Abstract
CRISPR/Cas9 genome editing technology, particularly cytosine base editing (CBE) systems, emerges as a powerful tool for precise genomic modification in plants, offering transformative applications across agricultural and forestry research and breeding programs. However, current CBE systems in poplar exhibit low efficiency and imprecise base substitutions, and optimization of base editing systems specifically for poplar remains a significant challenge. To address these limitations, we engineer a high-efficiency poplar CBE system (hyPopCBE) by integrating the MS2-UGI system, fusing Rad51 DNA-binding domain, and modifying the nuclear localization signal. Through stepwise optimization, we develop hyPopCBE-V4, which exhibits a synergistic effect in woody plants. Compared to the original hyPopCBE-V1, hyPopCBE-V4 improves C to T editing efficiency while reducing byproducts and exhibiting a narrower editing window. The proportion of plants with clean C to T edits (without byproducts) increases from 20.93% to 40.48%, and the efficiency of clean homozygous C to T editing rises from 4.65% to 21.43%. Using hyPopCBE-V1 and its variants, we induce Pro197Leu mutation in the herbicide target gene PagALS. Poplar lines with edits in all four PagALS homologues exhibit high resistance to tribenuron and nicosulfuron. This study employs a multi-component synergistic optimization strategy that specifically enhances the efficiency and precision of CBE editing in poplar while improving synchronous editing of alleles. Through editing the herbicide resistance gene PagALS, we obtain the herbicide-resistant poplar germplasm. Our research provides a more precise and efficient CBE tool for genetic modification in poplar that can also be applied to other forestry species, demonstrating its potential for advancing forestry research and breeding programs.
Similar content being viewed by others
Introduction
CRISPR/Cas9-based genome editing technologies, particularly CBE systems, have emerged as powerful molecular tools for precise genetic modifications in plants1. Unlike conventional CRISPR/Cas9 systems that rely on double-strand breaks (DSBs) and homology-directed repair (HDR), base editors enable direct nucleotide substitutions without requiring donor templates, significantly reducing the risk of unwanted insertions and deletions2. Cytosine base editors typically consist of a fusion of cytidine deaminase, nickase Cas9 (nCas9), and uracil glycosylase inhibitor (UGI), which collectively convert targeted cytosines (C) to thymines (T) with high precision. This technology has revolutionized plant breeding approaches by facilitating precise gene function studies and targeted trait improvement across various plant species, including Arabidopsis3, rice4, tomato5, maize6, rapeseed7, apple8, pear8, and poplar9,10.
Despite these advances, the application of base editing in woody plants faces challenges due to wide editing windows and byproduct generation. Current research efforts focus on optimizing CBEs to increase editing efficiency, reduce byproducts, and enhance specificity across various plant species. These optimization strategies primarily concentrate on improving deaminases, incorporating exogenous proteins, and enhancing nuclear localization11. Researchers have developed multiple CBEs using various cytidine deaminases, such as PmCDA112, hA3A/Y130F13, mini-Sdd714, and TadA-8e15, significantly improving the editing efficiency of plant CBEs. Another key strategy involves incorporating additional functional proteins to enhance the CBE system’s performance. During the cytosine base editing, deamination converts cytosine (C) to uracil (U), which is subsequently recognized and excised by the cellular repair mechanisms, potentially reducing editing efficiency and purity. To address this, researchers have introduced UGI fusion to nCas9, leading to enhanced CBE versions like BE3 and BE4, which significantly improved editing efficiency16, and the implementation of the MS2 system to increase UGI copies, which has shown marked improvements in C to T editing activity and purity in rice13,17. Furthermore, since cytidine deaminase primarily catalyzes the conversion of C to T on single-stranded DNA (ssDNA) substrates generated by nCas9, fusion of non-sequence-specific single-stranded DNA-binding domains (DBD), such as Rad51, to nCas9 has increased binding affinity and editing activity in plant and animal systems18,19. Additionally, enhancing nuclear localization has proven crucial for base editing efficiency. Recent studies have shown that the BPSV40NLS (bpNLS) is more effective than the traditional SV40NLS peptide for Cas protein expression. In Arabidopsis, using bpNLS on both the C-terminal and N-terminal of Cas9 significantly increased editing efficiency20. While in wheat and maize protoplasts, replacing the N-terminal SV40NLS of Cas9 and Cas12a with bpNLS enhanced the editing rate of ABE21.
To date, few studies have successfully employed base editing techniques in poplar. Li utilized A3A/Y130F-BE3 and PmCDA1-BE3 in poplar to achieve base editing9. However, their investigation reported on C to T and C to indel (insert and deletion) mutations without comprehensively analyzing other potential editing byproducts. Furthermore, these vectors exhibited low allelic editing efficiency across most target sites and a high incidence of indel mutations. Similarly, Yao achieved only 8% editing efficiency using pHEE901(BE3) with APOBEC-1 as the deaminase, with substantial occurrences of unintended G or A transitions and indel mutations10. These studies underscore the urgent need for optimized base editing systems tailored for woody plants.
During the plant seedling stage, weeds compete for spatial resources, restrict light exposure through shading, and deplete essential water and nutrients, significantly inhibiting seedling growth and development22. Current weed control methods include mechanical, manual, herbicide, and biological approaches22,23. Among these, herbicide control has emerged as the predominant strategy in forestry, particularly due to high labor costs and the extensive areas of nursery plantations. However, the sensitivity of young trees to herbicides can adversely affect their normal growth24. Consequently, developing herbicide-resistant woody plant varieties has become crucial in forestry research.
Acetolactate synthase (ALS) inhibitors are commonly used herbicides. ALS is a crucial enzyme in the biosynthesis of branched-chain amino acids (leucine, valine, and isoleucine). Point mutations in the ALS can confer herbicide resistance by eliminating the inhibitory effect of these chemicals on plant growth25. Previous research has demonstrated that point mutations in the ALS gene (such as P197, P186, and P185) can confer dominant resistance to ALS inhibitors. Consequently, CBE has been successfully employed to edit herbicide-specific targets in the ALS gene across various plant species, including Arabidopsis, soybean, maize, and peanut, resulting in the development of herbicide-resistant varieties26. Additionally, Malabarba et al. successfully edited the ALS gene in apple and pear, conferring resistance to chlorsulfuron herbicide, demonstrating that this strategy can also be applied to woody plant species8.
