Abstract
Head motion during magnetic resonance imaging (MRI) examinations of patients with autism spectrum disorder (ASD) can influence the identification of brain differences as well as early diagnosis and precise MRI-based interventions for ASD. This study aims to address head motion issues in resting-state functional MRI (rs-fMRI) data by comparing various correction methods. Specifically, we evaluate the independent component analysis-based automatic removal of motion artifacts (ICA-AROMA) against traditional preprocessing pipelines, including head motion realignment parameters and global signal regression (GSR). Our dataset consisted of 306 participants, including 148 individuals with ASD and 158 participants with typical development (TD). We find that ICA-AROMA, particularly when combined with GSR and physiological noise correction, outperformed other strategies in differentiating ASD from TD participants based on functional connectivity (FC) analyses. The correlation of quality control with functional connectivity (QC-FC) is statistically significant in proportion and distance after applying each denoising pipeline. The mean FC between groups is significant for Yeo’s 17-Network in each denoising strategy. ICA-AROMA head motion correction outperformed other strategies, revealing more significant FC networks and distinct brain regions linked to the posterior cingulate cortex and postcentral gyrus. This suggests ICA-AROMA enhances fMRI preprocessing, aiding ASD diagnosis and biomarker development.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder presenting with typical or repetitive body movements1,2, which contribute to increased head motion during magnetic resonance imaging (MRI) examinations3,4. These head motion scans yield spurious findings and/or reduce detection of the actual disorder to some degree. Functional MRI (fMRI) assessments are often hampered by the consequently poor signal-to-noise ratio (SNR) and low reproducibility, raising questions regarding the clinical utility of this technique.
A large number of personalized investigations are required to interpret results derived from individualized fMRI measurements. Every brain is unique, and the variability in brain characteristics is estimable by accurate and reliable fMRI analyses, which are of major importance in the field of neuroscience. The ability to characterize the brain organization of individuals is critical to biomarker discovery, and holds great promise in revealing the basis of brain disorders and potential treatments.
The process of effectively extracting noise can influence the reliability and conduct of any given clinical trial. Denoising is a potentially promising but not fully developed strategy to overcome poor SNR and improve the accuracy and reliability of fMRI examinations. Digital filtering, motion correction realignment, and spatial smoothing are frequently used procedures in data preprocessing but show limited denoising ability. Optimization of MRI analysis can address many of the concerns associated with motion-related artifacts. Early approaches, such as volume realignment and temporal censoring5, have been widely used to mitigate motion-related noise. These methods, including framewise displacement (FD) measures and regression of signals from white matter (WM) and cerebrospinal fluid (CSF), and principal component analysis (PCA)6,7,8 have formed the foundation of fMRI preprocessing pipelines. More recent advancements, such as independent component analysis-based techniques like ICA-AROMA9, have improved upon traditional methods by offering a more automated and refined approach to removing motion-related artifacts. These newer techniques allow for greater preservation of the signal of interest while reducing the impact of head motion, particularly in studies of neurodevelopmental disorders like ASD. Human neuroimaging offers a unique and promising avenue in the study of neurodevelopmental disorders (NDDs) such as ASD, attention-deficit hyperactivity disorder (ADHD), and Tourette’s syndrome (TS)10. based on previous research such the data due to motion >2 mm, such as ADHD (42%) and NDDs (5–10%) will be excluded. However, to the best of our knowledge, no previous study has performed head motion research on the ASD dataset.
We assume that different methods of head motion removal may produce different resting-state local connectivity. This distinction is especially important when studying networks involved in ASD, such as the posterior cingulate cortex (PCC). The PCC is a central component of the default mode network (DMN), associated with social cognition and self-referential processes—functions often disrupted in ASD. Given the role of the PCC in ASD, we hypothesize that different motion correction methods may yield varying connectivity patterns. Therefore, our research group has tried to identify the characteristics of ASD head motion. This study applied ICA-AROMA in the ASD and TD groups to explore the differences between them in head motion correction.
