Abstract
In Arabidopsis, effector-triggered immunity (ETI) against avirulent Pseudomonas syringae pv. tomato (Pst) correlates with the rapid, Dicer-Like 1 (DCL1)-dependent nuclear accumulation of a 31-nt 5'-tRNA fragment derived from Asp-tRNA (tRF31Asp2). Several tRFs, including tRF31Asp2, are induced at early stages of infection and associate with AGO2 in the nucleus. Infiltrating Arabidopsis leaves with synthetic tRF31Asp2 induces over 500 defense-associated genes, conferring immunity against virulent and avirulent Pst as well as aphids, while tRF31Asp2 depletion compromises resistance to avirulent Pst. The biological activity of tRF31Asp2 requires its 5' sequence and predicted stem-loop structure, and its loading into AGO2 or related clade members may contribute to activating defense responses. Chromatin affinity precipitation-sequencing revealed that tRF31Asp2 binds specific sequences in defense genes and the Gypsy superfamily of LTR retrotransposons, particularly at their primer binding sites (PBS). tRF31Asp2 binding appears to modulate transcriptional reprogramming, inducing neighboring tRF-responsive defense genes while suppressing active retrotransposons. Since Gypsy retrotransposon proliferation is primed by tRNA binding at PBS, tRF31Asp2 may exploit a similar mechanism to coordinate defense responses. Together, these findings reveal a role for DCL1 and tRF31Asp2 in regulating plant immunity and transcriptional dynamics at defense-associated loci and retrotransposons.
Similar content being viewed by others
Introduction
Plants resist pathogens that breach their passive defenses by detecting conserved Pathogen-Associated Molecular Patterns (PAMPs) and activating PAMP-triggered immunity (PTI). Some pathogens have evolved effectors that, following transport into host cells, facilitate evasion/suppression of PTI1. The low resistance observed in plants infected with these pathogens is termed basal immunity. In turn, plants have evolved resistance (R) proteins that interact with their cognate pathogen-encoded effector(s) and induce a robust defense response termed effector-triggered immunity (ETI)2,3. Interestingly, most transcripts induced during ETI also accumulate during PTI and basal immunity, albeit with weaker or delayed transcriptional dynamics4,5. Recent reports also revealed that the two-tiered immune cascades, ETI and PTI, share a significant number of downstream components, leading to mutual reinforcement6,7.
RNA silencing is a critical mechanism whereby noncoding small RNAs (sRNAs) regulate gene expression at either the post-transcriptional level, by modulating RNA stability/translatability, or at the transcriptional level, via DNA/histone modification or chromatin remodeling8. sRNAs are generated mostly by ribonuclease III-like enzymes, termed Dicer-like (DCL) proteins in plants6. Following loading onto Argonaute (AGO) proteins to form RNA-induced silencing complexes (RISC), the sRNA guide strand directs the RISC to complementary RNA targets for post-transcriptional or transcriptional gene silencing9. In Arabidopsis (Arabidopsis thaliana), infection with avirulent bacterial pathogen Pseudomonas syringae pv. tomato (Pst) carrying the effector avrRpt2 (Pst_avrRpt2) significantly induces AGO2, which together with its homologs AGO3 and AGO7 is required for full ETI10.
In addition to their conventional role in the translational machinery, transfer RNAs (tRNA) exhibit various noncanonical functions such as chromatin organization, DNA repair, and immune responses11,12. For instance, a 2′-O-Methyltransferase that modifies tRNA anticodon has been found to play a role in Arabidopsis resistance to Pst13. Interestingly, sRNAs derived from tRNAs (tsRNAs) are also identified as critical regulatory molecules14 and found in essentially all species ranging from bacteria to humans15. Several classes of tsRNAs have been identified. tRNA-halves (~35 nts), generated via cleavage at the anticodon loop by a ribonuclease such as angiogenin, inhibit protein synthesis by targeting translation machinery16,17,18. The remaining classes, collectively termed tRNA-derived fragments (tRFs), include 5′-tRFs, 3′-tRFs, and i-tRFs19. tRFs (<~32 nts) are generated by nucleases like Dicer and RNase Z, and associate with AGOs to mediate post-transcriptional gene silencing14. Furthermore, tRFs can be categorized based on their cleavage sites. For example, tRF-5a, tRF-5b, and tRF-5c belong to the group of 5′-tRFs, which are produced by cleaving the D-loop, D-stem, and anticodon stem, respectively12,20.
Short tRFs that resemble microRNAs in size are associated with Argonaute (AGO) proteins, performing functions similar to microRNAs15,21. In human HEK293T cells, for example, upregulating 3′-tRFs leads to the widespread repression of target genes21. Rhizobial 3′-tRFs use host AGO1 to repress negative symbiotic regulators in the host22. tRFs are also shown to be associated with transposons; 3′-tRFs hinder the replication of endogenous retroviruses (ERVs) by targeting its primer-binding site (PBS)23, while long 5′-tRFs, especially tRF-5c, in mouse sperm and embryonic stem cells (ESCs) suppress MERVL retrotransposons24. Given this mounting evidence of tsRNAs’ significance in biology, this study presents an unique function of a long 5′-tRF dependent on DCL1 in plant immunity and provides its underlying mechanical mechanism, in which the tRF physically associates target genes to modulate their transcription.
Results
DCL1, a putative positive regulator in defense responses
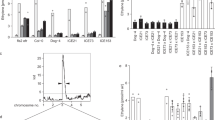
We previously showed that some Arabidopsis ago and dcl mutants are compromised for basal resistance to virulent Pst and/or ETI to avirulent Pst_avrRpt225. Bacterial growth and the expression of the defense gene Pathogenesis-related 1 (PR1) were monitored in both dcl1-7 and wild-type (WT) plants to confirm the impact of DCL1 loss on ETI with minimally affecting basal resistance. dcl1-7 supported significantly higher levels of Pst_avrRpt2 than WT at 3 day-post-inoculation (dpi), whereas growth of Pst in these plants was marginally higher (Fig. 1a). PR1 expression was significantly dampened in Pst_avrRpt2-inoculated dcl1-7 vs WT at 6 h-post-inoculation (hpi); however, it was marginally different at later points, or at any timepoint after Pst inoculation (Fig. 1b). Thus, DCL1 may play an early role in the induction of defense responses, though its direct impact is uncertain given its extensive function in sRNA biogenesis.
a Bacterial growth in wild type (WT) and dcl1-7 at 0 and 3 day-post-infection (dpi) with Pst containing or lacking AvrRpt2 (Pst_avrRpt2 or Pst, respectively); rps2 is an ETI control. The mean±RSE (n ≥ 9) from three independent experiments are presented. b Relative PR1 transcription levels measured by qRT-PCR are presented as mean±SD (n = 3). c Size distribution of nuclear sRNAs (11–100 nts) in WT and dcl1-7 at 1 h-post-infection (hpi) with Pst_avrRpt2 or buffer (mock); the y-axis represents a normalized read count in RPM. d Percentage of tRNA-aligned reads in 71–100 nts sRNAs. e Normalized read counts of tRNA-derived 71–100 nts RNAs as mean±SE (n = 3). Significance between indicated pairs is noted; *P < 0.05; **P < 0.01; ***P < 0.001 (t-test).
The transcriptomes of mock-, Pst-, and Pst_avrRpt2-inoculated Arabidopsis were analyzed at multiple time points to investigate transcriptional reprogramming following pathogen infection. Pair-wise comparisons revealed that the number of differentially expressed genes (DEGs) in Pst_avrRpt2 vs mock and Pst_avrRpt2 vs Pst peaked at 6 hpi, whereas those in Pst vs mock peaked at 24 hpi (Supplementary Fig. 1a). Interestingly, most of the upregulated DEGs in Pst_avrRpt2 vs Pst (for simplicity, termed as ETI-DEGs hereafter) at 6 hpi, which mainly belonged to “response to biotic stimulus/stress” (Supplementary Fig. 2), displayed little overlap with those at other timepoints (Supplementary Fig. 1b, c). Another transcriptome study similarly concluded that ETI-DEGs peak at 6 hpi26; note that this report used 50% of the inoculum used in our study. Since early ETI-associated responses occur within minutes of inoculation27, the machinery responsible for ETI-DEG induction presumably functions between these key ETI events.