In this study, we conducted a systematic and comprehensive analysis of A3A/Y130F-CBE editing outcomes in poplar to elucidate specific editing patterns of the CBE system in woody plants. To address the persistent challenges of low target efficiency and imprecise editing in woody species. We developed hyPopCBE, a CBE system specifically engineered for poplar. We systematically optimized the A3A/Y130F-CBE system through three key modifications: incorporating the MS2-UGI system, integrating the Rad51 DNA-binding domain, and modifying the nuclear localization signal, resulting in the development of hyPopCBE-V2, V3, and V4 variants. Our strategy involves the synergistic integration of these components, as optimally demonstrated in hyPopCBE-V4. This system represents a multi-component optimization of CBE specifically engineered for woody plant species. Our experiments demonstrated that these modifications synergistically enhanced C to T editing efficiency while reducing byproduct formation, with significant improvements in both allelic and homozygous editing efficiencies. To validate the practical application of our system, we targeted the PagALS Pro197 site to generate herbicide-resistant poplars and evaluated the engineered plants for resistance to tribenuron and nicosulfuron. Among all variants tested, hyPopCBE-V4 exhibited superior performance, showing high potential for developing new poplar varieties and advancing plant breeding programs. This research not only contributes to plant biotechnology but also has important implications for forestry management and sustainable agriculture. By providing a more efficient and precise genome editing tool for woody plants, our work establishes a foundation for tree breeding innovations and enables new approaches to exploring complex traits in forest trees.
Results
Selection of sgRNA targeting PagALS homologs and construction of CBE base editing vectors
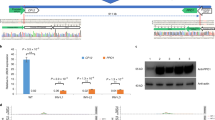
To identify ALS genes in poplar, we used the coding sequence of the Arabidopsis ALS gene (AT3G48560, https://www.arabidopsis.org/) as a query sequence and performed homology gene prediction using the genomic data of poplar hybrid 84K (Populus alba x P. tremula var. glandulosa) (https://www.ncbi.nlm.nih.gov/)27. Four genes homologous to AtALS were identified: PagALS-A01, PagALS-G01, PagALS-A02, PagALS-G02 (Fig. 1a). Sequence alignment revealed high similarity (>90%) among these four PagALS proteins (Supplementary Fig. 1). Quantitative real-time PCR analysis of gene expression in roots, stems, and leaves of wild-type 84K poplar showed significantly higher expression levels of PagALS-A01 and PagALS-A02 compared to PagALS-G01 and PagALS-G02 (Fig. 1b), suggesting PagALS-A01 and PagALS-A02 may play a predominant role in poplar. To generate herbicide-resistant poplar, we targeted the Pro197 site using the CBE system to replace the proline with leucine in all four PagALS homologs. Considering the high sequence similarity among the four PagALS homologs, we designed an sgRNA targeting all four genes simultaneously (Fig. 1c).
a Chromosomal positions of PagALS homologs in the poplar genome. b Relative expression levels of four PagALS homologous genes across root, stem, and leaf tissues of wild-type poplars. Expression is normalized to the lowest level observed in G02 stem tissue (set as 1). Error bars represent SD. n = 3 biologically independent tissue samples. Statistical comparisons were performed using t-test for comparisons of each sample group against the G02 stem control group. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001). c Design of a sgRNA targeting all four PagALS genes, showing the target sequence and PAM site. d Schematic representations of four iteratively optimized base editing vectors (hyPopCBE-V1 to V4) used in this study, highlighting key components and modifications.
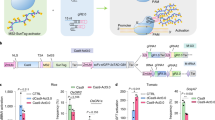
We constructed the hyPopCBE-V1 vector based on the A3A/Y130F-BE3 CBE system9, using the Ubi promoter to drive the fusion protein A3A/Y130F deaminase-nCas9-UGI, and the AtU3 promoter to drive sgRNA (Fig. 1d). To enhance precision and allelic editing efficiency, we created three variants. First of all, we developed hyPopCBE-V2 by incorporating the MS2-UGI system. We designed a fusion protein, MCP-UGI, composed of the MS2 coat protein (MCP) and UGI protein, connected by a flexible glycine-serine peptide linker (GSGSGSGSGS, 5XGS linker). This MCP-UGI fusion was added to the C-terminus of the original A3A/Y130F deaminase-nCas9-UGI construct. To ensure proper localization, we added SV40NLS to both the N-terminus of MCP and the C-terminus of UGI. The A3A/Y130F deaminase-nCas9-UGI and MCP-UGI proteins were linked using the thosea asigna virus 2A (T2A) self-cleaving peptide, allowing for their co-expression and subsequent separation. To facilitate the recruitment of additional MCP-UGI fusion proteins, we modified the sgRNA scaffold by inserting MS2 binding sequences at the 13th and 50th nucleotide positions. This comprehensive design, integrating the MS2-UGI system components, was designated as hyPopCBE-V2 (Fig. 1d). In hyPopCBE-V3, we aimed to improve DNA-binding and nuclear localization. We inserted a poplar codon-optimized human Rad51 DBD sequence (Rad51 DBD) with a GS linker between the A3A/Y130F deaminase and nCas9, and replaced the C-terminal SV40NLS of A3A/Y130F deaminase with a bpNLS signal (Fig. 1d). Finally, hyPopCBE-V4 integrated all modifications from hyPopCBE-V2 and hyPopCBE-V3, combining the MS2-UGI system, Rad51 DBD, and optimized NLS (Fig. 1d).
To determine whether the hyPopCBE-V1 and variants could successfully induce base substitution in plant, we introduced them with sgRNA targeting NbPDS into tobacco leaves via Agrobacterium-mediated transient overexpression (Supplementary Fig. 2a). Hi-TOM 2.0 analysis revealed that all potential cytosine sites within the protospacer were editable, with editing frequencies highest at C7, followed by C6, C5, C12, and C4, indicating a broader edit window for all variants (Tables 1–4, Supplementary Fig. 2b–e). We observed not only C to T conversions but also byproducts, including C to G, C to A, and C to indel mutations. The C to T editing efficiencies at various cytosine sites differed among the vectors. For the four vectors, the C to T editing efficiencies at various cytosine sites were as follows: 0.05%–4.78%, 0.10%–6.10%, 0.05%–9.33%, and 0.05%–6.33% (Tables 1–4). Therefore, hyPopCBE-V1 and its variants demonstrated successful induction of editing in plants, and optimized vectors effectively increased the C to T editing efficiency.