Results
We used Pearson correlations to quantify the associations between subject-specific mean FD and FC estimates for each edge, yielding edge-specific QC-FC values estimated across the entire sample for each group. The proportion of edges where the QC-FC correlation was statistically significant (p < 0.05, uncorrected) as well as the median absolute QC-FC correlation after applying each denoising pipeline are shown in Fig. 1A. Given the multiple-comparison corrections, we also show a corrected version in Fig. 1A. A histogram of all QC-FC correlations across seven sites is shown for each strategy (Fig. 1B). The QC-FC correlation of each edge as a function of the distance metricis shown statistically in Fig. 2. Since each network contained tens of thousands of edges, the resulting scatter plots appear as a dense point cloud, which masked the mean-level trends in Fig. 2A. The mean and standard deviation of QC-FC correlations with 10 equiprobable bins are shown in Fig. 2B.
A Proportion of QC-FC correlations (p < 0.05, uncorrected), Pearson’s correlations, and FDR-corrected values (p < 0.05). It shows the relationship between ASD patients’ head motions and their brains’ functional connectivity. B QC-FC correlation histograms. In each denoising strategy, the histogram shows the correlations among seven sites for each group.
The association between Euclidean distance measurements and the QC-FC correlation for each edge was obtained using Spearman’s rank correlation coefficients. Upward-pointing triangles denote TD, and purple downward-pointing triangles ASD. A QC-FC distance-dependent scatterplots. B QC-FC distance dependence arranged in bins, and QC-FC correlations split into 10 equiprobable bins (using equally spaced quantiles to define bins) based on the distances between nodes.
QC-FC correlations were statistically significant (p < 0.05, uncorrected and FDR corrected) in proportion and distance after applying each denoising pipeline, as was the mean FC (p < 0.01, uncorrected and FDR corrected) for Yeo’s 17-Network parcellation in each denoising strategy. The different cortical surfaces showed significant differences between the ASD and TD groups with the change of strategy. For example, there were radar plots of mean FC for Network 11 and cortical surface plots of mean FC for Network 11 (Fig. 3). In comparison with the TD group, significant differences in FC networks in the ASD group were found for each strategy (Fig. 4).
In each denoising strategy, the cortical surface plot denotes the mean FC (after Fisher’s r-to-z transform) between network 11 (related with posterior cingulate cortex) and the rest of networks in Yeo’s 17-Network parcellation for each group. The correlation ranges from -0.5 to 1.1(A: HMP+2Phys; B: HMP+2Phys+GSR; C: ICA-AROMA+2Phys; D: ICA-AROMA+2Phys+GSR).
For each FC between two networks in Yeo’s 17-Network parcellation, the box plot shows the distribution and significance of FC between two groups among the four denoising strategies (A: HMP+2Phys, uncorrected; B: HMP+2Phys+GSR, uncorrected; C: ICA-AROMA+2Phys, uncorrected; D: ICA-AROMA+2Phys+GSR, uncorrected; E: ICA-AROMA+2Phys+GSR, FDR corrected). In each grid, the left box represents the results of the TD group, and the right box represents the results of the ASD group. When the two groups show a significant difference, the block shows a yellow background and star symbol (*denotes p < 0.05, **denotes p < 0.01, ***denotes p < 0.001). X-axis and y-axis labels denote the network number in Yeo’s 17-Network parcellation. For network 6 which mainly represent the postcentral frontal, the significantly different regions (p < 0.01) between two groups across four strategies ranged from None, 11, 4-14, and 4-11 networks, as denoted by the red boxes. For network 11 which mainly represent the PCC, the significantly different regions (p < 0.01) between the two groups in the four strategies were ranged from None, 6-7-8, 8-12, and 6-7-8-12-16-17 networks, as denoted by green boxes. After applying FDR correction, significant results were observed only under ICA-AROMA+2Phys+GSR strategy, as shown in Fig. 4E.
Discussion
AROMA analysis is a reliable technique in the ASD and TD groups
ICA-AROMA uses independent component analysis to identify participant-specific motion-related components, which can increase the sensitivity to the signal of interest9. For the ASD and TD datasets, incorporating ICA-AROMA into the preprocessing pipeline yielded a significantly lower proportion of correlations between head motion and functional connectivity (FC) across different brain regions compared to other motion correction methods. Specifically, after FDR correction, ICA-AROMA plus 2Phys in the ASD group showed the lowest values across all strategies. That means that the ICA-AROMA was an effective method for head motion correction with ASD. The reason all these strategies could be compared and analyzed is that their QC-FC were derived from an approximately normal distribution (Fig. 2). Additionally, there was no significant difference in QC-FC correlation distributions between the ASD and TD groups with ICA-AROMA. Each strategy produced significant differences in the TD group, but not in the ASD group.
