Abstract
Social identity differences are crucial for gregarious animals, impacting survival and social development. This is particularly evident in humans, where social stratification, cultural divides, and ethnic differences influence societal dynamics. Social recognition memory plays a central role in this process, maintaining social order by allowing individuals to distinguish familiar members within their group. Notably, social recognition memory exhibits differences: within a group, individuals form detailed memories of each member (individualized memory), while for out-group members, a more generalized memory of the entire group forms (categorized memory). Although this phenomenon has been explored in human studies, current research techniques and methods have limited investigations into the underlying neural mechanisms, especially their plasticity and regulatory mechanisms. This study utilizes mice to establish an experimental model for investigating differences in social recognition memory and its neural basis. We demonstrate that mice also exhibit social identity-driven memory recognition patterns. Mice form individualized memories for same-strain individuals but categorized memories for different strains, and the type of social recognition memory could be regulated by oxytocin level of ventrolateral periaqueductal gray. These findings demonstrate that oxytocin and its receptors in the ventrolateral periaqueductal gray are essential for constructing and plastically regulating intergroup social memory in mice.
Similar content being viewed by others
Introduction
Social recognition memory (SRM), a key component of episodic memory, allows animals to identify and remember individual conspecifics1. This ability forms the foundation for various social behaviors, enabling group members to build relationships and adjust interactions to maintain social stability2,3,4,5.
In the animal kingdom, efficient cooperative relationships rely heavily on the ability to quickly distinguish familiar individuals, especially within the same social group6. Humans exhibit this phenomenon through the “other-race effect,” where they more accurately recognize and remember faces of their own race7. This distinction can even lead to differences in empathy and prosocial behavior between in-group and out-group members8,9,10,11,12,13. While research on this phenomenon in humans has progressed, understanding the underlying neural mechanisms remains limited due to methodological constraints. Nevertheless, using rodents as model organisms to study and understand the underlying neural mechanisms of this phenomenon appears to be a viable approach.
Rodent studies, particularly those using mice, have extensively explored SRM, primarily focusing on short-term memory within a single social group14. Social information processing in mice likely involves a dedicated neural circuit encompassing the olfactory system, amygdala, lateral septum, hippocampus, and other brain regions15,16. Recent research suggests the involvement of specific structures like the anterior insular cortex (aiC) and the CA2 region of the hippocampus in recognizing novel individuals17,18,19,20. Additionally, the amygdala appears to play a crucial role in long-term social memory.
However, existing studies have largely overlooked the potential for intergroup social recognition in mice. Just like humans with different ethnicities, mice naturally form social groups based on their strains. This raises the intriguing question: do mice, like humans, exhibit differential social recognition between groups? Furthermore, can this recognition be influenced by increased positive interactions, similar to the observed reduction in implicit bias in humans 11,12,13? Exploring the plasticity of intergroup social recognition in mice offers valuable insights. Ultimately, such behavioral studies pave the way for uncovering the underlying neural mechanisms.
The periaqueductal gray (PAG), a midbrain structure critical for defense, predation, and escape behaviors in mammals21,22,23,24, has recently emerged as a player in social behavior regulation. Tac1 neurons within the PAG project directly to oxytocin neurons in the hypothalamus, promoting social interaction25. Conversely, activating the CA3-PAG neural circuit in socially dysfunctional mice restores social novelty26. Additionally, the PAG plays a role in recognizing social odors, allowing mice to strategically avoid risks27. These findings highlight the PAG’s involvement in regulating social interactions, potentially mediated by oxytocin. Our published findings reveal that significant differences in PAG activity during inter-strain social interactions in mice, suggesting its role in processing social information between groups28. Building on this knowledge, this study aims to investigate differential SRM between mouse strains and its underlying neural mechanisms using mice as a model organism.
This investigation will address the following key questions: 1. Do mice exhibit differentiated SRM between strains? 2. Is SRM between mouse strains susceptible to modification through experience? 3.What are the neural mechanisms underlying differential SRM in mice? By unraveling the neural basis of strain-dependent social recognition, this study sheds light on the complex interplay between social behavior and intergroup interactions. This knowledge can potentially inform future research on social bias and intergroup relations across different species, including humans.
Results
C57 mice exhibit differences in SRM between mice of the same and different strains
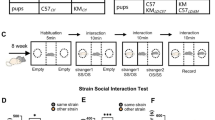
We first used a five-trial social memory experiment to detect whether C57 mice have different SRM patterns for mice of different strains. Same with the traditional five-trial social memory experiments, the same stimulus mouse A was used in the first four trials of the five-trial experiment, and the unfamiliar stimulus mouse B was used in the fifth trial. By comparing the interaction time of the test mouse with the stimulus mouse in the first, fourth, and fifth trials, we divided SRM into the following three modes: 1. Individualized memory: There is a significant difference between the first trial interaction time and the fourth trial interaction time, and there is also a significant difference between the fourth trial interaction time and the fifth trial interaction time; 2. Categorized memory: There is a significant difference between the first trial interaction time and the fourth trial interaction time, but there is no significant difference between the fourth trial interaction time and the fifth trial interaction time; 3. Unable to form SRM: There is no significant difference between either the interaction time of the first trial and the fourth trial, or between the fourth trial and the fifth trial. In all experiments, C57 mouse were used as test mouse, and either C57BL/6 (C57) or Kunming (KM) mouse were used as same strain or other-strain stimulus mouse.
The results showed that C57 mice can form individualized memory for mice of the same-strain. That is, with the introduction of the same stimulus mouse in the first four trials, the interaction time between the test mouse and the stimulus mouse gradually decreased (t1 vs. t4: P = 0.0001, n = 8). After the fifth trial a new stimulus mouse was introduced, the interaction time was significantly increased (t4 vs. t5: P = 0.0325, n = 8) (Fig. 1A, B). However, the SRM pattern was quite different when the KM mouse was used as stimulus mouse. With the introduction of the same KM mouse, the interaction time decreased significantly (t1 vs. t4: P = 0.0002, n = 8), but after introducing a novel stimulus KM mouse in the fifth trial, the interaction time between the test mouse and the stimulus mouse was not significantly different from that in the fourth trial (t4 vs. t5: P = 0.1969, n = 8) (Fig. 1A, B). The above results indicated that C57 mice show different SRM patterns to mice of the same and different strains. In particular, the C57 mice could form individualized recognition memory for same-strain mice, but did not seem to notice that a new stimulus KM mouse was replaced in the fifth trial. That is, similar to “face blindness” in humans, C57 may not be able to form individualized memory for other-strain mice.
A Five-trial social recognition memory paradigm for male C57BL/6 mice: Trials 1–4 involved repeated exposure to a single stimulus mouse (same-strain: SS1; other-strain: OS1). Trial 5 introduced a novel stimulus mouse (same-strain: SS2; other-strain: OS2) to assess memory discrimination. B Statistical analysis of interaction times for the five-trial social memory experiment in A. Male C57 mice developed individualized SRM for the same strain and group recognition memory for the different strain. Interaction times for C57 mice with the same strain in trial 1, trial 4 and trial 5 (n = 8). C The seven-trial social memory experimental paradigm for male C57 mice interacting with KM mice, where the same mouse was used for the first six trials, and a novel mouse was introduced in the seventh trial. D Statistical analysis of interaction times for the seven-trial experiment in C. After extending to seven trials, male C57 mice did not develop individualized recognition memory for KM mice and maintained group recognition memory. There was a significant difference in interaction times between trial 1 and trial 4, but no difference between trial 4 and trial 5 (n = 7). E Schematic of a five-trial experiment using 5 different KM stimulus mice, meaning each of the 5th trials involved a different KM novel mouse. F Statistical analysis of interaction times for the five-trial experiment in E. C57 mice could not distinguish between different KM novel mice (n = 8). G Five-trial social memory experimental paradigm for recognition of same and different strains, where KM mice were used for the first four trials, and a C57 mouse was introduced in the fifth trial. H Statistical analysis of interaction times for the five-trial experiment in G. C57 mice could distinguish between KM mice and C57 mice (n = 8). I Five-trial social recognition memory paradigm for female C57BL/6 mice. J Female C57 mice developed individualized SRM for the same strain(female) and group recognition memory for the different strain(femal) (n = 14). Data displayed as mean±S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001. T1: Trial 1, T4: Trial 4, T5: Trial 5, T6: Trial 6, T7: Trial 7.