Whether DCL1-associated sRNAs regulate ETI-induced transcriptional reprogramming was assessed by characterizing nuclear-localized sRNAs (<100 nts) in mock- and Pst_avrRpt2-inoculated WT and dcl1-7 at 1 hpi. Size-distribution analysis showed that, regardless of pathogen inoculation, dcl1-7 accumulated more RNAs of ≤28 nts, whereas WT accumulated more RNAs of ≥70 nts (Fig. 1c). Additionally, while the nuclear RNA distribution profile was not substantially altered in dcl1-7 after Pst_avrRpt2 inoculation, a ~ 2-fold increase in 74 nts and 76/77 nts RNAs was observed in pathogen- vs mock-inoculated WT; a significant increase also was observed in 85/86 nts. Examining >70 nts RNA revealed that many originated from tRNA genes; note that full-length Arabidopsis tRNAs are 71 to 92 nts. Strikingly, tRNA reads in the nuclei of Pst_avrRpt2-inoculated WT, but not dcl1-7, rose to nearly 10% of the total sRNA population by 1 hpi (Fig. 1d). Dramatic pathogen-induced, DCL1-dependent inductions were observed for a subset of tRNAs (Fig. 1e).
Early rise of DCL1-dependent tRFs in ETI
Since nuclear tRNA levels rose during ETI triggered by Pst_avrRpt2, we assessed whether nuclear tsRNAs also increased. Due to tRNA gene redundancy, a sequence-centered analysis, termed MINTmap19, was used to characterize nuclear tsRNAs of 11 to 40 nts in mock- and Pst_avrRpt2-inoculated WT and dcl1-7 at 1 hpi. Of the four tsRNA classes, 5′-tRFs and 5′-halves predominated; of these, only 5′-tRFs were induced by Pst_avrRpt2 in a DCL1-dependent manner (Supplementary Fig. 3a). Size distribution analysis of 5′-tRFs revealed peaks at 16 and 31 nt, with the 31 nts tRFs exhibiting DCL1-dependent accumulation after Pst_avrRpt2 inoculation (Fig. 2a). The 31 nts tRFs were primarily derived from a few tRNAs, with AspGTC predominating (Fig. 2b). The pathogen- and DCL1-dependent accumulation of a 31 nts 5′-tRF derived from tRNAAsp2GTC (designated tRF31Asp2) is illustrated in Fig. 2c; this tRF was largely responsible for the increase in AspGTC and generated from cleavage at anticodon stem, which belongs to tRF-5c. This DCL1-dependent increase of tRF31Asp2 in response to Pst_avrRpt2 was also independently confirmed via stem-loop quantitative RT-PCR (Supplementary Fig. 4a) and northern blot analysis (Supplementary Fig. 4b). In addition to avrRpt2, other ETI triggering effectors, avrRpm1 and avrRps4, were also found to induce tRF31Asp2 comparatively (Supplementary Fig. 4c). We also assessed if DCL1 is directly involved in biogenesis of tRF31Asp2 in vitro. Although DCL1 produced the expected 46 nts 5′ end fragment during the processing of pri-miR166g28, the production of 5′-tRFs at 31 nts by DCL1 was quite limited (indicated by an arrow in Supplementary Fig. 4d). This observation suggests that the reaction may require an extra cofactor to reach its maximum potential.
a–c sRNA-seq analysis of nuclear 5′-tRFs in Pst_avrRpt2- or mock-inoculated WT and dcl1-7 at 1 hpi. d–f Nuclear AGO-associated 5′-tRFs in Pst_avrRpt2-, Pst-, or mock-inoculated HA-AGO1 and HA-AGO2 transgenic Arabidopsis at 1 hpi were identified by sRIP-seq via α-HA; WT was the negative control. The y-axis represents a normalized read count in RPM. Size distribution of 5′-tRFs (a, d). Normalized read counts of 31 nt-long 5′-tRFs originated from each tRNA isodecoder (b, e) and of a representative Pst_avrRpt2-inducible, DCL1-dependent 5′-tRF (tRF31Asp2) (c, f). The mean ± SE is presented (n = 3, a–c; n = 2, d–f). Significance from mock noted for highlighted samples only; *P < 0.05; **P < 0.01; ***P < 0.001 (t-test).
Reactive oxygen species (ROS), an early molecule elevated during the defense responses, has been shown to increase the level of tsRNAs18. Thus, we investigated if the reverse could be true by analyzing the changes in ROS levels following the treatment of tRF31Asp2. While flg22, an epitope derived from a bacterial PAMP flagellin, induced ROS production, both tRF31Asp2 and its mutant exhibited no significant elevation over 24 h (Supplementary Fig. 4e, f). This observation suggests that ROS probably acts as an upstream signaling mediator for tsRNAs.
Since AGOs are effector proteins for some tRFs and tRNA-halves29, the possibility that they interact with nuclear-localized tRFs was assessed. Of the ten Arabidopsis AGOs, AGO1 and AGO2 were selected as they are the most highly expressed in mature leaves25, and AGO2 was implicated in ETI8. Additionally, marginal increases in nuclear-localized AGO2 were noted following pathogen inoculation (Supplementary Fig. 5). Analysis of the 5′-tRF profile from nuclear-localized AGO1- and AGO2-bound sRNAs, also known as RIP (RNA immunoprecipitation)-seq, in mock-, Pst- and Pst_avrRpt2-inoculated WT at 1 hpi revealed major peaks at 16 and 31 nts (Fig. 2d). Infection with Pst_avrRpt2, but not Pst, substantially increased the abundance of AGO1/2-bound 16 nts tRFs and AGO2-bound 31 nts tRFs. Similar to the nuclear tsRNAs (Fig. 2b), AGO2-bound 31 nts tRFs were derived from a subset of tRNAs, with AspGTC predominating (Fig. 2e). Likewise, Pst_avrRpt2-induced accumulation of AGO2-associated AspGTC tRFs was largely due to increases in tRF31Asp2 (Fig. 2f). A previous RIP-seq of nuclear AGO1 revealed that 21 nts sRNAs starting with ‘U’ are the major interactors29. Consistent with this report, AGO1 associated preferentially with 21 nts sRNAs starting with ‘U’, whereas AGO2 displayed an elevated association with those with ‘A’ (Supplementary Fig. 6b). Additionally, when assessing the fold enrichment before and after IP via AGO1/2, larger tRFs (>28 nts) were predominantly enriched (Supplementary Fig. 3c, d). Furthermore, miRNAs known to interact with nuclear AGO129 showed specific enrichment in AGO1 IP samples (Supplementary Fig. 3e), confirming the IP specificity. Thus, these findings suggest that some tRFs, including tRF31Asp2, induced by Pst_avrRpt2 are also associated with AGO2 in the nucleus.
tRF-triggered immunity
Synthetic tRF31Asp2 RNA was infiltrated into Arabidopsis leaves to investigate the role of tRF31Asp2 in defense responses. tRF31Asp2 induced PR1 expression in a dose-dependent manner (Supplementary Fig. 7a). A time course revealed that PR1 expression in infiltrated leaves peaked at 24 hpi; an increase in systemic, untreated leaves was observed at 48 hpi (Fig. 3a). We also found that tRF31SerGCT and tRF31ValCAC, two 31 nts 5′-tRFs that were not induced by Pst_avrRpt2, did not induce PR1 (Supplementary Fig. 7b). Pretreating WT with tRF31Asp2 vs mock led to a ~ 43% reduction in Pst growth at 3 dpi while the greater reduction (~75%) was observed in Pst_avrRpt2 (Fig. 3b). An even greater reduction was observed in tRF31Asp2-pretreated dcl1-7, suggesting that tRF31Asp2 may be more effective in complementing dcl1, which lacks the capability to generate immunity-related tRFs, compared to WT. To expand the analysis, the ability of tRF31Asp2 to induce aphid resistance was also tested. Aphid resistance was enhanced significantly in WT pretreated with tRF31Asp2 but not buffer. While ago2/3/7 showed increased resistance to aphids, tRF31Asp2 did not provide any additional improvement in resistance (Fig. 3c). This suggests that the aphid resistance induced by tRF31Asp2 is dependent on the AGO2 clade. However, we cannot rule out the possibility of additional factors contributing to aphid resistance. It should be noted that miRNA-regulated camalexin by AGO1 was shown to be crucial in aphid resistance30. Together, these findings indicate that tRF31Asp2 infiltration induces immune responses.
a PR1 expression in local and distal leaves at the indicated times after tRF31Asp2 (1 µM) or buffer infiltration was examined by qRT-PCR. b Bacterial growth in WT, dcl1-7, and ago2/3/7 pretreated with tRF31Asp2, tRF31Asp2_mSS, or mock at 0 and 3 dpi with Pst and Pst_avrRpt2; the mean±RSE (n = 12) from three independent experiments is presented. c tRF31Asp2 promotes enhanced resistance to aphids. The green peach aphid (GPA) numbers at 5 dpi on WT and ago2/3/7 leaves were pretreated with tRF31Asp2, tRF31Asp2_mSS; untreated (No) and the buffer (PBS)-treated WT used as a control. Plants were initially infested with three adult apterous aphids/plant after 24 hpi. The same experiment was conducted twice with similar results. The mean ± SE (n = 7 to 10) is presented. d Predicted secondary structure of full-length Asp-2-tRNAGTC and its 31 nt-long 5′-tRF (tRF31Asp2) by RNAfold. e PR1 induction by tRF31Asp2 and its mutants at 24 hpi was examined by qRT-PCR; relative transcription levels are presented as the mean ± SD (n = 3). Bar colors correspond to target sites in (d). f Antisense oligonucleotides or buffer were infiltrated into leaves 1 day before distal-infection with Pst_avrRpt2; PR1 was assessed at 1 dpi by qRT-PCR; the mean ± SD (n = 4). Three independent experiments were performed with similar results. g PR1 expression in distal leaves of short tandem target mimic (STTM) and empty vector (EV) lines, and WT at 24 hpi with Pst_avrRpt2 or mock was examined by qRT-PCR; naive is without any treatment; the mean ± SE (n = 4). h, i PR1 induction in dcl and ago mutants at 24 hpi responding to 1 (h) and 20 (i) µM of tRF31Asp2 and its mock control. The mean ± SE (n ≥ 4) is presented. j Bacterial growth in WT, ago2-1, and AGO2 complementation lines (DDD and DAD are WT and a slicer mutant, respectively) in ago2-1 at 0 and 3 day-post-infection (dpi) with Pst_avrRpt2 with the mean ± SE (n = 4); rps2 is an ETI control. Significance for indicated pairs is noted; *P < 0.05; **P < 0.01; ***P < 0.001 (t-test except for c where Tukey’s HSD test was used).
