Abstract
Limb regeneration in the Mexican axolotl relies on the dedifferentiation of mature limb cells into blastema cells, which gain the ability to respond to patterning signals that guide tissue regeneration. While limb nerves are essential to make the blastema cells competent to pattern, the mechanisms remain unclear due to the complex and overlapping signals in amputated limbs. To overcome this challenge, we developed the Competency Accessory Limb Model (CALM), a simplified limb regeneration assay to study the induction and maintenance of patterning competency. Using CALM, here we show specific temporal windows during which cells acquire competency and associate this state with distinct H3K27me3 chromatin signatures. Furthermore, a combination of FGF and BMP signaling is sufficient to induce patterning competency in limb wound cells, and the ErBB signaling pathway is a downstream epigenetic target of these signals. These findings offer new insights into the molecular regulation of regenerative patterning.
Similar content being viewed by others
Introduction
The question of why some tetrapod species can regenerate complex limb structures while humans and other mammals cannot remains a key question in the regenerative biology field. In humans, regenerative potential is restricted to the most distal phalange of the digit1,2. Years of research on mammalian limb injury models show that the regenerative abilities of “non-regenerative” limb cells can be enhanced by altering their environment; however, we now know that their transcriptional state and ability to contribute to the regenerated tissue does not match that of “regenerative” cells from the tip of the mammalian digit3,4,5. This revelation indicates that non-regenerative mammalian cells do not become fully competent to respond to regenerative signals. Yet, we have little understanding of the cellular and molecular aspects leading to this intrinsic deficiency. Understanding how the limb cells of tetrapods that can fully regenerate limbs, such as the Mexican axolotl, become competent to key regenerative signals will provide a basis to compare with mammals and identify future therapeutic targets.
Regeneration of missing limb tissue in both mice and axolotl relies on the development of a transient regenerative organ known as the blastema. In the axolotl, the blastema is predominantly composed of mature limb cells that dedifferentiate into a limb progenitor-like state6. These blastema cells can respond to a variety of signals that play key roles in regeneration: chemoattractive signals that attract dedifferentiating cells onto the severed end of the limb7, mitogenic and antiapoptotic signals that promote the accumulation and proliferation of undifferentiated cells8,9,10,11, and signals that either maintain an undifferentiated state or promote the redifferentiation of specific tissues12,13. Competency to regenerative signals that support these cell behaviors are also apparent in mouse digit blastemas14,15,16,17,18. However, one key difference between the axolotl and mouse, is the ability of the axolotl blastemas cells to organize into the complex tissues and structures of the missing limb. To do so, these cells also become competent to different pattern cues, which are generated by specific populations of blastema cells derived from the limb connective tissues in the anterior and posterior sides of the limb19,20.
Much work has focused on the molecular underpinnings of axial specification of blastema cells during limb regeneration19,21,22,23. These studies have unveiled that blastema cells from anterior and posterior sides of the limb generate and respond differently to unique patterning cues including Fgf8 and Shh, respectively19,20,24,25. This cellular heterogeneity makes it particularly challenging to study the molecular underpinnings of patterning competency since, presumably, blastema cells from anterior and posterior sides of the limb have different competencies to respond to patterning signals. To address this issue, we leveraged a simplified in vivo regeneration paradigm known as the nerve deviation (ND), which provides a platform to study patterning competency in blastema cells from discrete A/P limb locations.
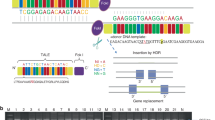
The ND paradigm, a key step in the well-established Accessory Limb Model (ALM), is based on the generation of an ectopic regeneration-permissive environment created by deviating a limb nerve bundle into a full thickness limb skin wound (Fig. 1)26. As opposed to amputation blastemas that naturally express multiple patterning genes from all of the limb axes19, ND-generated blastemas express the ones that are consistent with the position of the wound on the A/P limb axis19,20. For example, anterior-located ND blastemas (ND-A) activate the expression of anterior patterning genes including Alx4 and Fgf8, while posterior-located ND blastemas (ND-P) abundantly express Shh and Hand2 (Fig. 1)19,20. Since ND-blastemas express the patterning signals from one side of the A/P axis, they are unable to generate the A/P signaling feedback loop that is required to generate limb pattern (Fig. 1)19,26. However, if provided with tissue or patterning signals from the complementary side of the A/P axis, the ND-blastemas can pattern and generate ectopic limb structures19,20,25,26,27,28. This basic observation shows that while the ND-blastemas do not naturally generate all of the A/P patterning signals, they are competent to respond to these signals. Therefore, the simplified A/P-cell contributions in ND-generated blastemas, along with the ability to control the dosage and timing of different patterning cues, poise this system as an ideal platform to dissect the A/P-specific patterning competencies.
(Left boxes) Nerve deviations (ND) are performed by surgically deviating the branchial nerve bundle (yellow) to a lateral full thickness skin wound on the limb, which leads to the formation of a blastema (Endo et al.26). In the case of an anterior positioned ND (ND-A), within 7-days of surgery, there is a significant increase in the expression of “anterior” limb patterning genes, such as Alx4. Posterior positioned NDs (ND-P), exhibit a significant increase in the expression of “posterior” limb patterning genes, including Shh (Vieira et al.20). In both ND-A and ND-Ps, the blastema eventually regresses. (Right boxes) The Competency Accessory Limb Model (CALM) utilizes retinoic acid treatment to assess whether a ND-A or ND-P wound site has achieved competency to pattern limb structures. The achievement of patterning competency of the ND wound tissue can be identified in two ways; (1) location-specific changes in A/P patterning gene expression and (2) (in anterior-located tissue only) the formation of ectopic limb patterns (Vieira et al.20).
In the current study we used a derivative of the ND-system to begin to answer key unknowns about patterning competency including when and how patterning competency is induced and the molecular attributes of this cellular property. Our objective was, rather than focusing on a specific patterning signal, to determine how and when the cells were broadly competent to respond to patterning cues. Here, we define “patterning competency” as the broad capacity of cells to respond, both in patterning gene expression and morphogenically, to limb patterning cues. Evaluating patterning competency, however, requires a tool that impacts both A- and P-derived blastema cells and can be temporally controlled.
To address this, we utilized exogenous Retinoic Acid (RA) as a tool to identify patterning competent limb tissue. Previous research has established that treatment of blastema, but not uninjured, limb tissues with RA results in robust reprogramming of proximal/distal, and anterior/posterior pattern information leading to significant changes to the final pattern of the limb structures29,30,31,32,33,34. Our previous studies on ND-generated blastemas show that RA treatment leads to the suppression of Shh in ND-Ps, which eventually regress, while ND-As exhibit ectopic expression of Shh, and eventually generate complete, fully patterned, ectopic limbs (Fig. 1)20,28. Importantly, these observations indicate that RA impacts patterning in both A and P blastema cells, and that at least the ND-A blastemas are broadly competent to respond to the patterning cues sufficient to pattern complete limbs. Additionally, the effects of RA treatment on patterning can be detected within hours35, and RA can easily be administered at any time during experimentation. We therefore leveraged the phenomenological responses to RA treatment in the context of the ND system to assay for the broad competency of the injured limb tissue to pattern. We have called this assay the Competency Accessory Limb Model (CALM) to distinguish it from the simple ND-system (Fig. 1).
In this study, we used the CALM as a platform to generate foundational knowledge by (1) characterizing the specific timing of patterning competency induction and maintenance, (2) identifying specific nerve-dependent signals sufficient for induction, and (3) using ChIP-seq and CUT&RUN technologies to identify chromatin modifications induced in wounded cells by these signals. Our data reveals that the acquisition of patterning competency is a gradual, multi-day process that occurs specifically in innervated limb wound cells and is induced by a combination of FGF and BMP signaling. Our data also identifies the H3K27me3 chromatin signatures associated with patterning competency and which of these marks are dependent on FGF/BMP signaling. Last, we have identified the ErBB pathway as a target of H3K27me3 regulation downstream of FGF/BMP signaling in patterning competent cells. These observations provide direct insights into the molecular regulation of patterning competency in limb cells. Importantly, while we have employed the CALM to study broad patterning competency, this assay can easily be modified to unravel region-specific competencies to specific limb patterning cues in the future.
Results
Requirement of limb nerves for induction of patterning competency
The question of which environmental factors are minimally sufficient to induce patterning competency in mature limb cells has not been completely answered. We had previously shown that 7-day post-surgery ND-A blastemas had both transcriptional and morphological responses to RA treatment20,28, while multiple labs have shown that mature limb tissue does not20,33,36. Although this supports the idea that innervation is required to induce patterning competency in the limb wound cells, whether wounding alone was sufficient to induce this property had not been directly tested (Fig. 2a–c).
a Experimental design and treatment scheme of ND-A and A-wounds samples evaluated using the CALM assay. b Heat map representing qRT-PCR data of A/P patterning gene expression in DMSO (−) or RA (+) treated wounds and ND-As (positive control) relative to uninjured limb skin. Scale bar indicates expression relative to Ef1α (N = 3–5 biological replicates per sample group, p < 0.05 using the unpaired t-test) Black * indicates significant change in expression between RA and DMSO within the same injury-type, and yellow * indicates significant change in expression between RA treated groups. c Histogram representing the % of total ALM sites (from a) that exhibit the different ectopic growth phenotypes 9-weeks after grafts were implanted. The tissues were treated with RA (+,1-,3-,7-days) or DMSO (−) prior to grafting to the host ALM. (N = 8–18 biological replicates per sample group—refer to Supplementary Data 1.1). d Generation and treatment scheme of MB-staged blastemas 6-, 12-, or 24-h following treatment with RA or DMSO. e Histogram representing qRT-PCR data of Shh expression relative to Ef1α from the MB-blastema samples described in (d) (N = 5 biological replicates per sample group, *p ≤ 0.05 using the unpaired t-test, error bars are SEM). f Sample generation and treatment scheme of CALM-P samples over time (6-, 7-, or 8-days). g Histogram representing the qRT-PCR data for Shh expression relative to Ef1α in the samples described in (f) (N = 4 biological replicates, *p ≤ 0.05, **p ≤ 0.01 using the unpaired t-test, error bars are SEM). h Experimental set up to document RARE-eGFP reporter expression in ND-A wounds (N = 15 animals per group). i Representative live image (Green fluorescent channel overlayed on brightfield) time-courses of RA (top) and DMSO (bottom) treated ND-A’s from 3- to 6-days post-surgery. Wound edge is indicated with black dotted line and nerve bundle is highlighted (when visible) with yellow dotted line. Blue arrows indicate groups of GFP+ cells in ND-wound site visible starting at 5-days post ND-surgery.