Effective AROMA is helpful in efforts to discover different network functional areas in ASD
Identifying brain abnormalities in ASD is critical for early diagnosis and intervention. More recent studies have identified brain differences using resting-state fMRI (rs-fMRI), which provides a promising alternative to traditional task-based fMRI for studying the large-scale organization of typically and atypically developing brain networks11. This is particularly useful for young participants, where task-based paradigms can be more difficult to implement consistently. In such participants, the regions of identification may depend on the kind of head motion correction applied by author during clinical trials12.
With the same methods, Regional Homogeneity (ReHo) for fMRI, the results differed while the head motion correction methods used varied. The results differed in decreased regions (right superior temporal sulcus, right inferior and middle frontal gyri, bilateral cerebellar crus I, right insula, and right postcentral gyrus), increased regions (right thalamus, left inferior frontal and anterior subcallosal gyrus and bilateral cerebellar lobule VIII) and cingulate and medial frontal underconnectivity related with the default mode network13,14. Different software packages, such as Analysis of Functional NeuroImages software (AFNI)13, offer alternative approaches to head motion correction, which can impact the results of functional connectivity analyses. In particular, variations in motion correction methods across tools like AFNI and ICA-AROMA may lead to different interpretations of functional brain networks, underscoring the importance of selecting the most appropriate method for the study at hand. This conclusion could also be drawn from ICA for fMRI with different head motion correction methods15,16,17. Besides, the group of identical head motion correction methods displayed similar results for insula connectivity15,17. In this paper, different strategies were found to influence different regions in fMRI analysis. The number of increased FC networks showed significant (p < 0.05) differences between two groups (ASD > TD). The number of regions identified in the four strategiesincluded3 (p < 0.05), 13 (10 instances: p < 0.05, 3 instances: p < 0.01), 6 regions (4instances: p < 0.05, 2instances: p < 0.01), and 13 regions (7instances: p < 0.05, 6instances: p < 0.01). Decreased FC networks were significantly different (p < 0.05) between the two groups (ASD < TD). The various numbers of regions among the four strategies are from 8 (p < 0.05), 11 (9 instances: p < 0.05, 2instances: p < 0.01), 25 regions (18 instances: p < 0.05, 7instances: p < 0.01) and 16 regions (9 instances: p < 0.05, 7instances: p < 0.01).If the dataset was preprocessed with new methods such as the Driven Wavelet or Root Mean Square of Displacement, the functional areas were indicated in various aspects18. An identical strategy was carried out in this study. Moreover, the different data sources were another reason for the variable results. For example, for the same dataset, ABIDE, and same head motion correction methods and volume censoring, the functional regions did not align with differences in clinical and behavioral traits in individuals with ASD19 (Table 1).
Effective head motion correction can broaden the adaptation to society among those with ASD
Recent studies have indicated that head movement dynamics (displacement and velocity) were greater in children with ASD than in children without ASD20. This finding provided a quantitative foundation for previous clinical reports, similar to this study. In this study, there was no significant difference in the head motion parameter FD_Jenk_mean between the ASD and the TD groups (P > 0.05). Children with ASD may use head movement to modulate their perception of social scenes19.
The posterior cingulate cortex (PCC) plays a key role in cognitive, social, and emotional processing as well as in early neural development, supporting complex interactions across different functional networks. The changes in local and long-term connectivity in the resting state are usually related to ASD. This study also found changes in FC as PCC seed mainly distributed across networks 11 and 16. In relation to networks 11 and 16, which mainly represent the PCC, the significantly different regions (p < 0.01) between the two groups in the four strategies were ranged from None, 6-7-8, 8-12, and 6-7-8-12-16-17 networks, as denoted by green boxes in Fig. 4. ASD is characterized by social and communication barriers, as well as restricted and repetitive behaviors and interests. These brain regions overlapped with several components of the so-called “social brain,” such as the medial PFC, PCC, and amygdala, which are important for affective aspects of social processing20. Clearly, the networks connected with PCC increased with various methods of head motion removal. ICA-AROMA+2Phys+GSR was found to reveal a greater number of significant connectivity differences between the ASD and TD groups compared to other methods, highlighting its effectiveness in distinguishing functional brain connectivity patterns associated with ASD. In terms of regional metrics, only the dorsal part of the PCC showed local underconnectivity21. The reduced functional coupling between the posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC) may represent a core neuropathology of ASD, independent of the effects of medication. This disrupted connectivity in the default mode network (DMN) has been observed in multiple studies and is thought to contribute to the social and cognitive impairments characteristic of ASD. The functional importance of these resting-state abnormalities to ASD and directions of future studies are discussed at the end of this section. In comparison with TD controls, the right medial paracentral lobule exhibited abnormal functional connectivity was observed across adolescent and middle-aged samples with ASD, suggesting that this abnormality could be persistent in ASD. In this paper, in network 6 (mainly representing the postcentral frontal), the significantly different regions (p < 0.01) between two groups across four strategies ranged from None, 11, 4-14, and 4-11 networks with the red box in Fig. 4.