A stem-loop in the tRF structure
tRF31Asp2 has a predicted secondary structure with a stem-loop whose location differs from the tRNA D arm (Fig. 3d). We generated synthetic RNAs bearing mutations at four sites (Supplementary Fig. 7c) to characterize which regions of tRF31Asp2 are important for biological activity. Additionally, the effect of deleting/adding nts to the 3′ end of tRF31Asp2, based on the sequence of its tRNAAsp2GTC, was assessed (Supplementary Fig. 7d). tRF31Asp2_mSS with three altered nts in the 5′ single-strand region (Supplementary Fig. 7b) was substantially less effective at inducing PR1 expression (Fig. 3e) and immunity against Pst_avrRpt2, Pst and aphids (Fig. 3b, c). However, the decreased biological activity did not correlate with reduced AGO2 binding, which was comparable in tRF31Asp2_mSS and tRF31Asp2 (Supplementary Fig. 7e). tRF31Asp2_mStem1, with mutations disrupting the predicted stem structure, also was compromised for PR1 induction (Fig. 3e). However, tRF31Asp2_rStem1, containing compensatory mutations restoring the stem structure, induced PR1 to comparable levels as tRF31Asp2. Mutations in the predicted loop (tRF31Asp2_mLoop) and alterations in tRNA D-loop-associated nts (tRF[31Asp2_mStem2) did not substantially alter PR1 induction. Adding 4 nts to the 3′ end of tRF31Asp2 also did not significantly affect PR1 induction, whereas trimming 3 or more nts from this end, which impacts the stem-loop structure, abolished PR1 induction (Supplementary Fig. 7d). Whether an oligonucleotide containing the antisense sequence of tRF31Asp2 (RC_tRF31Asp2) could suppress Pst_avrRpt2-induced PR1 expression was assessed. Pretreating leaves with RC_tRF31Asp2 prior to infecting lower leaves with Pst_avrRpt2 suppressed systemic PR1 expression, whereas RC_tRF31Asp2_mSS, the antisense sequence of tRF31Asp2_mSS, did not (Fig. 3f). Consistent with this observation, short tandem target mimic (STTM) lines targeting tRF31Asp2, not tRF31Asp2_mSS, displayed reduced systemic PR1 induction (Fig. 3g). Together, these results suggest that the predicted stem-loop structure and the 5′ sequence of tRF31Asp2 are important for its biological activity.
Dependence of tRF31Asp2 immunity on the AGO2 clade
We tested whether tRF31Asp2 induces immune responses in the dcl or ago mutant backgrounds. All dcl and ago single mutants largely exhibited WT-level PR1 induction, but higher-order mutants defective either for AGO1 or AGO2 clade members (ago1/5/10 or ago2/3/7, respectively) showed significantly lower levels of PR1 induction (Fig. 3h). Interestingly, when a greater amount of tRF31Asp2 was administered to these triple mutants, only ago2/3/7 failed to induce PR1 (Fig. 3i). This observation suggests that while the AGO1 clade might be implicated in tRF signaling, the AGO2 clade could plays a relatively more predominent role in this process.
AGO2 requires its catalytic site for target suppression through slicing31. We investigated the role of AGO2’s slicing catalytic site in its immunity function and found that AGO2 with a slicer mutation (DAD) failed to complement the compromised immune response to Pst_avrRpt2 in ago2 (Fig. 3j). Together with the RIP-seq data, these results suggest that loading of tRF31Asp2 into AGO2 or its clade members in a catalytically active form could contribute to trigger defense responses.
tRF31Asp2-induced defense genes
To identify tRF31Asp2-induced DEGs, transcriptome analyses were performed (Supplementary Fig. 8a). Comparing the transcriptomes of i) WT treated with tRF31Asp2 vs mock, ii) WT treated with tRF31Asp2 vs tRF31Asp2_mSS, and iii) WT vs ago2/3/7 treated with tRF31Asp2 identified 810 DEGs present in all three combinations (tRF-DEGs, hereafter) (Supplementary Fig. 8b, c). The majority of these DEGs (503 of 810) also were identified as ETI-DEGs (Supplementary Fig. 8d). These tRF-DEGs mostly belong to “response to stress” such as biotic stimulus (Supplementary Fig. 9). Recent reports revealed that ETI and PTI share a significant number of downstream components6,7 and identified genes that are unique and overlapping to ETI and PTI6. Interestingly, the majority of tRF-DEGs belong to a group in which ETI and PTI are shared (Supplementary Fig. 8e).
Association of tRFs with Gypsy’s PBS and its homologous sequence in defense genes
We investigated the possibility that tRF31Asp2 physically interacts with Arabidopsis chromatin by infiltrating biotinylated tRF31Asp2 or tRF31Asp2_mSS into WT and ago2/3/7 leaves and performing ChAP (Chromatin Affinity Precipitation)-seq analysis. In WT, normalized reads showed that tRF31Asp2 interacted with chromatin significantly more than tRF31Asp2_mSS, particularly around the genic area, with these interactions further diminished in ago2/3/7 mutants (Supplementary Fig. 10a). Using MACS232, we identified ChAP-seq peaks by comparing reads from streptavidin beads with those from non-interacting IgG beads. The number of ChAP-seq peaks was significantly reduced in tRF31Asp2_mSS relative to its WT counterpart and again further decreased in ago2/3/7, suggesting that the AGO2 clade may facilitate the interaction of tRF31Asp2 with chromatin. Interestingly, a considerable number of ChAP-seq peaks were identified within transposon regions (Supplementary Fig. 11a). The greatest number of transposon-localized ChAP-seq peaks that also contained the tRF31Asp2 sequence, based on FASTA analysis with an E score of <0.01, were identified in the Gypsy and Copia superfamilies of long-terminal repeat (LTR)-retrotransposons (Supplementary Fig. 11b). Within the Gypsy superfamily, ChAP-seq peaks with the tRF31Asp2 sequence were most prevalent in members of ATGP1 (Supplementary Fig. 11c). Strikingly, the single-stranded 5′ region of tRF31Asp2 exhibited perfect complementarity to the Gypsy primer binding site (PBS) (Fig. 4b). Binding of Gypsy PBS by the 3′ end of tRNAs primes its proliferation33. Since the 3′ end of Asp-2-tRNAGTC is complementary to the 5′ sequences of tRF31Asp2, tRF31Asp2 might likewise bind these retrotransposons in a sequence-specific manner.
a Induction patterns in ETI-DEGs (lower) and non-ETI-DEGs (upper) are shown; the y- and x-axes show normalized read counts from RNA-seq of tRF31Asp2-treated WT, and the mean values of three controls, respectively. b The primer binding site of Gypsy retrotransposons is highly homologous to tRF31Asp2. c Physical distance of tRF-DEGs and control (non-tRF-DEGs) from the nearest tRF31Asp2-binding ChAP-seq peaks. Data are represented as boxplots where the middle line and the dot are respectively the median and the mean, the lower and upper hinges correspond to the first and third quartiles, and the upper/lower whisker extends from the hinge to the largest/smallest value no further than 1.5 × IQR (inter-quartile range). The y-axis shows the physical distance in bps. Significance for indicated pairs is noted; ***P < 0.001 (t-test). d Induction patterns of ETI-DEGs between with and without tRF binding. The y- and x-axes show normalized read counts from Pst_avrRpt2 and Pst at 6 hpi. Significance for indicated slopes is noted; ***P < 10−16 (Chow test). e Induction patterns of transcriptionally active Copia and Gypsy LTR-retrotransposons. The y- and x-axes show normalized read counts tRF31Asp2-treated WT and its mock control.