Comprehensive analysis of cytosine modification patterns and efficiency across hyPopCBE vector variants in poplar
To investigate base editing precision and efficiency in woody plants, we generated transgenic poplar hybrid 84K using the hyPopCBE series vectors containing PagALS sgRNA. Through Agrobacterium-mediated transformation, we obtained 244 regenerated poplar plants, of which 169 were confirmed as transgene-positive. Sanger sequencing analysis revealed that 148 transgenic plants exhibited successful editing events, providing a robust dataset for our analysis (Table 5, Supplementary Tables 1–4).
We first assessed the overall editing efficiency across the 148 edited poplar plants for each of the four hyPopCBE vectors. All four vectors demonstrated high performance. The hyPopCBE-V1 vector exhibited a high editing efficiency of 90.70%. Notably, the optimized vectors maintained comparable high efficiencies: hyPopCBE-V3 at 88.37%, hyPopCBE-V4 at 85.71%, and hyPopCBE-V2 at 85.37%. These results indicate that all four variants of the hyPopCBE system retain strong editing capabilities in poplar, with only minor variations in overall efficiency (Table 5).
To elucidate the specific cytosine modification patterns within the protospacer region for each vector, we conducted a comprehensive statistical analysis of editing events. This analysis revealed a total of 1122 cytosine modifications across 148 edited poplars. For all transgenic plants, we sequenced ~800 bp regions containing the protospacer using PagALS-A01/A02/G01/G02-F/R primers. Importantly, all editing events were confined to cytosines within the protospacer region(C1, C7, C8, C10, and C13) (Fig. 2a, Supplementary Tables 5–8), with no modifications detected at non-cytosine sites or in the surrounding non-protospacer sequences. This high specificity is crucial for precise genetic modifications and minimizes the risk of off-target effects.
a The frequencies of cytosine substitutions at different target positions across all edited plants. b Sequencing chromatograms showing different cytosine editing outcomes in the PagALS target region. “Ref” indicates the reference sequence. “Red arrows” mark edited cytosine positions, illustrating C to T, C to G, and C to del (deletion) conversions.
The 1122 cytosine modification events comprised 839 substitution events and 283 deletion events. Analysis of editing efficiency at each cytosine target locus revealed that C8 exhibited the highest editing efficiency (38.59%, 433/1122), followed by C7 (31.64%, 355/1122,), C13 (18.72%, 210/1122), C10 (10.96%, 123/1122), and C1 (0.09%, 1/1122). This distribution shows that C7 and C8 were the most frequently edited sites. (Fig. 2a, Supplementary Tables 5–8). Interestingly, this pattern aligns with our findings presented here in tobacco, suggesting a conserved mechanism across different plant species.
Among the 1122 total editing events observed, 763 were C to T conversions, which are the desired outcome of our base editing system. Specifically, C to T conversions were most frequent at C8 (42.99%, 328/763), with decreasing frequencies at C7 (35.91%, 274/763), C13 (15.47%, 118/763), C10 (5.50%, 42/763), and C1 (0.13%, 1/763). This alignment between overall editing and C to T conversion patterns suggests a consistent preference of our editing system across different types of modifications (Fig. 2a). While C to T conversions were the predominant outcome, we also observed three types of editing byproducts across the four vectors: C to G, C to A, and C to Del (deletions) (Fig. 2b). Among these, C to Del was the most frequent byproduct, while C to A was the least common. The presence of these byproducts highlights the ongoing challenge in base editing systems to maximize desired conversions while minimizing unintended modifications.
For base editing systems, especially in poplar genomes with multiple homologous genes27,28, the primary challenge lies not in improving overall editing efficiency, but in enhancing the precision of editing—specifically, increasing C to T conversions while reducing byproducts. The C to T editing efficiencies varied across the vectors: hyPopCBE-V1(61.17%, 178/291), hyPopCBE-V2(68.62%, 234/340), hyPopCBE-V3(61.04%, 152/249) and hyPopCBE-V4(82.23%, 199/242) (Fig. 3a, Supplementary Tables 5–8), These data indicate that hyPopCBE-V4 achieved the highest C to T conversion efficiency, showing an improvement over the original hyPopCBE-V1 vector. However, while these numbers indicate the overall C to T conversion rate, they don’t fully capture the precision of the editing process. To gain a more comprehensive understanding of editing accuracy, we examined the proportion of plants that underwent clean C to T editing without any byproducts. The results were as follows: hyPopCBE-V1(20.93%, 9/43), hyPopCBE-V2(21.95%, 9/41), hyPopCBE-V3(24.91%, 12/43) and hyPopCBE-V4(40.48%, 17/42) (Table 5). These results demonstrate an improvement in precise editing across our optimized vectors. In our samples, hyPopCBE-V4 produced plants with clean C to T edits at a rate of 40.48%, while the original hyPopCBE-V1 showed a rate of 20.93%. Our analysis revealed that while hyPopCBE-V1 demonstrated high overall cytosine conversion capabilities, it also produced a high level of byproducts and exhibited a broad editing window, making it challenging to obtain genes that can be edited correctly at the target site. In contrast, the optimized vectors, particularly hyPopCBE-V4, not only maintained high overall editing efficiency but also substantially improved editing precision. These findings underscore the effectiveness of our optimization strategies in enhancing both the efficiency and precision of base editing in poplar.