We first characterized the transcriptional responses of anterior-located lateral wounds treated with RA or vehicle (DMSO) and compared them to the responses of ND-A blastemas. Using qRT-PCR, we measured the relative abundance of limb A/P patterning genes, including Alx4, Shh, Hand2, Fgf8, and HoxD10 in the limb tissues 7-days post RA treatment (Fig. 2b). We found that while the ND-A blastemas showed significant differences in A/P gene expression between RA and control groups, these differences were not apparent in the lateral wound tissue (Fig. 2b). The only gene that showed detectable differences in expression in the wound tissue was HoxD10. However, the shift in expression was both modest and in the opposite direction of the ND-A blastemas.
We also characterized the morphogenic response of anterior-located lateral wounds to RA (Fig. 2c). Limb nerves are required for the generation of accessory limbs26. Therefore, to evaluate whether RA treatment altered the morphogenic potential of the wound tissue, the treated tissue was labeled with DiI as a lineage tracer and grafted into a host ALM (anterior). As a positive control, we grafted ND-A blastemas 1-, 3-, and 7-days post RA treatment, and found the most robust morphogenic responses were elicited by the 7-day tissue grafts (Fig. 2c and Supplementary Data 1.1). In contrast, the lateral-wound tissue grafts (21-days post RA treatment) did not induce accessory limbs (Fig. 2c and Supplementary Data 1.1). Together, these data show that wounding alone is insufficient to induce patterning competency. Moreover, it further establishes the CALM (ND surgery followed by RA treatment) as a valid system to test the patterning competency of limb wound tissue.
Timing of the induction of patterning competency
Once we established that nerves were required for the induction of patterning competency in limb wounds, we next determined the timing of when cells in this tissue achieved this cellular state. This required us to test for competency within short windows of time, which was not possible with the previously described methods to analyze the CALM (A/P expressional shifts and ectopic limb induction) that take between 7 days and 9 weeks to occur (Fig. 2a, b)20. To address this issue, we first identified the earliest time point following RA treatment when a response in patterning competent cells could be detected (Fig. 2d, e). A previous study on amputation blastemas had qualitatively observed fast (within hours) shifts in Shh expression following RA35. To quantify this, we used qRT-PCR on a time-series of mid-bud (MB) staged blastemas treated with RA or vehicle. A significant decrease in Shh expression was detectable within 6-h (Fig. 2e), and the fold difference in expression increased from 4- to 42-fold 24 h post-RA treatment (Fig. 2e, Supplementary Data 8). This fast expressional change provided us with a means to evaluate responsiveness to RA treatment in relatively short time windows following the ND surgery. Therefore, we used qRT-PCR to analyze the expression of Shh in a daily time series of ND-P sites following RA treatment (CALM-P).
The first time point that Shh (and other posterior patterning genes) could be detected by qRT-PCR was 6-days post-ND surgery in animals 5–7 cm snout-to-tip (Supplementary Fig. 1a–f). We therefore evaluated the expressional responsiveness of the surgery site to RA treatment 6-, 7-, and 8-days post ND-surgery (Fig. 2f, g) in the same size range axolotl. We detected significant shifts in Shh between the RA and control-treated tissue at the earliest (6-day) time point (Fig. 2g).
To further characterize the timing of competency induction, we assessed the expression of transgenic RARE-eGFP reporter induction in the context of the CALM-A (Fig. 2h, i). Dim GFP fluorescence was detectable close to the nerve bundle in the CALM-A sites 5-days post-surgery and increased substantially in the surrounding tissue by 6-days post-surgery (Fig. 2i). Combined, these data indicated that it takes wound tissue between 5 and 6 days to achieve patterning competency in 5–7 cm long animals.
Maintenance of patterning competency in the blastema
The acquisition of patterning competency (5–6 days) correlates with the time it takes for an early (undifferentiated) blastema to form (Fig. 2). As the blastema continues to develop, the cells begin to redifferentiate in the region closest to the stump, and farthest from the apical epithelial cap (AEC)37,38. We reasoned that the property of patterning competency could be specific to the undifferentiated blastema cells, since we and others had also previously shown that the pattern information in differentiated limb tissue and redifferentiating blastema tissue is not altered by RA treatment20,33. To test this idea, we used qRT-PCR to evaluate the expressional response of amputation-generated blastema tissue to RA treatment as it progressed from the early bud (EB) stage (undifferentiated) to the late bud (LB) stage composed of spatially restricted undifferentiated (apical) and redifferentiating (basal) cells (Fig. 3a, b). We detected significant shifts in Shh and Fgf8 (A/P patterning genes) expression in only the EB and apical-LB samples (Fig. 3b). Surprisingly, all RA-treated tissue samples, including the mature limb tissue, showed an increase in the expression of Meis2 (Fig. 3b), a gene involved in proximal/distal (Pr/Di) patterning in developing and regenerating limbs39. This data shows that while RA treatment elicited unique transcriptional responses in differentiated and undifferentiated limb tissue, both tissues are, in fact, responsive to the treatment.
a Experimental set-up to analyze the expressional responses of different-staged amputation blastemas when treated with RA or DMSO (early bud (EB), apical late bud (Ap. LB), basal LB (Ba. LB), and mature (Mat.) tissue treated with either RA or DMSO via qRT-PCR. Red lines indicate the locations of amputations. Blue line represents the border between the basal and apical regions of the LB blastema. b Histograms representing the qRT-PCR data of Shh, Alx4, Fgf8, and Meis2 (normalized to Ef1a expression) in treated mature (Mat.), EB, apical-LB (Ap. LB), and basal-LB (Ba.-LB) blastemas (N = 4–6 biological replicates, using the Unpaired t-test relative to EB, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, error bars are SEM). c Description of CALM-P treatment scheme and sample generation following an upper-arm denervation surgery (red line) or sham surgery. d Histogram representing the qRT-PCR data of the relative expression of Shh (normalized to Ef1a expression) in the CALM-P samples described in “C”. “S” indicates sham surgery samples, “D” indicates denervated samples. (N = 5 biological replicates, using the Unpaired t-test, *p < 0.05, error bars are SEM). e Description of near-wound denervated (red line) CALM-A treatment scheme and sample generation. f Histogram representing the % total of CALM-A surgeries that led to different ectopic growth responses in sham (S) and near-wound denervated (D) CALM-A blastemas treated with RA (+) or DMSO (−) (N = 20 biological replicates per sample—refer to Supplementary Data 1.1).
To further evaluate the negative correlation between redifferentiation and loss of patterning competency, we performed the CALM assay on blastemas that were manipulated to differentiate prematurely. Previous research has shown that severing the limb nerve results in inhibition of blastema growth and premature redifferentiation in the amputation and ND-induced blastemas40,41,42,43. Since the CALM-P assay depends on shifts in Shh expression, we first validated whether Shh expression could be detected in denervated blastemas at the time point when changes in the specification and differentiation state had been previously identified. We found that Shh expression remains elevated in ND-P blastemas 4-days post-denervation and is undetectable by 7-days (Supplementary Fig. 1h, i). Gross analysis of the 4-day post-denervation blastemas showed the expected inhibition of growth43 (Supplementary Fig. 2a–c). Additionally, since changes in the specification and redifferentiation had been previously detected in blastemas 4 days post-denervation41,43, we used this time point for both CALM-P and CALM-A assays.
The CALM-P was performed on denervated and sham-denervated blastemas (Fig. 3c, d). We found that the RA treatment resulted in significant changes in Shh expression in the denervated CALM-P blastemas. However, the fold reduction was more modest than that of CALM-P with sham denervation surgeries (5-fold in the denervated versus 17-fold in the sham) (Fig. 3d and Supplementary Data 8).
We next tested the impact of denervation on the morphogenic potential of CALM-A blastemas (Fig. 3e, f). However, evaluating the morphogenic potential via accessory limb formation necessitated a method that would temporarily remove nerves, since nerves are required for regenerative outgrowth26,44. To address this, for this single experiment we severed the nerve bundle at the edge of the ND wound site to allow regrowth into the blastema (Fig. 3e), rather than performing the typical denervation surgery at the brachial plexus (as we did in Fig. 3d and Supplementary Fig. 2g)41, which would have a long-term effect on the tissue. While both denervated and sham-denervated CALM-A blastemas formed accessory limbs (Fig. 3f), there was a significant decrease (p ≤ 0001; chi-squared) in the % of the accessory limbs with a complex pattern in the denervated samples (from 86% to 54%).
Together, the expression data from the developing blastemas (Fig. 3b) and analysis of denervated CALM-P and CALM-A blastemas (Fig. 3c–f) indicate that patterning competency is maintained in the undifferentiated blastema cell population.
