Abstract
Endometrial cancer (EC) remains a lethal gynecological malignancy with limited therapeutic options owing to unresolved pathogenesis. Cellular senescence acts as a key barrier against tumorigenesis in cancer cells, thus investigating its role in EC progression represents a pivotal research avenue to address these challenges. This study reveals the critical role of cellular senescence in EC progression through multi-omics profiling and functional validation. The integrative analysis of RNA-seq and clinical datasets identified Na+/H+ exchanger 7 (NHE7) as a prognostic biomarker that was significantly overexpressed in EC tissues. Functional studies demonstrated that NHE7 overexpression drives proliferation, cell motility, and cell cycle progression while suppressing senescence-associated markers and cytokine secretion. Conversely, NHE7 knockdown reversed these oncogenic phenotypes. Mechanistically, NHE7 binds to a cAMP-related transcription factor, thereby increasing GRIN2B expression to elevate intracellular Ca²⁺ levels influx, which delays cell senescence and promotes cancer progression in vitro and in vivo. Our findings suggest that NHE7 plays a crucial role in delaying cellular senescence and advancing EC progression through the cAMP pathway, uncovering critical mechanistic drivers of EC pathogenesis and highlighting actionable therapeutic targets.

Similar content being viewed by others
Introduction
Endometrial cancer (EC), a prevalent gynecological malignancy of the endometrial epithelium, poses a significant threat to women’s health globally1. In 2020, it was reported that 417,336 EC cases worldwide, making it the sixth most common female cancer2. Most cases occurred in women aged 65–75, with racial, socioeconomic, and geographical disparities playing crucial roles in morbidity and mortality3. While 67% of patients are diagnosed early, with an 81% 5-year survival rate, those with stage IVA and IVB disease have only 17% and 15% 5-year survival rates, respectively4. EC encompasses distinct histological and molecular subtypes: Type I (estrogen-linked, low-grade) exhibits a better prognosis, while Type II (non-estrogen, high-grade) has a poorer one. Type I ECs mainly include grade I/II endometrioid adenocarcinomas, while Type II ECs encompass grade III endometrioid adenocarcinomas, serous clear cell, undifferentiated, and carcinosarcomas5. Despite the significant advancements in our understanding of the biological heterogeneity of EC through the efforts of the Cancer Genome Atlas (TCGA), identifying these individuals, understanding their specific pathogenesis, performing the appropriate surgeries, and administering the optimal adjuvant therapies to enhance their outcomes remain the foremost challenge in this disease area6.
Senescence, a cellular response to stress and injury, involves stable withdrawal from the cell cycle and gradual physiological decline, characterized by reduced repair capabilities, weakened immune responses, hormonal imbalances, and increased disease susceptibility7. This senescence-associated proliferation arrest (SAPA) is accompanied by the hypersecretory senescence-associated secretory phenotype (SASP), which encompasses a diverse array of factors, such as pro-inflammatory cytokines (including IL-1β, IL-6, IL-8, and TNF-α), growth factors (IGFBP7 and VEGF), proteases (MMPs), chemokines, and various other components8. This complex interplay enables senescent cells to play context-dependent beneficial or detrimental roles in different physiological and pathological settings9. The major mediators of SAPA are cyclin-dependent kinase (CDK) inhibitors 1 and 2A (commonly known as p21 and p16INK4a), which block CDK-cyclin complexes formation at the G1-S transition10. CDK phosphorylation of retinoblastoma (Rb) family members triggers the release of E2F, a core cell cycle transcription factor, and its subsequent activation11. In addition to the telomere shortening discovered by Hayflick and Moorhead in 196112, various stress stimuli like oncogenic13, oxidative14, lysosomal/endoplasmic reticulum stress15,16, and nutrient depletion17 can trigger senescence.
The entrance of cancer cells into senescence is a formidable barrier to tumorigenesis, effectively mitigating the risk of cancer development18. Notably, a study in bladder carcinoma provided direct evidence that the overexpression of p53, a tumor suppressor, can activate the rapid onset of senescence in tumor cells by inducing the expression of β-D-galactoside19, a senescent-specific marker. Regarding mechanisms, upon p53 activation, Rb and retinoblastoma-like proteins 1 and 2 (often called p107 and p130, respectively) collaborate to inhibit the transcription of genes involved in this phase transition, promoting cellular senescence20. Furthermore, oncogene-induced senescence is a senescence program triggered by the activation of oncogenes, such as NRASG12V and BRAFV600E. These oncogenes, which are often mutated or abnormally expressed, play key roles in altering cell proliferation, driving the microenvironment surrounding senescent cells, and initiating complex tissue-wide signaling cascades. Pioneering studies showed that melanocytic naevi triggered by oncogenic BRAF mutations typically remain senescent for decades21, thereby evading the progression into melanoma22. It is well known that cancer cells enhance glucose consumption for growth and survival, relying on glycolysis (“Warburg effect”), producing ATP and H+23. This creates an acidic extracellular, alkaline intracellular pH (pHi) gradient, aiding tumor growth, cell death evasion, and invasiveness24. Interestingly, emerging evidence has demonstrated that numerous modulators of ion homeostasis contribute to organismal aging, like iron homeostasis25, local H2 release26, and calcium homeostasis27. Notably, it is well-established that calcium channels regulate numerous “hallmarks of cancer”28, and modulate membrane potential29, the transmembrane voltage due to ion gradients across cell membranes. Nevertheless, there is no direct evidence linking these modulators to cancer-associated senescence. Given that most EC cases were found in women aged 65–75, it is evident that senescence plays a significant role. Many factors influence the progression of EC, including genetic mutations, hormonal imbalances, and environmental exposures5. However, the intricate relationship between EC and senescence remains unclear. In this study, we conducted an in-depth analysis of the differentially expressed genes (DEGs) in EC using the TCGA database, identified a DEG associated with senescence, and then explored how this gene regulates cancer-related signaling pathways and impacts senescence, aiming to reveal their interplay in EC and identify potential therapeutic targets.
Results
Increased NHE7 expression in EC correlates with poor prognosis and senescence
To identify potential therapeutic targets for EC, this study analyzed RNA-seq data and clinical information from the TCGA database (Supplementary Data 5). EC-related DEGs were shown in a volcano plot, with senescence-associated genes, including Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1), MAX Dimerization Protein 1 (MXD1), 24-Dehydrocholesterol Reductase (DHCR24), Na+/H+ exchanger 7 (NHE7) and so on highlighted (Fig. 1A). The above DEGs were significantly enriched in tumor-related pathways such as p53, cyclic adenosine phosphate (cAMP), and calcium signalings (Fig. 1B, C). To investigate the relationship between EC-related DEGs and senescence, 721 genes were identified by intersecting those DEGs with a senescence-related gene set from the literature (Fig. 1D)30. Univariate Cox regression analysis revealed that 15 intersecting genes were significantly associated with poor prognosis (Fig. 1E). NHE7 was prioritized for further study due to its association with poor prognosis and senescence in EC and its role in maintaining the acidic tumor microenvironment31.
Transcriptome sequencing analysis was performed to identify DEGs in EC and normal samples from TCGA database. A The DEGs between EC and normal samples were analyzed, and their distribution was depicted using a volcano plot (the genes related to senescence have been marked on the diagram). The biological processes and signaling pathways in which DEGs were enriched were identified by B KEGG and C GO enrichment analysis. D The overlap of EC DEGs with senescence-related genes from the literature was visualized with a Venn diagram. E The intersection genes were analyzed using univariate Cox analysis (p < 0.001). F The box plots were used to illustrate the expression levels of NHE7 in EA and SC tumor tissues or healthy tissues. G Survival curve analysis was used to assess the prognostic effect of high or low NHE7 expression in tumor patients.