Interestingly, tRF31Asp2-associated peaks were also found in the genic area of many defense-associated genes, including PR1, PR2, and PR5; all the PR-associated peaks were statistically significant (Supplementary Fig. 10b), raising an intriguing possibility that this physical interaction could have resulted in the activation of defense genes and immune responses.
Proximity of tRF sites to their DEGs
We speculated that tRF31Asp2 might similarly regulate other tRF-DEG expression via interaction with its complementary sequence, which might be located in recognizable or degenerated/unrecognizable transposons; note that transposons are recognized as a critical driver involved in evolving transcriptional networks in plants and animals34,35. To assess this possibility, the distance between genes and their nearest ChAP-seq peak was assessed. Interestingly, analysis of ChAP-seq peaks revealed that the peaks were significantly closer to tRF-DEGs (Fig. 4c) than those not, suggesting that defense genes induced by tRF31Asp2 may associate with tRF31Asp2 itself. We then separately examined ETI-DEGs with and without the tRF overlap. While not all defense genes physically interacted with tRF31Asp2, ETI-DEGs that tRF31Asp2 bound exhibited a significantly greater fold induction compared to non-bound counterparts (Fig. 4d). In other words, genes that interact with tRF31Asp2 are typically silent under normal conditions but are strongly activated upon defense induction. These findings suggest that tRF31Asp2 may function as a transcriptional enhancer, facilitating the expression of otherwise dormant defense genes during ETI.
In contrast to this activating function, some tRFs were shown to suppress retrotransposons23,24. We, therefore, examined the changes of transcriptionally active LTR-retrotransposons and found that tRF31Asp2 largely suppressed these transposons, particularly Gypsy (Fig. 4e). These observations suggest that the tRF binding might be important in inducing neighboring tRF-DEGs while suppressing active retrotransposons.
Discussion
In this study, we have shown that tRF31Asp2 is a DCL1-dependent sRNA rapidly induced well before the ETI transcriptomic peak. Treatment of tRF31Asp2 alone was sufficient to initiate immune responses robust enough to immunize Arabidopsis against avirulent and virulent Pst as well as aphids. The increased resistance exhibited against aphids by tRF31Asp2 (Fig. 3c) indicates that this tRF may not be exclusively targeted towards ETI. Recent reports suggest the mutual potentiation between ETI and PTI6,7. Interestingly, a majority of tRF-DEGs belonged to a list of genes that are common to both ETI and PTI (Supplementary Fig. 8e), suggesting that tRF31Asp2 might play a role in both pathways, although ETI requiring quicker kinetics could be more impacted.
tRF31Asp2 bound chromatin at a specific sequence present in Gypsy LTR-retrotransposons and defense genes. The abundant presence of tsRNAs and their likely interacting partners, LTR-retrotransposons, in all plant systems examined14,35 indicates that the positive regulatory role found herein may be ubiquitous. Although the origin of binding sites in defense genes is uncertain, there is a possibility that these sites are degenerated sequences that originated from LTR-retrotransposons. The analysis supports this speculation that some transcriptional enhancers may have originated from transposons36. Interestingly, ETI-DEGs with the tRF31Asp2 binding appeared to be induced more steeply (Fig. 4d) than those without, suggesting that this tRF is involved in further accelerating transcriptional induction. RNA activation (RNAa), which is the process of activating transcription through sRNAs, involves the recruitment of RNA polymerase II and associated factors to target genes37,38. This is achieved through the binding of 21 nts double-stranded RNAs with mammalian AGO2, which is similar to Arabidopsis AGO1. By interacting with the promoters of target genes, these sRNAs function as enhancers, inducing target genes. Our observations, therefore, suggest that tRF31Asp2 may use a similar transcriptional activation mechanism. Consistent with earlier reports23,24,39, the role of suppressing transposons was also observed for transcriptionally active Gypsy LTR-retrotransposons (Fig. 4e). Altering the level of tRFs derived from tRNAGlyGCC, another tRF found to be induced by Pst_avrRpt2 (Fig. 2b, e), was shown to be closely correlated with pancreatic cancer development in mice40. Interestingly, tRFGlyGCC, which has the same size as tRF31Asp2, increases in abundance during mouse sperm maturation and has been found to inhibit the endogenous retroelement MERVL and its neighboring genes24. Therefore, understanding how these tRFs induce and suppress target genes will be crucial in this emerging tRF biology.
Understanding DCL1’s role in immunity (Fig. 1) is complex due to its involvement in generating various miRNAs influencing defense responses41. Furthermore, the pleiotropic phenotypes of dcl1, affecting growth and flowering42, further complicate assessing the specific impact of the tRF on defense responses despite its evident involvement in plant immunity when tRF31Asp2 triggers robust defense responses (Figs. 3 and 4). Nonetheless, after exposure to Pst_avrRpt2, both nuclear tRF31Asp2 and its likely precursor, tRNAAsp2GTC, exhibited DCL1-dependent increases (Figs. 1e, 2c and Supplementary Fig. 4), suggesting the import of the full-length tRNA and its cleavage to tRFs in the nucleus may depend on DCL1. In addition, the notable presence of tRF31Asp2 that did not trigger defense responses prior to pathogen exposure (Fig. 2c and Supplementary Fig. 4) suggests that DCL1 may play a role in generating the defense-activated form of tRF31Asp2. Consistent with this notion, murine Dicer was shown to relocate to the nucleus when exposed to DNA damage43. Additionally, human Dicer has been demonstrated to facilitate the nuclear import of a reporter protein by interacting with importins44. Notably, tRNAs undergo nuclear import during virus infection or amino acid deprivation in human and yeast cells, known as tRNA retrograde nuclear import45,46. Therefore, investigating the potential involvement of DCL1 in the retrograde import of tRNAs presents an intriguing avenue for tRF research.
Finally, our antisense and STTM approaches targeting tRF31Asp2 marginally suppress the systemic PR1 induction (Fig. 3f, g), whereas the delivery of tRF31Asp2 significantly induced hundreds of defense genes (Fig. 4a). These findings suggest that there may be additional tRFs involved in assisting defense responses or that these knock-down techniques are inadequate in significantly disrupting defense signaling because of the plentiful presence of other immunity-related tRFs. Nonetheless, the ability to modulate these tRFs to enhance or suppress defense responses indicates that gaining a deeper understanding of these immunity-related tRFs could lead to new biotechnological approaches for managing disease in agriculture.
Methods
Plant materials and growth conditions
Plants were grown in soil at 22 °C, 60% relative humidity, and in a 16 h light period. All Arabidopsis lines used in this study are in the Col-0 background. The following mutant lines were obtained from the Arabidopsis Stock Center or the research groups in indicated references: ago1-2747, ago2-1 (SALK_003380), ago3-2 (SALK_005335), ago4-248, ago5-1 (SALK_063806), ago6-3 (SALK_106607), ago749, ago8-1 (SALK_139894), ago9-2 (SALK_112059), ago10-2 (SALK_047336), ago2/3/710, dcl1-750, dcl2-1 (SALK_064627), dcl3-1 (SALK_005512), dcl4-2 (GABI_160G05), dcl2/3/4 (CS16391), HA-AGO131, HA-AGO231 and HA-AGO2-DAD31. ago1/5/10 was generated from ago1-27, ago5-1, and ago10-2. HA-AGO1 (pAGO1::3HA-AGO1 in ago1-25), HA-AGO2 (pAGO2::3HA-AGO2 in ago2-1) and HA-AGO2-DAD (pAGO2::3HA-AGO2-DAD in ago2-1) lines51 are transgenic Arabidopsis carrying HA-tagged AGO1/AGO2 under their own promoters. tRF31Asp2_STTM and tRF31Asp2_mSS_STTM transgenic Arabidopsis, respectively carrying pFGC5941-PacI-STTM-tRF31Asp2_STTM and pFGC5941-PacI-STTM-tRF31Asp2_mSS_STTM, were generated as described in ref. 51; oligonucleotides used in the vector constructions are listed in Supplementary Table 1 and Arabidopsis carrying pFGC5941-PacI was used as an empty vector control.
Total and small RNA extraction, semiquantitative/quantitative RT-PCR, and northern blot analysis
Total RNAs were isolated using the TRIzol reagent (Invitrogen). 1 μg of total RNAs were treated by DNase I (NEB), and cDNAs were then synthesized by M-MuLV reverse transcriptase (NEB).
For small RNA preparation, 3.5-week-old WT and dcl1-7 plants were infiltrated with 106 cfu/ml of Pst and Pst_avrRpt2 for 1 hour. After nuclei were isolated as described below, the small RNAs were prepared using the mirVana miRNA Isolation Kit (ThermoFisher).