Global signal regression
GSR was included as an optional variable in our analyses to account for confounding factors related to non-neural signals. However, it is important to note that GSR can potentially introduce artifacts into the functional connectivity measures, including an increase in instances of negative connectivity relationships or anticorrelations22. This phenomenon may arise from the removal of global fluctuations that could represent true neural activity, thereby distorting the interpretation of connectivity patterns23.
In our study, while GSR helped mitigate some motion-related noise, its application may have influenced the observed connectivity results, particularly in networks such as the default mode network (DMN). Therefore, caution should be exercised when interpreting our findings, as the presence of negative correlations may not solely reflect genuine neural dynamics but could also be a byproduct of GSR23. Future studies should consider the impact of GSR more thoroughly and explore alternative methods for addressing global signal fluctuations to provide a clearer understanding of functional connectivity in autism spectrum disorder (ASD).
Limitations
The analysis methods used for interpreting fMRI data can affect the reliability of fMRI results, and the key problem influencing this aspect is the modeling signal. In two previous ASD experiments, models were trained by using different datasets, each with their own limitations. Additionally, the proposed AROMA method focuses only on ASD, but could be transferred to identifying other sites from different geographic regions. The analysis focused on Yeo’s 17 networks, which include regions such as the posterior cingulate cortex (PCC) that are involved in multiple networks, for example, network 11 and network 16 are both related to the PCC area. This overlap can complicate the interpretation of connectivity results, as networks may share some dynamics while operating independently. Therefore, while we identified significant connectivity differences, the potential influence of these overlaps on our findings should be considered.
In this study, we proposed a novel ICA-AROMA method for head motion correction for the ASD fMRI dataset. This method shows better performance in decreasing head motion. In comparison with the traditional method, this method shows a greater number of meaningful brain regions for distinguishing ASD from TD. The results show that the ICA-AROMA method is an effective approach for processing fMRI data among those with ASD and affects the results of fMRI, which can facilitate the examination of meaningful networks such as PCC and postcentral gyrus. Evaluations using this method could serve as a precondition of reliable results in MRI clinical trials and the basis of interventions for ASD.
Methods
Participants
The structural and resting-state functional MRI data in this study were selected from the Autism Brain Imaging Data Exchange (ABIDE)24. Specifically, the data were obtained from participants enrolled at 7 sites across USA and China, including 38 participants at Site 1, 26 at Site 2, 36 at Site 3, 58 at Site 4, 56 at Site 5, 36 at Site 6, and 56 at Site 7. All participants were categorized into ASD or TD groups for each site (Table 2). The study protocol approved by the Medical Ethics Committee of the Chinese People’s Liberation Army General Hospital was fully disclosed to all participants and their guardians, and written informed consent was obtained from each participant’s guardian according to the provision of the Declaration of Helsinki.
Preprocessing
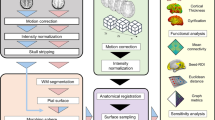
The preprocessing pipelines for structural and resting-state functional MRI data are derived from the code at https://github.com/lindenmp/rs-fMRI3. The corresponding code will be uploaded to Github(https://github.com/haililihai/ASD_HeadMotionCorrection). Running in the prepro directory, prepro.m is a general preprocessing script that can preprocess every participant’s structural and functional images, process multiple head movement correction algorithms, and calculate the functional connectivity based on different brain maps.
Structural MRI preprocessing
T1-weighted high-resolution structural images were processed using the following steps: (1) removal of the neck by using FSL’s robustfov; (2) segmentation of the native T1-weighted image into Gray Matter (GM), WM, and Cerebrospinal Fluid (CSF) probability maps by using SPM8’s New Segment routine; (3) nonlinear spatial transformation of the T1-weighted image to MNI space by using Advanced Normalization Tools (ANTs)25 with default settings (using the antsRegistrationSyN.sh script); and (4) application of the nonlinear transforms derived from the previous step to the WM, CSF, and GM masks.