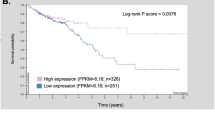
Boxplot compared NHE7 expression in EC samples vs. healthy samples, showing higher levels in tumors (p = 0.071) (Fig. 1F). TCGA EC data were used to categorize tumor samples into high-expression and low-expression groups based on NHE7 median expression level (Supplementary Data 6). Survival analysis further demonstrated that patients with high NHE7 expression were associated with poor prognosis (p = 0.0021) (Fig. 1G). A clinical correlation analysis showed that NHE7 expression levels were significantly associated with clinical variables, including age and tumor stage (p < 0.05) (Supplementary Table 4). These bioinformatic findings were validated through Immunohistochemistry (IHC) analysis, which confirmed elevated NHE7 expression in EC tissues compared to normal and atypical hyperplasia tissues. Conversely, the expression of senescence-promoting proteins (p16 and p53) was reduced in cancer tissues and showed an inverse correlation with NHE7 levels in EC tissues (Fig. 2A, B). These results suggest that NHE7 plays a critical role in EC progression and highlight NHE7’s potential as a therapeutic target.
A IHC assay was used to detect the expression of NHE7, p16, and p53 in normal, atypical hyperplasia, and cancer tissues (Scale bar: 50 μm, n = 3). The main image is magnified 400×, with an inset showing a 40× magnified view. A value of p below 0.05 is considered significant, *p < 0.05, **p < 0.01. B IHC assay was used to detect the expression of NHE7, p16, and p53 in cancer samples with high, medium, and low NHE7 expression (Scale bar: 50 μm, n = 3). *p < 0.05, **p < 0.01.
NHE7 promotes EC cell proliferation, accelerates the cell cycle, and delays senescence
To evaluate the role of NHE7 at the cellular level, we altered its expression by NHE7 overexpression or knockdown in EC cell lines (HEC-1-A and Ishikawa cells) and verified its expression efficiency by Western blotting (WB) assays for subsequent in vitro verification (Fig. 3A). Through Methyl thiazolyl tetrazolium (MTT) assay, we observed a significant increase in EC cell proliferation after NHE7 overexpression but a notable decrease after its knockdown (Fig. 3B). Consistently, NHE7 overexpression increased the migration and invasion in HEC-1-A and Ishikawa cells, while knockdown of NHE7 led to the opposite trend in migration and invasion (Fig. 3C). Apoptosis assays showed that NHE7 overexpression reduced the proportion of apoptotic cells, and NHE7 knockdown confirmed this finding (Fig. 3D).
HEC-1-A and Ishikawa cells with overexpression or knockdown of NHE7 were established, and the migration, invasion, and apoptosis experiments were conducted on the treated cells. A WB assay was used to verify the overexpression and knockdown efficiency of NHE7 in HEC-1-A and Ishikawa cells. β-actin served as the loading control. A value of p below 0.05 is considered significant, **p < 0.01. B MTT assay was used to detect the effect of NHE7 the effect of NHE7 on proliferation in HEC-1-A and Ishikawa cells. A value of p below 0.05 is considered significant, *p < 0.05, **p < 0.01. C The migration and invasion abilities of HEC-1-A and Ishikawa cells with overexpression or knockdown of NHE7 were detected through Transwell experiments (Scale bar: 100 μm). **p < 0.01. D Flow cytometry was used to detect the cell apoptosis status in treated HEC-1-A and Ishikawa cells. All data came from three repeated experiments (n = 3). *p < 0.05, **p < 0.01.
To understand NHE7’s effect on EC cell growth, we examined several pivotal checkpoints in the EC cell cycle, the foundation of cell proliferation, after altering NHE7 expression. The data revealed that upregulating NHE7 expression in EC cells led to accelerated cell cycle progression, especially in the S phases. Conversely, NHE7 silencing resulted in cell cycle arrest, mainly in the G1 phase (Fig. 4A). Given the pivotal role of the cell cycle in senescence7 and D-Galactose-induced accelerated senescence32, we next investigated the potential impact of NHE7 on senescence in EC cells using β-galactosidase staining. Our results demonstrated that NHE7 overexpression reduced the number of senescent EC cells, as evidenced by a decrease in β-galactosidase-positive staining compared to D-Galactose-induced senescence, whereas NHE7 silencing had the opposite effect, increasing the number of senescent EC cells (Fig. 4B). Without D-Galactose-induction, the NHE7-silenced group still showed increased cellular senescence compared to unsilenced group (Supplementary Fig. 1). To confirm our observations of NHE7-mediated senescence, we measured the protein expression levels of key regulators that promote senescence (p53, p21, and p16)10,19. Our results demonstrated that NHE7 overexpression down-regulated the expression of p53, p21, and p16, whereas NHE7 silencing up-regulated their expression (Fig. 4C). Subsequently, we analyzed the release of cytokines (IL-6, IL-1β, MMP3, and VEGF) in EC cells following NHE7 overexpression or silencing to investigate the potential link between NHE7 and the SASP8. The data showed that NHE7 overexpression significantly decreased the expression of these cytokines; NHE7 silencing increased their levels (Fig. 4D), further supporting its role in delaying senescence. Our findings indicate that NHE7 can promote EC cell proliferation, accelerate the cell cycle, delay EC cell senescence, and thus drive the occurrence and progression of EC.
A Flow cytometry was used to detect the effect of NHE7 on the cell cycle in HEC-1-A and Ishikawa cells. A value of p below 0.05 is considered significant, *p < 0.05, **p < 0.01. B β-galactosidase staining was used to detect the effect of NHE7 on senescence in HEC-1-A and Ishikawa cells (Scale bar: 50 μm). *p < 0.05, **p < 0.01. C WB assay was used to detect the expression of p53, p21, and p16 proteins in HEC-1-A and Ishikawa cells. *p < 0.05, **p < 0.01. D RT-qPCR assay was used to detect the mRNA expression of VEGF, IL-6, IL-1β, and MMP3 in HEC-1-A and Ishikawa cells. All data came from three repeated experiments (n = 3). *p < 0.05, **p < 0.01.
NHE7-driven EC progression is associated with the cAMP signaling pathway and GRIN2B-mediated calcium upregulation
To elucidate the mechanism underlying NHE7-mediated EC progression, we identified 2065 DEGs between the NHE7 high and low-expression groups in 550 EC samples from the TCGA database, of which 631 genes were up-regulated and 1434 genes were down-regulated, illustrated in a volcano plot (Fig. 5A). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses indicated that these DEGs were significantly enriched in signal transduction pathways such as the cAMP and calcium signalings (Fig. 5B, C), which are vital second messengers for cellular processes like proliferation, differentiation, and apoptosis33. Given cAMP and calcium signaling’s importance in tumor progression and senescence34,35, we cross-analyzed calcium signaling genes in the KEGG database with NHE7-related DEGs in EC. Among the 254 calcium signaling genes and 226 cAMP signaling genes, there were 8 intersecting DEGs, as presented by the Venn diagram (Fig. 5D). Notably, the presence of glutamate Ionotropic Receptor NMDA Type Subunit 2B (GRIN2B) in the intersection genes and its positive correlation with NHE7 attracted our attention (Fig. 5D, E). The GRIN2B-encoded GluN2B subunit is essential for NMDA receptors (NMDAR), a ligand- and voltage-gated channel. Activating these receptors allows Ca²⁺ influx, crucial for calcium signaling36, which can be disrupted via aberrant GRIN2B expression37. To validate our bioinformatics findings, we measured intracellular Ca2+ levels in EC cells with altered NHE7 levels, where overexpression of NHE7 slightly increased Ca2+ levels and silencing of NHE7 mildly decreased Ca2+ levels (Fig. 6A). Moreover, NHE7 overexpression in EC cells led to increased protein expression of calmodulin 1 (CALM1) and phosphorylated calcium/calmodulin-dependent protein kinase II (p-CAMKII), the calmodulin protein that serves as a marker for calcium signaling38,39, while NHE7 silencing resulted in decreased expression (Fig. 6B). More importantly, NHE7 overexpression significantly increased GRIN2B levels in mRNA and protein in EC cells; NHE7 knockdown significantly decreased them (Fig. 6B, C). Interestingly, Protein Kinase A Catalytic Subunit (PKACA), the main catalytic subunit of the cAMP signaling pathway molecule CAMP-dependent protein kinase (PKA)40, exhibited similar protein changes (Fig. 6B). cAMP level measurements also confirmed these results in EC cells with NHE7 overexpression or silencing (Fig. 6D).