Real-time quantitative RT-PCR was performed using HOT FIREPol EvaGreen qPCR Mix Plus (Solis BioDyne) with the primers listed in Supplementary Table 1. Relative expression level was calculated from the difference between the threshold cycle (Ct) values of reference and target genes52. The reference gene is Tip41-like (AT4G34270)53 for Fig. 1, and RHIP1 (AT4G26410) for the rest53.
Stem-loop RT-PCR was performed by following the procedure as described in ref. 54 to assess the levels of tRF31Asp2. Nuclear sRNA served as a template for reverse transcription, using a stem-loop RT primer targeting the six nucleotides at the 3′ end of the tRF. The RT product was amplified using a tRF31Asp2-specific forward primer and a universal reverse primer. Real-time quantitative PCR was performed as described above. Semiquantitative PCR was conducted using DreamTaq DNA Polymerase (Fisher). U6, a small nuclear RNA, was utilized as a reference gene and input control for qPCR and semi-qPCR, respectively55.
Northern blot procedure was carried out as described in ref. 56 with some modifications. Nucleus was prepared from 5 g of homogenized tissue as described below, and its total RNA was prepared using the mirVana™ miRNA Isolation Kit (Fisher). 175 ng of total RNA was separated on a denaturing 15% polyacrylamide gel. The gel was cut into two parts: the upper part containing larger-sized RNAs was stained with SYBR gold for use as a loading input, while the other part was transferred to a nylon membrane (Roche). Transfer was carried out at 15V for 90 min in a 1x TBE solution at 4 °C. The membrane was cross-linked using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC; Sigma). To prepare this, the membrane and saturated Whatman paper were soaked in freshly prepared EDC cross-linking reagent, wrapped in Saran wrap, and incubated at 60 °C for 2 h. Next, residual cross-linking solution was removed by thoroughly rinsing the membrane with ddH2O and the membrane was placed into hybridization bottle with the RNA side inward. The membrane was pre-hybridized in ULTRAhyb (ThermoFisher) buffer at 55 °C for at least 30 min in hybridization oven. A denatured (95 °C for 1 min) DIG-labeled LNA-DNA probe for tRF31Asp2 (5 nM) was hybridized at 55 °C overnight with slow rotation. The hybridized membrane was washed extensively at room temperature using Low Stringent Buffer (twice, 15 min each), High Stringent Buffer (twice, 5 min each) and Washing Buffer (once for 10 min). The membrane was then blocked in Blocking Buffer for 5 hours at room temperature and incubated in an anti-DIG antibody (Roche, 1:15,000) for 45 min. The membrane was washed in DIG Washing buffer four times for 15 min each and incubated in a chemiluminescence substrate, CSPD (Roche), for 5 min in a room temperature, followed by dark incubation at 37 °C for 15 min. The presence of tRF was detected by imaging using an Azure imaging station.
Nucleus enrichment
Nucleus-enriched and -depleted fractions were prepared as previously described in ref. 57 with the following modifications. Around 1 g leaves were homogenized in liquid N2 using pre-chilled mortar and pestle followed by homogenization in 5 mL of NIB [250 mM sucrose (Sigma), 10 mM NaCl (ThermoFisher), 15 mM PIPES pH 6.8 (ThermoFisher), 0.8% Triton X-100 (Sigma) and 0.1 mM PMSF (Sigma)] with 1x Protease Inhibitor Cocktail (ThermoFisher) at 4 °C. This extract was centrifuged at 300 x g for 5 min at 4 °C to remove the debris and filtered through a double layer of Miracloth (Sigma). Nuclei-enriched fraction was obtained by centrifuging at 1500 x g for 10 min; its supernatant was nucleus-depleted fraction. For the nucleus-depleted fraction, the supernatant was centrifuged again at 1500 x g for 5 min and 200 µL of the supernatant was collected in a new Eppendorf tube. For the nuclei-enriched fraction, the pellet was washed 3–4 times at 1500 x g for 2 min to remove any residual green color. Both the nuclei-enriched and depleted fractions were mixed with 4x SDS sample preparation buffer for western blot. Immunoblotting (IB) analysis with α-HA (1:5000) (Novus) was performed to track the AGO proteins; α-phosphoenolpyruvate carboxylase antibody (α-PEPC, 1:25,000) (Rockland) and α-histone H3 antibody (1:25,000) (Abcam) were used to ensure depletion and enrichment of nuclei, respectively.
IB (immunoblot) and AB (affinityblot) analysis
IB and AB blot analyses were performed as described in ref. 58. For IB, transferred proteins on PVDF (ThermoFisher) membrane were probed with indicated antibodies at 4 °C for 12 h. For AB, transferred tRF31Asp2 and tRF31Asp2_mSS oligos on Hybond-N+ membrane (ThermoFisher) were probed with α-streptavidin-HRP (ThermoFisher) at 4 °C for 12 h. For AB, separated biotin labeled tRF31Asp2 and tRF31Asp2_mSS oligos using 15% Urea-PAGE were transferred on Hybond-N+ membrane (ThermoFisher) then, probed with α-streptavidin-HRP (ThermoFisher) at 4 °C for 12 h. These blots were visualized via chemiluminescence using ECL2 (ThermoFisher).
Quantification of bands on images
The band intensities in IB/northern blots and stem-loop semiquantitative PCR were quantified using ImageJ59. Mean grey values of the target and loading input bands were calculated by subtracting the background. For the northern blot and the stem-loop semiquantitative PCR, the band intensity ratio of α-DIG/SYBR gold and tRF31Asp2/U6 was presented, respectively. For IB blot, mean grey values for both α-HA and α-H3 were separately calculated, and the ratio of α-HA/α-H3 was presented.
Pathogen infection assay
3.5-week-old Arabidopsis plants were used for the resistance assay. Three leaves of each plant were syringe-infiltrated from the abaxial side with a suspension of Pst_avrRpt2 or Pst at 105 cfu/ml. Bacterial growth was determined at 0- and 3-days post-infection (dpi) as described in ref. 25. Pst along with its avirulent strain carrying the effectors avrRpt2 (Pst_avrRpt2), was cultured overnight at 28 °C in King’s B medium (10 g protease peptone, 0.75 g K2HPO4, 10 ml 50% glycerol per 500 ml), supplemented with 2 mM MgSO4 and appropriate antibiotics (50 μg/ml rifamycin or kanamycin). Leaves from 3.5-week-old plants were hand-inoculated with bacterial suspensions at a concentration of 105 cfu/ml using a needleless syringe. To measure in planta bacterial growth, two leaves were collected as a set, weighed, and ground in 500 μl of ddH2O. Serial dilutions of the homogenate were plated on LB medium containing 50 μg/ml kanamycin. The plates were incubated at 28 °C, and bacterial colony-forming units (cfu) were counted at 0 and 3 days post-inoculation.
Nucleotide Infiltration
RNA and DNA oligos delivered to 3.5-week-old Arabidopsis leaves are listed in Supplementary Table 1. These oligos in 1x PBS were denatured at 95 °C for a few seconds and renatured by gradually cooling to 25 °C at 5 °C/min. Renatured nucleotides were syringe-infiltrated into the abaxial side of the leaves. Delivery of a tRF RNA nucleotide into the nucleus was initially confirmed by using a 5′ 6-fluorescein (FAM)-conjugated one as shown in Supplementary Fig. 12; sRNAs were extracted from the nucleus-enriched fraction of WT plants infiltrated with FAM-conjugated tRF44Asp2 oligo, as described above, and visualized by SYBR gold staining for general RNAs and FAM-specific fluorescence scanning in Typhoon FLA 9000 (GE) for FAM-oligos.
Treatment of the antisense nucleotides on leaves distally infected by Pst_avrRpt2
Three leaves were infected with Pst_avrRpt2 at 107 cfu/ml for one day. Uninfected leaves were then treated with 0.2 and 1 μM of RC_tRF31Asp2 and RC_tRF31Asp2_mSS oligos, respectively, for 24 h; 1x PBS buffer was used as a negative control.
Plant immunization assay
Leaves of 3.5-week-old Arabidopsis plants were infiltrated with 1 μM of tRF31Asp2 or tRF31Asp2_mSS oligos for one day. The same leaves were then infected with Pst and Pst_avrRpt2 at 105 cfu/ml. Bacterial growth was determined at 0 and 3 dpi.
Aphid propagation and bioassay
The green peach aphid (GPA) colony was reared as previously described in ref. 60. Briefly, the GPA was reared on an equal mixture of commercially available radish (Raphanus sativus) and mustard (Brassica juncea) plants (Main Street Seed & Supply) in a Percival growth chamber with a 14 h/10 h (light/dark) photoperiod, 160 μE m−2 s−1, 22 °C, and 50% to 60% relative humidity. Three-week-old Arabidopsis WT and ago2/3/7 plants were used for aphid bioassays. Plants were infiltrated with the tRF nucleotides as described above. Three adult apterous GPAs were placed in a 1.5 cm diameter clip cage on leaves one dpi with tRF31Asp2 or tRF31Asp2_mSS. The total numbers of GPAs (adults + nymphs) were counted after five days. Statistical analysis was performed using PROC GLIMMIX in SAS 9.3 (SAS Institute) and multiple comparison analyses were carried out using Tukey’s HSD test.
