 10x Barcode, (iii) a 10 nt Unique Molecular Identifier (UMI), and (iv) a poly-dT primer sequence were released and combined with nucleus lysate and Master Mix. Following this, Barcoded, full-length cDNAs were reverse transcribed from poly-adenylated mRNA. Magnetic beads coated with silane were utilized to remove any residual biochemical reagents and primers from the post-GEM reaction mixture. Afterward, a PCR was conducted to amplify full-length, barcoded cDNAs for library construction. Next, the R1 primer sequence was added to the molecules during the GEM incubation process. Subsequently, the library construction process involved adding P5, P7, a sample index, and R2 primer sequence through a series of steps, including End Repair, A-tailing, Adaptor Ligation, and PCR. The resulting libraries included the P5 and P7 primers which are used in Illumina bridge amplification. The sequencing process was performed by Gene Denovo Biotechnology Co. using the NovaSeq 6000 system (Illumina in San Diego, CA, USA). The Illumina-ready sequencing libraries were generated using the Single Cell 3’ Protocol. A Single Cell 3’ Library consisted of typical Illumina paired-end structures, which commenced and terminated with P5 and P7. Within Read 1, the Single Cell 3’ 16 bp 10x Barcode and 10 bp UMI were encoded, while Read 2 was employed to sequence the cDNA fragment. Sample index sequences were incorporated as the i7 index read. Read 1 and Read 2 served as standard Illumina® sequencing primer sites in paired-end sequencing.
Bulk RNA-seq
In bulk RNA-seq, each group comprised three samples: SC sample from the L4 lumbar enlargement segment of a mouse after unilateral SNL or sham, each DRG sample pooled from the L3-L5 DRGs of three mice 10 days after SNI or sham procedures (Supplementary Table 1). Total RNA was isolated using the Trizol reagent kit (Invitrogen, Carlsbad, CA, USA), and evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and RNase-free agarose gel electrophoresis. Next, mRNA was enriched utilizing Oligo(dT) beads and then fragmented using a fragmentation buffer and reverse transcribed into cDNA using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB #7530, New England Biolabs, Ipswich, MA, USA). The purified double-stranded cDNA fragments were subjected to end-repaired, followed by the addition of an A base and ligation to Illumina sequencing adapters. The ligation reaction was purified using the AMPure XP Beads (1.0×). Afterward, the ligated fragments underwent size selection through agarose gel electrophoresis and PCR amplified. The cDNA library was sequenced using Illumina Novaseq 6000 by Gene Denovo Biotechnology Co. (Guangzhou, China).
Analysis of ST data
The Space Ranger count module was utilized to perform alignment, filtering, barcode counting, and UMI enumeration, creating a feature-barcode matrix and cluster identification. Seurat 4.2.1 was used to normalize, estimate the variance of raw filtered data, and identify the most variable genes. PCA was implemented for dimensionality reduction, and t-SNE and UMAP visualization were utilized to display data. The FindAllMarkers() function was used to analyze marker genes in each cell subpopulation. The Pearson correlation coefficients between different regions were calculated using the cor() function.
Analysis of snRNA-seq data
10X Genomics Cell Ranger software (Version 3.1.0) was utilized to transform raw BCL files into FASTQ files, alignment and counts quantification. For downstream analysis, the outcomes were imported into Seurat (Version 4.2.1)65.
Cells that exhibited an unusually high number of genes (≥7500) or a mitochondrial gene percentage of (≥5%) were promptly eliminated from the analysis. Furthermore, doublet GEMs were identified and excluded by the application of the DoubletFinder (Version 2.0.3) tool66. The data obtained from all samples were merged using Seurat’s merge function, followed by the normalization process using ScTransform. Dimensionality reduction clustering was performed using the RunPCA and RunTSNE functions. The final number of principal components (PCs) was determined to be 20, based on the inflection point of the ElbowPlot function. The core cell clusters were identified using Seurat’s FindClusters function with a clustering resolution of 1.5. Cell types were manually annotated based on representative marker genes for each cell type, according to the clustering results. The subset() function was used to classify the neurons further and set the clustering resolution at 0.8. The clustered results were then merged with the non-neuronal subpopulation for further analysis. Astrocytes and microglia were separated using subset() function with clustering resolution 1.7 and 1, respectively. FindAllMarkers function was used to analyze marker genes between cell subpopulations.