A The volcano plots were used to illustrate the distribution of 2065 DEGs identified between the high-expression and low-expression groups of NHE7 from the TCGA database. The biological processes and signaling pathways in which DEGs were enriched were identified by (B) KEGG and (C) GO enrichment analysis. D The overlap of DEGs in the calcium signaling pathway and cAMP signaling pathway with DEGs significantly enriched in NHE7 expression was visualized with a Venn diagram. E The Pearson correlation method was used to illustrate the relationship between GRIN2B and NHE7.
A Flow cytometry was used to detect the effect of NHE7 on Ca2+ levels in HEC-1-A and Ishikawa cells. A value of p below 0.05 is considered significant, *p < 0.05, **p < 0.01. B WB assay was used to detect the expression of GRIN2B, CALM1, CAMKII, p-CAMKII, and PKACA proteins in HEC-1-A and Ishikawa cells. *p < 0.05, **p < 0.01. C RT-qPCR assay was used to detect the mRNA expression of GRIN2B in HEC-1-A and Ishikawa cells. *p < 0.05, **p < 0.01. D An Elisa kit was used to detect cAMP levels in EC cells that overexpress or silence NHE7. All data came from three repeated experiments (n = 3). *p < 0.05, **p < 0.01.
Next, we established a xenograft EC model in nude mice using Ishikawa cells transfected with Lenti-sh-NHE7 or control, and confirmed stable NHE7 knockdown efficiency by WB assay to investigate NHE7’s role in vivo (Fig. 7A). Monitoring of tumor volume and weight revealed that NHE7 knockdown resulted in smaller and lighter tumors than control (Fig. 7B, C). Additionally, IHC staining for Ki67 (a cell proliferation marker) showed reduced positivity in Lenti-sh-NHE7 tumor tissues (Fig. 7D). Detection of Ca2+ levels in NHE7 knockdown tumor tissues showed changes in ion homeostasis, with a tendency towards lower Ca2+ levels (Fig. 7E). To gain insights into the relationship between NHE7 and SASP in vivo, ELISA analyses of the supernatant obtained after grinding tumor tissue homogenate revealed changes in the SASP expression, with increases in IL-6, IL-1β, MMP3, and VEGF expression within the NHE7-silenced group (Fig. 7F). Finally, WB analysis showed different protein expression patterns in senescence regulation and signaling pathways, with notably up-regulated p53, p16, and p21 expression, and down-regulated GRINB, CALM1, and PKACA expression in the Lenti-sh-NHE7 tumor tissues derived from tumors from the xenograft tumor models (Fig. 7G). Our findings suggest that the cAMP signaling pathway and GRIN2B-mediated Ca2+ upregulation contribute to NHE7-delayed senescence to drive EC malignant progression.
The mice were subcutaneously injected with cells stably transfected with NHE7 knockdown, or with the control Ishikawa cells (5 × 106/200 μL) (n = 6). A WB assay was used to verify the transfected efficiency of NHE7 in Ishikawa cells (n = 3). A value of p below 0.05 is considered significant, **p < 0.01. B, C Once the tumors reached a volume of 1500 mm³, all mice were euthanized. B Tumor tissues were isolated from the mice and then C the volume and weight of the tumors were recorded (n = 6). *p < 0.05, **p < 0.01. D IHC assay was used to detect the expression levels of Ki67 in tumor tissues (Scale bar: 50 μm, n = 6). **p < 0.01. E The Ca2+ levels in tumor tissue were detected using a kit (n = 3). *p < 0.05. F ELISA was used to detect the IL-6, IL-1β, MMP3, and VEGF levels in the supernatant of tumor tissue homogenates (n = 3). **p < 0.01. G WB assay was used to detect the expression of p53, p21, p16, GRIN2B, CALM1, and PKACA in tumor tissues (n = 3). **p < 0.01.
Antagonizing or knocking down GRIN2B reverses NHE7-mediated senescence resistance of EC cells
For further validation, memantine, a GRIN2B antagonist41, was used in Ishikawa EC cells to investigate NHE7-induced senescence resistance. Our data showed that memantine normalized elevated Ca2+ levels in NHE7-transfected cells, partly restoring calcium homeostasis and reversing NHE7 effects (Fig. 8A). After D-galactose-induced senescence and NHE7 overexpression transfection, memantine treatment partially restored senescence-associated β-galactosidase activity inhibited by NHE7 overexpression (Fig. 8B). Cell cycle analysis showed that the D-galactose + NHE7 group increased S and G2 phase cells vs. the D-galactose group, indicating proliferative resistance to senescence. Adding memantine reversed this, shifting cells to the G1 phase, suggesting a reversal toward a senescent cellular state (Fig. 8C). Subsequent MTT assay to assess cell proliferation also confirmed this result (Fig. 8D). Additionally, WB or qPCR analysis showed altered senescence/SASP-related molecule expression in D-galactose + NHE7 vs. D-galactose group, with decreased levels of p53, p21, p16, IL-6, IL-1β, MMP3, and VEGF, and increased levels of GRIN2B, CALM1, and PKACA. The addition of memantine partially reversed these changes, except for PKACA (Fig. 8E, F). Further, Ishikawa cells were transfected with siR-GRIN2B or siR-NC, confirming its knockdown efficiency via WB assay (Fig. 9A). Similarly, our data showed that the knockdown of GRIN2B partially reversed NHE7-induced senescence resistance in EC cells, reflecting in calcium levels, β-galactosidase activity, and cell cycle distribution (Fig. 9B–D). This also inhibited proliferation, altered senescence-associated molecule expressions, and reduced SASP-related molecule expressions, which was caused by NHE7-induced senescence resistance (Fig. 9E–G). Collectively, both the GRIN2B antagonist and GRIN2B knockdown effectively reverse NHE7-induced senescence resistance in EC cells, highlighting GRIN2B’s crucial role in NHE7-regulating senescence of EC.
A Flow cytometry was used to detect the effect of NHE7 overexpression and memantine treatment (20 µmol/L) on Ca2+ levels in Ishikawa cells. Ishikawa cells were treated with D-galactose to induce senescence (20 µmol/L). A value of p below 0.05 is considered significant, *p < 0.05. B β-galactosidase staining was used to detect the effect of NHE7 overexpression and memantine treatment (20 µmol/L) on senescence in Ishikawa cells (Scale bar: 50 μm). A value of p below 0.05 is considered significant, **p < 0.01. C Flow cytometry was used to detect the effect of NHE7 overexpression and memantine treatment (20 µmol/L) on the cell cycle in Ishikawa cells. *p < 0.05. D MTT assay was used to detect the effect of NHE7 overexpression and memantine treatment (20 µmol/L) on the proliferation in Ishikawa cells. **p < 0.01. E WB assay was used to detect the expression levels of p53, p21, p16, GRIN2B, CALM1, and PKACA in Ishikawa cells with NHE7 overexpression and memantine treatment (20 µmol/L). *p < 0.05, **p < 0.01. F RT-qPCR assay detected the mRNA expression of VEGF, IL-6, IL-1β, and MMP3 in Ishikawa cells with NHE7 overexpression and memantine treatment (20 µmol/L). All data came from three repeated experiments (n = 3). *p < 0.05, **p < 0.01.