In vitro transcription and labeling of RNA, and DCL1 processing assay
A pre-tRNAAsp2 (AT5G59055) was amplified using pT7-tRNA_AspGTC_F and tRNA_AspGTC_R primers and cloned into pJET1.2 (Fisher). This plasmid was used to synthesize an amplicon by PCR with pT7- F and tRNA_AspGTC_R primers, which was used for generating a pre-tRNAAsp2GTC in vitro using the HiScribe T7 high-yield RNA synthesis kit (NEB). The pre-tRNAAsp2GTC was treated with DNase I and calf intestinal alkaline phosphatase, labeled with γ−32PATP using T4 polynucleotide kinase (PNK), and gel-purified.
Recombinant DCL1 (0.05 pmol), HYL1 (0.1 pmol), SE (0.1 pmol) and RNA substrates at 500 counts per minute after PNK labeling were added to 20 μl assay buffer containing 20 mM Tris-HCl/7.5, 50 mM KCl, 4 mM MgCl2, 1 mM DTT, 5 mM ATP, 5 mM GTP and 1 U/μl Superase-In RNase Inhibitor (ThermoFisher). The final pooled concentration of NaCl and KCl was ~70 mM, of which ~20 mM salt was from protein dialysis buffer and RNA-dissolved buffer. The reconstitution assay was carried out at 37 °C for 1 hr. The reactions were stopped by adding 1 volume TBE-Urea sample buffer (8 M Urea, 2 mM Tris-HCl/7.5, 20 mM EDTA, bromophenol blue/xylene cyanol), being heated at 95 °C for 10 min and then being chilled on ice. The reactions were loaded into 10% denaturing polyacrylamide gel and visualized with a phosphor imaging plate (GE Healthcare).
mRNA-Sequencing library preparation
For antibacterial transcriptome analysis, 3.5-week-old Arabidopsis WT leaves were collected at 0, 1, 6, 24 and 48 hpi with Pst_avrRpt2 or Pst at 106 cfu/ml was used to generate Illumina-compatible sequencing libraries. 1 μg of mRNAs enriched by Poly(A) Magnetic Isolation Module (NEB) were reverse-transcribed, end-repaired, and adapter-ligated via NEBNext Ultra II RNA Library Prep Kit for Illumina (NEB). These libraries were sequenced using an Illumina HiSeq 4000 at the University of Texas at Austin Genomic Sequencing and Analysis Facility.
For RNA-seq with tRF, 1 μM of tRF31Asp2 and tRF31Asp2_mSS oligos were infiltrated into 3.5-week-old WT and ago2/3/7 plants for one day, and their RNAs were extracted as described above. A paired-end sequencing was run using BGISEQ-500 with a read length of 100 bp (BGI, Shenzhen, China).
sRNA-Sequencing library preparation
The nuclear sRNAs were treated by 10 U of T4 polynucleotide kinase (NEB) at 37 °C for 40 min to remove cyclic phosphate at the 3′ end. Small RNA libraries were prepared by the QIAseq miRNA Library Kit for Illumina (Qiagen) following manufacturer protocol. A single-end sequencing was run by using BGISEQ-500 with a read length of 100 bp (BGI, Shenzhen, China).
RIP-Sequencing library preparation
106 cfu/ml of Pst DC3000 with and without avrRpt2 was infiltrated into 3.5-week-old HA-AGO1, HA-AGO2 lines for 1 h; WT plants were used as a negative control. Around 10 g of leaf tissue was crosslinked in 1% formaldehyde at room temperature for 20 min and ground with liquid N2. Nuclei extract prepared as described above were co-immunoprecipitated with α-HA-conjugated gel (Sigma). Immunoprecipitated HA-AGO1 and HA-AGO2 proteins were eluted using HA peptides (GenScript). After reverse-crosslinking and digestion by proteinase K (NEB), purified sRNAs were subjected to sRNA-seq library preparation following the protocol described above. sRNAs purified from input fractions before co-IP with α-HA were used as an input control. sRNA-seq libraries were sequenced on an Illumina MiSeq in 2 × 100 bp paired-end mode except for the input control, using DNBSEQ-G400 with a read length of 2 × 100 bp paired-end mode (BGI, Shenzhen, China).
Chromatin-affinity precipitation (ChAP)-Sequencing
Biotin-labeled tRF31Asp2 and tRF31Asp2_mSS at 5 nmole were infiltrated into 3.5-week-old WT and ago2/3/7 plants for 1 h; uninfiltrated WT plants were used as controls. ChAP was performed as described61 with the following minor modifications. Collected tissues were crosslinked with 1% formaldehyde at room temperature for 15 min and ground with liquid N2. Nuclei isolated as described above were lysed in the lysis buffer (50 mM HEPES pH 7.5, 140 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS and 25 U/ml RNase Inhibitor) and sonicated (Diagenode, Bioruptor Pico) for the 10 cycles of 30 s on/off interval. Biotin-bound DNA was affinity-purified from nuclear lysate using Dynabeads M-280 streptavidin (ThermoFisher) overnight at 4 °C. As a negative control, DNA was pulled down using MagnaBind Goat Anti-Rabbit IgG (Fisher). After reverse cross-linking and digestion by proteinase K (NEB), DNAs were recovered and used for the ChAP-seq library using NEBNext Ultra II DNA Library Prep kit (NEB). Pair-end sequencing was performed using BGISEQ-500 with a read length of 100 bp (BGI, Shenzhen, China).
ROS measurement
Leaf discs (5-mm diameter) from 3.5-week-old soil-grown Arabidopsis plants were incubated overnight with water in 96-well plates to eliminate the wounding effect. ROS burst was measured by a luminol-based assay as described62 in the presence of tRF31Asp2 (1 μM), tRF31Asp2_mSS (1 μM), flg22 (0.1 μM), or water by using Synergy H1 plate reader (Biotek).
RNA-seq data analysis
Reads from RNA-seq of Pst treatment were trimmed for the adapter (AGATCGGAAGAGCACACGTCTGAACTCCAGTCAC) via cutadapt (v2.10)63. The adapters of raw reads were also trimmed for RNA-seq of tRF treatment by BGI-Genomics. The clean reads were aligned to the Arabidopsis genome (Araport11)64 using HISAT2 (v2.1.0)65. Beyond the default parameters for RNA-seq of Pst treatment, the paired-end alignment options were set ‘--no-mixed, --no-discordant’ in the case of tRF-RNA-seq. Alignment results were sorted using samtools (v1.10)66 and fed into HT-seq (v0.13.5)67 for read counting. The read coverage tables produced by HT-seq were fed into DESeq268 to generate a normalized expression table using DESeq2’s median of ratios normalization method. Principal component analysis (PCA) was performed using “prcomp” implemented in R. The differentially expressed genes (DEGs) were then identified following the pipelines of DESeq2. For RNA-seq of Pst treatment, DEGs were identified using Pst_avrRpt2 and Pst as the numerator and the denominator, respectively. Upregulated DEGs were identified using a p-adjusted value less than or equal to 0.01 with a log2 fold change greater than 1. For RNA-seq of tRF, three sets of DEGs (p-adjusted value ≤ 0.05; log2 fold-change ≥ 1) were identified using WT treated with tRF31Asp2 as the numerator and the following three controls as denominators: WT treated with mock, WT treated with tRF31Asp2_mSS, and ago2/3/7 treated with tRF31Asp2. Upregulated tRF-DEGs were identified from the common set of all three DEGs identified from the three comparisons described above.
Analysis of tRNAs, tRFs, and tRNA-halves in sRNA and RIP sequence datasets
Reads from nuclear sRNA-seq and RIP-seq were trimmed of adapter sequences (AACTGTAGGCACC) via cutadapt (v2.10). We used 642 Arabidopsis nuclear tRNA genes annotated in GtRNAdb269. For analyzing the presence of tRNAs in reads larger than 70 nts, Bowtie2 (v2.2.9)70 was used to map reads to the tRNA sequences using the option ‘--no-unal’. The tRNA-aligned read counts were generated for each tRNA isoacceptor and size.
For the tRFs and tRNA-halves analysis, reads were mapped using MINTmap software19. Each tRF and tRNA-half sequence was grouped based on sequence, tRNA isoacceptor, tRF type, and length, as per MINTmap annotation. Normalized counts (RPM) were generated by using total reads as a denominator. Ambiguous alignments that can also align with non-tRNA sequences were removed from the analysis. Over 98% of tRF reads were tRNA-exclusive alignments.