C57 mice fail to form individualized SRM for KM mice and instead form categorized SRM
To address the possibility that C57 mice require a longer time to form individualized memory of KM mice, we extended the five-trial experiment to a seven-trial experiment. By comparing the interaction times between the sixth and seventh trial, we aimed to determine if C57 mice could form individualized SRM of KM mice after prolonged repetitive interaction (Fig. 1C). The results showed that extending the interaction time did not enable C57 mice to establish individualized SRM for KM mice (t6 vs. t7: P = 0.6188, n = 7) (Fig. 1D). Next, in the five-trial experiment, we replaced the stimulus mouse in each trial with five different KM stimulus mice (Fig. 1E). The results showed that the interaction time of C57 mice with the different KM stimulus mice gradually decreased (t1 vs. t5: P = 0.0083, n = 8) (Fig. 1F). These results indicated that C57 mice indeed have difficulty distinguishing individual KM mice. To further validate the previous findings, we introduced a modification in the fifth trial by substituting the stimulus mouse with a C57 mouse, while continuing to use a KM mouse as the stimulus in the first four trials. (Fig. 1G). The results showed that C57 mice could distinguish between KM mice and C57 mice (t1 vs. t4: P < 0.0001, t4 vs t5: P = 0.0014, n = 8) (Fig. 1H). Together these results confirmed that C57 mice cannot distinguish between individual KM mice, but instead recognize them as a group. In other words, C57 mise form categorized SRM for other-strain mice.
We additionally assessed SRM in female C57 mice (Fig. 1I, J). Similar to their male counterparts, female mice demonstrated the capacity for individualized memory of same-strain conspecifics (female), as evidenced by significantly increased social interaction time in Trial 5 compared to Trial 4 (T4 vs T5: P = 0.0335, n = 14; Fig. 1J). However, consistent with male behavioral patterns, female mice failed to establish individualized memory for other-strain individuals(female), showing no significant difference in interaction duration between Trial 4 and Trial 5 (T4 vs T5: P = 0.8179, n = 14; Fig. 1J). Given this identical strain-dependent behavioral phenotype across sexes, we subsequently focused our investigations on male subjects for all further experiments.
Cross-fostering Induces a Shift from Individualized to Categorized SRM of Same-Strain Mice
Previous studies have shown that people who have more contact with out-group members in their early upbringing have significantly lower implicit bias towards those out-group members29,30. This suggests that postnatal living environments can influence social recognition of out-group individuals. Therefore, we attempted to change the SRM patterns of C57 mice towards KM mice by increasing their interaction during the rearing process. We adopted a traditional method to manipulate postnatal living environments: cross-fostering31. In this study, we primarily used two cross-fostering paradigms for C57 mice: C57 maternal cross-fostering and KM maternal cross-fostering. In C57 maternal cross-fostering, KM pups up to 3 days old were introduced into the cage of age-matched C57 pups and were raised by C57 dams (mothers were C57 mice, C57 pups and KM pups were mixed-reared; C57 pups under C57 maternal cross-fostering conditions are denoted as C57BD:C57) (Fig. 2A). In KM maternal cross-fostering, C57 pups up to 3 days old were introduced into the cage of age-matched KM pups and were raised by KM dams (mothers were KM mice, and C57 pups and KM pups were mixed-reared; C57 pups under KM maternal cross-fostering conditions are denoted as C57LD:KM) (Fig. 2B). These cross-fostering paradigms aimed to investigate whether early exposure to outgroup members (KM mice) during a critical developmental period could modify the social recognition patterns of C57 mice towards KM mice.
A, B Schematic of cross-fostering. Within three days after birth, C57 pups were given to a KM mother for mixed rearing, or KM pups were given to a C57 mother for mixed rearing. C57BD:C57: The mother is a C57 mouse, and the offspring of C57 and KM mice are reared together. BD: Birth Dam. C57LD:KM: The mother is a KM mouse, and the offspring of C57 and KM mice are reared together. LD: Lactating Dam. C The five-trial social memory experimental paradigm for cross-fostered offspring mice, where the same C57 or KM mouse was used for the first four trials, and a novel mouse with the same strain was introduced in the fifth trial. D Statistical analysis of interaction times between C57BD:C57 mice and same or other strain mice in a five-trial experiment. C57BD:C57 mice can form individualized memory for the same strain (n = 8) and group memory for the different strain (n = 6). E Statistical analysis of interaction times between C57LD:KM mice and same or other strain mice in a five-trial experiment. C57LD:KM mice form group memory for the same strain (n = 8) and also form group recognition memory for the different strain (n = 8). Data displayed as mean±S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001. T1: Trial 1, T4: Trial 4, T5: Trial 5.
The cross-fostering lasted until weaning and SRM pattern was then evaluated in adulthood (8 weeks of age) (Fig. 2C). The results revealed that cross-fostering did not affect the individualized recognition memory of C57BD:C57 mice for C57 mice (t1 vs t4: P = 0.045, t4 vs t5: P = 0.0135, n = 8), and they remained unable to form individualized memory for KM mice, exhibiting only categorized memory (t1 vs t4: P = 0.001, t4 vs t5: P = 0.5132, n = 6) (Fig. 2D). These findings suggested that simply introducing KM pups into the early rearing environment of C57 mice is insufficient to induce individualized memory for other-strain mice in C57 mice. Interestingly, C57LD:KM mice, which were reared by KM mothers, exhibited a striking shift in their SRM patterns for C57 mice, transitioning from the original individualized memory to categorized memory (t1 vs t4: P = 0.0308, t4 vs t5: P = 0.2650, n = 8). Notably, they maintained categorized memory for other-strain KM mice, failing to form individualized memory (t1 vs t4: P = 0.0003, t4 vs t5: P = 0.5168, n = 8) (Fig. 2E). Given that C57LD:KM mice were unable to form individualized memory for non-littermate C57 mice, we investigated whether they could form individualized memories for their littermate C57 mice (Fig. 3A). The results showed that the interaction time remained unchanged between the fourth and fifth trial (t1 vs t4: P = 0.002, t4 vs t5: P = 0.3172, n = 6) (Fig. 3B), indicating that C57LD:KM mice could not form individualized memory even for their same-strain littermates. In summary, cross-fostering could alter the SRM of C57 mice, although C57BD:C57 retained their differential social recognition patterns for C57 and KM mice, C57LD:KM mice lost their individualized memory for C57 mice, shifting to categorized memory.
A Schematic of the behavioral paradigm for a five-trial experiment to test the SRM of C57LD:KM mice for same-strain mice within the same litter, where the same C57 mouse from the same litter was used for the first four trials, and a different C57 mouse from the same litter was introduced in the fifth trial. B Statistical analysis of interaction times for the five-trial experiment in Figure A. The SRM pattern of C57LD:KM mice for same strain mice within the same litter is group recognition memory (n = 6). C Schematic of the behavioral paradigm for a five-trial experiment to test the SRM of C57LD:KM mice in distinguishing between same-litter and different-litter same-strain mice, where the same C57 mouse from the same litter was used for the first four trials, and a C57 mouse from a different litter was introduced in the fifth trial. D Statistical analysis of interaction times for the five-trial experiment in Figure C. C57LD:KM mice can distinguish between same-litter and different-litter same strain mice (n = 6). E Schematic of the behavioral paradigm for a five-trial experiment to test the social memory of cross-fostered mice for same-litter and different-litter heterospecific KM mice, where the same KM mouse from the same litter was used for the first four trials, and a KM mouse from a different litter was introduced in the fifth trial. F Statistical analysis of interaction times for the five-trial experiment in E. Both C57LD:KM mice and C57BD:C57 mice can distinguish between same-litter and different-litter KM mice (n = 6). Data displayed as mean±S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001. T1: Trial 1, T4: Trial 4, T5: Trial 5.
Cross-fostered C57 mice can discriminate between littermates and non-littermates KM mice
In the aforementioned experiment, all stimulus mice were either all non-littermates or all littermates. Therefore, we next tested whether C57 mice could discriminate between littermates and non-littermates after cross-fostering. Since C57BD:C57 mice retained normal individualized recognition memory for same-strain mice but C57LD:KM mice lost this ability, we first tested whether C57LD:KM mice could discriminate between littermates and non-littermates of the same-strain. We used littermate C57 mice as stimulus mice for the first four trials and switched to non-littermate C57 mice for the fifth trial (Fig. 3C). The results showed that C57LD:KM mice could discriminate between C57 mice from littermates and non-littermates (t1 vs t4: P = 0.0038, t4 vs t5: P = 0.0328, n = 6) (Fig. 3D). Next, we determined whether cross-fostered C57 mice could discriminate between littermate and non-littermate KM mice. We replaced the stimulus mice with littermate KM mice for the first four trials and switched to non-littermate KM mice for the fifth trial (Fig. 3C). The results showed that both C57BD:C57 and C57LD:KM mice could discriminate between littermate and non-littermate KM mice (C57BD:C57: t1 vs t4: P = 0.0002, t4 vs t5: P = 0.0265, n = 6; C57LD:KM: t1 vs t4: P = 0.0492, t4 vs t5: P = 0.0127, n = 6) (Fig. 3E, F).These results also suggested that after cross-fostering, C57 mice divide KM mice into two social groups based on whether they are littermates, indicating that there is plasticity in the social group division of mice when performing SRM.