Resting-state functional MRI preprocessing
Resting-state functional images were processed with the following steps:(1) removal of the first four volumes of each acquisition; (2) slice-time correction implementation in SPM8; (3) two-pass realignment of all volumes to the first volume (first pass) and then to the mean volume (second pass) by using SPM8; (4) co-registration of EPI data to the native, cropped, high-resolution structural image through rigid-body registration by using ANTs; (5) application of the nonlinear transform derived from the T1-weighted image-processing pipeline to the co-registered EPI data by using ANTs; (6) linear detrending of the spatially normalized BOLD time series; and (7) intensity normalization of the EPI data to a modewith 1000 units.
Noise extraction
Head motion parameters (HMPs)
The two-pass realignment of resting-state functional images yielded six time series describing in-scanner movement along six dimensions—three translational axes of X, Y, and Z, and three rotational axes of pitch, roll, and yaw.
White matter and cerebrospinal fluid (CSF) signals
WM and CSF tissue masks segmented in structural image preprocessing were eroded to ensure minimal contamination with GM voxels7. The averaged time series from all WM voxels and all CSF voxels in the masks were extracted as mean WM and CSF signals.
Global signal regression
Global signals were calculated by averaging across all voxels in the functional images by using participant-specific masks that covered the entire brain. These masks were generated by considering the union of two whole-brain masks created using FSL’s bet function to the spatially normalized EPI and T1-weighted images created during preprocessing.
ICA-AROMA. ICA-AROMA was applied to each participant’s BOLD data separately25. This method automatically identifies and removes motion-related artifacts from functional images by using FSL’s MELODIC to first decompose the data into spatially Independent Components (IC) before applying a predetermined, theoretically motivated classifier to identify ICs as noise or signal. Specifically, ICs were classified as motion-related if any of the following criteria were true: (1) more than 10% of the IC voxels were located within the CSF; (2) the IC time series had a high frequency content greater than 35%, where high frequency was defined as the fraction of the Nyquist frequency at which higher frequencies explain 50% of the total power between 0.01 Hz and the Nyquist frequency; or(3) ICs exceeded a decision boundary from a two-dimensional Linear Discriminant Analysis classifier. Unlike the other methods, ICA-AROMA requires data to be spatially smoothed before noise correction. As such, for all strategies that included ICA-AROMA, spatial smoothing was performed immediately before noise correction rather than after bandpass filtering.
Denoising strategies
Four strategies were applied to the preprocessed functional images: (1) 6HMP+2Phys, in which six HMPs and WM/CSF signals were regressed. (2) 6HMP+2Phys+GSR, regression of global signals was added to the steps listed above. (3) ICA-AROMA+2Phys, motion-related noise was removed by ICA-AROMA, after which WM/CSF signals were regressed. (4) ICA-AROMA+2Phys+GSR, global signals were also regressed along with WM/CSF signals. If k denotes an arbitrary number of additional regressors estimated automatically by the denoising method, which can vary from person to person, the number of regressors are 2+k and 3+k for ICA-AROMA+2Phys and ICA-AROMA+2Phys+GSR, respectively. Next, additional functional image-processing steps were applied, including bandpass filtering between 0.008 and 0.08 Hz with the fast Fourier transform, and spatial smoothing with a 6-mm FWHM kernel. Notably, ICA-AROMA required smoothing prior to noise correction.
Network construction
After preprocessing, functional connectivity networks were constructed by using Yeo’s 17-Network parcellations26. For each region, the mean time series across all voxels constituting the region was multiplied by its GM probability. Subsequently, Pearson correlation coefficients were calculated between each pair of regional averaged time series. These correlations can be represented as a network, where edges connecting pairs of nodes (brain regions) represent correlation coefficients between the resting-state fMRI time series. These networks underwent Fisher’s r-to-z transformation to normalize the correlation distribution and facilitate group comparisons.
QC-FC correlations
To measure the efficacy of each approach in removing motion-related variance, we compared the proportion of edges where this QC-FC correlation was statistically significant (p < 0.05, uncorrected and corrected FDR) as well as the median absolute QC-FC correlation after applying each denoising pipeline. To investigate the distribution of QC-FC correlations, we compiled a histogram of all QC-FC correlations across the seven sites used in each strategy. We also compared the differences between groups and strategies.