For the fold-of-enrichment analysis, reads from input samples were mapped and normalized using MINTmap software19, as described above. The fold-of-enrichment was then calculated by dividing the normalized values of the post-IP samples by those of the input samples.
ChAP-seq data analysis
Pair-end reads were adapter-trimmed (forward: GATCGGAAGAGCACACGTCTGAACT, reverse: GATCGGAAGAGCGTCGTGTA) and quality-trimmed (3:25) via cutadapt (v2.10) and trimmomatic (v0.36), respectively. Trimmed reads were aligned to the Arabidopsis genome (Araport 11)64 using Bowtie2 (v2.2.9)70 with the options ‘--no-unal, --no-discordant, --end-to-end, --no-mixed’. Only unique alignments aligned to the nuclear genome were retained. Peaks were identified by MACS271 with default parameters, and filtered with q-value ≤ 0.05 using aligned reads between biotin-binding streptavidin beads and non-targeted IgG beads for tRF31Asp2 and tRF31Asp2_mSS. Four biologically independent batches were separately analyzed to call peaks that associated significantly with tRF31Asp2 and tRF31Asp2_mSS, relative to the non-targeted control. Reproducible peaks found in a minimum of two independent biological replicates were merged as primary peaks.
We investigated the relationship between these primary peaks and transposons (TEs). The annotation of TEs was based on Araport11 updated on March 202164. TEs shorter than 1000 bp were excluded from the analysis. First, we analyzed the classification of (TEs) that overlapped with the primary ChAP-peaks. The search for sequence similarity between tRF31Asp2 and the peaks was performed using FASTA with the option ‘U’ for tRF31Asp2, allowing an RNA-DNA alignment. The peaks carrying significantly homologous sequences (E score of 0.05 or lower) were further analyzed for the classification of overlapping TEs. In addition, we also explored the long terminal repeat retrotransposons (LTR-RTs) associated with the tRF31Asp2 binding sites. The LTR-RTs were identified using both LTRharvest72 and LTRfinder73, and filtered following the LTR_retriever pipeline74. The potential tRF31Asp2 binding sites were analyzed for the LTR-RTs that overlapped with ChAP-peaks. Only the LTR-RTs with significant tRF31Asp2 binding sequences located between the 5′-LTR and GAG gene were retained for sequence alignment among LTR-RTs and tRF31Asp2.
We surveyed the relationship between genes and ChAP-peaks. The ChAP-seq reads mapped within gene body and upstream/downstream regions were calculated and normalized (RP10M) within 10 bp windows around the TSS (transcription starting site) and TTS (transcription termination site) of tRF-DEGs identified as described above. All of the four batches were merged together. The physical distances between genes and ChAP-peaks were calculated using bedtools (v2.26.0) of the closest distance between tRF31Asp2 binding sites and genes were made for TE and non-TE associated tRF31Asp2 binding sites, as well as tRF-DEGs and non-tRF-DEGs, respectively.
Sequence homology, RNAfold and 5′ terminal nucleotides analysis
FASTA (v36.3)75 was used to obtain an E-value (the number of expected hits of similar quality found just by chance) in sequence homology to 13 nts of 5′ single-strand sequence of tRF31Asp2. The tRF sequence was aligned to ChAP-peak sequences identified above with the option ‘-U’ allowing an RNA-DNA alignment.
RNAfold76 was used to predict secondary structures of tRNA and its fragments with the default parameters.
Trimmed reads in 21 nts were aligned to the TAIR10 reference genome using Bowtie2 (v2.2.9)70. Subsequently, reads aligned to rRNA, tRNA, snRNA, and snoRNA were eliminated using samtools (v1.10)66. Then, 21 nts reads mapping to miRNA and siRNA after excluding those present in the WT control were analyzed for the 5′ terminal nucleotide.
Reagent details
Detailed information on the reagents used in this study, including vendor names and catalog numbers, is provided in Supplementary Table 2.
Statistics and Reproducibility
For statistical analysis, unpaired Student’s t-tests or unpaired One-way ANOVA with Tukey post hoc tests were used to compare two different conditions. The following values were used: ***p < 0.001; **p < 0.01; *p < 0.05. GO enrichment analysis was performed using goProfiles77 to identify significantly enriched GO categories. Only GO categories with a false discovery rate (FDR) of less than 0.01 were considered significant and are presented in the results. The Wilcoxon rank sum test was employed to compare the expression levels of the PR1/2/5 genes between different treatment groups. This non-parametric test was selected to assess differences in gene expression without assuming a normal distribution of the data. Fisher’s LSD post-hoc test was conducted to determine which specific groups (non-TE or TE) showed significant differences after the initial ANOVA analysis indicated overall group differences. The significance level was set at p < 0.05. The n value, ranging from 2 to 12, was provided to indicate the number of samples or independent experiments. All experiments were independently reproduced with similar outcomes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Deep-sequencing data have been deposited in the SRA database and processed data from the ChAP-seq (bed alignment files, bw genome browser files, and narrowPeak peak-calling files) have been deposited to the GEO database. These SRA and GEO data are accessible through BioProject number PRJNA788635 whose processed data at the final step are provided as Supplementary Data 1–6. All the data, the main text, or the supplementary materials are available from the corresponding author on request. Numerical source data for all graphs in the manuscript can be found in Supplementary Data 7. Uncropped images are presented in Supplementary Fig. 13.
Code availability
No custom computer code or algorithm was used in this study.
References
Toruno, T. Y., Stergiopoulos, I. & Coaker, G. Plant-Pathogen Effectors: Cellular Probes Interfering with Plant Defenses in Spatial and Temporal Manners. Annu Rev. Phytopathol. 54, 419–441 (2016).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Jubic, L. M., Saile, S., Furzer, O. J., El Kasmi, F. & Dangl, J. L. Help wanted: helper NLRs and plant immune responses. Curr. Opin. Plant Biol. 50, 82–94 (2019).
Tsuda, K., Sato, M., Stoddard, T., Glazebrook, J. & Katagiri, F. Network properties of robust immunity in plants. PLoS Genet 5, e1000772 (2009).
Tsuda, K. & Somssich, I. E. Transcriptional networks in plant immunity. N. Phytol. 206, 932–947 (2015).
Yuan, M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109 (2021).
Ngou, B. P. M., Ahn, H. K., Ding, P. & Jones, J. D. G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115 (2021).
Bologna, N. G. & Voinnet, O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev. Plant Biol. 65, 473–503 (2014).
Vaucheret, H. Plant ARGONAUTES. Trends Plant Sci. 13, 350–358 (2008).
Zhang, X. et al. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393(*)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol. Cell. 42, 356–366 (2011).
Jimeno, S., Balestra, F. R. & Huertas, P. The Emerging Role of RNA Modifications in DNA Double-Strand Break Repair. Front Mol. Biosci. 8, 664872 (2021).
Su, Z., Wilson, B., Kumar, P. & Dutta, A. Noncanonical Roles of tRNAs: tRNA Fragments and Beyond. Annu Rev. Genet 54, 47–69 (2020).
Ramirez, V. et al. A 2’-O-Methyltransferase Responsible for Transfer RNA Anticodon Modification Is Pivotal for Resistance to Pseudomonas syringae DC3000 in Arabidopsis. Mol. Plant Microbe Interact. 31, 1323–1336 (2018).
Fangfang, J. & Zhigang, G. Emerging role of a novel small non-coding regulatory RNA: tRNA-derived small RNA. ExRNA 1, 39 (2019).
Kumar, P., Anaya, J., Mudunuri, S. B. & Dutta, A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 12, 78 (2014).
Fu, H. et al. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 583, 437–442 (2009).
Levitz, R. et al. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J. 9, 1383–1389 (1990).
Thompson, D. M. & Parker, R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J. Cell Biol. 185, 43–50 (2009).
Loher, P., Telonis, A. G. & Rigoutsos, I. MINTmap: fast and exhaustive profiling of nuclear and mitochondrial tRNA fragments from short RNA-seq data. Sci. Rep. 7, 41184 (2017).
Hou, J., Li, Q., Wang, J. & Lu, W. tRFs and tRNA Halves: Novel Cellular Defenders in Multiple Biological Processes. Curr. Issues Mol. Biol. 44, 5949–5962 (2022).
Kuscu, C. et al. tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA 24, 1093–1105 (2018).
Ren, B., Wang, X., Duan, J. & Ma, J. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science 365, 919–922 (2019).
Schorn, A. J., Gutbrod, M. J., LeBlanc, C. & Martienssen, R. LTR-Retrotransposon Control by tRNA-Derived Small RNAs. Cell 170, 61–71.e11 (2017).
Sharma, U. et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396 (2016).
Pujara, D. S. et al. Imaging-based resistance assay using enhanced luminescence-tagged Pseudomonas syringae reveals a complex epigenetic network in plant defense signaling pathways. Mol Plant Microbe Interact 34, 990–1000 (2021).