Periaqueductal gray may be involved in the construction of SRM between mouse strains
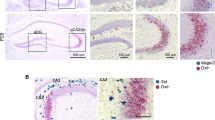
Accurate identification of members outside the group in a complex social environment and adjusting one’s own behavior based on social memory is crucial for individual survival and development32,33. The vlPAG (ventrolateral periaqueductal gray) is involved in the integration and execution of active and passive defense behaviors in mammals34,35, and it also plays an important role in the construction of working memory36. Therefore, we hypothesize that the vlPAG may also be involved in the construction of SRM between mouse strains. Therefore, we tested whether there was a difference in vlPAG brain region activity when mice interacted with same-strain and other-strain mice. Within 90 min following completion of Trial 1 (after a 10-min interaction session with either OS or SS stimulus mice), mice were sacrificed for immunofluorescence staining of immediate expression c-Fos protein in the vlPAG of mice (Fig. 4A), and the number of c-Fos-positive cells was counted to characterize the level of vlPAG brain region activity during interaction (Fig. 4B-D). The results showed that the expression of c-Fos in the vlPAG of C57 mice in control group (without social interaction) was very low, while it increased after interacting with stimulate mice (Ctl vs. SS: P < 0.0001; Ctl vs. OS: P = 0.0113; Ctl:n = 4, SS and OS: n = 6). Meanwhile, the result showed that the c-Fos expression was significantly higher when C57 mice interacted with same-strain C57 mice than when they interacted with other strain KM mice (SS vs. OS: P = 0.0331) (Fig. 4D). This result suggested that the vlPAG may be involved in the construction of differentiated SRM between mouse strains.
A Experimental timeline for c-Fos immunohistochemistry: Subject mice underwent 10-min social interaction followed by a 1.5-hour post-interaction interval before brain extraction. B Basal c-Fos expression in the vlPAG of untreated control mice (Ctl). Representative micrograph showing constitutive neuronal activation patterns (scale bar = 200 μm; inset: 20 μm). C Schematic of c-Fos expression in the vlPAG after mice interact with mice of same-strain mice. D Schematic of c-Fos expression in the vlPAG after mice interact with mice of other-strain mice. E Statistical graph of the number of c-Fos positive neurons in the vlPAG after mice interact with same or other strain mice. The number of c-Fos positive neurons in the vlPAG of C57 mice after interacting with same stain mice is significantly higher than after interacting with other-strain mice (Ctl: n = 4, SS/OS: n = 6). Data displayed as mean±S.E.M. *P < 0.05, ***P < 0.001.
Strain-specific oxytocin dynamics in C57BL/6 mice vlPAG during social interaction
Previous studies in both humans and rodents have established the critical role of oxytocin in social memory formation37, and given that the PAG receives direct oxytocinergic projections25, we investigated whether PAG oxytocin participates in SRM processing. To examine potential strain-dependent differences in vlPAG OXT dynamics during social interaction, we employed an oxytocin-sensitive fiber photometry approach. Specifically, we injected an OXT-specific sensor (rAAV-hSyn-OT1.0) into the vlPAG 38,39, enabling real-time monitoring of oxytocin fluctuations via in vivo fiber photometry. We recorded and analyzed OXT levels during sniffing episodes with SS and OS stimulus mice (Fig. 5A). Photometry signals were z-score normalized, with sniffing onset aligned to time zero (t = 0). The analysis window spanned from −2 s to +3 s relative to sniffing initiation (Fig. 5B, C). The results showed opposing OXT response patterns: SS interactions triggered significant OXT increases while OS interactions caused decreases, with response divergence beginning at −0.2 s and lasting until +1.5 s (Fig. 5D, E). Quantitative analysis of area under the curve (AUC) revealed: significantly elevated OXT during SS interactions versus baseline (P < 0.0001, n = 7; Fig. 5D), significantly suppressed OXT during OS interactions (P < 0.0001, n = 7; Fig. 5E), and significantly higher OXT responses in SS versus OS encounters (P < 0.0001, n = 7; Fig. 5F). These results demonstrate that 1) vlPAG oxytocin levels differ substantially between SS and OS interactions, 2) the opposing response patterns (activation versus suppression) may encode strain-specific SRM, and 3) PAG oxytocin likely contributes to differential social memory formation across mouse strains.
A Fiber photometry recording paradigm: Subject mice interacted with stimulus mice for 10 min, with simultaneous recording of social behaviors. Sniffing episodes were behaviorally annotated (left). Magnified view of OXT microinjection site verification in the vlPAG (right; scale bar = 100 μm). B Z-score-normalized calcium signal traces aligned to sniffing onset (−2 to −3 s), visualized as a heatmap. C Average z-scored calcium signal trajectories across all trials. D, E Signal analysis comparing baseline (−1.5 to −0.2 s before-sniffing) and response periods (−0.2 to 1.5 s after-sniffing). Significant differences were observed between same-strain (SS: n = 124 sniffing episodes) and other-strain (OS: n = 127) interactions (n = 7 mice; ****P < 0.0001). F Statistical quantification of response window (−0.2–1.5 s) signal amplitudes for SS and OS groups (****P < 0.0001). Data displayed as mean±S.E.M.
Oxytocin receptors in the PAG are involved in the retrieval of SRM in mice
Previous studies have shown that maternal care is crucial for oxytocin secretion in offspring, and oxytocin is involved in the construction of social memory in mice40,41, and there are a large number of oxytocin receptors in the vlPAG42. Therefore, we chose to focus on whether oxytocin receptors in the PAG are involved in regulating the SRM in mice.
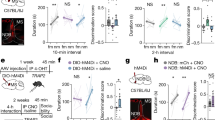
We first tested whether inhibiting OXT receptors would affect the SRM of mice. We microinjected oxytocin receptor antagonists Atosiban or L-371,257 into the vlPAG of mice 30 min before the five-trial experiment (Fig. 6A). The results showed that whether 50 μM Atosiban or 20 μM L-371,257 was injected, C57 mice showed no difference in interaction time with familiar and unfamiliar mice in the first, fourth, and fifth trials of the five-trial experiment (Atosiban, SS: t1 vs t4: P = 0.1097, t4 vs t5: P = 0.6401, n = 8. Atosiban, OS: t1 vs t4: P = 0.6248, t4 vs t5: P = 0.9498, n = 7. L -371,257, SS:t1 vs t4: P = 0.0813, t4 vs t5: P = 0.9069, n = 7. L -371,257, OS: t1 vs t4: P = 0.5685, t4 vs t5: P = 0.3248, n = 8. (Fig. 6B, C). The above results indicated that when the oxytocin receptors in the PAG brain region were inhibited, C57 mice cannot form categorized memory or individualized memory for either same-strain or other-strain mice, which meant that the oxytocin receptors in the vlPAG were critical for mice to form SRM.
A Administration paradigm of oxytocin receptor inhibitors, with a microinjection of the drug into the vlPAG 30 min before the five-trial experiment. B Statistical analysis of interaction times between C57 mice with microinjections of Atosiban or L-371,257 in the PAG and same strain stimulus mice. C57 mice administered Atosiban (n = 8) or L-371,257 (n = 7) showed disrupted SRM for same strain mice. Administration of saline (n = 6) did not affect the SRM of C57 mice. C C57 mice administered Atosiban (n = 7) or L-371,257 (n = 8) showed disrupted SRM for other strain mice. Administration of saline (n = 6) did not affect the SRM of C57 mice. D Administration paradigm of oxytocin receptor inhibitors, with a microinjection of the drug into the vlPAG 30 min before the stimulus mouse was changed in the fifth trial of the five-trial experiment. E C57 mice administered Atosiban (n = 6) or L-371,257 (n = 6) before the fifth trial showed disrupted individualized SRM for same strain mice. Administration of saline (n = 6) did not affect the SRM of C57 mice. Data displayed as mean±S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001. T1: Trial 1, T4: Trial 4, T5: Trial 5.