Analysis of bulk RNA-seq data
RNA differential expression analysis was conducted between two distinct groups utilizing limma67 and edgeR68, with criteria: fold change ≥2 and false discovery rate below 0.05.
Relatedness analysis
To analyze the relatedness of neuron subclusters, the mean expression per gene (with CPM expression >0) was calculated for each cluster in R, and the dist() function was used to generate a Euclidean-based distance metric for all pairwise combinations of clusters. Hierarchical clustering was performed utilizing complete agglomeration via the hclust() function.
CellTrek
We employed the Celltrek69 package to retrace the cell types found in snRNA-seq datasets. Specifically, we utilized the “traint” function to co-embed the snRNA-seq datasets with ST datasets. The coembedded traint data was then subjected to Celltrek. For visualization, we used the celltrek_vis tool and evaluated colocalization with the “sColoc” function.
SCENIC analysis
SCENIC R package was applied to infer the TF network70. The pipeline consisted of three stages. Initially, we identified the gene co-expression network through GENIE371. Subsequently, each module was refined by RcisTarget based on a regulatory motif in proximity to the transcription start site. We retained networks where the TF-binding motif was enriched among its targets, while target genes lacking direct TF-binding motifs were excluded, resulting in retained networks called regulons. Finally, we measured the activity of each regulon for each single-cell using the AUCell R package and scored them with AUC scores.
Cell communications
Cell communication analysis was performed utilizing CellChat (Version 1.5.0)72. To compare the communications between sham and PNI groups, the CellChat objects were merged using the functions liftCellChat() and mergeCellChat().
STlearn
The ST data for L_R Interactions was analyzed using STlearn73. We extracted ligand and receptor genes from enriched L_R pairs identified by stLearn analysis on ST data. Next, we intersected these genes with the DEGs identified by bulk RNA-seq of the SC from sham and SNL mice 10 days after surgery to identify the deregulated L_R pairs. Biological Process (BP) analysis was performed with ClueGO (P < 0.01)74.
Pathway activity analysis
Area under the curve (AUC) was evaluated using AUCell v1.1870. The genes in each ST-spot of ST data or in each cell of snRNA-seq data were sorted by expression level using the AUCell_buildRankings() function. For Pathway activity analysis, we used mouse canonical pathways from MsigDb (M2:CP). Using the AUCell_calcAUC() function, we calculated the percentage of genes within each geneset that appeared among the top 5% of expressed genes in each ST-spot or cell and allocated it as an AUC score for the corresponding geneset in each ST-spot or each cell. The AUC scores represented the percentage of genes derived from each M2:CP pathway found among the top 5% of expressed genes in each ST-spot or each cell.
Immunofluence
Immunofluorescence staining was conducted on 10-μm sections of the lumbar DRG and 30-μm sections of the SC lumbar enlargement3. The DRG or SC slices were incubated overnight at 4 °C with one or two (in double labeling experiments) of the primary antibodies against the following proteins: Complement component 1q (C1Q, rabbit, 1:200, Abcam, Cambridge, UK, ab182451, RRID:AB_2732849), Choline transporter 1 (CHT1, rabbit, 1:200, Millipore, Chicago, USA, ABN458), Protein kinase C gamma (PKC γ, SC-211, rabbit, 1: 500, Santa Cruz Biotechnology, CA, USA, RRID:AB_632234), Neuronal nuclear protein (NeuN, mouse, 1:1000, Millipore, Chicago, USA, MAB377, RRID:AB_2298772), Glial fibrillary acidic protein (GFAP, mouse, 1:1000, Millipore, Chicago, USA, MAB360, RRID:AB_11212597), Ionized calcium-binding adaptor molecule 1 (IBA-1, goat, 1: 500, Abcam, Cambridge, UK, ab5076, RRID:AB_2224402), Calcitonin gene-related peptide (CGRP, goat, 1:300, Abcam, Cambridge, UK, ab36001, RRID:AB_725807), and the 200 kD neurofilament protein (NF200, mouse, 1:2000, Millipore, Chicago, USA, MAB5266, RRID:AB_2149763). After 1 hour of incubation at room temperature, the sections were washed with PBS three times for 15 min each and then incubated with the following secondary antibodies: donkey anti-rabbit 594 (1:500, Invitrogen, CA, USA, A-21207, RRID:AB_141637), donkey anti-mouse 488 (1:500, Invitrogen, CA, USA, A-21202, RRID:AB_141607), donkey anti-goat 488 (1:500, Invitrogen, CA, USA, A32814, RRID:AB_2762838). Biotinylated isolectin B4 (IB4, L2140, 1:50, Sigma, Darmstadt, Germany, RRID:AB_2313663) was used to mark small non-peptidergic sensory neurons and afferent, followed by streptavidin-Fluorescein isothiocyanate (FITC) (S3762, Sigma, Darmstadt, Germany) to develop signals. After immunostaining, the nuclei were stained with DAPI (28718-90-3, 0.1 μg/mL, Sigma, Darmstadt, Germany). Finally, the sections were imaged by a Leica Thunder system (LEICA DM6 B, Wetzlar, Germany) and a Nikon Ni-E microscope (Nikon ECLIPSE Ni-E, Tokyo, Japan).