Mine, A. et al. The Defense Phytohormone Signaling Network Enables Rapid, High-Amplitude Transcriptional Reprogramming during Effector-Triggered Immunity. Plant Cell 30, 1199–1219 (2018).
Cui, H., Tsuda, K. & Parker, J. E. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev. Plant Biol. 66, 487–511 (2015).
Zhu, H. et al. Bidirectional processing of pri-miRNAs with branched terminal loops by Arabidopsis Dicer-like1. Nat. Struct. Mol. Biol. 20, 1106–1115 (2013).
Liu, C. et al. Arabidopsis ARGONAUTE 1 Binds Chromatin to Promote Gene Transcription in Response to Hormones and Stresses. Dev. Cell 44, 348–361.e347 (2018).
Kettles, G. J., Drurey, C., Schoonbeek, H. J., Maule, A. J. & Hogenhout, S. A. Resistance of Arabidopsis thaliana to the green peach aphid, Myzus persicae, involves camalexin and is regulated by microRNAs. N. Phytol. 198, 1178–1190 (2013).
Carbonell, A. et al. Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell 24, 3613–3629 (2012).
Liu, T. Use model-based Analysis of ChIP-Seq (MACS) to analyze short reads generated by sequencing protein-DNA interactions in embryonic stem cells. Methods Mol. Biol. 1150, 81–95 (2014).
Schorn, A. J. & Martienssen, R. Tie-Break: Host and Retrotransposons Play tRNA. Trends Cell Biol. 28, 793–806 (2018).
Cordaux, R. & Batzer, M. A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet 10, 691–703 (2009).
Galindo-Gonzalez, L., Mhiri, C., Deyholos, M. K. & Grandbastien, M. A. LTR-retrotransposons in plants: Engines of evolution. Gene 626, 14–25 (2017).
Judd, J., Sanderson, H. & Feschotte, C. Evolution of mouse circadian enhancers from transposable elements. Genome Biol. 22, 193 (2021).
Kwok, A., Raulf, N. & Habib, N. Developing small activating RNA as a therapeutic: current challenges and promises. Ther. Deliv. 10, 151–164 (2019).
Li, L. C. et al. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. USA 103, 17337–17342 (2006).
Martinez, G., Choudury, S. G. & Slotkin, R. K. tRNA-derived small RNAs target transposable element transcripts. Nucleic Acids Res. 45, 5142–5152 (2017).
Pan, L. et al. Inflammatory cytokine-regulated tRNA-derived fragment tRF-21 suppresses pancreatic ductal adenocarcinoma progression. J. Clin. Invest. 131 https://doi.org/10.1172/JCI148130 (2021).
Qiao, Y. et al. Small RNAs in Plant Immunity and Virulence of Filamentous Pathogens. Annu Rev. Phytopathol. 59, 265–288 (2021).
Schauer, S. E., Jacobsen, S. E., Meinke, D. W. & Ray, A. DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci. 7, 487–491 (2002).
Burger, K. & Gullerova, M. Nuclear re-localization of Dicer in primary mouse embryonic fibroblast nuclei following DNA damage. PLoS Genet 14, e1007151 (2018).
Doyle, M. et al. The double-stranded RNA binding domain of human Dicer functions as a nuclear localization signal. RNA 19, 1238–1252 (2013).
Zaitseva, L., Myers, R. & Fassati, A. tRNAs promote nuclear import of HIV-1 intracellular reverse transcription complexes. PLoS Biol. 4, e332 (2006).
Kramer, E. B. & Hopper, A. K. Retrograde transfer RNA nuclear import provides a new level of tRNA quality control in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 110, 21042–21047 (2013).
Morel, J. B. et al. Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant cell 14, 629–639 (2002).
Agorio, A. & Vera, P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 19, 3778–3790 (2007).
Montgomery, T. A. et al. Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133, 128–141 (2008).
Lang, J. D., Ray, S. & Ray, A. sin 1, a mutation affecting female fertility in Arabidopsis, interacts with mod 1, its recessive modifier. Genetics 137, 1101–1110 (1994).
Tang, G. et al. Construction of short tandem target mimic (STTM) to block the functions of plant and animal microRNAs. Methods 58, 118–125 (2012).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Hong, S. M., Bahn, S. C., Lyu, A., Jung, H. S. & Ahn, J. H. Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiol. 51, 1694–1706 (2010).
Varkonyi-Gasic, E. Stem-Loop qRT-PCR for the Detection of Plant microRNAs. Methods Mol. Biol. 1456, 163–175 (2017).
Matoušková, P. et al. Reference genes for real-time PCR quantification of messenger RNAs and microRNAs in mouse model of obesity. PLoS One 9, e86033 (2014).
Kim, S. W. et al. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic acids research 38, e98 (2010).
Mang, H. G. et al. Abscisic Acid Deficiency Antagonizes High-Temperature Inhibition of Disease Resistance through Enhancing Nuclear Accumulation of Resistance Proteins SNC1 and RPS4 in Arabidopsis. Plant cell 24, 1271–1284 (2012).
Kang, H. G. & Klessig, D. F. Salicylic acid-inducible Arabidopsis CK2-like activity phosphorylates TGA2. Plant Mol. Biol. 57, 541–557 (2005).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. methods 9, 671–675 (2012).
Louis, J. et al. Discrimination of Arabidopsis PAD4 activities in defense against green peach aphid and pathogens. Plant Physiol. 158, 1860–1872 (2012).
Hassan, T. et al. Isolation and identification of cell-specific microRNAs targeting a messenger RNA using a biotinylated anti-sense oligonucleotide capture affinity technique. Nucleic Acids Res 41, e71 (2013).
Smith, J. M. & Heese, A. Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods 10, 6 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 17 https://doi.org/10.14806/ej.17.1.200 (2011).
Cheng, C. Y. et al. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 89, 789–804 (2017).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Chan, P. P. & Lowe, T. M. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 44, D184–D189 (2016).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Feng, J., Liu, T., Qin, B., Zhang, Y. & Liu, X. S. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 7, 1728–1740 (2012).
Ellinghaus, D., Kurtz, S. & Willhoeft, U. LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinforma. 9, 18 (2008).
Hsu, C. C. et al. Identification of high-copy number long terminal repeat retrotransposons and their expansion in Phalaenopsis orchids. BMC Genomics 21, 807 (2020).
Ou, S. & Jiang, N. LTR_retriever: A Highly Accurate and Sensitive Program for Identification of Long Terminal Repeat Retrotransposons. Plant Physiol. 176, 1410–1422 (2018).
Pearson, W. R. & Lipman, D. J. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85, 2444–2448 (1988).
Denman, R. B. Using RNAFOLD to predict the activity of small catalytic RNAs. Biotechniques 15, 1090–1095 (1993).
Sanchez-Pla, A., Salicrú, M. & Ocana, J. Statistical methods for the analysis of high-throughput data based on functional profiles derived from the gene ontology. J. Stat. Plan Inference 137, 3975–3989 (2007).
Acknowledgements
We thank Daniel.F. Klessig, Georg Jander, D’Maris Dempsey, Chang-Sik Oh, and Angela H. Kang for critical reading on the manuscript, Hailing Jin for ago2/3/7, James C. Carrington for HA-AGO1 and HA-AGO2 lines, and Dittmar Hahn, Bhavya Doddavarapu and Udayangani Jayasekara for technical assistance. This work was supported by the National Science Foundation (IOS-1553613, H.K.; DBI-1546890, Q.Y.; IOS-1845588, J.L.; MCB 2139857 to X.Z.) and the National Health Institute (R01GM112722, J.K.), the US Department of Agriculture National Institute of Food and Agriculture (Hatch Project TEX0-2-9374, Q.Y.).
Author information
Authors and Affiliations
Contributions
S.I.K., Y.B., H.L., D.S.P., P.S.B., J.M., H.C., P.K.Z., P.K., X.Y., and H.G.K. performed the research; H.G.K., S.I.K., Y.B., D.S.P., P.S.B., H.L., J.M., J.K., J.L., X.Z., and Q.Y. analyzed the data; H.G.K. conceived and designed research, and wrote the manuscript, with input from S.I.K., Y.B., H.L., D.S.P., J.M., X.Z., J.L., and Q.Y.
Corresponding author
Ethics declarations
Competing interests
H.G.K. and S.I.K. are named inventors on a patent application related to the subject matter of this publication. The patent, US 2023/0112054, has been published and is currently under review.
Peer review
Peer review information
This manuscript has been previously reviewed at another Nature Portfolio journal. Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: David Favero. A peer review file is available. This document only contains reviewer comments and rebuttal letters for versions considered at Communications Biology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, SI., Lyu, H., Pujara, D.S. et al. A nuclear tRNA-derived fragment triggers immunity in Arabidopsis. Commun Biol 8, 533 (2025). https://doi.org/10.1038/s42003-025-07737-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07737-1