Synergistic effects of UGI enrichment and Rad51 DBD fusion improve the base editing precision and editing efficiency in poplar
Building upon our comprehensive analysis of hyPopCBE-V1 and variants’ performance, we observed that our optimization strategies successfully enhanced C to T editing efficiency while reducing byproduct formation. To elucidate the specific improvements conferred by each optimization, we performed a detailed statistical analysis of modifications at each cytosine position for each vector, allowing us to discern the unique editing profiles and characteristics of hyPopCBE-V1 and its optimized variants (Fig. 3a, Supplementary Tables 5–8).
hyPopCBE-V2, which incorporates the MS2-UGI system, appeared to show improvements in C to T conversion efficiency at all targeted cytosine positions compared to hyPopCBE-V1 (Fig. 3a, b). Specifically, we observed an increase of 15.14%, 6.80%, 4.66%, and 7.74% at the C7, C8, C10, and C13 cytosine sites, respectively. Moreover, hyPopCBE-V2 showed lower levels of editing byproducts (range: 11.11%–19.43%, mean: 15.27%) compared to hyPopCBE-V1 (range: 14.11%–18.28%, mean: 16.03%) (Fig. 3a, c). Our analysis indicated that hyPopCBE-V2 reduced C to G editing efficiency at most cytosine sites, while C to Del mutations remained similar between constructs (Fig. 3d, e). These findings suggest that the addition of MS2-UGI enhances both editing efficiency and accuracy.
In contrast, hyPopCBE-V3, which features the addition of Rad51 DBD and the replacement of SV40NLS with bpNLS, did not increase overall editing events or C to T editing efficiency (Fig. 3a, b). However, it demonstrated a decrease in byproduct editing efficiency (range: 11.56%–16.20%, mean: 13.87%) compared to hyPopCBE-V1 (Fig. 3a, c). Similar to hyPopCBE-V2, hyPopCBE-V3 reduced C to G editing efficiency at most cytosine sites. Interestingly, for C to Del mutations, hyPopCBE-V3 showed an increase at C7 and C8 sites but a decrease at C10 and C13 sites (Fig. 3d, e). These results indicate that the modifications in hyPopCBE-V3 primarily improved editing precision by reducing byproduct edits.
Furthermore, hyPopCBE-V4, which combines all optimization strategies, displayed a unique editing profile that suggests a synergistic effect of the combined modifications. It showed increased C to T editing efficiency at C7 and C8 sites (by 15.73% and 9.76%, respectively) but decreased efficiency at C10 and C13 sites (by 1.54% and 8.72%, respectively) compared to hyPopCBE-V1 (Fig. 3a, b). This pattern indicates that the synergistic use of MS2-UGI, Rad51 DBD, and bpNLS in hyPopCBE-V4 not only enhanced C to T editing efficiency but also narrowed the C to T editing window, Given that the aim of base editing is to achieve precise modifications at specific sites, this narrowing of the high-efficiency editing window is beneficial for achieving precise modifications at specific target sites. Furthermore, hyPopCBE-V4 demonstrated decreased byproduct editing efficiency (range: 4.76%–9.93%, mean: 6.33%) (Fig. 3a, c). Both C to G and C to Del mutations were reduced at most cytosine sites(Fig. 3d, e). Additionally, the proportion of fragment deletions in hyPopCBE-V4 was decreased, with only 12 deletions observed compared to 32 deletions in hyPopCBE-V1 (Table 6). These results collectively demonstrate that the synergistic combination in hyPopCBE-V4 improved editing precision by decreasing byproduct formation and specifically reducing the proportion of fragment deletions. In conclusion, our optimization efforts have yielded improvements in both editing efficiency and precision. Each hyPopCBE variant demonstrated unique strengths, with hyPopCBE-V4 emerging as promising for practical applications due to its synergistic combination of enhanced editing efficiency, reduced byproducts, and a narrowed editing window. This synergistic effect highlights the potential of combining multiple optimization strategies to achieve improved base editing outcomes in complex plant genomes, particularly in woody species like poplar.
Optimization enhances allelic and homozygous editing efficiency in poplar base editing
Following our comprehensive analysis of site-specific editing patterns, we investigated the effects of our optimizations on allelic and homozygous editing efficiency in poplar. Our genomic analysis revealed that the poplar hybrid 84K contains four PagALS homologous genes, forming two pairs of allelic genes: PagALS-A01 with PagALS-G01, and PagALS-A02 with PagALS-G02. Among 148 transformed plants, we observed editing events in 455 PagALS homologous genes.
To assess the efficiency of our optimized systems, we focused on the proportion of plants exhibiting clean C to T conversions without byproducts in both allelic and homozygous editing scenarios. For allelic editing, we analyzed 192 pairs of edited alleles across 127 plant lines. The efficiency of effective allelic editing (C to T conversions only, without byproducts) showed improvements: 27.91%, 46.34%, 30.23%, and 45.24% for hyPopCBE-V1, V2, V3, and V4, respectively (Table 7, Supplementary Tables 9–12).
Similarly, for effective homozygous editing (C to T conversions in all four homologous genes without byproduct), we observed an enhancement in efficiency. The efficiency of effective homozygous editing increased from 4.65% in hyPopCBE-V1 to 12.20%, 6.98%, and 21.43% in V2, V3, and V4, respectively (Table 7, Supplementary Tables 9–12). These results demonstrate that our optimizations enhanced the precision of both allelic and homozygous gene editing by reducing byproducts. Notably, hyPopCBE-V4 demonstrated improvements in both categories, showing considerably higher efficiency in allelic editing and even more pronounced enhancement in homozygous editing efficiency compared to the original hyPopCBE-V1. This increase in editing efficiency underscores the effectiveness of our combined optimization strategies, particularly in achieving clean, byproduct-free edits in the complex genome of poplar.
To assess the specificity and safety of our vectors, we also evaluated the off-target effects of hyPopCBE and its variants. Using the CRISPR-GE software (http://skl.scau.edu.cn), we predicted potential off-target sites for the sgRNAs targeting PagALS, focusing on sites with the highest scores and fewer than 5 mismatched bases. We analyzed the top three potential off-target sites with the highest scores. Sequencing was performed on these sites in three randomly selected transgenic lines for hyPopCBE-V1 and its variants. The results revealed no differences between edited plants and wild-type plants at these potential off-target sites, suggesting a low off-target mutation efficiency of hyPopCBE-V1 and its variants in poplar (Table 8). In conclusion, these results corroborate and extend our findings, demonstrating improvements in both editing efficiency and precision for the poplar PagALS gene through optimization of the hyPopCBE system.