Global changes in H3K27me3 correspond with patterning competency
The above experiments show that the induction of patterning competency in limb wound cells occurs gradually (5–6 days), is dependent on innervation, and correlates with the undifferentiated blastema tissues. However, the molecular underpinnings of patterning competency remained unknown. We reasoned that identifying changes in the epigenome that are associated with patterning competency would provide valuable insight since chromatin restructuring has been shown to occur in limb cells when they contribute to the blastema45,46,47. We focused on the inhibitory mark H3K27me3, which is regulated by the Polycomb Repressor Complex 2 (PRC2), because of its well-established association with embryonic and regenerative limb patterning and maintenance of pattern information in the mature limb tissues48,49,50,51.
We first validated the specificity of a commercially available H3K27me3-specific antibody on axolotl samples using quantitative western blot and immunofluorescence on developing embryos and normal, denervated, and EZH2- (enzymatic activity of PRC2) inhibited limb blastemas (Supplementary Fig. 3). Using this validated antibody, we performed chromatin immunoprecipitation sequencing (ChIP-seq) on anterior-located mature limb, lateral wound, and ND-A blastema (patterning competent) samples (Fig. 4a). To identify stepwise changes in H3K27me3 marks, we compared data from the lateral wounds vs. mature limb tissue and data from ND-A vs. lateral wounds. The normalized enrichment scores (NES) of GO biological process (GOBP) terms formed four distinct clusters where the largest cluster (C2) corresponded with limb patterning (Fig. 4b and Supplementary Data 2.1). Multiple limb patterning genes (Shh, Ptch2, En1, Fzd5 and Fgf8) were observed in the list of the top 30 increased pathways enriched in the ND-A to lateral wound comparison (Fig. 4c).
a Sample generation scheme for H3K27me3 ChIP-sequencing analysis. b Heatmap of normalized enrichment scores of GO Biological Process Terms from Gene Set Enrichment Analysis (GSEA) in comparisons of H3K27me3 ChIP-seq data from Wound (W) relative to Mature (Mat.) and ND-A relative to Wound samples. Arrows indicate the direction of each comparison (refer to Supplementary Data 2.1). Data is segregated into four clusters (black circles 1–4) using K-means clustering. c Heat map of log2 fold change (log2FC) values of statistically significant genes that were pathway enriched in the GSEA. Limb patterning genes are indicated with blue font (refer to Supplementary Data 2.4). d Heatmap of log2FC values of limb patterning genes with statistically significant differences in H3K27me3 enrichment in each comparison (refer to Supplementary Data 2.5-2.6). e, f Heatmaps of log2FC values of cell cycle and immune and stress response-related genes with statistically significant differences in H3K27me3 enrichment in each comparison (full data sets and gene lists located in Supplementary Data 2.5 and 2.7-2.8). The top 2 most significant genes in each cluster are indicated in bold.
This prompted us to further analyze the stepwise changes in H3K27me3 on these and other limb patterning genes that had significant enrichment changes in the samples. We found that most of the limb patterning genes show decreased or no change in H3K27me3 enrichment in the lateral wounds compared to mature limb tissue. In contrast, almost all the patterning genes showed an increase in the ND-A blastemas compared to the lateral wound (Fig. 4d). These observations indicate that H3K27me3 enrichment is dynamically regulated on patterning genes in limb cells transitioning into patterning competent cells. In addition to the gene groups associated with limb patterning, we identified the cell cycle and immune and stress response groups as targets of H3K27me3 regulation in patterning competent cells (Fig. 4e, f and Supplementary Data 2.7-8).
Using the CALM to identify specific nerve signals required and sufficient for patterning competency
Multiple complex processes including wound healing, immune signaling, cell migration, and de-differentiation are ongoing in the limb tissue during blastema formation, and almost all of them are dependent on the limb nerves. Therefore, while the above experiment identified the gene regulations that are present in patterning competent cells in the H3K27me3 ChIP-seq analysis, it is likely that some of the genes identified are not directly associated with the property of patterning competency. To address this and provide deeper insight into the molecular mechanisms regulating patterning competency, we sought the specific upstream signals that induce patterning competency and the epigenetic changes that require these signals in patterning competent cells.
Previous studies have shown that treatment of limb wounds with gelatin beads soaked in a combination of BMP2, FGF8, and FGF2, which are factors that are expressed by the limb nerves52,53, are sufficient to induce responsiveness to exogenous RA20. Therefore, we tested whether all of these factors (BMP2 + FGF2 + FGF8) are required to induce competency or whether a combination of only two factors (FGF2 + FGF8, BMP2 + FGF8) is sufficient for competency in the context of the CALM (Fig. 5a–c).
a Treatment scheme of lateral limb wound samples: wounds were treated with beads soaked in different combinations of FGF and BMP growth factors (GF-Bead) and 7-days (7D) later injected with RA. Subsequently, wounds were evaluated for patterning competency as in the CALM. b Histograms of qRT-PCR data of relative Alx4 and Shh expression in treated wounds described in (a) (GF-Beads: PBS control, FGF2 + FGF8 (F2F8), BMP2 + FGF8 (B2F8), and BMP2 + FGF2 + FGF8 (B2FF)). Gene expression is relative to Ef1α (N = 3 biological replicates per sample group; black * indicates significant change in expression relative to PBS treated wounds using the unpaired t-test, *p < 0.05,**p < 0.005, error bares are SEM). c Images of ectopic growth limb phenotypes in the GF-Bead treated lateral wounds (described in a) 9–12 weeks following RA treatment (scale = 2 mm). N = 20–28 biological replicates for each sample group—refer to Supplementary Data 1.2. d FGF and BMP inhibitor treatment scheme for ND-A tissue. e Heat map representing qRT-PCR data of the relative expression of BMP- (Msx1 and Msx2) and FGF- (Prrx1) dependent genes (normalized to Ef1a expression) in the inhibitor treated ND-As (described in d) relative to uninjured limb tissue. Samples are labeled as follows: (+) = DMSO control, BMPi = LDN193189, FGFI = SU5402, BMPi + FGFi = cotreated with LDN193189 and SU5402. N = 3–5 biological replicates per sample group, black * indicates significant difference in expression relative to DMSO (+) treated ND-A using the Mann–Whitney t-test, *p < 0.05). f (right) Heat map of Normalized Enrichment Scores of GO Biological Process Terms from Gene Set Enrichment Analysis (GSEA) of H3K27me3 CUT&RUN data from the treated ND-As relative to DMSO treated ND-As (full gene lists and GOBP lists are provided in Supplementary Data 3.1-6). Scale bar indicates log2FC in H3K27me3 enrichment. K-means clustering identified 6 clusters (black circles 1–6). (right) Plots of the enrichment scores for the top pathway enrichment hits from the ND-A samples treated with both BMP and FGF inhibitors within the three largest clusters. g Log2FC values of 30 most significant “Additive” genomic regions (flipped enrichment trend only with both inhibitors) in the treated ND-As relative to A-lateral wounds. The top 5 most significant gene regions are indicated in bold font. h List of the top 5 GO Biological Processes from the PANTHER Overrepresentation Test (release 20230705) of “Additive” genomic regions. Full gene lists and associated PANTHER analysis are provided in Supplementary Data 3.13-14.
Gelatin beads soaked in the different combinations of growth factors were implanted directly below the wound epithelium of anterior-located lateral limb wounds (2-days post wounding), and animals were subsequently treated with RA 7-days later (Fig. 5a). While all of the growth factor-soaked beads led to the formation of an ectopic bump in the limb wound sites, only the ones with B2FF and B2F8 showed a significant increase in Alx4 and Shh expression compared to the negative control (PBS-soaked beads) (Fig. 5b) and resulted in the generation of accessory limbs (Fig. 5c). We further analyzed the accessory limbs and found that they can regenerate and induce ectopic limbs when grafted into an anterior-located ALM host site, confirming that stable/determined posterior identity had been established in the CALM-A tissue (Supplementary Fig. 4). Together, these data show that BMP2 with FGF8 are required and minimally sufficient to induce patterning competency in lateral limb wound tissue.
Now that we had identified the sufficient pathway ligands, we could determine which of the nerve-dependent changes in H3K27me3 were specifically associated with the acquisition of patterning competency. We first validated the efficacy of a BMP-signaling inhibitor (LDN193189, BMPi) and an FGF-signaling inhibitor (SU5402, FGFi) in the context of the ND-A by performing an expressional analysis using qRT-PCR on transcriptional targets of these pathways; Msx1, Msx2 (BMP targets) and Prrx1 (FGF target) (Fig. 5d, e). Our data shows that the inhibitors significantly suppressed the expression of their respective signaling targets compared to the DMSO control ND-As (Fig. 5e).
Therefore, we next performed CUT&RUN for H3K27me3 on ND-As treated with DMSO, BMPi, FGFi, or both inhibitors combined and anterior-located lateral limb wounds (Fig. 5d, f–h). With the resulting data we performed differential abundance and GSEA analysis and found that the NES of GOBP terms segregated into 12 clusters when the treated ND-A samples were compared with the lateral wounds (Supplementary Fig. 5a). As in the H3K27me3 ChIP-seq analysis (Fig. 4), limb patterning, cell cycle, and immune-related terms were strongly represented in these clusters (Supplementary Fig. 5 and Supplementary Data 3.1-6).