It is currently believed that the construction of memory is mainly divided into three stages: memory formation, memory consolidation, and memory retrieval43. Since the metabolic cycles of the oxytocin receptor antagonists Atosiban and L-371,257 in mice are relatively long44,45, injecting either drug 30 min before the five-trial experiment will have an effect on all three stages of social memory construction. Therefore, we further tested whether oxytocin is necessary for the memory retrieval stage. We changed the administration time to the memory retrieval stage (after the fourth trial and before the fifth trial of the five-trial experiment) (Fig. 6D). The results showed that whether Atosiban or L-371,257 was injected, the mice could not form individualized or categorized memory for same-strain mice, while injection of saline has no influence on SRM of C57 mice (Atosiban, SS: t1 vs.t4: P = 0.0162, t4 vs.t5: P = 0.5478, n = 6; L-371,257, SS: 1 vs.t4: P = 0.0006, t4 vs.t5: P = 0.4202, n = 6; Saline, SS: t1 vs.t4: P < 0.0001, t4 vs.t5: P = 0.0125, n = 6) (Fig. 6E). These results demonstrate that neither atosiban nor L-371,257 impaired the recall of familiar conspecifics in C57BL/6 mice, as evidenced by the absence of increased interaction time with the Trial 5 stimulus mouse (which would have been expected if the mouse was perceived as novel, similar to Trial 1). Instead, both oxytocin receptor antagonists appear to selectively disrupt the formation of individualized SRM for same-strain mice. Consequently, while treated mice retained memory of familiar individuals, they failed to discriminate the Trial 5 stimulus mouse as a novel conspecific.
Oxytocin concentration in the PAG region determines the pattern of SRM in mice
The above results showed that inhibiting oxytocin receptors in the vlPAG interfered with the formation of SRM, indicating that oxytocin in the vlPAG is very important for the formation of SRM. Therefore, we further tested whether the differential SRM of mice for same strain and other strain mice is related to the concentration of oxytocin in the vlPAG. Previous studies have suggested that the effect of oxytocin on social memory is influenced by its local concentration, that is, the effects of high or low concentrations of oxytocin on the social memory of mice are different46,47. Therefore, we tested the effects of three different concentrations of oxytocin (100 μM, 1 μM, and 0.01 μM) on the strain-specific SRM of mice. The results showed that administering 100 μM and 1 μM concentrations of oxytocin to the vlPAG region of C57 mice before the five-trial experiment disrupted the individual memory of mice for same-strain mice, causing their SRM pattern to switch to categorized memory (100 μM OXT, SS: t1 vs.t4: P = 0.0063, t4 vs.t5: P = 0.3352, n = 6; 1 μM OXT, SS: t1 vs.t4: P = 0.0024, t4 vs.t5: P = 0.5649, n = 8); while 0.01 μM of oxytocin did not affect the individualized memory of C57 mice (0.01 μM OXT, SS: t1 vs.t4: P = 0.0039, t4 vs.t5: P = 0.0498, n = 6; Saline, SS: t1 vs.t4: P = 0.0036, t4 vs.t5: P = 0.0415, n = 6) (Fig. 7A, B). For mice of a different strain, similar to those of the same-strain, injection of three concentrations of oxytocin into the vlPAG did not affect the categorized memory of C57 mice for KM mice. At the same time, three concentrations of oxytocin did not enable C57 mice to form individualized memory for KM mice (100 μM OXT, OS: t1 vs.t4: P = 0.0382, t4 vs.t5: P = 0.9975, n = 6; 1 μM OXT, OS: t1 vs.t4: P = 0.0001, t4 vs.t5: P = 0.7409, n = 7; 0.01 μM OXT, OS: t1 vs.t4: P = 0.0119, t4 vs.t5: P = 0.8532, n = 8; Saline, OS: t1 vs.t4: P = 0.0005, t4 vs.t5: P = 0.3081, n = 6) (Fig. 7C). Previous studies have shown that the timing of oxytocin administration may influence its effectiveness48. Therefore, we varied the timing of oxytocin administration to the retrieval stage of social memory, that is, between the fourth and fifth trials of the five-trial experiment (Fig. 7D). The results showed that only 0.01 μM oxytocin, but not 1 μM or 100 μM oxytocin, successfully induced individualized SRM of C57 mice for KM mice (0.01 μM OXT: t4vs t5: P = 0.0017, n = 6; 100 μM OXT: t4 vs t5: P = 0.3571, n = 6; 1 μM OXT: t4 vs t5: P = 0.0736, n = 6) (Fig. 7E). In summary, these results proved that the OXT level in vlPAG influence the SRM. Especially, a slight increase in OXT levels in the vlPAG during the memory retrieval stage might be crucial for the formation of individualized memory.
A Schematic of the behavioral paradigm for a five-trial experiment with oxytocin administration. Different concentrations of oxytocin are microinjected into the vlPAG of mice 30 min before the experiment. B Statistical analysis of interaction times between mice injected with different concentrations of oxytocin in the vlPAG and same strain mice. C57 mice injected with 100 μM (n = 6) and 1 μM (n = 8) oxytocin showed a shift from individualized memory to categorized memory for same strainmice, while 0.01 μM oxytocin and saline did not affect the SRM of C57 mice (n = 6). C Statistical analysis of interaction times between mice injected with different concentrations of oxytocin in the vlPAG and other strain mice. All three concentrations of oxytocin did not affect the categorized SRM of C57 mice for other strain mice (n ≥ 6). D Schematic of the behavioral paradigm for a five-trial experiment. Mice are administered the drug in the vlPAG after completing the fourth interaction, and a novel stimulus mouse is introduced 30 min after administration. E Statistical analysis of interaction times between C57 mice and KM mice in the five-trial experiment. After microinjection of 0.01 μM oxytocin into the PAG, C57 mice developed individualized recognition memory for KM mice, while 100 μM and 1 μM oxytocin or saline did not change the SRM of C57 mice for KM mice (n = 6). Data displayed as mean±S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001. T1: Trial 1, T4: Trial 4, T5: Trial 5.
Oxytocin’s effect on SRM in mice is not due to its influence on social novelty
Since the five-trial experiment is based on social novelty, therefore if the social novelty of mice is affected, it will also cause changes in the results of the five-trial experiment. Therefore, we further tested the effect of microinjection of oxytocin or oxytocin receptor antagonists in the vlPAG on the social ability and social novelty of mice (Fig. 7A). In the behavioral experiment to detect social ability, when facing social stimuli (a stimulus mouse in a wire cage) and non-social stimuli (an empty wire cage), the interaction time of mice with microinjection of oxytocin and oxytocin receptor antagonists in the vlPAG was significantly higher with the stimulus mouse than with the empty cage, indicating that the social ability of the mice was not affected (100 μM OXT: P < 0.001, n = 6; 1 μM OXT: P < 0.001, n = 6; 0.01 μM OXT: P < 0.001, n = 6; Atosiban; P = 0.0033, n = 6; L-371,257: P = 0.0216, n = 6;) (Fig. 8B). In the behavioral experiment to detect social novelty, the interaction time of mice with three concentrations of oxytocin microinjected in the PAG with unfamiliar mice was significantly higher than that with familiar mice (100 μM OXT:P = 0.004, n = 6; 1 μM OXT: P < 0.0001, n = 6; 0.01 μM OXT: P = 0.002, n = 6), indicating that oxytocin does not affect the social novelty of mice. However, mice injected with oxytocin receptor antagonists in the PAG showed a lack of social novelty, as evidenced by no significant difference in their interaction time with unfamiliar or familiar mice (Atosiban: P = 0.1120, n = 6; L-371257: P = 0.6954, n = 6) (Fig. 8C).
A Schematic of the behavioral paradigm for the three-chamber social test to assess sociability and social novelty in mice. B Statistical analysis of interaction times between the test mouse and an empty cage (Empty cage, E) and a novel mouse (Novel mouse, N) (n = 6). Oxytocin and oxytocin receptor antagonists did not affect the social ability of mice. C Statistical analysis of interaction times between the test mouse and a novel mouse (Novel mouse, N) or a familiar mouse (Familiar mouse) (n = 6). Social novelty was disrupted in mice when oxytocin receptors were inhibited, while oxytocin did not affect social novelty in mice (n = 6). Data displayed as mean±S.E.M. *P < 0.05, ***P < 0.001, ****P < 0.0001.