A Ishikawa cells were transfected with GRIN2B-targeting siRNA (siR-GRIN2B) or a non-targeting control (siR-NC). WB assay was used to verify the transfected efficiency of GRIN2B in the Ishikawa cells. Ishikawa cells were transfected with GRIN2B-targeting siRNA (siR-GRIN2B) or a non-targeting control (siR-NC) and then treated with D-galactose (25 mg/mL) to induce senescence. A value of p below 0.05 is considered significant, **p < 0.01. B Flow cytometry was used to detect the effect of NHE7 overexpression combined with GRIN2B knockdown on Ca2+ levels in Ishikawa cells. *p < 0.05. C β-galactosidase staining was used to detect the effect of NHE7 overexpression combined with GRIN2B knockdown on senescence in Ishikawa cells (Scale bar: 50 μm). **p < 0.01. D Flow cytometry was used to detect the effect of NHE7 overexpression combined with GRIN2B knockdown on the cell cycle in Ishikawa cells. *p < 0.05, **p < 0.01. E MTT assay detected the effect of NHE7 overexpression combined with GRIN2B knockdown on the proliferation in Ishikawa cells. *p < 0.05, **p < 0.01. F WB assay detected the expression levels of p53, p21, p16, GRIN2B, CALM1, and PKACA in Ishikawa cells with NHE7 overexpression and GRIN2B knockdown. *p < 0.05, **p < 0.01. G RT-qPCR assay detected the mRNA expression levels of VEGF, IL-6, IL-1β, and MMP3 in Ishikawa cells with NHE7 overexpression and GRIN2B knockdown. All data came from three repeated experiments (n = 3). *p < 0.05, **p < 0.01.
NHE7 activates the cAMP-CREB pathway to upregulate GRIN2B, influencing calcium signaling and driving EC progression
Since NHE7-related DEGs enriched the cAMP pathway and NHE7 upregulation boosted PKACA expression in EC, NHE7-transfected Ishikawa cells treated or untreated with BLU0588, the PKACA inhibitor42, were used for further mechanistic exploration. Flow cytometry showed higher Ca2+ levels than controls in NHE7-transfected cells, whereas BLU0588 treatment partially reversed this effect, suggesting NHE7 may affect calcium signaling via the cAMP pathway (Fig. 10A). Using the JASPAR database for binding site prediction, there was strong binding between GRIN2B promoter region and cAMP-responsive element binding protein (CREB), a cAMP signaling downstream transcription factor43 (Fig. 10B). Additionally, we examined the phosphorylation level of CREB in the EC cells. WB analysis showed that NHE7 overexpression increased PKACA, P-CREB, and CREB levels, whereas BLU0588 treatment reversed this (Fig. 10C). Consequently, we proposed a reasonable hypothesis: NHE7 promotes GRIN2B expression via the cAMP-CREB pathway to modulate Ca2+ levels.
A Flow cytometry was used to detect the effect of NHE7 overexpression combined with BLU0588 treatment (5 nmol/L) on Ca2+ levels in Ishikawa cells. A value of p below 0.05 is considered significant, *p < 0.05. B The JASPAR database (http://jaspar.genereg.net/) predicted potential binding sites between the GRIN2B promoter region and CREB. C WB assay detected the expressions of PKACA, CREB, and pCREB in Ishikawa cells with NHE7 overexpression and BLU0588 treatment (5 nmol/L). *p < 0.05, **p < 0.01. D The Dual-luciferase reporter assay detected the luciferase intensity of the pGLO or GRIN2B WT in Ishikawa cells with NHE7 overexpression and BLU0588 treatment (5 nmol/L). *p < 0.05, **p < 0.01. E In Ishikawa cells with overexpressed NHE7, the potential binding site of CREB to the GRIN2B promoter underwent mutation. A dual-luciferase reporter assay was conducted to measure the luciferase activity of each group. **p < 0.01. F The binding interaction between NHE7 and CREB was confirmed through a ChIP assay. **p < 0.01. G IHC assay was used to detect the expression levels of PKACA and GRIN2B in tumor tissues with different NHE7 expressions (Scale bar: 50 μm). The main image is magnified 400×, with an inset showing a 40× magnified view. All data came from three repeated experiments (n = 3). *p < 0.05, **p < 0.01.
We first conducted a dual-luciferase reporter assay in NHE7-overexpressed Ishikawa cells to validate this hypothesis. The result showed NHE7-regulated GRIN2B promoter activity via the cAMP pathway, as NHE7 overexpression increased luciferase activity of the wild-type GRIN2B (GRIN2B WT) promoter, which could be reversed by BLU0588 treatment (Fig. 10D). Another dual-luciferase reporter assay further confirmed that NHE7 regulates GRIN2B promoter activity and its regulatory sites. Specifically, luciferase activity significantly increased in cells with overexpressed NHE7 and the GRIN2B WT promoter. However, no significant difference was observed in cells with overexpressed NHE7 and the mutant GRIN2B (GRIN2B MUT) promoter (Fig. 10E). Subsequent Chromatin immunoprecipitation (ChIP) experiments using CREB antibodies showed significant enrichment of the GRIN2B promoter region in NHE7-overexpressed cells, indicating NHE7 activates the cAMP-CREB cascade to enhance GRIN2B expression (Fig. 10F). Preliminary analysis of cAMP signaling indicator (PKACA) and GRIN2B expression in EC clinical samples with different NHE7 expressions. IHC analysis confirmed that NHE7 expression positively correlated with cAMP pathway activation and GRIN2B upregulation (Fig. 10G). In summary, our findings provide strong evidence that NHE7 activates the cAMP-CREB signaling pathway to upregulate GRIN2B expression, impacting calcium signaling and ultimately promoting EC progression.
Discussion
Cellular senescence is a natural barrier to tumorigenesis18. Almost three decades ago, the accumulation of SA-β-gal-positive senescent cells in the skin of older adults was reported44. Later, senescent hallmarks were found in premalignant skin lesions, specifically human melanocytic naevi22,45, sparking a major interest in exploring senescence in neoplastic and malignant tissues. The study presented herein provides evidence that NHE7 regulates the levels of Ca2+ to delay senescence in EC, ultimately promoting cancer progression, elucidating mechanisms underlying EC progression, and suggesting potential therapeutic targets for treating this disease.