RNAscope
Fixed SC and DRG were cut into 30 μm and 14 μm-thick sections, respectively, to be subjected to RNA-Scope using the RNAscope™ 2.5 HD Detection Reagents-RED [#322360, Advanced Cell Diagnostics (ACD), Newark, CA]. Generally, sections underwent steps of baking, hydrogen peroxide and protease plus treatment sequentially and then hybrid with Probes targeting mouse Tgfb1 (#407751, ACD, Newark, CA), Tgfbr1 (#406201, ACD, Newark, CA), Ly86 (#579381, ACD, Newark, CA), or Adam8 (#1047421-C1, ACD, Newark, CA). Following the signal amplification, Fast RED-A and Fast RED-B were used for signal detection. Immediately after, these sections underwent additional immunofluorescence experiment under light-shielded conditions with primary antibodies targeting ATF3 (rabbit, Santa Cruz Biotechnology, CA, USA, sc-188) or IBA-1 (goat, 1: 500, Abcam, Cambridge, UK, ab5076). Images were collected with the Leica Thunder system (LEICA DM6 B, Wetzlar, Germany).
qRT-PCR
Total RNA was extracted from the SC using TRIzol reagent (No. 15596018, Invitrogen). The cDNA was synthesized from 500 ng total RNA using the Hifair II1strand cDNA synthesis super mix kit (11123ES60p, Yeasen, Shanghai, China). The Hieff UNICON qPCR SYBR Green Master Mix kit (11200ES08, Yeasen) was used to perform PCR with primer sets: Ly86 forward 5-CTG CCC TCC TTG TGT GGA TTC-3; Ly86 reverse 5′-TGG AAC ACT GGT CAA TGG AAA G-3; Gapdh forward 5′-AGG TCG GTG TGA ACG GAT TTG-3; Gapdh reverse 5′-TGT AGA CCA TGT AGT TGA GGT CA-3′. The Roche LightCycler480II machine was utilized for the PCR process, 95°C (30 s) and 40× [95°C (5 s), 60°C (30 s), 72°C (30 s)]. The melting point curve verifies the specificity of each PCR product. The 2−△△Ct method was used for relative quantitative analysis.
Luciferase assay
To identify the promoter activity of Penk, HEK293T cells cultured in the 12-well culture plates were transfected with 0.5 µg plasmid pGL3-basic (Promega, Madison, WI, USA) or pGL3-Penk-promoter constructed by Genecreate (Wuhan Genecreate Biotechnology Engineering Co., Ltd., Wuhan, China) that encompasses a 1500 bp region preceding the transcription start site. We also transfected the pGL3-Penk-promoter plasmid with control GFP- or HOXB9 overexpression plasmids (Genecreate, Wuhan, China) into 293 T cells to investigate the role of HOXB9 on Penk transcription. After 48 h of transfection, the firefly activities were measured using the Dual-Luciferase® reporter assay system (E1910, Promega, Madison, USA) according to the introduction in Infinite E Plex multimode microplate reader (Tecan, Männedorf, Switzerland).