In summary, microinjection of oxytocin in the vlPAG does not alter the social ability or social novelty of mice, indicating that low-dose OXT given in the memory retrieval stage can indeed promote the formation of individualized memory of KM mice in C57 mice. At the same time, the above results also indicated that the oxytocin receptor in the vlPAG played a key role in social novelty. Therefore, for the phenomenon of social memory loss in C57 mice after inhibition of OXT receptors, it cannot be ruled out that the social memory deficits caused by oxytocin inhibitors are due to their effect on the social novelty of mice.
Discussion
In this study, we found that C57 mice show different SRM patterns for same-strain mice (C57 mice) and other-strain mice (KM mice), they form individualized SRM for same-strain mice but only categorized memory for other-strain mice. In addition, we found this SRM could be affected by postnatal rearing environment. And furthermore, the oxytocin level in the PAG brain region determines the memory pattern of mice in the process of forming SRM, that is, the formation of individualized social memory is based on the OXT level in the PAG brain region during the memory retrieval stage (Fig. 9).
C57BL/6 mice exhibit distinct social recognition memory (SRM) patterns depending on conspecific strain identity. They develop individualized SRM for genetically identical conspecifics while forming categorical SRM representations for mice of different strains. Pharmacological intervention using specific receptor antagonists into the ventrolateral periaqueductal gray (vlPAG) can modulate these memory patterns, effectively transforming individualized SRM for same-strain conspecifics into categorical memory. Furthermore, neurochemical manipulation through microinjection of 0.01 μM oxytocin into the vlPAG during memory retrieval phases demonstrates the capacity to convert categorical SRM for heterospecific mice into individualized memory formations (Created in BioRender.com).
SRM refers to the ability of animals to recognize and remember familiar conspecifics. SRM is the basis of a variety of social behaviors, and gregarious animals must adjust their behavior in a timely manner in response to SRM to adapt to complex social environments18,49. SRM helps people remember others’ information, behavioral characteristics and social roles, so that they can behave more appropriately in social interactions and promote cooperation and harmony. Good interpersonal relationships and social cooperation are the keys to success, and SRM is the foundation of this ability. It enables individuals to better understand others, predict their behavior, thereby reducing misunderstandings and conflicts, improving communication effects, and promoting teamwork and the achievement of common goals. Therefore, SRM plays an indispensable role in the smooth progress of social interaction and cooperation.
Previous studies have found that people differentiate social recognition for others in different social groups, and therefore have a stronger implicit preference and stronger tendency to cooperate with members within the group, showing stronger altruism towards them50,51. The neural mechanism of this phenomenon has been gradually revealed. However, research on humans is limited by its technical limitations, and it is currently not possible to study the neural mechanism and plasticity of this phenomenon at the subcortical region and neurotransmitter level. In this project, we used mice as model animals to establish a research paradigm for studying the neural mechanism of differentiated SRM across social groups. The results showed that C57 mice can form individualized recognition memory for C57 mice, that is, they can distinguish individual C57 mice. This individualized recognition memory exists stably whether the mice are littermates or not, which is consistent with previous reports in the literature52,53. However, C57 mice do not exhibit individualized recognition, but only categorized recognition memory for KM mice. This phenomenon is similar to the “face blindness” phenomenon in humans54, which means that C57 mice will recognize different KM mice as the same and remember them as a group but cannot distinguish between different KM mice. This is similar to the pattern of categorized memory observed in humans. In addition, through specific experimental training, such as actively interacting with members of external groups, people’s facial recognition of members of external groups can be enhanced50,55. In mice, we also found that their SRM patterns can be affected by the postnatal rearing environment. Mixing C57 and KM mice for rearing within 4 weeks after birth can help C57 mice recognize KM mice socially. C57 mice that grow up in such a growth environment can distinguish between littermates and non-littermates of KM mice. However, they still cannot form individualized recognition memory for even KM littermates. But at least after cross-fostering, C57 divides KM mice into two social groups, littermates and non-littermates. That is to say, SRM can undergo plastic changes due to alteration of individual’s postnatal growth environment and social interaction experience. The above results provided a behavioral basis for future exploration of inter-group differentiated SRM using mice as model animals.
Moreover, in this study we found that the oxytocin level in vlPAG plays a key role in determining the pattern of SRM. Oxytocin is a classic neuropeptide, named for its ability to cause uterine contractions. In recent years, its role in social interaction has gradually attracted attention and has become a research focus. When the oxytocin receptor in the hippocampus of mice is specifically knocked out, the formation of social memory in mice will be hindered56. In whole-cell recordings of pyramidal cells in the CA2 subregion of the hippocampus, activation of the oxytocin receptor can adjust the potassium ion current of the M channel by activating the PLC signaling pathway. This leads to rapid depolarization of the pyramidal cells57. When the oxytocin receptor is repeatedly activated, it modulates synaptic plasticity in neurons58,59, thereby participating in the construction of social memory. However, Regarding the role of oxytocin in the PAG, previous studies have primarily focused on its analgesic functions60, whether oxytocin in vlPAG also participates in the regulation of social memory is currently unknown.
In this study, we observed significant differences in oxytocin levels within the vlPAG of C57BL/6 mice following same-strain versus other-strain social interactions, with same-strain interactions eliciting markedly higher oxytocin release. However, our findings demonstrate that oxytocin’s role in the vlPAG is complex and context-dependent. First, the establishment of individualized SRM in the PAG appears to require precisely timed and dosed oxytocin release. Specifically, the diminished oxytocin levels during C57-KM interactions were insufficient to support SRM formation. Notably, supra-physiological oxytocin administration (0.01 μM) during the memory retrieval phase successfully established individualized SRM for other-strain (KM) mice in C57 subjects, while higher concentrations not only failed to induce cross-strain SRM but also disrupted existing same-strain SRM. Furthermore, our results indicate that oxytocin signaling is essential for baseline sociability in C57 mice. Pharmacological blockade of oxytocin receptors using L-371,257 or atosiban abolished both SRM formation and social novelty preference—a prerequisite for the five-trial paradigm’s validity. This raises an important caveat: the observed SRM deficits following oxytocin antagonism may represent secondary consequences of impaired social novelty detection rather than direct effects on memory consolidation.
PAG is mainly known for its downstream control of defensive behavior. However, there is growing evidence that it is also involved in feeding behavior and social interaction61,62,63,64. Regarding its function in sensory-related emotional processing, PAG is one of the main targets of the brainstem and regulates the ascending anterolateral pathway of somatic pain sensation65. In addition, it is also involved in regulating the emotional aspects of pain and social processes with a strong emotional touch component, such as mother-infant bonding63,66. Overall, current evidence supports PAG as an important hub for ascending and descending pathways. In other words, the periaqueductal gray (PAG) may function as an integrative hub, receiving top-down inputs primarily from higher-order emotional regulatory regions (e.g., prefrontal cortex, amygdala, and hypothalamus) and exerting bottom-up control via projections to medullary and pontine motor-related nuclei (such as the locus coeruleus). This circuitry enables multidimensional integration of affective, autonomic, and motor systems62,67. Therefore, the research results of this study suggest that the role of oxytocin in the PAG brain region in the regulation of social behavior should be given more attention in the future. In particular, consistent with previous studies, we also found that there is a threshold effect in vlPAG. That is, when a specific stimulus exceeds the threshold level, it will trigger the corresponding mouse behavior. Previous studies have shown that there is a monosynaptic excitatory connection from medial superior colliculus (mSC) to dorsal periaqueductal gray(dPAG) neurons. This connection is necessary for escape behavior and provides a synaptic threshold for the activation of dPAG. Due to the high level of mSC network activity, the synaptic threshold can be overcome by short-term synaptic facilitation and repetitive excitation within mSC, which will enhance and maintain the synaptic drive to dPAG, thereby calculating the decision and intensity of the animal’s escape. Our study indicates that the oxytocin level within the vlPAG appears to be subject to a temporally conditioned threshold for the generation of individual recognition memory. It is imperative that a precise dose of oxytocin be administered to the vlPAG region at a critical juncture, specifically during the memory retrieval phase, to facilitate the formation of such memory. Subsequent inquiries must address the underlying mechanisms that govern the release of oxytocin from the hypothalamus to the vlPAG during social interactions between mice of both inbred and outbred strains. Furthermore, it is necessary to elucidate the nature of the signals projected by the vlPAG to its downstream neural circuits following the reception of suprathreshold levels of oxytocin, which ultimately culminate in the establishment of individual recognition memory in mice. These questions represent pivotal challenges for future research.