Cancer cells divide and proliferate uncontrollably to drive their progression46, and the alkaline intracellular environment of cancer cells is pivotal for facilitating their uncontrolled proliferation47. Cancer cells enhance the expression or activity of membrane proton transporters to elevate cytosolic pHs, such as Na+/H+ exchangers (NHEs), carbonic anhydrases, monocarboxylate transporters 1 and 4, and Na+-driven HCO3-exchangers, with NHEs playing a central role48,49. The NHE family includes nine isoforms (NHE1-9) that regulate intracellular pH by exchanging hydrogen for sodium50. They are found in various cellular locations, such as the plasma membrane, the Golgi apparatus, and endosomes, to influence cellular function and cancer progression by modulating pH51. For instance, NHE1 causes cell swelling and membrane tension, activating the transient receptor potential cation vanilloid 4 (TRPV4) and enhancing calcium influx, which promotes breast cancer cell migration52. Recent evidence shows that organellar sequestration of H+ also regulates pHi in tumor acidification studies, beyond plasma membrane transporters31,53. Galenkamp et al. showed that NHE7 maintains alkaline pHi in pancreatic ductal adenocarcinoma (PDAC) by supporting trans-Golgi network acidification. Doxycycline-induced NHE7 knockdown in established xenografts resulted in tumor growth arrest, implying NHE7’s protumorigenic role in PDAC54. 5-(N-ethyl-N-isopropyl)-Amiloride, which inhibits NHE7, exhibits anti-cancer effects in non-small cell lung cancer by disrupting cancer stem cell activity and reducing PD-L1 expression55. However, the role of NHEs in EC has not been clarified. Our study innovatively linked EC-related DEGs to senescence-associated genes, finding 721 common genes hinting at a cancer-senescence link. Among them, NHE7 was identified as a potential target due to its role in maintaining acidic tumor microenvironments, poor cancer prognosis, and its association with senescence. Although Voltage-Gated Calcium Channels56, Store-Operated Calcium Channels57, Calmodulin-Dependent Kinases (CAMKs)58, Protein Kinase C59, and so on certainly have a role in the disease progression of EC, we preferred to focus on NHE7-mediated signaling pathways around tumor cell senescence mechanisms. Strikingly, bioinformatic analyses and IHC staining showed NHE7’s importance in promoting EC progression, and its expression negatively correlated with EC senescence. Functional analysis revealed that NHE7 promoted EC cell proliferation by accelerating the cell cycle and delaying senescence. β-galactosidase staining supported these findings. Further exploration found that NHE7-regulated senescence in EC cells by modulating the expression of key senescence-associated markers such as P53, P21, and P1610,19. Specifically, overexpression of NHE7 suppressed senescence, while its silencing promoted it. Furthermore, NHE7 influenced the release of SASP cytokines (IL-6, IL-1β, MMP3, VEGF), impacting the tumor microenvironment and cell senescence8. This underscores NHE7’s complexity and potential as a therapeutic target in EC.
Our in-depth exploration of NHE7 in EC samples sourced from the TCGA database has illuminated its profound impact on cAMP and calcium signaling pathways, with NHE7-related DEGs enriched in these pathways. Both calcium and cAMP signaling are crucial in cancer and senescence33,60. In cancer, abnormal calcium levels drive proliferation, invasion, and drug resistance28, while cAMP signaling affects tumor growth, differentiation, apoptosis, and metabolism61. Anti-cancer drugs target these signaling pathways to inhibit tumor cells. Calcium and cAMP homeostasis disruptions in senescence lead to physiological abnormalities, but modulating these pathways may delay senescence, enhance cell survival and antioxidants, and influence age-related diseases34,62. A long-recognized synergistic relationship between cAMP and calcium signaling was also evident in EC. Our findings indicated that NHE7 activated both cAMP and calcium signaling pathways, leading to upregulation of cAMP-associated PKACA levels, as well as increases in calcium-related Ca2+, GRIN2B, and CALM1 levels, and downregulation of senescence-related factors expression. Conversely, NHE7 silencing reversed these effects, which has also been confirmed in our in vivo experiments. Interestingly, our results further demonstrated that both the application of a GRIN2B antagonist and the knockdown of GRIN2B expression effectively counteract the senescence resistance, calcium levels, cell cycle distribution, and proliferation induced by NHE7 in EC cells. GRIN2B encodes GluN2B, an essential subunit of NMDARs. These receptors play a crucial role in glutamatergic transmission, synaptic plasticity, and neural development by forming ion channels permeable to Na+, K+, and Ca2+, thereby being vital for signaling and function36,63. The existing data have shed light on the pathophysiological mechanisms of GRIN2B-related neurodevelopmental disorders, linking specific variants to clinical symptom development. For instance, L-serine treatment in pediatric patients with LoF GRIN2B variants showed improvements in motor, behavioral, and social functions following daily L-serine supplementation (~500 mg/kg) for 17 months64. However, GRIN2B research in cancer is currently at the screening and prediction stage65,66. Our study is the first to elucidate the role of GRIN2B in EC to the best of our knowledge, highlighting its pivotal role in NHE7-regulated senescence in EC cells, suggesting that NHE7’s anti-senescent effects are partially mediated by upregulating GRIN2B.
In terms of mechanism, we predicted and validated the interaction between the GRIN2B promoter region and CREB. The data suggested that NHE7 up-regulated GRIN2B promoter activity via cAMP-CREB signaling, thereby influencing calcium signaling. Overall, our study provides compelling evidence that NHE7 upregulates GRIN2B expression through the cAMP-CREB signaling pathway, affecting calcium homeostasis to delay the senescence of EC cells, ultimately promoting EC progression. To date, no published literature or research has explicitly elucidated the mechanisms underlying targeting NHE7 or GRIN2B in endometrial cancer, according to the information available to us. However, based on the findings of this study, we propose the following perspectives for exploration: 1) Target feasibility: Overexpression of NHE7 promoted cell proliferation, accelerated cell cycle progression, and down-regulated the expression of senescence markers, whereas NHE7 knockdown induced cellular senescence. Antagonism or knockdown of GRIN2B attenuated the effects of NHE7 on senescence marker expression. Collectively, these results validate the potential of NHE7 and GRIN2B as therapeutic targets. 2) Administration approaches: the development of small-molecule inhibitors or monoclonal antibody-based therapeutics delivered via intravenous injection represents a viable strategy for targeting these proteins. 3) Off-target effects and safety considerations: NHE7 inhibition may potentially induce electrolyte imbalances or gastrointestinal adverse effects67, necessitating tissue-specific delivery systems or highly selective drug design to mitigate risks. Given GRIN2B’s predominant expression in the central nervous system37, its targeting might precipitate cognitive impairments or epileptiform symptoms. Risk mitigation strategies could include localized administration or development of blood-brain barrier-impermeable compounds. Certainly, future research endeavors are vital to delve deeper into the clinical significance of targeting NHE7 and GRIN2B as potential therapeutic strategies for treating EC. For instance, alongside the notable presence of cAMP and Ca2+ signaling in NHE7-related DEGs, our database analysis revealed a significant enrichment of immune-related pathways, thereby highlighting avenues for future investigation.
Conclusion
Our study unveils a previously uncharacterized regulatory mechanism in EC to our knowledge, demonstrating that NHE7 upregulates GRIN2B expression through the cAMP-CREB signaling pathway. This regulatory mechanism impacts intracellular calcium homeostasis, effectively delaying the senescence of EC cells and ultimately contributing to cancer progression. Our findings underscore the indispensable roles of NHE7 and GRIN2B in EC and highlight the promising potential of therapeutic interventions targeting this pathway.
Methods
Bioinformatic analyses
RNA-seq data and clinical information for EC from the TCGA database (https://portal.gdc.cancer.gov/) were analyzed, including 550 tumors (403 Endometrioid adenocarcinoma, EA; 136 Serous cystadenocarcinoma, SC; 11 EC cancer without cancer label) and 35 healthy samples. This study complies with the regulations of China’s Human Genetic Resources Administration. All ethical regulations relevant to human research participants were followed. DEGs between tumor and healthy groups were identified using “DESeq2” in R software (R; Version R4.4.2), with screening criteria of fdrFilter = 0.05 and logFCfilter = 2, and presented in a volcano plot. GO and KEGG enrichments were performed using the “clusterProfiler” package in R. The overlap of EC DEGs with senescence-related genes from the literature30 is visualized in a Venn diagram. Univariate Cox regression analysis was then conducted on the intersected genes using the “survival” package in R, with a significance threshold of p < 0.001. Tumor samples were grouped by high or low NHE7 expression based on the median expression of NHE7 to assess its correlation with clinical characteristics (age and stage) using SPSS software (Version 26). NHE7 expression in EA and SC tumors compared to healthy samples was plotted as a boxplot using the “ggplot2” package in R. Survival curve was created in the “survival” and “survminer” packages in R.