Mutation of the PagALS gene to create herbicide-resistant poplar
To validate the practical application of our optimized base editing system, we evaluated the herbicide resistance of the edited poplar lines. The ALS genes play a crucial role in plant growth, and their partial or complete loss of function can impair plant development. We generated 169 positive transgenic editing poplar lines, of which 122 exhibited the expected P197L mutation. However, 101 lines showed unexpected editing byproduct mutations in at least one PagALS gene (Fig. 4a) and were excluded from further analysis. The remaining 21 poplar lines contained only the desired P197L substitution without any other types of byproduct edits. Further characterization of these lines revealed varying degrees of editing of homologs: five lines had all four PagALS homolog genes edited, three lines had three homolog genes with P197L substitution and one wild-type allele, five lines had two edited homologs and two wild-type alleles, and eight lines had only one PagALS homolog gene edited with three wild-type alleles remaining (Fig. 4b, Supplementary Tables 13–16).
a Representative sequencing chromatograms showing various Pro197 mutations in PagALS homologs. Mutations include amino acid substitutions, deletions, and premature stop codons. “Ref” indicates the reference sequence. b P197 amino acid changes across PagALS homologs (A01, A02, G01, G02) in different edited plant lines. Plants are grouped based on the number of successfully edited homologs. c Time-course comparison of the edited line 1#58 and wild-type (WT) potted plants treated with 3000 mg/L tribenuron. Images show plant status before treatment and 30 days after herbicide application, with close-up images of leaf morphology at the 30-day timepoint. Scale bar represents 10 cm. d In vivo herbicide resistance assay comparing growth of edited (1#58) and WT tissue-cultured seedlings after 30 days of culture on medium supplemented with 0.5 mg/L nicosulfuron. Terminal bud and stem segment growth are displayed. Scale bar represents 1 cm.
Among these, the five lines with all four PagALS homologs edited (P197L mutation) were confirmed by Sanger sequencing and amino acid change analysis to exhibit genetic uniformity at the target sites. From these homozygous-edited lines (1#58, 4#10, 4#12, 4#37, and 4#64), we selected mutant plant line 1#58 as a representative for herbicide resistance experiments. We used tribenuron-methyl at 3000 mg/L (a concentration significantly higher than the field-recommended dosage of 150 g/ha) as the treatment concentration to compare resistance between wild-type and mutant plants. Using this concentration, we evaluated the herbicide resistance of edited poplar in greenhouse conditions. Both mutant (three stem segment-derived clones of line 1#58) and wild-type poplars, all grown for 3 months after transplanting from tissue culture to soil, were subjected to direct spray treatment with tribenuron-methyl herbicide at a concentration of 3000 mg/L. The application was administered at a 45-degree angle from the apex, with each plant receiving 50 mL per application. Treatments were conducted at 2-day intervals for a total of seven applications. After 30 days of treatment, wild-type poplar displayed leaf wilting, leaf abscission, apical leaf necrosis, and eventual mortality, whereas the three stem segment-derived clones of line 1#58 continued to thrive (Fig. 4c).
To further evaluate herbicide resistance, we tested an additional herbicide, nicosulfuron, to compare endogenous absorption. We excised 2–3 cm stem segments from tissue-cultured seedlings of both wild-type and mutant plants and subsequently inserted these segments into rooting medium incorporated with 0.5 mg/L nicosulfuron. Over a 30-day treatment period, apical buds regenerated in wild-type poplar was suppressed, but the edited poplar displayed markedly better growth than the wild type, evident in both apical buds and stem segments, indicating the development of herbicide resistance (Fig. 4d). These findings not only validate the efficacy of our optimized base editing system but also demonstrate its potential for developing herbicide-resistant poplar varieties suitable for field application.
Discussion
The CRISPR/Cas9-based base editing system has emerged as a powerful tool for plant genetic improvement, heralding a new era in modern plant breeding29. However, the application of existing base editing systems in poplar has been hindered by low targeting efficiency and imprecise base substitutions. Previous studies have indicated that the A3A/Y130F deaminase outperforms PmCDA1 and APOBEC-1 deaminase, exhibiting high editing efficiency in poplars9. In this study, we employed the cytosine base editor A3A/Y130F-BE3, named as hyPopCBE-V1, achieving a high base editing efficiency of 90.70% in poplars. Despite this high efficiency, a critical challenge in base editing is the production of unwanted byproducts. Our results revealed that hyPopCBE-V1 exhibited a low rate of clean C to T edits without byproducts (20.93%). hyPopCBE-V1 also demonstrated broad editing windows when using either tobacco or poplar as experimental materials. Our research demonstrates that the optimization of vectors through either individual or combined use of MS2-UGI and Rad51 DBD proteins enhanced C to T editing efficiency, reduced the generation of byproducts. The potential of MS2-UGI and Rad51 DBD to enhance allele gene and homozygous gene C to T editing efficiency was highlighted, especially with the hyPopCBE-V4 vector. These findings provide strategies and methodologies for genome editing in the complex genomes of woody plants, which present unique challenges compared to herbaceous model species due to their high heterozygosity, complex genomes, and long generation times.
A significant challenge in base editing strategies is the occurrence of unintended modifications, or byproducts, which can compromise the precision of gene alterations. For instance, Li observed C to indel byproduct editing efficiencies of up to 19% using the A3A/Y130F deaminase9, while Yao noted a considerable amount of editing byproducts when using pHEE90-BE3 in poplars, significantly limiting the application of base editing in this species10. We conducted a detailed investigation using hyPopCBE-V1 in poplar trees to further clarify the editing patterns of CBE in poplars. Our results corroborate these findings, showing that hyPopCBE-V1 produced a high proportion of editing byproducts (C to G, C to A, C to indel) in poplars. To address this issue, we explored the potential of the MS2-UGI system, which has been shown to reduce byproduct rates and increase editing purity in rice by enriching UGI13. Our optimized version, hyPopCBE-V2, incorporating the MS2-UGI system, successfully increased C to T editing efficiency while decreasing the proportion of editing byproducts in poplars. This improvement demonstrates the transferability of the MS2-UGI system’s benefits across different plant species and highlights its potential for enhancing base editing precision in woody plants. While the MS2-UGI system has been previously applied in herbaceous crops, our work provides evidence of its efficacy in woody plants, expanding the toolkit available for non-model species with complex genomes.