Materials and methods
Animals
The experimental animals used in this experiment were C57B.L/6 J mice and KM mice (Kunming mice, an outbred stock experimental mice derived from Swiss mice). C57B.L/6 J female mice and KM female mice were used to provide the rearing environment, and 8-week-old C57B.L/6 J male mice and KM male mice were used for behavioral experiments. The animal experiments and animal handling procedures were approved by the Animal Ethics Committee of Kunming Medical University (Ethical Approval number: KMMU2020006), which was provided by the Experimental Animal Center of Kunming Medical University (experimental animal licence number: SCXK (Dian) K-2020-0004). We have complied with all relevant ethical regulations for animal use. The living environment of the mice was as follows: temperature 22–25 °C; humidity 40–70%; light intensity 12 h (7:00-19:00, automatically controlled); noise intensity in the rearing room <60 dB; food and water were provided in sufficient quantities. The care and handling of mice complied with the Guiding Principles for the Use of Animals in Experimental Research (1985) formulated by the Council for the International Organization of Medical Sciences, with the aim of reducing the number of experimental animals and reducing the pain of experimental animals.
Cross-fostering
In this experiment, the experimental mice were divided into two primary groups: the normal environment rearing group and the cross-fostering group. The normal environment rearing group served as the control group. The cross-fostering group was further subdivided based on the maternal lineage of the mice: C57 maternal line cross-fostering and KM maternal line cross-fostering. Cross-fostering was conducted within 24–72 h postpartum using age-matched litters of KM and C57BL/6 J mice, with a birth interval of ≤72 h between litters. After ensuring age-matched offspring, we replaced half of the total number of C57 pups with an equal number of KM pups. To facilitate the cross-fostering, we took bedding with the scent of C57 female mice from their cage and wrapped the same number of KM neonatal mice, within 24 h of birth, in this bedding. These KM neonates were then placed in an incubator set to 35 °C for 30 min. After 30 min, each KM neonate, carrying the scent of the C57 female mouse, was introduced into the C57 female mouse’s breeding cage one at a time (each neonate was placed away from the C57 female’s nest, and we waited until the C57 female carried the KM neonate back to her nest before introducing the next one). Once all KM neonates had been retrieved by the C57 female and returned to her nest, the breeding cages were returned to their original breeding room. The behavior of the female mice was observed every 30 min. If the C57 female mouse remained calm, this indicated a successful cross-fostering. The procedure for the KM maternal line cross-fostering was identical.
Five trial social memory test
The five-trial experiment is a classic behavioral paradigm used to test social memory in mice. Prior to the experiment, the mice require environmental acclimatization, which involves bringing them from the breeding room to the behavioral observation room once a day for three consecutive days. Considering the mice’s circadian rhythm, each adaptation session is scheduled around 12 o’clock and lasts for one hour. After adaptation, the mice are returned to the breeding room. The apparatus used in the five-trial experiment is an acrylic rectangular box measuring 60 cm in length, 40 cm in width, and 22 cm in height. The box is divided into three chambers by two transparent acrylic plates with a partition in the middle. Each side chamber contains a cylindrical metal fence restraint cage, approximately 15 cm high and 9 cm in diameter. This experiment primarily utilizes the middle and left chambers. After the experiment commences, the mice are placed in the middle chamber, the left partition is opened, and the test mice are allowed to explore freely for 5 min. Following exploration, the mice are gently driven back to the middle chamber and the partition is closed. SS1/OS1 (Stranger 1) is then placed into the metal restraint cage. After positioning, the left partition is opened, and the mice are allowed to interact freely for 5 min. The mice are then gently driven back to the middle chamber, the partition is closed, and the stimulus mouse is removed from the restraint cage. Next, the left partition is opened, and the mice are allowed to freely explore the apparatus for 10 min. The above experiment is then repeated three times. After removing Stranger 1, the stimulus mouse, for the fourth trial, the test mouse is removed, and the apparatus is cleaned with 75% alcohol to eliminate any residual scent of the test mouse. After cleaning, the subject mouse is returned to the middle chamber, the left partition is opened, and the subject mouse is allowed to explore freely for 10 min. After exploration, the subject mouse is gently driven back to the middle chamber, and the left partition is closed. Another stimulus mouse, SS2/OS2 (Stranger 2), is placed in the left metal cage. The left partition is opened, and the test mouse is allowed to interact freely for 5 min. After the interaction, the mouse is removed, and the apparatus is cleaned with 75% alcohol. For the social memory experiment involving other strain (OS) mice, the C57 stimulus mice are replaced with KM mice, while other steps remain consistent with the aforementioned procedure. After the experiment, all mice are returned to their original cages and transferred back to their original breeding rooms2. 7-trial SRM protocol (Fig. 1C): This protocol extends the 5-trial paradigm, with trials 1–6 involving repeated interactions with the same stimulus mouse (Stranger 1), while trial 7 introduces a novel stimulus mouse (Stranger 2). Each 5-min interaction session is separated by 10-min inter-trial intervals. 5-trial stranger alternation assay (Fig. 1E): Each trial features the sequential introduction of novel stimulus mice (Stranger 1–5) to assess dynamic social memory. All interactions are conducted under identical parameters (5-min duration, 10-min inter-trial intervals).
Three-chamber social interaction test
The three-chamber social interaction test was used to test the social ability and social novelty of mice. The device used in the social experiment is an acrylic rectangular box with a length of 60 cm, a width of 40 cm, and a height of 22 cm. The box is divided into three chambers by two transparent acrylic plates with partitions in the middle. There is a cylindrical metal fence restraint cage about 15 cm high and 9 cm in diameter in each side chamber. The test mice were adapted for three days before the test. The adaptation time was around 12 o’clock and lasted for 1 hour. Social experiments are mainly divided into three stages. In the first stage, the subject mouse was placed in the middle room of the device, the partitions on both sides of the device were opened, and the mice were allowed to explore freely in the device for 10 min. After the exploration was completed, the mice were gently driven back to the middle room and the partitions on both sides were closed. board; in the second stage, in order to eliminate the impact of the order of placement on the social interaction of mice, randomly select one side of the chamber to put the stimulus mouse Stranger A. After placing it, open the partitions on both sides and allow the mice to interact freely for 5 min. The interaction is completed. Finally, the mice were gently driven back to the middle room and the partition was closed; in the third stage, the stimulation mouse Stranger B was placed in the restraint cage on the other side, and then the partitions on both sides were opened to allow the mice to interact freely for 5 min. After the experiment, the mice were taken out, the device was cleaned with 75% alcohol, the mice were returned to the original cage and returned to the original breeding room56.
Cannula implantation surgery
Anesthetize the mouse with a 60 mg/kg dose of pentobarbital sodium administered intraperitoneally. Gently touch the mouse’s cornea with a cotton swab; the absence of a corneal reflex indicates successful anesthesia. Apply erythromycin ointment to the mouse’s eyes to prevent drying and light damage during the surgery. Use a shaver to remove the hair from the mouse’s scalp and then fix the mouse in a mouse holder. Disinfect the mouse’s scalp with 75% alcohol. After disinfection, use a scalpel to incise the scalp and expose the dura mater. Aspirate 0.1 mL of normal saline with a syringe and carefully inject it subdural. Once a small bulge forms under the dura mater, use scissors to cut open the dura mater, completely exposing the skull. If there is still dura mater adhering to the top, wipe it with 3% hydrogen peroxide solution until the skull sutures are clearly visible. Surround the incised wound with absorbent cotton to prevent bleeding. Level the mouse’s skull, and use a well-prepared glass electrode for positioning. Adjust the glass electrode to the mouse’s bregma position, zero the coordinates, and then move the glass electrode to the lambda position, adjusting the mouse’s head so that the bregma and lambda are at the same height. Then, move the glass electrode the same distance left and right from the bregma to ensure that the skull is level from side to side. Determine the coordinates of the periaqueductal gray matter (AP: −4.8 mm; ML: 0.5 mm; DV: −2.9 mm, from Bregma) using the mouse brain atlas. Mark the projection position of the periaqueductal gray matter on the skull surface with ink. Drill through the skull with a skull drill, taking care not to penetrate the skull. Drill two screw holes away from the projection position (do not penetrate the skull). Fix the screws into the screw holes, then use a holder to implant the cannula into the mouse’s brain and secure it with dental cement. After the cannula is implanted, insert the pin cap, place the mouse on a heating pad, and wait for the mouse to fully recover from anesthesia before returning it to its original cage68.