To identify H3K27me3 enrichment patterns that were dependent on BMP and/or FGF signaling in patterning competent cells, we first made broad comparisons between the inhibitor- to DMSO-treated ND-As NES (Fig. 5f). We identified 6 clusters, the largest of which (C2) consisted of terms with decreased enrichment in the FGFi-treated samples and increased enrichment in the BMPi-only sample (Fig. 5f and Supplementary Data 3.7-11). The top pathway enrichment hits within the three largest clusters included GOBPs for homologous recombination, viral gene expression, and neuron fate specification (Fig. 5f). Additionally, identification of the top 30 significant pathway-enriched gene regions from these comparisons consisted of genes involved in limb patterning (Tbx1, Tbx2, Tbx3, Gli2, Msx1, Hoxb8), limb development (Ror2, Mmp14, Osr2, ChrD, Cxcl12, Nr4a2) and neural-related (Dmrt3, Lhx3, Ndnf, Ntrk2, Gata3, Cntn2, Tlx3, Slc6a4, Nhlh2, Ptprs, Nefl) (Supplementary Data 3.12).
Because the induction of patterning competency requires both BMP and FGF ligands (Fig. 5a–c), we reasoned that the H3K27me3 patterns that require both BMP and FGF signaling in ND-As would likely provide the most relevant insights into the regulatory targets that are specifically associated with this property. We therefore refined our analysis to identify the gene regions that showed different enrichment trends in the treated ND-A blastemas vs. lateral wound comparison. We defined FGF-specific (flipped enrichment trend in FGFi and BMPi+FGFi (BiFi) samples compared to DMSO), BMP-specific (flipped enrichment trend in BMPi and BiFi samples compared to DMSO), co-dependent (flipped in all inhibitor-treated groups compared to DMSO), and additive (only flipped trend when both pathways were inhibited) trends (Supplementary Fig. 6).
PANTHER analysis of the regions that showed FGF-specific dependent marks were almost exclusively neural-related GO terms (Supplementary Fig. 6a, a’ and Supplementary Data 5.1-2). BMP-specific dependent regions included neural-related and WNT signaling-related to dorsal/ventral (D/V) limb patterning GO terms (Supplementary Fig. 6b, b’ and Supplementary Data 5.3-4). Co-dependent marks were diverse, including RA receptor-, WNT signaling-, and cell cycle regulation-related GO terms (Supplementary Fig. 6c, c’ and Supplementary Data 5.5-6). Last, the most significant GO terms associated with additive type modifications were ErBB signaling related (Fig. 5g, h and Supplementary Data 3.13-14). PANTHER analysis of the genomic regions that exhibited different patterns from the above groups of interest, including nerve-dependent marks that are independent of FGF and BMP signaling, can be found in Supplementary Fig. 6d-e’ and Supplementary Data 5.7-10).
Deep analysis of FGF and BMP-dependent marks on limb patterning genes
Since a third of the top 20 pathway-enriched genes that have FGF- and BMP-dependent changes in H3K27me3 enrichment consisted of limb patterning genes (Supplementary Fig. 5c and Supplementary Data 4.2), we reasoned that this group was an important regulatory target in patterning competent cells. However, it was not clear whether the marks on regions associated with specific limb axes were dependent on one or both of these pathways. Therefore, we performed a more in-depth analysis of the genomic regions regulated in the limb patterning genes that showed significant changes in the treated ND-A tissues relative to the lateral wounds (Fig. 6). Each gene region identified was categorized as FGF-specific, BMP-specific, codependent/additive, FGF/BMP independent, or other based on the criteria described above (Fig. 6a). This strategy allowed us to identify specific regions of patterning genes where the regulation of H3K27me3 occurred and determine whether the marks were dependent on FGF and/or BMP signaling. Only the genes that had more than three regions with significant changes were analyzed further.
a Simplified gene maps of genes associated with A/P, D/V, and Pr/Di limb patterning where the general locations of regions with significant nerve-dependent changes in H3K27me3 enrichment are compared to lateral wounds are indicated with rectangles. Green boxes indicate regions that flipped enrichment trend in FGFi (LND193189) treated ND-As compared to DMSO treated. Pink boxes indicate regions that flipped enrichment trend in BMPi (SU5402) treated ND-As compared to DMSO treated. Yellow boxes indicate regions that either had additive or co-dependent changes in inhibitor treated samples. Orange boxes indicate regions that exhibited changes in enrichment in the inhibitor groups that do not fit with the above definitions. Gray boxes indicate changes in enrichment that occur independently of FGF and BMP signaling. Numbered white rectangles indicate promoters (P) and exons (1-1+n). b Heat maps of log2FC values for regions in which differential H3K27me3 abundance were detected in limb patterning genes associated with Anterior-Posterior, Dorsal-Ventral, and Proximal-Distal limb patterning in ND-A samples compared to lateral wounds. Regions are displayed in 5’ to 3’ order. Complete data used to generate a and (b) provided in Supplementary Data 6.
All the patterning genes that had significant changes in H3K27me3 enrichment contained regions that were sensitive to FGF and/or BMP signaling (Fig. 6). To determine whether there were trends in the locations where marks were identified, we documented the location of “hits” on simple maps of each gene analyzed (Fig. 6a). The largest number of FGF/BMP sensitive regions were identified in A/P patterning genes, many of which were located within the first intron (Fig. 6a).
The impact of BMP and/or FGF inhibition on the enrichment of H3K27me3 differed depending on the location within the genes (Fig. 6b). For example, intron one of Grem1 had increased enrichment for H3K27me3 in the inhibitor-treated samples, while many of the intergenic regions had the opposite trend (Fig. 6b and Supplementary Data 6.1). In contrast, intron one of Gli1 and Gli2 showed decreased enrichment following inhibitor treatment, and marks in regions outside of these positions did not have obvious enrichment trends (Fig. 6b and Supplementary Data 6.1). These data show that FGF and BMP signaling are required for the regulation of H3K27me3 enrichment on limb patterning genes, particularly those involved with A/P patterning, in patterning competent limb cells.
Discussion
Understanding how limb cells become competent to respond to key regenerative signals is an essential step in identifying future targets for human regenerative therapies. The goal of this research was to better resolve how axolotl limb cells become broadly competent to respond to patterning cues, which is essential for organizing the regenerating tissue into complex limb structures.
A challenge to studying patterning competency in amputation-generated blastemas is the contribution of cells from the different limb axes, particularly from the A/P axis, because they both generate and respond differently to limb patterning cues19,25,54,55. To address this, we leveraged the nerve-deviation (ND) surgical paradigm, which limits the A/P cellular contribution in the blastema to one side of the axis. To distinguish limb cells that have acquired patterning competency from the other limb cells, we treated the tissue with exogenous Retinoic Acid (RA), which changes both patterning gene expression and the morphogenic potential in limb cells that have acquired this property. We combined the ND-system with RA as an assay for patterning competency and named it the Competency ALM (CALM) to distinguish it from the simple ND-system.
In the current study, we have used the CALM assay to (1) determine the minimal environmental factors and the timing of patterning competency induction, (2) identified stepwise changes in the epigenetic mark, H3K27me3, when adult limb cells transition to patterning competency, and (3) determined the sufficiency for specific BMP and FGF ligands in patterning competency induction and identified the H3K27me3 marks that depend on these signals. Since little was previously known about patterning competency, our main objective was to provide knowledge about this cellular property as a foundation for future investigations.
One key finding from our experiments was the unexpectedly long time, between 5- and 6-days post-injury, that it takes for the mature limb cells to acquire patterning competency. Why it takes so long is not clear and understanding what is happening within the cells during this transition will provide critical information about how patterning competency is induced. One possibility is that the nerve-based signals like FGF and BMP-ligands described here, set off a chain reaction of events that take days to complete. These events could be molecular signaling and/or epigenetic in nature. If this is the case, it could be possible to activate the chain further down the line to increase the fidelity and/or decrease the time it takes to induce patterning competency. Alternatively, it is possible that FGF and BMP proteins need to accumulate to a critical threshold to induce the cellular responses that lead to competency. Careful characterization of the cellular responses (expressional and epigenetic) over time to competency-inducing signals, including FGF/BMP, will both shed light on the upstream mechanism and the nature of patterning competency as a cellular property. In addition, whether uninjured limb cells are naturally responsive to FGF and BMP signaling or require a wound environment to become competent to these signals is an important unanswered question.
Regardless of the upstream mechanism, we have just begun to understand the changes that happen in the limb cells as they become competent. For example, here we show that patterning competency in limb wound cells is nerve-dependent and correlates with (1) cellular dedifferentiation, (2) changes in the H3K27me3 signatures, and (3) increased expression of A/P patterning genes. Further experiments will be needed to determine whether these play a direct role in patterning competency.
While there are certainly many potential models that could explain how patterning competency is induced, multiple pieces of evidence indicate that the role of epigenetics should be included in these models. First, large-scale changes in chromatin structure that correlate with the timing that patterning competency is acquired have been previously documented (Supplementary Fig. 3)45,51,56. Large-scale chromatin modifications have been shown to be essential for the reprogramming of differentiated cells into iPSCs in culture57,58,59. Therefore, it is likely that changes in the epigenome of limb cells play a role in dedifferentiation. However, whether all or some of these changes are associated with patterning competency had not been investigated. To begin to understand the epigenetic modifications associated with the induction of competency, here we identified changes in H3K27me3 downstream of nerve signaling that specifically required FGF and BMP signaling. This repressive epigenetic mark was chosen because of its role in the maintenance of positional memory in differentiated cells50,51 and the regulation of patterning gene expression in the developing limb field48,60.
We found many nerve-dependent changes in H3K27me3 that occur independently of the signals that induce patterning competency (Supplementary Fig. 6). These changes are likely associated with the different activities that rely on nerve signaling during blastema formation. However, we also identified H3K27me3 patterns that were specifically dependent on FGF/BMP signaling in patterning competent cells. In addition to the RA pathway, our PANTHER analysis identified the WNT and ERBB signaling pathways as highly significant epigenetic targets downstream of FGF/BMP. All three pathways are associated with nerve-dependent processes during the early stages of blastema formation36,61,62. We have also found FGF/BMP-dependent marks on limb patterning genes, especially those involved with A/P patterning. While further investigation is required to evaluate the role of these marks and pathways, these studies show the shifts in the epigenome that are specific to patterning competency that can be used to inform future studies.