ssDNA binding proteins are known to stabilize ssDNA, potentially extending the time window available for deamination reactions and DSB repair18. To leverage this property and enhance deaminase activity, we fused Rad51 DBD in hyPopCBE-V3. Contrary to previous reports in mammalian systems, which showed a 257-fold increase in editing efficiency with Rad51 DBD18. Our results did not demonstrate an improvement in editing efficiency. However, we observed a decrease in the byproduct rate, aligning with findings in rice where the addition of Rad51 DBD to AncBE4max-NG reduced the average indel byproduct rate from 2.3% to 0.93%19,30. Interestingly, while previous research suggested that Rad51 DBD fusion could expand the editing window, our study showed a narrowing of the editing window. This discrepancy may be attributed to the inherent differences between woody and herbaceous plants, highlighting the need for species-specific optimization of base editing systems. The observed narrowing of the editing window may actually be advantageous for poplar base editing, as it could potentially reduce off-target effects and increase precision. This unexpected finding contributes valuable knowledge to the broader plant genetics community by demonstrating that editing components may function differently across plant taxa, cautioning against the direct transfer of tools between model organisms without proper validation. Future research should focus on elucidating the impact of Rad51 DBD on the editing window in different plant systems. Previous research has also focused on the precision of the editing window by optimizing linker proteins31, replacing deaminases32, and fusing phage peptides33 to restrict the editing window, thereby achieving precise base editing. Our findings suggest that while Rad51 DBD may have a less pronounced impact on editing efficiency in plant systems compared to mammalian models, it effectively narrows the editing window and reduces byproducts. This innovation in our research investigates the performance of mainstream CBE modification strategies in poplar, highlighting the importance of species-specific optimization, particularly for complex plant genomes such as poplar.
Our results reveal an intriguing synergistic effect between Rad51 DBD and the MS2-UGI system in the hyPopCBE-V4 vector. While Rad51 DBD alone did not improve base editing efficiency, its combination with MS2-UGI in hyPopCBE-V4 led to an enhancement in C to T editing efficiency, particularly in allele-specific and homozygous gene editing. This synergistic effect was not observed when either component was used individually. The observed synergy can be explained by the complementary roles of these components. Rad51 DBD, containing 114 DNA-binding residues, is known to increase the proportion of HDR during DNA repair19. Although this property alone did not enhance editing efficiency in our poplar system, it likely created a more favorable environment for precise editing. Concurrently, the MS2-UGI system, which we demonstrated to reduce byproduct formation, likely improved editing fidelity. When combined in hyPopCBE-V4, these components appear to function synergistically: Rad51 DBD potentially stabilizes the DNA repair process and creates a more accessible chromatin environment, while MS2-UGI ensures higher editing purity. This combination not only improved overall editing efficiency but also reduced undesired byproducts, as evidenced by the fewer deletions observed in plants edited with hyPopCBE-V4. These findings underscore the complexity of optimizing base editing systems in plants, particularly in woody species like poplar. It highlights that while individual optimizations may not always yield improvements, their strategic combination can lead to substantial enhancements in editing outcomes. Furthermore, this synergistic effect emphasizes the importance of exploring multi-component strategies in the development of efficient and precise gene editing tools for complex plant genomes.
To further enhance base editing efficiency in poplar, we replaced the N-terminal SV40NLS of nCas9 with bpNLS in the hyPopCBE-V3 and hyPopCBE-V4 vectors. However, this modification did not improve overall editing efficiency, contrary to findings in some other plant species. This discrepancy may be due to species-specific differences in nuclear import mechanisms or Cas9 protein expression levels. Future research should focus on measuring Cas9 protein expression levels to better understand the impact of NLS modifications on genome editing efficiency in poplar.
The simultaneous editing of homologous genes is crucial for effective genome modification, especially in polyploid species like poplar. Our analysis of the editing efficiency in four PagALS homologous genes revealed that while hyPopCBE-V1 achieved high overall editing rates, it exhibited low efficiency in clean allele-specific and homozygous gene C to T editing. The optimized variants, especially hyPopCBE-V4, showed improvements in editing fidelity, enhancing the precision of allelic and homozygous editing. These results provide strong evidence that the incorporation of MS2-UGI or Rad51 DBD can indeed enhance the effective C to T editing efficiency of alleles and homologous genes in poplar. This finding has important implications beyond poplar, as many economically important plant species are polyploid and require efficient editing of multiple gene copies to achieve desired phenotypes. Our optimized system offers a potential solution to this challenge faced by the broader plant genetics community.
Interestingly, our statistical analysis revealed higher editing efficiencies in PagALS-A01 and PagALS-G01 compared to PagALS-A02 and PagALS-G02 across all vector editing statistics. Given that the sequence contexts of these four genes differ only by a few SNPs, this bias is unlikely to be explained solely by sequence variations. Instead, differences in chromatin states may play a crucial role in determining editing efficiency. Recent research has indicated that increasing chromatin accessibility can improve overall editing efficiency and homologous gene editing efficiency34,35. This observation of differential editing efficiency across highly similar homologous genes highlights the critical role of chromatin state in determining editing outcomes, a finding with implications for polyploid crop improvement beyond tree species. Further investigation into the relationship between chromatin state and editing efficiency in these specific PagALS genes could provide valuable insights for improving base editing in poplar.
To demonstrate the practical applicability of our optimized hyPopCBE system, we successfully created herbicide-resistant poplar lines. The edited poplar 1#58 with simultaneous P197L mutations across all four PagALS genes exhibited significant resistance to 3000 mg/L tribenuron. This high level of resistance demonstrates significant potential for field application. Additionally, herbicide-resistant poplar tissue-cultured seedlings showed resistance to 0.5 mg/L Nicosulfuron, further validating the system’s versatility. The efficacy of herbicide resistance conferred by ALS mutations varies across species and mutation types. For instance, in peanuts, mutations such as P197F, P197C, P197I, and P197S confer different levels of resistance to tribenuron-methyl36. While in rice, mutations like P197A, P197S, P197Y, and P197F exhibit distinct resistance patterns to various herbicides37. In this study, we specifically investigated the PagALS P197L mutation and demonstrated its ability to confer significant resistance to both tribenuron and nicosulfuron in poplar, though the herbicide resistance profiles of other P197 mutation variants in poplar await further investigation. It’s worth noting that in some species, ALS often exists in allelic forms, and resistance to herbicides shows an additive effect between alleles. For example, in oilseed rape, homozygotes of BnALS1 and BnALS3 mutants exhibit higher herbicide resistance than heterozygote38. Similarly, in maize, single-gene mutants of ZmALS1 were resistant to five times the recommended dose, while allelic mutants survived up to fifteen times the recommended dose6. While our study focused on homozygous mutants of PagALS, future research could explore the resistance profiles of various heterozygous editing types to further optimize herbicide resistance in poplar. Future studies should include multiple independent transformation events to robustly validate the reproducibility of the herbicide resistance phenotype. Notably, this study demonstrates the development of herbicide-resistant germplasm in poplar using base editing technology, offering insights for its production and application.