Intracranial drug administration
Anesthetize the mouse using isoflurane through a small animal anesthesia machine. First, place the mouse in the anesthesia chamber and adjust the airflow to maximum and the anesthetic concentration to 1.5-2%. Once the mouse is anesthetized, secure it to the anesthesia mask and reduce the airflow to 0.1. Expel the air from the microsyringe with liquid paraffin, and after expelling the air, draw the drug into the microsyringe. At this point, there should be a clear demarcation line between the drug and the liquid paraffin. Fix the mouse to the mouse adapter, remove the cap from the microinjection cannula, and secure the inner injection tube to the cannula using the cannula locking nut (AP: −4.8 mm; ML: 0.5 mm; DV: −2.9 mm, from Bregma). Use a microinjection pump to slowly inject the drug (drug concentrations: Atosiban: 50 μM; L-371,257: 20 μM; Oxytocin: 100 μM, 1 μM, and 0.01 μM) into the periaqueductal gray matter of the mouse (at a rate of 0.1 μL/min, for a total volume of 0.5 μL). After the injection is complete, wait for five minutes, then remove the inner injection tube after the drug has been fully infused and reinsert the cap into the microinjection cannula. Return the mouse to its original cage after the injection is complete and proceed with the next step of the experiment once the mouse has fully recovered from anesthesia48.
Fiber photometry
Fiber photometry recordings were performed in 8-week-old C57BL/6 mice. Surgical procedures, including anesthesia and cannula implantation, followed cannula implantation surgery protocols for optical fiber implantation. Oxytocin-sensor-expressing recombinant adeno-associated virus (rAAV-hSyn-OT1.0; BrainCase Biotech) was stereotaxically injected into the ventrolateral periaqueductal gray (vlPAG) (coordinates: AP − 4.8 mm, ML ± 0.5 mm, DV − 2.9 mm; 300 nL total volume). After a 10-min diffusion period, a 0.25-mm-diameter fiber-optic ferrule (Inper Inc.) was slowly implanted 0.15 mm dorsal to the injection site. Postoperative recovery and housing conditions matched standard occlusive surgery protocols, with a 2-week recovery period prior to experimentation. Signal integrity was verified using a fiber photometry system (Inper Inc.). vlPAG oxytocin (OXT) dynamics were monitored at 470 nm (oxt-dependent signal) and 410 nm (isosbestic reference), with excitation intensity maintained at 50% to minimize photobleaching. Raw photometry data were processed using Inper Plot software (v1.6.5), including motion artifact correction and Z-score normalization. Following behavioral testing, mice were perfused for histological verification of viral expression and fiber placement. Fluorescence images were acquired using a VS120 virtual slide microscope (Olympus Corp.) with matched exposure parameters across samples.
c-Fos staining
c-Fos immunohistochemistry was performed to assess differential neuronal activation in the ventrolateral periaqueductal gray (vlPAG) of C57BL/6 mice during social interactions with conspecific (C57BL/6) or heterospecific (KM) mice. A two-chamber social interaction paradigm was employed, wherein subject mice underwent a single 10-min interaction session with either a same-strain (C57BL/6) or other-strain (KM) stimulus mouse. Following interaction sessions, subjects were returned to their home cages for a 90-min post-interaction interval to allow c-Fos protein expression to peak. Brains were subsequently harvested and processed for histological analysis. The mice were anesthetized with an intraperitoneal injection of pentobarbital sodium at a dose of 60 mg/kg. The anesthesia was deemed successful when there was no corneal reflex upon gentle stimulation with a cotton swab. The brains were then perfused and extracted from the anesthetized mice. Using the frozen sectioning method, brain slices of the mice were obtained (each slice with a thickness of 50 micrometers). The embedding medium on the brain slices was washed with PBS; followed by blocking with 10% bovine serum solution for 2 h. After blocking, the primary antibody (ab208942, Abcam, 1:200 dilution) was added, and the slices were placed in a 4 °C refrigerator overnight. After incubation, the slices were washed three times for 15 min each. Following the washes, the secondary antibody (ab7066, Abcam, 1:500 dilution) was added for 2 h. After the secondary antibody incubation, the slices were washed three times again. Finally, the slices were mounted with a DAPI-containing anti-fade mounting medium (ab104139, Abcam), and the entire process required protection from light. Following image acquisition, histological sections were analyzed using FIJI software (ImageJ 2.0). The ventrolateral periaqueductal gray (vlPAG) region was delineated by manually tracing its anatomical boundaries. c-Fos-positive cells within the defined region of interest (ROI) were manually quantified by researchers blinded to experimental conditions using FIJI’s cell counter plugin. Tissue area (mm²) for each section was calculated via FIJI’s automated pixel-to-metric conversion algorithms, allowing normalization of c-Fos+ cell counts to density measurements (cells/mm²).
Statistics and reproducibility
A minimum of six biological replicates were used in each experimental group. The data were analyzed using the GraphPad Prism 8 software package. All data were subjected to normality testing and homogeneity of variance tests. All data are presented as mean ± standard error of the mean (Mean ± SEM). For multiple group comparisons and pairwise comparisons, results were statistically analyzed using one-way analysis of variance (ANOVA) and t-tests(two-tailed). A P-value less than 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All source data are provided with this paper. The source data behind the graphs in the paper can be found in Supplementary Data 1.
References
Bluthé, R. M., Gheusi, G. & Dantzer, R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology 18, 323–335 (1993).
Wu, X., Morishita, W., Beier, K. T., Heifets, B. D. & Malenka, R. C. 5-HT modulation of a medial septal circuit tunes social memory stability. Nature 599, 96–101 (2021).
Okuyama, T. Social memory engram in the hippocampus. Neurosci. Res. 129, 17–23 (2018).
Leblanc, H. & Ramirez, S. Linking Social Cognition to Learning and Memory. J. Neurosci. 40, 8782–8798 (2020).
Wang, X. & Zhan, Y. Regulation of Social Recognition Memory in the Hippocampal Circuits. Front. Neural Circuits 16, 839931 (2022).
Walum, H. & Young, L. J. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 19, 643–654 (2018).
Herzmann, G., Ogle, O. & Curran, T. More elaborate processing of own-race faces and less elaborate processing of other-race faces contribute to the other-race effect in face memory. Br. J. Psychol. 113, 1033–1055 (2022).
Jacobs, S. & Tsien, J. Z. Adult forebrain NMDA receptors gate social motivation and social memory. Neurobiol. Learn. Mem. 138, 164–172 (2017).
Meissner, C. & Brigham, J. Thirty years of investigating the own-race bias in memory for faces: A meta-analytic review. Psychol. Public Policy Law 7, 3–35 (2001).
Zhou, Y. et al. Neural dynamics of racial categorization predicts racial bias in face recognition and altruism. Nat. Hum. Behav. 4, 69–87 (2020).
Chiang, M. C., Huang, A. J. Y., Wintzer, M. E., Ohshima, T. & McHugh, T. J. A role for CA3 in social recognition memory. Behavioural Brain Res. 354, 22–30 (2018).
Fu, F. et al. Evolution of in-group favoritism. Sci. Rep. 2, 460 (2012).
Jeon, Y. A., Banquer, A. M., Navangul, A. S. & Kim, K. Social group membership and an incidental ingroup-memory advantage. Q. J. Exp. Psychol. 74, 166–178 (2021).
Tang, Y. & Stoop, R. The petting factor: Oxytocin and social touch. Neuron 110, 909–911 (2022).
Dulac, C. & Torello, A. T. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat. Rev. Neurosci. 4, 551–562 (2003).
Dulac, C. & Wagner, S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev. Genet 40, 449–467 (2006).
Min, J. Y., Park, S., Cho, J. & Huh, Y. The anterior insular cortex processes social recognition memory. Sci. Rep. 13, 10853 (2023).
Chen, S. et al. A hypothalamic novelty signal modulates hippocampal memory. Nature 586, 270–274 (2020).
Smith, A. S., Williams Avram, S. K., Cymerblit-Sabba, A., Song, J. & Young, W. S. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 21, 1137–1144 (2016).
Gur, R., Tendler, A. & Wagner, S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol. Psychiatry 76, 377–386 (2014).
Lefler, Y., Campagner, D. & Branco, T. The role of the periaqueductal gray in escape behavior. Curr. Opin. Neurobiol. 60, 115–121 (2020).
Franklin, T. B. Recent Advancements Surrounding the Role of the Periaqueductal Gray in Predators and Prey. Front. Behav. Neurosci. 13, 60 (2019).
Li, Y. et al. Hypothalamic Circuits for Predation and Evasion. Neuron 97, 911–924.e915 (2018).