Statistics and reproducibility
The locally distinguishing algorithm of the Loupe Browser was used to conduct significant feature comparison analyses to distinguish each cluster from the cells or ST spots in the other selected clusters, and the p values of each feature were adjusted using the Benjamini-Hochberg correction for multiple tests. One-way ANOVA was employed to compare means among three or more independent groups. The differences between the two groups were analyzed using the Student’s t test. Data were presented as mean ± SD, with statistical significance defined as P < 0.05. GraphPad Prism is used for statistical analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data associated with this study are present in the main text or the Supplementary Materials. All materials and primary data used in this study are available upon request. The newly constructed plasmids, pAAV-MCS-Hoxb9-CDS (ID: 232237) and pGL3-Basic-Penk-promoter (ID: 232238) have been deposited in the Addgene repository. The raw sequence data of the ST sequencing, snRNA-seq, and bulk RNA-seq reported in this paper have been deposited in the Genome Sequence Archive at the National Genomics Data Center China75,76, National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA011548, OMIX008153, and OMIX008150). The primary datasets have been deposited in figshare and can be accessed via the following https://doi.org/10.6084/m9.figshare.2806916077. The numerical data for all manuscript graphs are in supplementary data 7 for main figures and supplementary data 8 for Supplementary Figs.
Code availability
All codes used to reproduce all analyses in this study are available at https://github.com/Jianglab1/SC_DRG-bulk_single_spatial78.
References
Colloca, L. et al. Neuropathic pain. Nat. Rev. Dis. Prim. 3, 17002 (2017).
Woolf, C. J. Pain modulation in the spinal cord. Front Pain. Res. 3, 984042 (2022).
Jiang, B. C. et al. NFAT1 orchestrates spinal microglial transcription and promotes microglial proliferation via c-MYC contributing to nerve injury-induced neuropathic pain. Adv. Sci. 9, e2201300 (2022).
Del Barrio, M. G. et al. A transcription factor code defines nine sensory interneuron subtypes in the mechanosensory area of the spinal cord. PloS One 8, e77928 (2013).
Koch, S. C., Acton, D. & Goulding, M. Spinal circuits for touch, pain, and itch. Annu Rev. Physiol. 80, 189–217 (2018).
Todd, A. J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 11, 823–836 (2010).
Knotkova, H. et al. Neuromodulation for chronic pain. Lancet 397, 2111–2124 (2021).
Jiang, B. C. et al. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J. Clin. Invest. 126, 745–761 (2016).
Liu, B. et al. Molecular and neural basis of pleasant touch sensation. Science 376, 483–491 (2022).
Guan, Z. et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 19, 94–101 (2016).
Bressan, D., Battistoni, G. & Hannon, G. J. The dawn of spatial omics. Science 381, eabq4964 (2023).
Maniatis, S. et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364, 89–93 (2019).
Hasel, P., Rose, I. V. L., Sadick, J. S., Kim, R. D. & Liddelow, S. A. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 24, 1475–1487 (2021).
Li, X. et al. Profiling spatiotemporal gene expression of the developing human spinal cord and implications for ependymoma origin. Nat. Neurosci. 26, 891–901 (2023).
Sathyamurthy, A. et al. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep. 22, 2216–2225 (2018).
Pillai, A., Mansouri, A., Behringer, R., Westphal, H. & Goulding, M. Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development 134, 357–366 (2007).
Niehaus, J. K., Taylor-Blake, B., Loo, L., Simon, J. M. & Zylka, M. J. Spinal macrophages resolve nociceptive hypersensitivity after peripheral injury. Neuron 109, 1274–1282 e1276 (2021).
Brewer, C. L., Styczynski, L. M., Serafin, E. K. & Baccei, M. L. Postnatal maturation of spinal dynorphin circuits and their role in somatosensation. Pain 161, 1906–1924 (2020).
Jiang, B. C., Liu, T. & Gao, Y. J. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharm. Ther. 212, 107581 (2020).
Milligan, E. et al. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur. J. Neurosci. 22, 2775–2782 (2005).
Renthal, W. et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 108, 128–144 e129 (2020).
Yang, L. et al. Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron 110, 1806–1821 e1808 (2022).
Wang, K. et al. Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain. Cell Res. 31, 904–918 (2021).
Zamponi, G. W., Striessnig, J., Koschak, A. & Dolphin, A. C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharm. Rev. 67, 821–870 (2015).
Liu, X. G., Pang, R. P., Zhou, L. J., Wei, X. H. & Zang, Y. Neuropathic pain: sensory nerve injury or motor nerve injury? Adv. Exp. Med. Biol. 904, 59–75 (2016).
Zang, Y. et al. Calpain-2 contributes to neuropathic pain following motor nerve injury via up-regulating interleukin-6 in DRG neurons. Brain Behav. Immun. 44, 37–47 (2015).
He, X. H. et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain 151, 266–279 (2010).