Beyond these observations, the differential responses of patterning competent vs. incompetent cells to exogenous RA provide “smoking gun” evidence pointing toward a role for epigenetics. For decades the limb regeneration field has known the treatment of limb blastema cells with RA stably reprograms the morphogenic potential of this tissue, while mature limb tissue is resistant to these effects29,30,32,33,34,63. The current study shows that limb wound tissue behaves much like mature limb tissue in this regard (Fig. 2b, c). We have also documented shifts in A/P patterning genes that accompany RA reprogramming of blastema cells, which does not happen in the differentiated limb tissues (Fig. 2b). Additional experiments are needed to evaluate whether Meis2, Tig1, or other Pr/Di patterning genes will show similar shifts in expression in the context of the CALM assay. However, one unexpected observation was the shift in the Pr/Di patterning gene, Meis2, expression occurs in both amputation blastemas and mature tissues treated with RA (Fig. 3b). This does not appear to be an anomaly. Previously published studies have seen a similar shift in the Pr/Di patterning gene Tig1 in both mature and regenerating limb tissue following RA treatment21. These expressional shifts in Pr/Di genes indicate that the mature limb tissue has the molecular machinery required to respond to exogenous RA, yet the morphogenic potential of this tissue remains unaltered. One reasonable interpretation of all of this is that differences in chromatin structure/accessibility in mature vs. patterning competent blastema tissue underly the differential expressional responses of these tissues to exogenous RA. Moreover, it shows that patterning competency cannot be simply defined by the ability to “respond” to RA. Rather, this ability needs to be accompanied by the appropriate cellular state to permit a morphogenic response.
It is worth noting here that the ability of both mature and patterning competent limb tissues to respond (expressionally) to RA, reveals a weakness in the CALM assay itself. That is, if expressional responses to RA are to be used as a proxy for patterning competency, as in the CALM-P, we will need to identify the responses that only occur in cells that have achieved this state. A more rigorous method of evaluation is the CALM-A, which focuses on changes in the morphogenic potential. However, because the output of this assay is the presence of accessory limb structures that form well after patterning competency is lost, the CALM-A cannot be easily used to study time-sensitive aspects of this property. In the current study, we documented shifts in Shh and Fgf8 expression that occur specifically in patterning competent limb tissue (Figs. 2b, d–g and 3 and Supplementary Fig. 1). These molecules are expressed by posterior and anterior blastema cells, respectively, and suffice as position-specific markers for patterning competency. Identifying a broad molecular marker for patterning competency that is present in all patterning-competent cells, regardless of their axial origin, will significantly streamline future studies. The H3K27me3 regulatory targets downstream of FGF/BMP, including members of the RA, WNT, and ErBB signaling pathways identified here are good candidates. Additionally, we advise researchers to perform both the expressional assays in the CALM-P with the morphogenic potential assay in the CALM-A whenever possible to rigorously test for patterning competency.
Last, the recent sequencing and ever-improving annotation of the axolotl genome64 and the development of many new transgenic axolotl lines have opened doors to investigate regenerative competency in ways that were never before possible. Combining these tools with classical surgical manipulations, which the axolotl model is especially amendable to, will provide key details about the basic biology of limb regeneration so that we can make informed comparative studies with mammalian limb injury models. In line with this, the epigenetic signatures that we have identified here that are specifically associated with the acquisition of patterning competency can be used as a resource for both the axolotl and mammalian limb regeneration research communities to identify new ways to enhance this property in mammalian limb cells.
Materials and methods
Animal husbandry and ethics statement
This study was conducted in accordance with the recommendations of the Guide for Care and Use of Laboratory Animals of the NIH, and all experiments were approved by the Institutional Animal Care and Use Committee of UMass Boston. Axolotl salamanders (Ambystoma mexicanum) of the wild type (RRID: AGSC_100), white (RRID: AGSC_101, golden albino (RRID: AGSC_102), GFP (RRID: AGSC_110), either spawned in house or obtained from the Ambystoma Genetic Stock Center at the University of Kentucky and a transgenic RARE-EGFP reporter strain (generously donated by J. Monaghan and P. Murawala)38, were used in the described studies. Animals were anesthetized using a 0.1% tricaine (TRS1, Pentair) solution. Animals ranged in size between 5–22 cm snout to tip, and all animals were sized matched (within 2–6 cm) for time-based experiments. Size ranges for each experiment are provided in Supplementary Data 7.
Animal surgeries
There were six types of surgeries performed on forelimbs during this study: (1) limb amputation, (2) denervation, (3) lateral limb wound, (4) nerve deviation (ND and CALM), (5) ALM, and (6) bead implantation. Forelimb amputations were performed by severing at the mid-stylopod level and trimming the bone. To denervate, a section of the brachial nerve was removed either at the brachial plexus (Fig. 3c, d and Supplementary Figs. 1h–g, 2, and 3) or at the wound edge of an ectopic blastema (Fig. 3e, f). Sham denervation surgeries were performed by making the same incisions as the corresponding denervation surgeries, without severing the nerve bundle.
Lateral limb wound sites were positioned on the anterior or posterior apices of the forelimb stylopod, and a 2 × 2 mm piece of full-thickness skin was carefully excised, ensuring minimal damage to the underlying tissues. To generate ectopic blastemas for ND- and CALM-based assays, the brachial nerve bundle was severed near the elbow and deviated into the center of the wound site (as in ref. 65). The ALM experiments from Fig. 2c were performed by harvesting anteriorly positioned CALM-A blastemas 1-, 3-, or 7-days post RA treatment (which was 8-, 10-, or 14-days post-initial surgery) and grafting them into ND-A 2-days post-surgery. To generate growth factor-induced ectopic blastemas, growth factor-soaked gelatin beads were implanted below the wound epithelium into lateral wound sites 2 days after surgery (as in ref. 20).
Animal treatments
Animals received three different types of pharmacological treatments during this study: (1) injections, (2) growth factor beads, and (3) soak treatments. For injection-based treatments, animals were dosed intraperitoneally with 30–50 μL 150 mg/kg of all-trans-RA (R2625, Sigma-Aldrich), 10 mg/kg of 3-deazaneplanocin A (13828, Cayman Chemical), or DMSO (Sigma-Aldrich) and kept in the dark for 2 days as described previously20. For growth factor beads, gelatin microsphere beads were prepared as previously described (as in ref. 66) and rehydrated in cocktails of 1X PBS, 300 ng/μL FGF2 + FGF8, 300 ng/μL BMP2 + FGF2 + FGF8, or 300 ng/μL BMP2 + FGF8 of each growth factor (BMP2:355-BM-050/CF, FGF8a:4745-F8-050, FGFbasic:233-FB-025/CF, R&D Systems) and implanted into the wound site as previously described20. For growth factor inhibitor soaking treatments, stock solutions of LDN193189 (A8324, ApexBio), SU5402 (A3843, ApexBio) were prepared in DMSO and diluted in 40% Holtfreter’s Solution (animal housing water) to working concentrations of 1 μM (as in ref. 13) and 15 μM (as in ref. 66), respectively.
Gene expression analysis
For experiments in which gene expression was assessed, tissues were collected in Tripure (Sigma-Aldrich) and pooled (2–4 harvested injury sites per tube) to generate each biological replicate. RNA was isolated using the NucleoSpin RNA XS kit (Macherey-Nagel) according to the manufacturer’s protocol. cDNA was then generated from these samples using the Transcription First Strand cDNA Synthesis Kit (Roche Diagnostics) according to the manufacturer’s protocol. Relative transcription levels of genes of interest were generated by performing qRT-PCR (AzuraQuant™ Green Fast qPCR Mix Lo-Rox, AzuraGenomics Inc.) using the Bio-Rad C1000 Touch Thermal Cycler (CFX96 Realtime System, Bio-Rad) and quantified using the Pfaffl method67. Mature limb tissue was used for the reference library. The forward and reverse primer sequences used are listed in Table 1. Each primer was validated by ensuring that the melting curve of the pilot reactions yielded a single amplimer, and their efficiencies were determined using 1:2 cDNA serial dilutions. Three technical replicates were performed for each biological replicate and averaged. The number of biological replicates per sample was 3 or greater, and the exact number for individual expressional analyses is specified in the figure legends.
Morphological phenotype assays
For the morphology assays in Figs. 2c, 3f, 5c and Supplementary Fig. 4, the wound sites were live imaged weekly using a stereoscope (Zeiss Discovery. V8 SteREO) until week 4 and subsequently biweekly until either pattern formation was completed or the blastemas regressed. For Figs. 2c, 3f and Supplementary Fig. 4, samples were harvested and stained with 0.04% Alcian Blue (Sigma-Aldrich) and 0.002% Alizarin Red (VWR) as described by Horton and Maden68. Samples were imaged in 80% glycerol (RPI) on a stereomicroscope. Prior to harvesting, the developing blastemas were imaged on a stereomicroscope using a fiber optic illuminator lamp (Micro Optical Solutions SCHOTT MLS) for lighting. In all cases, green and/or red channels were imaged using a Lumen Dynamics X-Cite Series 120Q fluorescent lamp where applicable.