Ogawa, S., Kow, L. M. & Pfaff, D. W. Effects of lordosis-relevant neuropeptides on midbrain periaqueductal gray neuronal activity in vitro. Peptides 13, 965–975 (1992).
Yu, H. et al. Social touch-like tactile stimulation activates a tachykinin 1-oxytocin pathway to promote social interactions. Neuron 110, 1051–1067.e1057 (2022).
Thirtamara Rajamani, K. et al. Oxytocin Activity in the Paraventricular and Supramammillary Nuclei of the Hypothalamus is Essential for Social Recognition Memory in Rats. Ssrn null, null (2022).
Dielenberg, R. A., Leman, S. & Carrive, P. Effect of dorsal periaqueductal gray lesions on cardiovascular and behavioral responses to cat odor exposure in rats. Behavioural Brain Res. 153, 487–496 (2004).
Peng, K. -z. et al. Neural mechanisms underlying strain preference behaviour and plasticity in mice. Sci. Rep. 15, 6566 (2025).
Yetkili, O., Abrams, D., Travaglino, G. A. & Giner-Sorolla, R. Imagined contact with atypical outgroup members that are anti-normative within their group can reduce prejudice. J. Exp. Soc. Psychol. 76, 208–219 (2018).
Borinca, I. et al. Dehumanization of outgroup members and cross-group interactions. Curr. Opin. Behav. Sci. 50, 101247 (2023).
Breed, M. D. & Moore, J. In Animal Behavior (eds Michael D. Breed & Janice Moore) 67-98 (Academic Press, 2012).
Wacker, D. W. & Ludwig, M. Vasopressin, oxytocin, and social odor recognition. Hormones Behav. 61, 259–265 (2012).
Tanimizu, T. et al. Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory. J. Neurosci. 37, 4103–4116 (2017).
Reis, F., Mobbs, D., Canteras, N. S. & Adhikari, A. Orchestration of innate and conditioned defensive actions by the periaqueductal gray. Neuropharmacology 228, 109458 (2023).
Vázquez-León, P., Miranda-Páez, A., Valencia-Flores, K. & Sánchez-Castillo, H. Defensive and Emotional Behavior Modulation by Serotonin in the Periaqueductal Gray. Cell. Mol. Neurobiol. 43, 1453–1468 (2023).
Kragel, P. A. et al. Functional Involvement of Human Periaqueductal Gray and Other Midbrain Nuclei in Cognitive Control. J. Neurosci. 39, 6180–6189 (2019).
Guastella, A. J., Mitchell, P. B. & Mathews, F. Oxytocin enhances the encoding of positive social memories in humans. Biol. Psychiatry 64, 256–258 (2008).
Qian, T. et al. A genetically encoded sensor measures temporal oxytocin release from different neuronal compartments. Nat. Biotechnol. 41, 944–957 (2023).
Li, L. et al. PVN-mPFC OT projections modulate pup-directed pup care or attacking in virgin mandarin voles. Elife 13 https://doi.org/10.7554/eLife.96543 (2024).
Scatliffe, N., Casavant, S., Vittner, D. & Cong, X. Oxytocin and early parent-infant interactions: A systematic review. Int. J. Nurs. Sci. 6, 445–453 (2019).
Zhan, S. et al. Oxytocin neurons mediate stress-induced social memory impairment. Curr. Biol. 34, 36–45.e34 (2024).
Son, S. et al. Whole-Brain Wiring Diagram of Oxytocin System in Adult Mice. J. Neurosci. 42, 5021–5033 (2022).
Meira, T. et al. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun. 9, 4163 (2018).
Sanu, O. & Lamont, R. F. Critical appraisal and clinical utility of atosiban in the management of preterm labor. Therapeutics Clin. Risk Manag. 6, 191–199 (2010).
Tunstall, B. J. et al. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. Plos Biol. 17, e2006421 (2019).
Dantzer, R., Bluthe, R. M., Koob, G. F. & Le Moal, M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology 91, 363–368 (1987).
Popik, P., Vetulani, J. & van Ree, J. M. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology 106, 71–74 (1992).
Li, X. H. et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep. 36, 109411 (2021).
Okuyama, T., Kitamura, T., Roy, D. S., Itohara, S. & Tonegawa, S. Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016).
Dasgupta, N. & Rivera, L. When Social Context Matters: The Influence of Long–Term Contact and Short–Term Exposure to Admired Outgroup Members on Implicit Attitudes and Behavioral Intentions. Soc. Cognition 26, 112–123 (2008).
Harrison, V. & Hole, G. J. Evidence for a contact-based explanation of the own-age bias in face recognition. Psychonomic Bull. Rev. 16, 264–269 (2009).
Ferguson, J. N., Young, L. J. & Insel, T. R. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 23, 200–224 (2002).
Hitti, F. L. & Siegelbaum, S. A. The hippocampal CA2 region is essential for social memory. Nature 508, 88–92 (2014).
Blandón-Gitlin, I., Pezdek, K., Saldivar, S. & Steelman, E. Oxytocin eliminates the own-race bias in face recognition memory. Brain Res. 1580, 180–187 (2014).
Page-Gould, E., Mendoza-Denton, R. & Tropp, L. R. With a little help from my cross-group friend: reducing anxiety in intergroup contexts through cross-group friendship. J. Personal. Soc. Psychol. 95, 1080–1094 (2008).
Tsai, T. C., Fang, Y. S., Hung, Y. C., Hung, L. C. & Hsu, K. S. A dorsal CA2 to ventral CA1 circuit contributes to oxytocinergic modulation of long-term social recognition memory. J. Biomed. Sci. 29, 50 (2022).
Liu, J. J., Eyring, K. W., König, G. M., Kostenis, E. & Tsien, R. W. Oxytocin-Modulated Ion Channel Ensemble Controls Depolarization, Integration and Burst Firing in CA2 Pyramidal Neurons. J. Neurosci. 42, 7707–7720 (2022).
Wang, F., Yin, X. S., Lu, J., Cen, C. & Wang, Y. Phosphorylation-dependent positive feedback on the oxytocin receptor through the kinase PKD1 contributes to long-term social memory. Sci. Signal 15, eabd0033 (2022).
Ripamonti, S. et al. Transient oxytocin signaling primes the development and function of excitatory hippocampal neurons. eLife 6, null (2017).
Liang, D. & Labrakakis, C. Multiple Posterior Insula Projections to the Brainstem Descending Pain Modulatory System. Int. J. Mol. Sci. 25 https://doi.org/10.3390/ijms25179185 (2024).
George, D. T., Ameli, R. & Koob, G. F. Periaqueductal Gray Sheds Light on Dark Areas of Psychopathology. Trends Neurosci. 42, 349–360 (2019).
Benarroch, E. E. Periaqueductal gray: An interface for behavioral control. Neurology 78, 210–217 (2012).
Motta, S. C., Carobrez, A. P. & Canteras, N. S. The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neurosci. Biobehav. Rev. 76, 39–47 (2017).
Silva, C. & McNaughton, N. Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? A comprehensive review. Prog. Neurobiol. 177, 33–72 (2019).
Basbaum, A. I., Bautista, D. M., Scherrer, G. & Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 139, 267–284 (2009).
Zelena, D., Menant, O., Andersson, F. & Chaillou, E. Periaqueductal gray and emotions: the complexity of the problem and the light at the end of the tunnel, the magnetic resonance imaging. Endocr. Regul. 52, 222–238 (2018).
Pessoa, L. Noncortical cognition: integration of information for close-proximity behavioral problem-solving. Curr. Opin. Behav. Sci. 55 https://doi.org/10.1016/j.cobeha.2023.101329 (2024).
Garrido Zinn, C. et al. Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc. Natl Acad. Sci. USA 113, E4914–E4919 (2016).
Author information
Authors and Affiliations
Contributions
Conceptualization, T.L.L., Z.X.M.; experiment performing, P.K.Z., L.J., Y.S.Y., X.Y.Y., Q.L.T., B.W.Y., T.L.L., Z.X.M.; data analysis, P.K.Z., L.J., T.L.L.; writing—original draft preparation, P.K.Z., L.J., T.L.L., Z.X.M.; writing—review and editing, Z.X.M., T.L.L.; funding acquisition, Z.X.M., T.L.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Christoph Anacker and Benjamin Bessieres. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Peng, K., Li, J., You, S. et al. Oxytocin in periaqueductal gray plasticly regulates strain-dependent social recognition memory in mice, modeling social identity. Commun Biol 8, 881 (2025). https://doi.org/10.1038/s42003-025-08307-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08307-1