DEGs between high/low NHE7 groups in EC tumor patients were identified using the “DESeq2” package in R and presented as a volcano plot. GO and KEGG enrichments were analyzed using the “clusterProfiler” package in R. cAMP and calcium signaling pathway genes from KEGG were intersected with NHE7-related DEGs, visualized in a Venn diagram. The correlation between gene enriched in the calcium signaling pathway and NHE7 expression in EC was analyzed using the Pearson method and visualized using the “ggstatsplot” package in R. Finally, the JASPAR database was used for the transcription factor binding profiles.
Ethical approval and consent to participate
Bioinformatics data were downloaded from public databases. The study involving patient data in this database was approved by the institutional ethics committee, and all patients provided informed consent.
The animal experiments were conducted by the “Guiding Principles in the Care and Use of Animals” (China) and approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University (AE20240029).
The clinical samples experiments conducted in this study were approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University (IRB-20230176-R) and strictly adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from all patients before the collection of clinical samples, ensuring that they fully understood and agreed to the use of their samples for research purposes.
Cell culture
The HEC-1-A (YDT-0225), Ishikawa (YDT-0302), and HEK-293T (YDT-0019) cell lines used in this study were purchased from INDIT Bio-Technology Co., Ltd. (Hangzhou, China). HEC-1-A cells were cultured in McCoy’s 5A medium (Thermo Fisher, USA, 16600082), while Ishikawa and HEK-293T cells were maintained in MEM medium (BDBIO, China, C11095500BT). All cell lines were incubated at 37 °C in a humidified atmosphere with 5% CO₂, and the culture media were supplemented with 10% fetal bovine serum (FBS, SERANA, China, s-FBS-x-015) and 1% penicillin/streptomycin (Gibco, USA, 15140-122). All experiments were performed with mycoplasma-free cells (Supplementary Data 4).
Cell transfection
To generate NHE7-overexpressing EC cell lines (the cultured HEC-1-A and Ishikawa cells in the “Cell culture” section), the pCDH-NHE7 (NM_001257291) and the empty pCDH vector (VT1480) were purchased from Youbio (Hunan, China). The human coding sequence (CDS) of NHE7 was synthesized, digested with restriction enzymes, and inserted into the pCDH vector to construct the overexpression plasmids. These plasmids were transfected into EC cells using Lipofectamine 2000 (Invitrogen, USA, 11668-019) and incubated for 24 h. For NHE7- or GRIN2B-silenced EC cell lines, siRNAs targeting NHE7 (siR-NHE7), GRIN2B (siR-GRIN2B), and a negative control siRNA (siR-NC) were purchased from Tsingke Biotechnology Co., Ltd. (Beijing, China). The above siRNAs were transfected into EC cells using Lipofectamine 2000 for 24 h. The sequences of these siRNAs are provided in Supplementary Table 1.
To create stable NHE7 knockdown Ishikawa cells, shRNA was designed based on the siR-NHE7 sequence (Lenti-sh-NHE7) or negative control (Lenti-sh-NC), coated into 293T cells to produce lentiviral particles. The viral supernatant was collected, filtered, and used to transfect Ishikawa cells with Lipofectamine 2000 for 24 h at 60%–70% transfection fusion. Following transfection, the medium was refreshed, and puromycin (2.5 µg/mL, Yuanye, China, S17055) was applied for 7–10 days to select stable clones. Finally, the transfection efficiency of the above cells was verified by Western blot assays.
MTT assay
HEC-1-A and Ishikawa cell lines were plated in 96-well plates at a density of 1 × 10⁴ cells per well and cultured for 24 h. Then, MTT solution (Sigma, USA, M2128) was added, and the cells were incubated for 3 h. The formazan crystals were dissolved in DMSO (Sigma, USA, D2650). Cell viability was assessed by measuring the optical density (OD) at 570 nm using a microplate reader (Thermo Fisher, USA, 51119570).
Flow cytometry assay
HEC-1-A and Ishikawa cells during logarithmic growth were collected, centrifuged, and washed with PBS. The cell cycle was detected using a cell cycle detection kit (Beyotime, China, C1052-50T). Briefly, the cells were fixed in 70% ethanol overnight, then stained with propidium iodide (PI, 50 μg/mL) and RNase A (100 μg/mL) before flow cytometry analysis (Beckman Coulter, USA, CytoFLEX) with a 488 nm laser. For calcium ion measurement, cells were trypsinized, stained with Fluo-4 AM (Beyotime, China, S1061S), and incubated at 37 °C. After centrifugation, the cells were resuspended in PBS and analyzed using a flow cytometer to detect green fluorescence at 490 nm excitation and 525 nm emission wavelengths.
WB analysis
Fresh frozen tumor tissues were thawed, minced, and homogenized in lysis buffer supplemented with 1 mM protease inhibitors. For cell samples, whole-cell lysates were prepared using a cell lysis buffer. Protein concentrations were determined using a BCA assay kit (Solarbio, China, PC0020). WB analysis was conducted following standard protocols68. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 8%–12%) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Thermo Fisher, USA, 88585). Protein-antibody complexes were detected using an ECL kit (Bio-Rad, USA, 1705060). The primary antibodies used are listed in Supplementary Table 2, with β-actin serving as the loading control. Grayscale values of protein bands were quantified using ImageJ software (National Institutes of Health, USA, Version 1.8.0).
β-Galactosidase stain assay
The cells were collected and fixed with 1% glutaraldehyde for 15 min, followed by three washes with PBS to remove residual fixative. The staining working solution was prepared according to the instructions of the β-galactosidase staining kit (Beyotime, China, C0602). After adding the staining solution, the cells were incubated overnight at 37 °C in the dark. Following incubation, the cells were washed with PBS three times for 5 min each time. The staining results were examined under a light microscope.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using Trizol reagent (Ambion, China, 15596018), followed by phase separation with chloroform and RNA precipitation with isopropanol (Acros Organics, USA, 327270010). The RNA pellet was washed with 75% ethanol, air-dried, dissolved in RNase-free water, and then quantified. cDNA synthesis was performed using the PrimeScript™ RT reagent Kit (TAKARA, Japan, RR037Q), and the resulting cDNA was stored at −80 °C. RT-qPCR was performed using the TB Green™ Premix Ex Taq™ II kit (TAKARA, Japan, RR820S), with β-actin as the reference gene. Relative expression levels were calculated using the 2−ΔΔCT method. The primer sequences are listed in Supplementary Table 3.
ChIP assays
Ishikawa cells were cultured and fixed with 1% formaldehyde, then lysed and sonicated. After centrifugation, the supernatant was divided into three groups (input for positive control). The experimental group followed the manufacturer’s instructions using a ChIP kit (Thermo Fisher, 26157) and an anti-CREB antibody (Thermo Fisher, MA1-083). The antibody was added and incubated with Protein A/G beads overnight at 4 °C. The beads were washed and eluted, followed by reverse cross-linking with 5 M NaCl at 65 °C for 4 h and Core Mix at 45 °C for 1 h. DNA was purified using a DNA purification kit (Promega, USA, AS1450). qPCR was then used to amplify using the designed GRIN2B promoter primers in the ChIP products (forward Primer: CTCTCCGTCGGTGCTGTTC; reverse Primer: ACCCCCTCACTCCTCTTGC).