Yin, Z. et al. APOE4 impairs the microglial response in Alzheimer’s disease by inducing TGFβ-mediated checkpoints. Nat. Immunol. 24, 1839–1853 (2023).
Tansley, S. et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat. Commun. 13, 843 (2022).
Duan, B., Cheng, L. & Ma, Q. Spinal circuits transmitting mechanical pain and itch. Neurosci. Bull. 34, 186–193 (2018).
Huang, T. et al. Identifying the pathways required for coping behaviours associated with sustained pain. Nature 565, 86–90 (2019).
Velazquez, K. T., Mohammad, H. & Sweitzer, S. M. Protein kinase C in pain: involvement of multiple isoforms. Pharm. Res. 55, 578–589 (2007).
Shi, T. J. et al. Phospholipase Cbeta3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain. Proc. Natl Acad. Sci. USA 105, 20004–20008 (2008).
Tozaki-Saitoh, H. et al. Transcription factor MafB contributes to the activation of spinal microglia underlying neuropathic pain development. Glia 67, 729–740 (2019).
Goldshmit, Y. et al. Different Fgfs have distinct roles in regulating neurogenesis after spinal cord injury in zebrafish. Neural Dev. 13, 24 (2018).
Li, X. J., Morgan, C., Goff, L. A. & Doetzlhofer, A. Follistatin promotes LIN28B-mediated supporting cell reprogramming and hair cell regeneration in the murine cochlea. Sci. Adv. 8, eabj7651 (2022).
Holmes, F. E. et al. Targeted disruption of the galanin gene reduces the number of sensory neurons and their regenerative capacity. Proc. Natl Acad. Sci. USA 97, 11563–11568 (2000).
Jankowski, M. P. et al. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 1256, 43–54 (2009).
Bonilla, I. E., Tanabe, K. & Strittmatter, S. M. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J. Neurosci. 22, 1303–1315 (2002).
Jiang, B. C. et al. G protein-coupled receptor GPR151 is involved in trigeminal neuropathic pain through the induction of Gbetagamma/extracellular signal-regulated kinase-mediated neuroinflammation in the trigeminal ganglion. Pain 162, 1434–1448 (2021).
Lu, Y., Ni, S., He, L. N., Gao, Y. J. & Jiang, B. C. Annexin A10 is involved in the development and maintenance of neuropathic pain in mice. Neurosci. Lett. 631, 1–6 (2016).
Richner, M. et al. Sortilin gates neurotensin and BDNF signaling to control peripheral neuropathic pain. Sci. Adv. 5, eaav9946 (2019).
Liu, H. X. & Hokfelt, T. The participation of galanin in pain processing at the spinal level. Trends Pharm. Sci. 23, 468–474 (2002).
Chen, Y. et al. Distinct neural networks derived from galanin-containing nociceptors and neurotensin-expressing pruriceptors. Proc. Natl. Acad. Sci. USA 119, e2118501119 (2022).
Li, J. & Chen, J. GADD45A induces neuropathic pain by activating P53 apoptosis pathway in mice. Genes Genomics 44, 1051–1060 (2022).
Ossipov, M. H. et al. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J. Neurosci. 22, 9858–9867 (2002).
Gautam, M. et al. Role of neurokinin type 1 receptor in nociception at the periphery and the spinal level in the rat. Spinal cord. 54, 172–182 (2016).
Chen, Z. F. A neuropeptide code for itch. Nat. Rev. Neurosci. 22, 758–776 (2021).
Otten, U., Goedert, M., Mayer, N. & Lembeck, F. Requirement of nerve growth factor for development of substance P-containing sensory neurones. Nature 287, 158–159 (1980).
Solinski, H. J. et al. Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Sci. Transl. Med. 11, eaav5464 (2019).
McCoy, E. S., Taylor-Blake, B. & Zylka, M. J. CGRPalpha-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PloS One 7, e36355 (2012).
Tansley, S. et al. Microglia-mediated degradation of perineuronal nets promotes pain. Science 377, 80–86 (2022).
Taylor, R. A. et al. TGF-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Invest 127, 280–292 (2017).
Wang, Y. et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci. Adv. 7, eabb6075 (2021).
Li, Z. et al. M-CSF, IL-6, and TGF-beta promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci. Adv. 7, eabb6260 (2021).
Sideris-Lampretsas, G. & Malcangio, M. Pain-resolving microglia. Science 376, 33–34 (2022).