Histology and immunostaining
Tissues were harvested, fixed overnight in 4% formaldehyde, decalcified in 10% EDTA (Amresco) for 5 days on an orbital shaker, and then moved into 30% sucrose (Fisher Scientific) for 3 days on an orbital shaker at 4 °C. Samples were then flash frozen in OCT (Sakura) and cut into 10 μm longitudinal tissue sections for immunodetection. After sectioning, the samples were warmed and then washed twice in PBS and once in 1X PBS-Tween to remove OCT. Samples for H3K27me3 detection were then pressure cooked for 2 min in sodium citrate buffer. Samples are then washed with diH2O. After blocking with 0.5% BSA, the samples were incubated overnight in a 1:300 solution of rabbit anti-histone H3K27me3 polyclonal antibody (A4039050, Epigentek) in PBS-Tween at 4 °C. Slides were washed and blocked for an additional 10 min before being incubated for 2 h at 4 °C in a 1:200 dilution of goat anti-rabbit IgG H&L secondary antibody (ab150080, Abcam). Samples were then stained with DAPI in PBS-Tween for 10 min prior to being washed in PBS-Tween and mounted with VECTASHIELD Antifade Mounting Medium. Additional information for antibodies is listed in Table 2.
Tissue sections were imaged on an Axio Observer Z1 inverted fluorescence microscope equipped with an EXCELITAS Technologies X-Cite 120 LED lamp, a 40X oil immersion objective with a Hamamatsu digital camera C11440 and an Apotome2 unit. The imaging was controlled by the Zen Pro 2.0 software package. Z-stacks were imaged using 0.24 μm intervals. Raw z-stacks were then converted into apotome calculation stacks using Zen software and exported to TIFF format files for analysis.
Some tissue sections were histologically stained (supplementary Fig. 2). These samples were prepared as described above for sectioning. The sections were then stains using Mallory’s triple stain for collagen and counterstained with Hematoxylin and Eosin.
Bioimage analysis and quantification
Images are preprocessed using Crop&Snip, as previously reported, custom ImageJ plug-in45, and run through H3K_CbyC, a new algorithm and MATLAB code that calculates the relative amount of fluorescently tagged protein present within a cell nucleus in large 3-dimensional image datasets (Supplementary Fig. 3). This works by Gaussian blurring the individual images within the DAPI channel, binarizing, setting the image to a black background, filling holes, and then recombining the individual layers back into a matrix. Small objects and under segmented nuclei are removed by volume filtering, and the number of nuclei is detected using a connected component. From the red channel, (specific to AB detection) pixels that overlap with the identified nucleus locations are counted, and the total fluorescence per nucleus within an image stack is calculated. Outlier z-stacks were removed from subsequent analysis. A total of 50–100 nuclei were included in the calculation of averages for each biological sample. Analyzed data are saved as a single csv format file for the entire data set. Crop&Snip and H3K_CbyC are available for download and use online (https://doi.org/10.5061/dryad.tqjq2bw8x)69.
Western blot
Samples were harvested and homogenized in 2X Laemmli Sample Buffer (Sigma-Aldrich), boiled at 100 °C for 3–5 min and spun down in a microcentrifuge. Protein supernatant was separated by SDS-PAGE using a BIO-RAD Mini-PROTEAN Tetra System at 100 V on a 10% acrylamide gel (40% Acrylamide– Thermo Fisher Scientific) with a 180 kDa protein ladder (Thermo Fischer Scientific). Samples were then transferred onto an Immuno-Blot PVDF membrane (BIO-RAD) using a BIO-RAD Mini-PROTEAN Tetra System at 100 V for 1 h and blocked overnight at 4 °C on an orbital shaker in a 5% BSA-PBS solution. Samples were then incubated at room temperature on an orbital shaker in a solution of 1:1000 primary antibody solution for rabbit anti-H3K27me3 (A4039050, Epigentek) and mouse anti-α-Tubulin (12G10– 6/9/19, DSHB) for 2 h (Table 2). Samples were then washed in PBS-Tween and incubated at room temperature on an orbital shaker for 1 h in a 1:5000 secondary antibody solution with IRDye® 800CW Donkey anti-Mouse IgG (926-68072, LI-COR Biosciences) and IRDye® 800CW Donkey anti-Rabbit IgG (925-32213, LI-COR Biosciences) (Table 2). Samples were then washed in PBS-Tween, stored in PBS, and imaged using a LI-COR ODYSSEY CLx. Gel images were quantified in ImageJ using the built-in Gel Analyzer tools.
Chromatin immunoprecipitation assay
For the preparation of samples for ChIP-seq, we used the protocol developed by O’Geen et al. and optimized it for the axolotl system70,71,72. Briefly, uninjured mature skin tissue, 7-day-old A-lateral wounds, and 7-day-old ND-A blastemas were collected from 10 limbs for each of the 3 biological replicates and fixed on ice in 1.5% formaldehyde for 20 min, gently inverting every 5 min. Samples were then pelleted and incubated in 125 mM glycine in PBS to remove fixative. Samples were washed with PBS and, after pelleting, were resuspended in 300 µl lysis buffer (50 mM TRIS-HCL pH 8.0, 1 mM EDTA, 0.1% SDS, 150 mM NaCl, 1% IGEPAL, and 0.5 mM DTT, with 1x Halt™ Protease and Phosphatase Inhibitor Cocktail (78440, Thermo Fisher Scientific)) and frozen at −80 °C overnight. Then, an additional 300 µl of lysis buffer was added, and the samples were homogenized using a needle. Cell lysates were sonicated on ice in a Branson Ultrasonics Sonifier™ S-450 (20% duty, 20% amplitude, 4 cycles of 5 seconds, 15 cycles of 10 s) and centrifuged at 14,000 × g for 10 min, and the supernatant was collected. Fifty microliters of sonicated supernatant was combined with 50 µl of ChIP-elution buffer (50 mM NaHCO3, 1% (w/v) SDS) and 12 µl of 5 M NaCl to prepare for purification by heating to 95 °C for 20 min, cooling, and then incubating with 1 μl of DNase-free RNase A (R6148-25 ml, Sigma) at 37 °C for 20 min. The DNA was then purified using the QIAquick PCR Purification Kit (Qiagen) and assessed spectrophotometrically for DNA concentration and by agarose gel electrophoresis for sonication efficiency. Five hundred nanograms of DNA, adjusted to a final volume of 100 µl with lysis buffer, of each sample was collected as input and stored at −20 °C.
Eight micrograms of DNA, adjusted to a final volume of 500 µl with lysis buffer, was then incubated with A/G Plus Agarose beads (Thermo Fisher Scientific) overnight. Immunoprecipitation was achieved by incubating the sample with the anti-H3K27me3 antibody (A4039050, Epigentek) overnight and precipitating with the aid of bovine serum albumin-blocked A/G Plus Agarose beads. Beads were then washed by inversion for 5 min twice with IP wash buffer 1 (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% (v/v) igepal, 0.25% (w/v) deoxycholic acid, 1 mM EDTA pH 8), twice with IP wash buffer 2 (100 mM Tris–HCl pH 9, 500 mM LiCl, 1% (v/v) igepal, 1% (w/v) deoxycholic acid) and once with IP wash buffer 3 (100 mM Tris–HCl pH 9, 500 mM LiCl, 150 mM NaCl, 1% (v/v) igepal, 1% (w/v) deoxycholic acid). The antibody/chromatin complex was eluted by incubating the bead complex with 100 μl elution while vortexing for 30 min.
Input samples were then thawed and processed along with the immunoprecipitated samples. All samples were centrifuged (12,000 × g, 30 s), and the supernatant was collected. Twelve microliters of 5 M NaCl was added to the supernatant and then incubated overnight at 67 °C and then with 1 μl of RNaseA at 37 °C for 20 min. One microliter of proteinase K was added (P8107S 800 U/ml NEB) for 1 h at 55 °C. DNA was purified by a QIAquick PCR Purification Kit and eluted into 40 μl of EB buffer.
CUT&RUN assay
For the CUT&RUN assay, uninjured mature limb tissue (DMSO treated), 7-day-old A-lateral wound tissue (DMSO treated), and ND-A blastema tissue for each treatment condition (DMSO, LDN193189, SU5402, and LDN193189 + SU5402 treated) was collected in quadruplicate from 2–3 limbs per sample. Wound epithelia were removed from the blastema mesenchyme, cells were counted on a hemocytometer, and 80,000–100,000 connective tissue cells were collected per sample. Three of the collected samples per condition were designated biological replicates, and the fourth was set aside as an input sample. Samples were processed following the manufacturer’s protocol for the CUT&RUN Assay Kit (86652, Cell Signaling), and 5 μL of the anti-H3K27me3 antibody (A4039050, Epigentek) was used for each biological sample in incubation. Input samples were fragmented using Cell Signaling’s MNase Input sample fragmentation protocol. Sample and input DNA was then purified using Cell Signaling DNA Purification Buffers and Spin Columns (CUT&RUN) (Cell Signaling) and eluted in 50 μL of DNA Elution Buffer.
Library preparation and sequencing
For the ChIP-seq samples, DNA libraries were prepared and barcoded to facilitate multiplexing using the Kapa DNA Hyper Prep Kit (07962355001, Roche). Samples were then validated using the Agilent 2100 Bioanalyzer system. Sequencing was conducted using the HiSeq 2500 System, rapid Run 1 ×51, per 2 lane FC. For the CUT&RUN samples, DNA libraries were prepared and barcoded for multiplexing using the Cell Signaling DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) (56795, Cell Signaling) and Multiplex Oligos for Illumina® (Dual Index Primers) (ChIP-seq, CUT&RUN) (47538, Cell Signaling). Samples were then validated using the Agilent 2100 Bioanalyzer system. Sequencing was conducted using the HiSeq 2500 System, rapid Run 1 ×51, per 2 lane FC.
Sequencing alignment and statistics
Alignment and statistics for the ChIP-seq and CUT&RUN assays were performed by the Harvard T.H., Chan Quantitative Biomedical Research Center (QBRC). FASTQ files were aligned to the reference genome AmexG_v6.0-DD with bwa and SAMtools to produce BAM files64,73,74.