QC-FC distance dependence
We estimated the distances between regions as the Euclidean distance between the stereotaxic coordinates of the volumetric centers of brain region pairs. For each edge, we then quantified the association between this measure of distance and the QC-FC correlation for that edge using Spearman’s rank correlation coefficient, ρ, due to the observed non-linear associations between QC-FC and distance. To visualize the relationship between QC-FC and distance, we also plotted the QC-FC correlation of each edge as a function of this distance metric. To provide a more interpretable visualization of QC-FC distance dependence, we divided the data into 10 equiprobable bins (using equally spaced quantiles to define bins) based on nodal distance and plotted the mean and standard deviation of QC-FC correlations in each bin.
Functional connectivity
To investigate which FC shows group differences under the four denoising strategies, we plotted the FC distributions of the two groups for each FC between two networks in Yeo’s 17-Network parcellation. Specifically, we computed Pearson correlation coefficients between the time series of centroid coordinates across different networks, which are provided by Freesurfer software (https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011). We tested the significance of group differences by using two-tailed two-sample t-tests with FDR multiple-comparison corrections. We also plotted circular graphs denoting significantly different FCs, and plotted the FCs between network 11 and other networks in Yeo’s 17-Network in standard surface (Freesurfer’s fsaverage) for different groups and denoising strategies.
Statistical analysis
SPSS 22.0 was used for demographic statistical analysis. Shapiro–Wilktest was used to evaluate the normality of continuous variables before data analysis, and data showing a normal distribution were presented as mean ± standard deviation and compared with an independent-sample t-test; data that did not show a normal distribution were presented as median (quartile) values and compared using the Mann–Whitney U test. The chi-square test or Fisher’s exact probability method was used to compare categorical variables between groups. P < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available on request from the corresponding author [S.J.L.].
Code availability
The preprocessing and statistical analysis codes are publicly available in Github: https://github.com/haililihai/ASD_HeadMotionCorrection (https://doi.org/10.5281/zenodo.5517104)3.
References
Lai, M. C., Lombardo, M. V. & Baron-Cohen, S. Autism [J]. Lancet 383, 896–910 (2014).
Dawson, G., Rieder, A. & Johnson, M. Prediction of autism in infants: progress and challenges [J]. Lancet Neurol. 22, 244–254 (2023).
Parkes, L., Fulcher, B., Yücel, M. & Fornito, A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI [J]. NeuroImage 171, 415–436 (2018).
Phạm, D., Mcdonald, D., Ding, L., Nebel, M. & Mejia, A. Less is more: balancing noise reduction and data retention in fMRI with data-driven scrubbing [J]. NeuroImage 270, 119972 (2023).
Power, J., Barnes, K., Snyder, A., Schlaggar, B. & Petersen, S. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion [J]. NeuroImage 59, 2142–2154 (2012).
Satterthwaite, T. D. et al. Functional maturation of the executive system during adolescence [J]. J. Neurosci. 33, 16249–16261 (2013).
Power, J., Schlaggar, B. & Petersen, S. Recent progress and outstanding issues in motion correction in resting state fMRI [J]. NeuroImage 105, 536–551 (2015).
Muschelli, J. et al. Reduction of motion-related artifacts in resting state fMRI using aCompCor [J]. NeuroImage 96, 22–35 (2014).
Pruim, R. et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data [J]. NeuroImage 112, 267–277 (2015).
Bednarz, H. & Kana, R. Advances, challenges, and promises in pediatric neuroimaging of neurodevelopmental disorders [J]. Neurosci. Biobehav. Rev. 90, 50–69 (2018).
Lau, W., Leung, M. & Lau, B. Resting-state abnormalities in Autism Spectrum Disorders: A meta-analysis [J]. Sci. Rep. 9, 3892 (2019).
Paakki, J. et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders [J]. Brain Res. 1321, 169–179 (2010).
Maximo, J., Keown, C., Nair, A. & Müller, R. Approaches to local connectivity in autism using resting state functional connectivity MRI [J]. Front. Hum. Neurosci. 7, 605 (2013).
Von Dem Hagen, E., Stoyanova, R., Baron-Cohen, S. & Calder, A. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions [J]. Soc. Cogn. Affect. Neurosci. 8, 694–701 (2013).