Construction of the xenograft tumor models in vivo
Animal experiments complied with China’s Guidelines for the Care and Use of Animals and were approved by the Ethics Committee of Zhejiang University Women’s Hospital (Approval No. AE20240029). We have complied with all relevant ethical regulations for animal use. Female BALB/c nude mice, aged 6–8 weeks, were obtained from Charles River (Beijing, China). They were kept under controlled conditions (specific pathogen-free) with a 12 h light/dark cycle, a room temperature of 20–22 °C, and humidity of 30%–70%. Following a 7-day acclimatization period, the mice were divided into two groups (n = 6): Lenti-sh-NC and Lenti-sh-NHE7. Ishikawa cells, either control or NHE7 knockdown (5 × 10⁶ cells/200 μL), were injected subcutaneously to establish xenograft tumor models. Tumor growth was monitored, and once the tumors in the control group reached ~1500 mm³, mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), euthanized via cervical dislocation, and tumors were surgically excised by incising along the tumor margins, then the tumor volumes and weights were recorded.
IHC assay
IHC was used to analyze the expression of target proteins in tumor tissues. Tissue samples were fixed in 4% paraformaldehyde for 1 week, dehydrated with ethanol and xylene, and embedded in paraffin. Sections (5 μm) were deparaffinized, rehydrated, and subjected to antigen retrieval. Primary antibodies (listed in Supplementary Table 2) were incubated overnight, followed by secondary antibody incubation and Hematoxylin and eosin (H&E, Solarbio, G1120) staining. The target protein expression was observed under a microscope and compared with the control group.
Dual-luciferase reporter gene assay
To investigate the regulatory effect of NHE7 on GRIN2B promoter transcriptional activity, overexpressing NHE7 Ishikawa cells or control cells were seeded into 24-well plates and cultured until they reached 80% confluence. Luciferase reporter plasmids containing either an empty vector (pGLO), the GRIN2B WT (sequence: GCACATGACGTG), or GRIN2B MUT (sequence: CACGTCATGTGC) promoter region (Supplementary Note 1) were purchased from Tsingke Biotechnology Co., Ltd. (Beijing, China). Using Lipofectamine 2000 transfection reagent following the manufacturer’s protocol, the above plasmids were respectively transfected into the treated Ishikawa cells. After 24 h of transfection, luciferase activity was measured using a Luciferase Assay Reagent kit (Merck, Germany, LUC1-1) in a 96-well plate according to the manufacturer’s protocol, and the relative luciferase data were recorded and calculated.
Ca2+ detection in the tumor tissues
Tumor tissue samples were collected, chopped into small pieces, and homogenized in PBS containing protease inhibitors. The homogenate was then centrifuged, and the supernatant was collected for analysis. Ca2+ concentration was measured using a calcium ion kit (Beyotime, China, S1063S).
ELISA assay
Cells were harvested, trypsinized, and seeded into plates. After 24 h of culture, the supernatant was collected to measure cAMP levels and SASP-related molecule concentrations using specific ELISA kits (MEIMIAN, China). Briefly, standards and samples were added into a 96-well plate, followed by the addition of specific antibodies from MEIMIAN (cAMP, No.: MM-0006H1; IL-6, No.: MM-0049H2; IL-1β, No.: MM-0181H2; MMP3, No.: MM-0108H2; VEGF, No.: MM-0115H2). The plate was incubated at 37 °C for 1 h. After washing with washing buffer to remove unbound antibodies, enzyme-labeled secondary antibodies were added, and the plate was incubated for another 30 min. After the reaction, substrate solution was added until visible color development occurred, followed by the addition of a stop solution to terminate the reaction. Finally, the absorbance at 450 nm (OD value) was measured using a microplate reader, and SASP-related molecules in the samples were calculated based on the standard curve.
Statistics and reproducibility
GraphPad Prism 6.0 (USA, SCR_002798) was used to analyze group differences using a t-test for two groups and one-way ANOVA for multiple groups. Continuous variables are reported as mean ± standard deviation (SD). Sample sizes were carefully determined based on experimental type and variability, ranging from n = 3 in vitro studies to n = 6 for longitudinal animal experiments. All key experiments were independently replicated at least three times by different researchers using fresh reagent batches, with consistent results across replicates. For animal studies, we followed ARRIVE guidelines with randomization and blinded outcome assessment. Statistical significance levels are denoted as *p < 0.05 and **p < 0.01.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article. Values for all data points found in graphs can be found in the “Supplementary Data 1” file. Uncropped blots are provided in Supplementary Fig. 2. The flow cytometry gating strategies are provided in Supplementary Data 2. The graphical abstract was created with BioRender.com, and the confirmation of publication and licensing rights for the graphical abstract is provided in Supplementary Data 3. Additional information or other data are available from the corresponding author upon reasonable request.
References
Amant, F. et al. Endometrial cancer. Lancet 366, 491–505 (2005).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Morice, P., Leary, A., Creutzberg, C., Abu-Rustum, N. & Darai, E. Endometrial cancer. Lancet 387, 1094–1108 (2016).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
Makker, V. et al. Endometrial cancer. Nat. Rev. Dis. Prim. 7, 88 (2021).
Jamieson, A. & McAlpine, J. N. Molecular profiling of endometrial cancer from TCGA to clinical practice. J. Natl Compr. Cancer Netw. 21, 210–216 (2023).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22, 340–355 (2022).
Lopes-Paciencia, S. et al. The senescence-associated secretory phenotype and its regulation. Cytokine 117, 15–22 (2019).
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 28, 436–453 (2018).
Arora, M. et al. Rapid adaptation to CDK2 inhibition exposes intrinsic cell-cycle plasticity. Cell 186, 2628–2643.e21 (2023).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Moiseeva, O., Bourdeau, V., Roux, A., Deschênes-Simard, X. & Ferbeyre, G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol. Cell. Biol. 29, 4495–4507 (2009).
Dörr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013).
Pluquet, O., Pourtier, A. & Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am. J. Physiol. Cell Physiol. 308, C415–C425 (2015).
Carroll, B. et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J. Cell Biol. 216, 1949–1957 (2017).
Schmitt, C. A., Wang, B. & Demaria, M. Senescence and cancer—role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 19, 619–636 (2022).
Sugrue, M. M., Shin, D. Y., Lee, S. W. & Aaronson, S. A. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl. Acad. Sci. USA 94, 9648–9653 (1997).
Sage, J., Miller, A. L., Pérez-Mancera, P. A., Wysocki, J. M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Liberti, M. V. & Locasale, J. W. The Warburg Effect: How does it benefit cancer cells?. Trends Biochem. Sci. 41, 211–218 (2016).
Webb, B. A., Chimenti, M., Jacobson, M. P. & Barber, D. L. Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677 (2011).
Zeidan, R. S., Han, S. M., Leeuwenburgh, C. & Xiao, R. Iron homeostasis and organismal aging. Ageing Res. Rev. 72, 101510 (2021).
Chen, S. et al. Local H(2) release remodels senescence microenvironment for improved repair of injured bone. Nat. Commun. 14, 7783 (2023).
Farfariello, V., Iamshanova, O., Germain, E., Fliniaux, I. & Prevarskaya, N. Calcium homeostasis in cancer: a focus on senescence. Biochim. Biophys. Acta 1853, 1974–1979 (2015).
Zheng, S., Wang, X., Zhao, D., Liu, H. & Hu, Y. Calcium homeostasis and cancer: insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 33, 312–323 (2023).
Baczkó, I., Giles, W. R. & Light, P. E. Resting membrane potential regulates Na(+)-Ca2+ exchange-mediated Ca2+ overload during hypoxia-reoxygenation in rat ventricular myocytes. J. Physiol. 550, 889–898 (2003).
Saul, D. & Kosinsky, R. L. Single-cell transcriptomics reveals the expression of aging- and senescence-associated genes in distinct cancer cell populations. Cells 10, 3126 (2021).
Ward, N. P. & DeNicola, G. M. The Golgi: keeping it unapologetically basic. Cancer Discov. 10, 768–770 (2020).