For woody plants, the regeneration process during transformation, whether through callus organogenesis or direct organogenesis pathways, may introduce chimeric editing events, a common challenge in woody plant transformation systems. While our stringent hygromycin selection strategy effectively eliminated non-transformed cells, the inherent characteristics of multi-cell origin regeneration in poplar could still lead to partial chimerism in early regeneration stages. In this study, we collected samples for sequencing during regenerated bud differentiation, therefore primarily focusing on editing efficiency during the transition from callus to regenerated buds. Although woody plants cannot eliminate chimerism through sexual reproduction selection methods, previous research has found that redifferentiation can effectively eliminate chimerism, and this approach has been successfully applied in apple trees8. Furthermore, as the base editor-containing T-DNA was stably integrated into the plant genome, there remains a potential risk of continuous editing. In this study, we conducted sequencing analyses on target plants at different developmental stages, confirming the stability of these edits. While the sustained expression of editing components theoretically poses a risk of recurrent editing, this risk is mitigated by the reduced complementarity between the edited protospacer and the sgRNA. Nevertheless, this issue still requires significant attention. To fundamentally address this problem, DNA-free strategies have become a key focus. Recently, several studies39,40 have reported DNA-free approaches for poplar, addressing potential long-term stability concerns, particularly considering the challenges of segregating editing components in perennial species.
In conclusion, the integration of MS2-UGI and Rad51 DBD in hyPopCBE-V4 achieves high editing efficiency and enhanced safety, establishing a robust base editing platform for woody plants. While these individual components have been tested in various contexts, our study demonstrates their synergistic effect in woody plants, providing an approach to improve base editing in complex plant genomes. This system provides a tool for gene function research and precision molecular breeding in trees, deepening our understanding of gene functions and advancing the precision and efficiency of tree breeding. The successful generation of herbicide-resistant poplar lines not only demonstrates the feasibility of applying the CBE in poplar but also highlights the potential of our optimized base editing system for creating valuable traits in woody plants. These advancements pave the way for more sustainable and efficient forestry practices, contributing to agricultural innovation and environmental sustainability.
Methods
Plant material and growth conditions
Nicotiana benthamiana seeds were sown in soil and cultivated under controlled environmental conditions with a photoperiod regime of 14 h light/10 h darkness at a constant temperature of 26 °C. Plants were grown for 4–5 weeks until they reached an appropriate developmental stage for Agrobacterium-mediated transient transformation experiments.
Hybrid Poplar 84K (Populus alba x P. tremula var. glandulosa) was maintained under sterile conditions in a tissue culture facility. Propagation of wild-type and mutant lines was achieved via stem segment regeneration. Stem segments (1–1.5 cm) were excised from in vitro-grown plantlets and inserted into rooting medium. The rooting medium consisted of 1/2 Murashige and Skoog (1/2 MS) medium supplemented with 30 g/L sucrose, 6 g/L agar, 0.05 mg/L naphthylacetic acid (NAA), and 0.05 mg/L indolebutyric acid (IBA), with pH adjusted to 5.8 prior to autoclaving. The cultures were maintained under controlled environmental conditions with a photoperiod of 14 h light/10 h darkness at a constant temperature of 26 °C. Plants were cultured for 6–8 weeks until they reached the appropriate developmental stage for Agrobacterium-mediated stable transformation experiments.
Transient overexpression of the CBE binary vector in N. benthamiana
For transient expression in Nicotiana benthamiana, Agrobacterium tumefaciens strain GV3101 (pSoup-p19) (CAT: AC1003, Weidi, China) harboring the CBE binary vector was cultured in YEB medium supplemented with Kanamycin (50 mg/L) and Rifampicin (50 mg/L) at 28 °C until reaching an optical density (OD600) of 0.8–1.0, followed by centrifugation at 4000 rpm for 15 min. Subsequently, the bacterial pellet was resuspended in Lloyd & McCown Woody Plant Basal Medium with Vitamins (WPM; PhytoTech Labs, cat. no. L449) liquid medium to attain an OD600 of 0.6–0.8, yielding the transformation-competent infection solution. Prior to injection, the bacterial culture was maintained under dark conditions for 2–3 h at room temperature. The leaves of 4- to 5-week-old N. benthamiana plants were then injected with the bacterial suspension of the GV3101 (pSoup-p19) strain carrying the CBE binary vectors. The injected N. benthamiana leaves were harvested 72 h post-injection, and leaf tissue samples ~1 cm² surrounding the injection site were collected for DNA extraction41.
Agrobacterium-mediated poplar stable transformation
For stable transformation of poplar, the engineered CBE vectors were introduced into GV3101 (pSoup-p19). The positive Agrobacterium cultures were cultivated on YEB medium supplemented with Kanamycin (50 mg/L) and Rifampicin (50 mg/L) at 28 °C to an optical density (OD600) of 0.6–0.8, followed by centrifugation at 4000 rpm for 15 min. Subsequently, the bacterial pellet was resuspended in WPM liquid medium to attain an OD600 of 0.4–0.6, yielding the transformation-competent infection solution.
For poplar transformation, hybrid 84K poplar tissue culture seedlings with a developmental period of ~6–8 weeks were aseptically harvested under sterile conditions. The second to fourth leaves (counting from apex to base) were excised into ~1 cm² fragments and immersed in the infection solution for 15 min. Excess solution was carefully removed using filter paper.