Mueller, S. et al. Convergent Findings of Altered Functional and Structural Brain Connectivity in Individuals with High Functioning Autism: A Multimodal MRI Study [J]. PloS one 8, e67329 (2013).
Bos, D. et al. Developmental differences in higher-order resting-state networks in Autism Spectrum Disorder [J]. NeuroImage Clin. 4, 820–827 (2014).
Guo, X. et al. Enhancement of indirect functional connections with shortest path length in the adult autistic brain [J]. Hum. brain Mapp. 40, 5354–5369 (2019).
King, J. et al. Generalizability and reproducibility of functional connectivity in autism [J]. Mol. autism 10, 27 (2019).
Martin, K. et al. Objective measurement of head movement differences in children with and without autism spectrum disorder [J]. Mol. autism 9, 14 (2018).
Subbaraju, V., Sundaram, S. & Narasimhan, S. Identification of lateralized compensatory neural activities within the social brain due to autism spectrum disorder in adolescent males [J]. Eur. J. Neurosci. 47, 631–642 (2018).
Leung, M. & Lau, W. Resting-state abnormalities of posterior cingulate in autism spectrum disorder [J]. Prog. Mol. Biol. Transl. Sci. 173, 139–159 (2020).
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B. & Bandettini, P. A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage 44, 893–905 (2009).
Murphy, K., Birn, R. M. & Bandettini, P. A. Resting-state fMRI confounds and cleanup. NeuroImage 80, 349–359 (2013).
Di Martino, A. et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism [J]. Mol. psychiatry 19, 659–667 (2014).
Catani, M. Thiebaut De Schotten, M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections [J]. Cortex; a J. devoted study Nerv. Syst. Behav. 44, 1105–1132 (2008).
Kong, R. et al. Comparison between gradients and parcellations for functional connectivity prediction of behavior [J]. NeuroImage 273, 120044 (2023).
Nair, S. et al. Local resting state functional connectivity in autism: site and cohort variability and the effect of eye status [J]. Brain imaging Behav. 12, 168–179 (2018).
Acknowledgements
I greatly thank Dr. Singh, Dr. Ajay K, Dr. McCausl and, Dr. Finnian Raymond, Dr. Kroshinsky Daniela and their teammates of MMSCI in Harvard Medical School. Their direction, understanding and great help make my study on a good path and my thesis continuously better. This study was supported by Project of National Key R&D Program of China (2022YFC2409404), Capital’s Funds for Health Improvement and Research (CFH2024-204) and the National Natural Science Foundation of China (81971603). The clinical trial registration is ChiCTR1800018616 and ChiCTR1800020467. Funding This work was supported by National Key R&D Program of China (grant numbers 2022YFC2409404), Capital’s Funds for Health Improvement and Research (grant numbers CFH2024-2-5024),National Natural Science Foundation of China (81971603), Clinical cohort research and integration platform for neuropsychiatric disorders: cohort collaborative research for children with autism spectrum disorder (grant numbers Z181100001518005). This work was supported by National Key R&D Program of China (grant numbers 2022YFC2409404), Capital’s Funds for Health Improvement and Research (grant numbers CFH2024-2-5024),National Natural Science Foundation of China (81971603), Clinical cohort research and integration platform for neuropsychiatric disorders: cohort collaborative research for children with autism spectrum disorder (grant numbers Z181100001518005).
Author information
Authors and Affiliations
Contributions
S.L. and H.L. conceptualized the project; S.L., L.Z. and Y.W designed research; J.G., S.L., Y.L., and Y.W. performed research; H.L., M.X., P.W. and Q.Y. contributed new reagents/analytic tools; S.L., and Y.L. analyzed data; and J.G., S.L. and H.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects were approved by the Medical Ethics Committee of the Chinese People’s Liberation Army General Hospital (No. S2018-218-01). The clinical trial registration number is ChiCTR1800018616 (2018.09.03) and ChiCTR1800020467 (2018.12.31).
Peer review
Peer review information
Communications Biology thanks David Cole and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Michel Thiebaut de Schotten and Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guan, J., Li, H., Yang, Q. et al. Improving brain difference identification in autism spectrum disorder through enhanced head motion correction in ICA-AROMA. Commun Biol 8, 473 (2025). https://doi.org/10.1038/s42003-025-07928-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07928-w