Azman, K. F. & Zakaria, R. D-Galactose-induced accelerated aging model: an overview. Biogerontology 20, 763–782 (2019).
Cooper, D. M., Mons, N. & Karpen, J. W. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374, 421–424 (1995).
Hansen, R. T. 3rd & Zhang, H. T. Senescent-induced dysregulation of cAMP/CREB signaling and correlations with cognitive decline. Brain Res. 1516, 93–109 (2013).
Farfariello, V. et al. TRPC3 shapes the ER-mitochondria Ca(2+) transfer characterizing tumour-promoting senescence. Nat. Commun. 13, 956 (2022).
Vieira, M., Yong, X. L. H., Roche, K. W. & Anggono, V. Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J. Neurochem. 154, 121–143 (2020).
Sabo, S. L., Lahr, J. M., Offer, M., Weekes, A. & Sceniak, M. P. GRIN2B-related neurodevelopmental disorder: current understanding of pathophysiological mechanisms. Front. Synaptic Neurosci. 14, 1090865 (2022).
Kobayashi, H. et al. Calm1 signaling pathway is essential for the migration of mouse precerebellar neurons. Development 142, 375–384 (2015).
LaMoia, T. E. et al. Cytosolic calcium regulates hepatic mitochondrial oxidation, intrahepatic lipolysis, and gluconeogenesis via CAMKII activation. Cell Metab. 36, 2329–2340.e2324 (2024).
Cao, B. et al. Structures of the PKA RIα Holoenzyme with the FLHCC Driver J-PKAcα or Wild-Type PKAcα. Structure 27, 816–828.e814 (2019).
Fedele, L. et al. Disease-associated missense mutations in GluN2B subunit alter NMDA receptor ligand binding and ion channel properties. Nat. Commun. 9, 957 (2018).
Schalm, S. S. et al. Evaluation of protein kinase cAMP-activated catalytic subunit alpha as a therapeutic target for fibrolamellar carcinoma. Gastro Hep Adv. 2, 307–321 (2023).
Ahmed, M. B., Alghamdi, A. A. A., Islam, S. U., Lee, J. S. & Lee, Y. S. cAMP signaling in cancer: a PKA-CREB and EPAC-centric approach. Cells 11, 2020 (2022).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367 (1995).
Gray-Schopfer, V. C. et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16?. Br. J. Cancer 95, 496–505 (2006).
Klein, C. A. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 20, 681–694 (2020).
Pouysségur, J., Franchi, A., L’Allemain, G. & Paris, S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 190, 115–119 (1985).
White, K. A., Grillo-Hill, B. K. & Barber, D. L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell Sci. 130, 663–669 (2017).
Acevedo-Olvera, L. F. et al. Inhibition of the Na+/H+ antiporter induces cell death in TF-1 erythroleukemia cells stimulated by the stem cell factor. Cytokine 75, 142–150 (2015).
Spugnini, E. P. et al. Proton channels and exchangers in cancer. Biochim. Biophys. Acta 1848, 2715–2726 (2015).
Reshkin, S. J., Cardone, R. A. & Harguindey, S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat. Anti Cancer Drug Discov. 8, 85–99 (2013).
Bera, K. et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature 611, 365–373 (2022).
Liu, B. et al. STAT3 associates with vacuolar H(+)-ATPase and regulates cytosolic and lysosomal pH. Cell Res. 28, 996–1012 (2018).
Galenkamp, K. M. O. et al. Golgi acidification by NHE7 regulates cytosolic pH homeostasis in pancreatic cancer cells. Cancer Discov. 10, 822–835 (2020).
Wang, B. Y., Shen, H. T., Lee, Y. L., Chien, P. J. & Chang, W. W. Inhibition of Na+/H+ exchanger (NHE) 7 by 5-(N-ethyl-N-isopropyl)-Amiloride displays anti-cancer activity in non-small cell lung cancer by disrupting cancer stem cell activity and downregulating PD-L1 expression. Am. J. Cancer Res. 13, 4721–4733 (2023).
Phan, N. N. et al. Voltage-gated calcium channels: novel targets for cancer therapy. Oncol. Lett. 14, 2059–2074 (2017).
Jiang, M. et al. Dysregulation of SOCS-mediated negative feedback of cytokine signaling in carcinogenesis and its significance in cancer treatment. Front. Immunol. 8, 70 (2017).
Racioppi, L. & Means, A. R. Calcium/Calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J. Biol. Chem. 287, 31658–31665 (2012).
Garg, R. et al. Protein kinase C and cancer: what we know and what we do not. Oncogene 33, 5225–5237 (2014).
Takahashi, A. et al. Induction of senescence by progesterone receptor-B activation in response to cAMP in ovarian cancer cells. Gynecol. Oncol. 113, 270–276 (2009).
Gao, Z., Lei, W. I. & Lee, L. T. O. The role of neuropeptide-stimulated cAMP-EPACs signalling in cancer cells. Molecules 27, 311 (2022).
Martin, N., Zhu, K., Czarnecka-Herok, J., Vernier, M. & Bernard, D. Regulation and role of calcium in cellular senescence. Cell Calcium 110, 102701 (2023).
Hansen, K. B. et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol. Rev. 73, 298–487 (2021).
Soto, D. et al. L-Serine dietary supplementation is associated with clinical improvement of loss-of-function GRIN2B-related pediatric encephalopathy. Sci. Signal. 12, eaaw0936 (2019).
She, X., Gao, Y., Zhao, Y., Yin, Y. & Dong, Z. A high-throughput screen identifies inhibitors of lung cancer stem cells. Biomed. Pharmacother. 140, 111748 (2021).
Yuan, Z., Wang, Y., Xu, S., Zhang, M. & Tang, J. Construction of a prognostic model for colon cancer by combining endoplasmic reticulum stress responsive genes. J. Proteom. 309, 105284 (2024).
Masereel, B., Pochet, L. & Laeckmann, D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur. J. Med. Chem. 38, 547–554 (2003).
Pillai-Kastoori, L., Schutz-Geschwender, A. R. & Harford, J. A. A systematic approach to quantitative Western blot analysis. Anal. Biochem. 593, 113608 (2020).
Acknowledgements
This study was financially supported by the Natural Science Foundation of Zhejiang Province (No. LQ20H160054; No. LQ20H160049). We are grateful to the Women’s Hospital, School of Medicine, Zhejiang University, for their ethical oversight and approval of our study. Our thanks also go to our colleagues and participants for their invaluable support and contributions throughout this research.
Author information
Authors and Affiliations
Contributions
Shizhou Yang designed the study, developed the methodology, conducted key experiments, analyzed the data, and drafted the original manuscript. Tao Shen participated in experimental design, carried out critical investigations, performed statistical analyses, and contributed to data interpretation. Minghua Yu conducted specialized validation experiments, performed detailed data analysis, and verified experimental results. Tingting Wu was responsible for collecting clinical samples, organizing datasets, and maintaining research records. Linhua Qian and Wu Liu collaborated on analytical procedures, conducted quality control assessments, and validated experimental findings. Ting Wang performed supplementary data analysis and assisted with results verification. Xiufeng Huang provided overall project supervision, secured research funding, contributed to manuscript revision, guided methodological development, and coordinated interdisciplinary collaboration. All co-authors assisted with reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Paul Kennedy and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Georgios Giamas, Johannes Stortz, and David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, S., Shen, T., Yu, M. et al. NHE7 drives endometrial cancer progression by delaying senescence through cAMP/CREB/GRIN2B axis-mediated Ca²⁺ influx. Commun Biol 8, 877 (2025). https://doi.org/10.1038/s42003-025-08296-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08296-1