Peak calling of aligned ChIP-seq and CUT&RUN libraries was performed with MACS2 with matching input libraries used as the background for each individual biological replicate. The replicated peaks were annotated in a strand neutral manner by HOMER using the reference GTF v6.0-DD. Peaks were aggregated across annotated genes to generate a gene-by-gene peak count. These peaks were then the median of ratios normalized by a defined cohort of gonad-specific and neuro-specific genes as housekeeping genes, which are listed in Table 3. The Bioconductor Package DESeq275 was used to perform statistical analysis on the contrasts between samples. Annotated genes were converted to human orthologs76. Gene set enrichment analysis was performed with GSEA77.
Sequencing data visualization
TSV files were converted into CSVs and loaded in R. Heatmaps were generated using the pheatmap library, and k-means clustering was performed using the pheatmap library78. Manual clustering was performed by sorting the first column of values, dividing the data set into positive and negative, and repeating. The subset columns were then re-appended together to generate the sorted dataset. Identifying subsets of genes associated with GO Terms of interest was conducted using the bioMART library in R to access the Ensemble Genes database79,80.
PANTHER analysis
Gene regions that were associated with BMP-dependent, FGF-dependent, co-dependent, additive, other, or independent modifications were compiled into a list, and the genes were run through the algorithm on pantherdb.org for PANTHER Overrepresentation Test (release 20230705) analysis81. The top 5 GO terms from each were considered.
Statistics and repeatability
Biological replicates (N) were defined as either a pooled sample for the qRT-PCR (3–4 individual blastemas per biological replicate), ChIP-seq (tissue—i.e. mature, wound, or blastema from ten limbs per biological replicate), and CUT&RUN (pooled tissue from 4–6 limbs per biological replicate) or an individual limb/blastema per biological replicate for Western Blot, immunofluorescence, tissue sections, and morphological phenotype experiments. Technical replicates for qRT-PCR were performed in triplicate. All unpaired t-tests, chi-squared tests, one-way ANOVAs, and associated post hoc testing were performed using GraphPad Prism 10 (all information regarding biological replicates, test-specific animal sizes, and statistical test results can be found in Supplementary Data 6 for all information on how tests were run). Statistical analysis for non-sequencing data can be found in Supplementary Data 8. Statistical analysis for the sequencing data was performed using the Bioconductor Package DESeq275. All error bars displayed throughout this study are the standard error of the mean.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data from the findings of this study are available within the paper and its Supplementary Information. The ChIP-seq FASTQ data were deposited into the SRA database under the accession number PRJNA1218456 and the CUT&RUN FASTQ data were deposited into the SRA database under the accession number PRJNA1218471. The Quantitative Biomedical Research Center at the Harvard T.H. Chan School of Public Health, Boston, MA performed bioinformatic processing (QC, alignment, peak-calling, and GSEA analysis) and provided us with the FASTQ files for SRA submission and the raw GSEA analysis for the ChIP-Seq and CUT&RUN which we used for subsequent analysis. We were not provided with graph files, such as BED files, but GSEA analysis, including differential abundance and statistics. This raw GSEA analysis supporting the ChIP-Seq and CUT&RUN are publicly available in the figshare repository, as part of this record: https://doi.org/10.6084/m9.figshare.2832950982. Morphological phenotype images supporting Figs. 2, 3f, 5c and Supplementary Fig. 4 are publicly available in the figshare repository, as part of this record: https://doi.org/10.6084/m9.figshare.2830682083. Densitometry images supporting Supplementary Fig. 3 are available in Supplementary Data 7. Immunofluorescent images and the associated Crop&Snip and H3K_CbyC codes supporting Supplementary Fig. 3 are publicly available in the dryad repository, as part of this record: https://doi.org/10.5061/dryad.tqjq2bw8x.70.
References
Douglas, B. S. Conservative management of guillotine amputation of the finger in children. J. Paediatr. Child Health 8, 86–89 (1972).
Illingworth, C. M. Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 9, 853–858 (1974).
Yu, L. et al. BMP9 stimulates joint regeneration at digit amputation wounds in mice. Nat. Commun. 10, 1–9 (2019).
Wu, Y. et al. Connective tissue fibroblast properties are position-dependent during mouse digit tip regeneration. PLoS ONE 8, e54764 (2013).
Storer, M. A. et al. Acquisition of a unique mesenchymal precursor-like blastema state underlies successful adult mammalian digit tip regeneration. Dev. Cell 52, 509–524.e9 (2020).
Gerber, T. et al. Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science 362, eaaq0681 (2018).
Currie, J. D. et al. Live imaging of axolotl digit regeneration reveals spatiotemporal choreography of diverse connective tissue progenitor pools. Dev. Cell 39, 411–423 (2016).
Albert, P. & Boilly, B. Effect of transferrin on amphibian limb regeneration: a blastema cell culture study. Rouxs Arch. Dev. Biol. 197, 193–196 (1988).
Boilly, B. & Albert, P. In vitro control of blastema cell proliferation by extracts from epidermal cap and mesenchyme of regenerating limbs of axolotls. Rouxs Arch. Dev. Biol. 198, 443–447 (1990).
Furukawa, S., Yamamoto, S., Kashimoto, R., Morishita, Y. & Satoh, A. Variable Shh and Fgf8 positioning in regenerating axolotl limb guarantees consistent limb morphogenesis in different limb sizes. bioRxiv https://doi.org/10.1101/2022.01.04.475010 (2022).
Mescher, A. L., White, G. W. & Brokaw, J. J. Apoptosis in regenerating and denervated, nonregenerating urodele forelimbs. Wound Repair Regen. 8, 110–116 (2000).
Ghosh, S., Roy, S., Séguin, C., Bryant, S. V. & Gardiner, D. M. Analysis of the expression and function of Wnt-5a and Wnt-5b in developing and regenerating axolotl (Ambystoma mexicanum) limbs. Dev. Growth Differ. 50, 289–297 (2008).
Vincent, E. et al. BMP signaling is essential for sustaining proximo-distal progression in regenerating axolotl limbs. Development 147, dev170829 (2020).
Han, M., Yang, X., Farrington, J. E. & Muneoka, K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development 130, 5123–5132 (2003).
Yu, L. et al. BMP signaling induces digit regeneration in neonatal mice. Development 137, 551–559 (2010).
Agrawal, V. et al. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng. Part A 17, 2435–2443 (2011).
Lee, J. et al. SDF-1α/CXCR4 signaling mediates digit tip regeneration promoted by BMP-2. Dev. Biol. 382, 98–109 (2013).
Johnston, A. P. W. et al. Dedifferentiated Schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell 19, 433–448 (2016).
Nacu, E., Gromberg, E., Oliveira, C. R., Drechsel, D. & Tanaka, E. M. FGF8 and SHH substitute for anterior–posterior tissue interactions to induce limb regeneration. Nature 533, 407–410 (2016).
Vieira, W. A. et al. FGF, BMP, and RA signaling are sufficient for the induction of complete limb regeneration from non-regenerating wounds on Ambystoma mexicanum limbs. Dev. Biol. 451, 146–157 (2019).
Oliveira, C. R. et al. Tig1 regulates proximo-distal identity during salamander limb regeneration. Nat. Commun. 13, 1141 (2022).
Kumar, A., Gates, P. B. & Brockes, J. P. Positional identity of adult stem cells in salamander limb regeneration. C. R. Biol. 330, 485–490 (2007).
Iwata, R., Makanae, A. & Satoh, A. Stability and plasticity of positional memory during limb regeneration in Ambystoma mexicanum. Dev. Dyn. 249, 342–353 (2020).
Otsuki, L., Plattner, S., Taniguchi-Sugiura, Y. & Tanaka, E. Molecular basis for positional memory and its reprogrammability in limb regeneration. bioRxiv https://doi.org/10.1101/2023.10.27.564423 (2023).
Vieira, W. A. et al. Integration failure of regenerated limb tissue is associated with incongruencies in positional information in the Mexican axolotl. Front. Cell Dev. Biol. 11, 1–14 (2023).
Endo, T., Bryant, S. V. & Gardiner, D. M. A stepwise model system for limb regeneration. Dev. Biol. 270, 135–145 (2004).
McCusker, C. D., Diaz-Castillo, C., Sosnik, J., Q. Phan, A. & Gardiner, D. M. Cartilage and bone cells do not participate in skeletal regeneration in Ambystoma mexicanum limbs. Dev. Biol. 416, 26–33 (2016).
McCusker, C., Lehrberg, J. & Gardiner, D. Position-specific induction of ectopic limbs in non-regenerating blastemas on axolotl forelimbs. Regeneration 1, 27–34 (2014).
Monkemeyer, J., Ludolph, D. C., Cameron, J. A. & Stocum, D. L. Retinoic acid-induced change in anteroposterior positional identity in regenerating axolotl limbs is dose-dependent. Dev. Dyn. 193, 286–294 (1992).
Ludolph, D. C., Cameron, J. A. & Stocum, D. L. The effect of retinoic acid on positional memory in the dorsoventral axis of regenerating axolotl limbs. Dev. Biol. 140, 41–52 (1990).
Thoms, S. D. & Stocum, D. Retinoic acid-induced pattern duplication in regenerating urodele limbs. Dev. Biol. 103, 319–328 (1984).
Crawford, K. & Stocum, D. L. Retinoic acid coordinately proximalizes regenerate pattern and blastema differential affinity in axolotl limbs. Development 102, 687–698 (1988).
Niazi, I. A., Pescitelli, M. J. & Stocum, D. L. Stage-dependent effects of retinoic acid on regenerating urodele limbs. Wilhelm Rouxs Arch. Dev. Biol. 194, 355–363 (1985).