Kohno, K. et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science 376, 86–90 (2022).
Nguyen, M. Q., Le Pichon, C. E. & Ryba, N. Stereotyped transcriptomic transformation of somatosensory neurons in response to injury. Elife 8, e49679 (2019).
Velmeshev, D. et al. Single-cell genomics identifies cell type-specific molecular changes in autism. Science 364, 685–689 (2019).
Slyper, M. et al. A single-cell and single-nucleus RNA-Seq toolbox for fresh and frozen human tumors. Nat. Med. 26, 792–802 (2020).
Jiang, B. C. et al. Promoted interaction of C/EBPalpha with demethylated Cxcr3 gene promoter contributes to neuropathic pain in mice. J. Neurosci. 37, 685–700 (2017).
Richner, M., Bjerrum, O. J., Nykjaer, A. & Vaegter, C. B. The spared nerve injury (SNI) model of induced mechanical allodynia in mice. J. Vis. Exp. 18, 3092 (2011).
Ma, L. et al. ZNF382 controls mouse neuropathic pain via silencer-based epigenetic inhibition of Cxcl13 in DRG neurons. J. Exp. Med. 218, e20210920 (2021).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337.e324 (2019).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Wei, R. et al. Spatial charting of single-cell transcriptomes in tissues. Nat. Biotechnol. 40, 1190–1199 (2022).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Huynh-Thu, V. A., Irrthum, A., Wehenkel, L. & Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS One 5, e12776 (2010).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Pham, D. et al. stLearn: integrating spatial location, tissue morphology and gene expression to find cell types, cell-cell interactions and spatial trajectories within undissociated tissues. bioRxiv https://www.biorxiv.org/content/10.1101/2020.05.31.125658v1 (2020).
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Chen, T. et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics 19, 578–583 (2021).
Members, C.-N. Database resources of the national genomics data center, China national center for bioinformation in 2022. Nucleic Acids Res. 50, D27–D38 (2022).
Jiang, B. C. Time-course analysis of Ly86 mRNA expression and the effect of HOXB9 on the Penk promoter. figshare https://doi.org/10.6084/m9.figshare.28069160 (2024).
Jiang, B. C. Codes used to reproduce all analyses in this study. Zenodo https://doi.org/10.5281/zenodo.14533892 (2024).
Acknowledgements
We would like to thank Jiang Cao, Xing Zhang, Zhi-Wei, Zhong, Han-Xiao Yu, and Yan Feng (the Second Affiliated Hospital of Zhejiang University) for their technical support. This research was supported by grants from National Nature Science Foundation of China (81971054, 82371216, 81874171, 82371217, 82071227, 82301386); Key Project of Zhejiang Provincial Natural Science Foundation of China (LZ23H090003, Z25H090013); National Clinical Key Specialty Construction Project of China (2021-LCZDZK-01); Natural Science Research Program of Zhejiang Province (LQ23H090010). The research is also supported by the programs of Leading Health Talents of Zhejiang Province No. 18 (2020), the China Postdoctoral Science Foundation (2022TQ0273), and the Zhejiang Province Postdoctoral Research Projects Selected Funding (ZJ2022073).
Author information
Authors and Affiliations
Contributions
B.C.J. and F.L.D. conceptualized the project and designed the experiments. F.L.D., B.C.J., L.N.Y., P.D.F., and Y.F.C. performed the bioinformatics analysis. J.X.R., X.H.B., J.Q.L., Y.T.D., D.L.C., H.H.S., Y.Z., and H.G. carried out immunofluorescence staining, RNA Scope, and qRT-PCR. D.Q.C., M.N.L., P.F.Z., and N.H. prepared NP models. W.X.S. performed the luciferase assay. B.C.J., F.L.D., M.Y., and Y.X.T. draft the article. B.C.J., Y.X.T., and M.Y. revised the paper. Y.X.M., L.F.M., M.Z.Y., X.J.Z., B.L.Z., Y.L., and D.Z. participated in revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, FL., Yu, L., Feng, PD. et al. An atlas of neuropathic pain-associated molecular pathological characteristics in the mouse spinal cord. Commun Biol 8, 70 (2025). https://doi.org/10.1038/s42003-025-07506-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07506-0
This article is cited by
-
Senescent Nociceptors: A Novel Therapeutic Target for Chronic Pain Treatment
Neuroscience Bulletin (2025)