Wallace, H. & Maden, M. Local action of vitamin A on amphibian limb regeneration. Experientia 40, 985–986 (1984).
Torok, M. A., Gardiner, D. M., Izpisúa-Belmonte, J. C. & Bryant, S. V. Sonic hedgehog (shh) expression in developing and regenerating axolotl limbs. J. Exp. Zool. 284, 197–206 (1999).
Monaghan, J. R. & Maden, M. Visualization of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Dev. Biol. 368, 63–75 (2012).
Tassava, R. A. & McCullough, W. D. Neural control of cell cycle events in regenerating salamander limbs. Am. Zool. 18, 843–854 (1978).
Duerr, T. J. et al. A constitutively expressed fluorescent ubiquitination-based cell-cycle indicator (FUCCI) in axolotls for studying tissue regeneration. Development 149, 1–13 (2022).
Mercader, N., Tanaka, E. M. & Torres, M. Proximodistal identity during vertebrate limb regeneration is regulated by Meis homeodomain proteins. Development 132, 4131–4142 (2005).
Monaghan, J. R. et al. Microarray and cDNA sequence analysis of transcription during nerve-dependent limb regeneration. BMC Biol. 7, 1 (2009).
McCusker, C. D. & Gardiner, D. M. Positional information is reprogrammed in blastema cells of the regenerating limb of the axolotl (Ambystoma mexicanum). PLoS ONE 8, e77064 (2013).
Mullen, L. M., Bryant, S. V., Torok, M. A., Blumberg, B. & Gardiner, D. M. Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Development 122, 3487–3497 (1996).
Satoh, A., Bryant, S. V. & Gardiner, D. M. Regulation of dermal fibroblast dedifferentiation and redifferentiation during wound healing and limb regeneration in the Axolotl. Dev. Growth Differ. 50, 743–754 (2008).
Singer, M. The influence of the nerve in regeneration of the amphibian extremity. Q. Rev. Biol. 27, 169–200 (1952).
Sosnik, J., Vieira, W. A., Webster, K. A., Siegfried, K. R. & McCusker, C. D. A new and improved algorithm for the quantification of chromatin condensation from microscopic data shows decreased chromatin condensation in regenerating axolotl limb cells. PLoS ONE 12, e0185292 (2017).
Taylor, A. J. & Beck, C. W. Histone deacetylases are required for amphibian tail and limb regeneration but not development. Mech. Dev. 129, 208–218 (2012).
Wei, X. et al. An ATAC-seq dataset uncovers the regulatory landscape during axolotl limb regeneration. Front. Cell Dev. Biol. 9, e651145 (2021).
Wyngaarden, L. A., Delgado-Olguin, P., Su, I. -h, Bruneau, B. G. & Hopyan, S. Ezh2 regulates anteroposterior axis specification and proximodistal axis elongation in the developing limb. Development 138, 3759–3767 (2011).
Andrey, G. et al. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science 340, 1234167 (2013).
Rinn, J. L. et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 (2007).
Kawaguchi, A. et al. A chromatin code for limb segment identity in axolotl limb regeneration. Dev. Cell 59, 2239–2253.e9 (2024).
Makanae, A., Hirata, A., Honjo, Y., Mitogawa, K. & Satoh, A. Nerve independent limb induction in axolotls. Dev. Biol. 381, 213–226 (2013).
Satoh, A., Makanae, A., Nishimoto, Y. & Mitogawa, K. FGF and BMP derived from dorsal root ganglia regulate blastema induction in limb regeneration in Ambystoma mexicanum. Dev. Biol. 417, 114–125 (2016).
Vieira, W. A. & McCusker, C. D. Hierarchical pattern formation during amphibian limb regeneration. BioSystems 183, 103989 (2019).
Vieira, W. A., Goren, S. & McCusker, C. D. ECM-mediated positional cues are able to induce pattern, but not new positional information, during axolotl limb regeneration. PLoS ONE 16, e0248051 (2021).
Hay, E. D. Electron microscopic observations of muscle dedifferentiation in regenerating Amblystoma limbs. Dev. Biol. 1, 555–585 (1959).
Wei, X. et al. Small molecule compound induces chromatin de-condensation and facilitates induced pluripotent stem cell generation. J. Mol. Cell Biol. 6, 409–420 (2014).
Gładych, M., Andrzejewska, A., Oleksiewicz, U. & Estécio, M. R. H. Epigenetic mechanisms of induced pluripotency. Wspolczesna Onkol. 1A, A30–A38 (2015).
Guo, S. et al. Nonstochastic reprogramming from a privileged somatic cell state. Cell 156, 649–662 (2014).
Williamson, I. et al. Anterior-posterior differences in HoxD chromatin topology in limb development. Development 139, 3157–3167 (2012).
Kawakami, Y. et al. Wnt/β-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 20, 3232–3237 (2006).
Farkas, J. E., Freitas, P. D., Bryant, D. M., Whited, J. L. & Monaghan, J. R. Neuregulin-1 signaling is essential for nerve-dependent axolotl limb regeneration. Development 143, 2724–2731 (2016).
Maden, M. The effect of vitamin A on the regenerating axolotl limb. Development 77, 273–295 (1983).
Schloissnig, S. et al. The giant axolotl genome uncovers the evolution, scaling, and transcriptional control of complex gene loci. Proc. Natl. Acad. Sci. USA 118, e2017176118 (2021).
Raymond, M. & Mccusker, C. D. The accessory limb model regenerative assay and its derivatives. in Salamanders. Methods in Molecular Biology (eds. Seifert, A. W. & Currie, J. D.) 217–233 (Springer Protocols, 2023).
Makanae, A., Mitogawa, K. & Satoh, A. Co-operative Bmp- and Fgf-signaling inputs convert skin wound healing to limb formation in urodele amphibians. Dev. Biol. 396, 57–66 (2014).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, 16–21 (2001).
Horton, C. & Maden, M. Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev. Dyn. 202, 312–323 (1995).
Raymond, M., Cherubino, M., Vieira, W., Manon, S. & McCusker, C. Nerve-dependent regulation of H3K27me3 during the induction of patterning competency in regenerating Axolotl limb cells [Dataset]. Dryad https://doi.org/10.5061/dryad.tqjq2bw8x (2025).
O’Geen, H., Echipare, L. & Farnham, P. J. Using ChIP-seq technology to generate high-resolution profiles of histone modifications. Methods Mol. Biol. 791, 265–286 (2011).
Edwards, N. S. & Murray, A. W. Identification of xenopus CENP-A and an associated centromeric DNA repeat. Mol. Biol. Cell 16, 1800–1810 (2005).
Buro, L. J., Shah, S. & Henriksen, M. A. Chromatin Immunoprecipitation (ChIP) to assay dynamic histone modification in activated gene expression in human cells. J. Vis. Exp. 1–4 https://doi.org/10.3791/2053 (2010).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. 1–3 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21 (2014).
Nowoshilow, S., Fei, J. F., Voss, S. R., Tanaka, E. M. & Murawala, P. Gene and transgenics nomenclature for the laboratory axolotl—Ambystoma mexicanum. Dev. Dyn. 251, 913–921 (2022).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Kolde, R. Package ‘pheatmap’: Pretty heatmaps. R Package. 1–8 (2012).
Smedley, D. et al. BioMart—biological queries made easy. BMC Genomics 10, 1–12 (2009).
Aken, B. L. et al. The Ensembl gene annotation system. Database 2016, baw093 (2016).
Mi, H. et al. Protocol update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 14, 703–721 (2019).
Raymond, M. J., Cherubino, M. A., Vieira, W. A., Manon, S. & McCusker, C. D. Gene set enrichment analysis output data from ChIP-seq and CUT&RUN experiments. figshare https://doi.org/10.6084/m9.figshare.28329509 (2025).
Raymond, M. J., Cherubino, M. A., Vieira, W. A., Manon, S. & McCusker, C. D. Raw images and montages for morphological phenotype experiments (.jpg format for size). figshare https://doi.org/10.6084/m9.figshare.28306820 (2025).
Acknowledgements
This work was supported by a grant awarded to C.M. (NICHD R15 Award 2R15HD092180-02) and by the Proposal Development Grant Program at UMass Boston. S.M. was supported in part by the following grants (NSF-Funded REU Program Award DBI01950051 to R. Skvirsky and NIH-funded Initiative for Maximizing Student Development (IMSD) Award R25GM076321). We also wish to thank S. Patalano-Salsman in the Center for Personalized Cancer Therapy Genomics Core at the University of Massachusetts Boston (grant support as part of the NIH/NCI U54 CA156734 award) in the generation of the ChIP-seq and CUT&RUN data and D. DeConti of the Quantitative Biomedical Research Center at the Harvard T.H. Chan School of Public Health, Boston, MA for assistance with bioinformatic processing (QC, alignment, and peak calling) of the ChIP-seq and CUT&RUN data.
Author information
Authors and Affiliations
Contributions
M.R., M.C., W.V. and C.M. contributed to the conceptualization of the project, experimental design, experimentation, data analysis, writing, and reviewing of the manuscript. S.M. and M.C. contributed to the experimentation, data analysis, and reviewing of the manuscript. All authors had access to all the data and were responsible for the decision to submit the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Simona Chera and Manuel Breuer. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Raymond, M.J., Cherubino, M.A., Vieira, W.A. et al. Neural regulation of H3K27me3 during the induction of patterning competency in regenerating Axolotl limb cells. Commun Biol 8, 659 (2025). https://doi.org/10.1038/s42003-025-08084-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08084-x
This article is cited by
-
Ependymoglial cells are critical for cortex regeneration in axolotls
Nature Communications (2026)