Following infection, the leaf explants underwent a sequential culture regime: first, a 3-day dark incubation in co-culture medium [WPM supplemented with 30 g/L sucrose, 6 g/L agar, 1 mg/L NAA, 2 mg/L Zeatin (ZT), and 100 μM acetosyringone (As)]. The explants were then transferred to callus induction medium (WPM containing 30 g/L sucrose, 6 g/L agar, 1 mg/L NAA, and 2 mg/L ZT) and maintained in darkness for 20–30 days. Subsequently, the developing tissue was cultured on shoot regeneration medium (WPM with 30 g/L sucrose, 6 g/L agar, 0.1 mg/L NAA, and 2 mg/L ZT) followed by root induction medium (WPM containing 30 g/L sucrose, 6 g/L agar, and 0.1 mg/L NAA) until resistant plantlets emerged. Harvested leaf tissues were subsequently processed for genomic DNA extraction to facilitate further molecular analyses.
Throughout the transformation process, carbenicillin (200–400 mg/L) was incorporated into the media to suppress Agrobacterium growth. Putative transformants were selected using hygromycin B (2 mg/L) as the selective agent. The successfully regenerated transgenic shoots and plantlets were subsequently utilized for molecular genotyping and phenotypic characterization42.
Real-time qPCR
Total RNA was extracted from 1-month-old roots, stems, and leaves of wild-type 84K poplar using the extraction kit DP441 (TIANGEN BIOTECH (BEIJING)CO., LTD.). RT-qPCR analyses were conducted using AceQ qPCR SYBR Green Master Mix (Vazyme, Q141-02) on an iQ5 Multicolor Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). Different primers for the four homologous genes were employed for amplification (Supplementary Table 17). The PCR conditions included an initial denaturation at 95 °C for 15 min, followed by 39 cycles of denaturation at 95 °C for 10 s, annealing at 58 °C for 20 s, and extension at 72 °C for 30 s. Data analysis was performed using iQ5 (Bio-Rad) software, and differences in gene expression were calculated using the 2−∆∆Ct method. β-actin served as an internal control to quantify the relative expression levels of genes in the samples. Source data for Real-Time qPCR are provided in Supplementary Data 1.
Vector construction
The primary strategy employed for constructing vectors in this study was Gateway Assembly, with the entry vectors and destination vectors obtained from Addgene (https://www.addgene.org). Each Gateway LR reaction comprised an attL5-attL2 sgRNA entry clone, an attL1-attR5 base editor entry clone, and an attR1-attR2 destination vector.
To prepare sgRNA entry clones, forward and reverse primers (Supplementary Table 17) were phosphorylated using T4 polynucleotide kinase (NEB, catalog #M0201*), annealed, and then ligated into pYPQ141B (Addgene #69291) after cleavage with the BsmB I enzyme(NEB, catalog #R0180S). For hyPopCBE-V2 and V4, the sgRNA entry clones required modifications to the pYPQ141B vector by adding an MS2 localization sequence to the sgRNA scaffold.
For the construction of base editor entry clones, we employed PYPQ265 (Addgene #164712) for hyPopCBE-V01 and an optimized version, PYPQ265-V2, for hyPopCBE-V2. The process of vector modification and subsequent constructions proceeded as follows: The PYPQ265 vector was digested with XmaI (NEB, catalog #R0180S) and NotI (NEB, catalog #R0189S) enzymes. A gene encoding UGI-T2A-MS2-UGI was synthesized by Tsingke and was codon-optimized for poplar. This synthesized UGI-T2A-MS2-UGI fragment was then ligated into the cleaved PYPQ265 vector using T4 DNA ligase (NEB, catalog #M0202), resulting in the construction of PYPQ265-V2. Similarly, both PYPQ265 and PYPQ265-V2 vectors were digested with NcoI (NEB, catalog #R3193L) and SbfI (NEB, catalog #R3642L). These vectors were then ligated with a synthesized bpNLS and Poplar codon optimization Rad51 DBD sequence, culminating in the creation of the PYPQ265-V3 and PYPQ265-V4 vectors, which served as the foundation for the subsequent development of hyPopCBE-V3 and hyPopCBE-V4.
The destination vector was pYPQ203 (Addgene #86207) containing the ZmUBI promoter for base editor expression. Both sgRNA entry clone and base editor entry clone recombination regions were confirmed by Sanger sequencing. Final T-DNA vectors were confirmed by restriction digestion with HindIII (NEB, catalog # R3104L) and SbfI (NEB, catalog # R3642L). The specific DNA sequences of the vectors are provided in Supplementary Note 1.
Deep amplicon sequencing and data analysis in Nicotiana benthamiana
Genomic DNA was extracted from transiently transformed N. benthamiana leaves using the Plant Genomic DNA Rapid Extraction Kit (Aidlab Biotechnologies Co., Ltd, China). Target sequences for deep sequencing analysis were amplified using NbPDS-F-hitom and NbPDS-R-hitom primers with Phanta® Max Super-Fidelity DNA Polymerase (Vazyme, China) to ensure high-fidelity amplification (Supplementary Table 17). The resultant PCR products were used for deep sequencing library preparation based on the two-round PCR strategy described by Liu et al.43. The prepared libraries were sent to Novogene Bioinformatics Institute (Beijing, China) for sequencing on an Illumina HiSeq X Ten platform. Comprehensive deep sequencing analysis was performed via the Hi-TOM system (http://www.hi-tom.net/hi-tom/), with a 1% data threshold43. Subsequent mutation analysis was conducted through the Hi-TOM 2.0 analytical platform (http://hi-tom.net/#/), following methodological principles delineated in the referenced literature44. Detailed Hi-TOM deep sequencing results are provided in Supplementary Data 2–6.
Mutation analysis for target sites in poplar
Regenerated shoots derived from callus cultures were collected for genomic DNA extraction using the Plant Genomic DNA Rapid Extraction Kit (Aidlab Biotechnologies Co., Ltd, China). Transgenic status was initially verified by amplifying the nCas9 gene fragment using nCas9-F/R primers. Positive transgenic lines were further subjected to target-specific amplification using PagALS-A01/A02/G01/G02-F/R primer pairs (Supplementary Table 17). Using the genomic DNA from verified positive buds as template, sequences encompassing the sgRNA target regions were amplified using Phanta® Max Super-Fidelity DNA Polymerase (Vazyme, China). The resulting PCR products were first subjected to Sanger sequencing, and samples exhibiting single peaks (excluding chimeric samples with double peaks) were purified via gel electrophoresis and ligated into the TA/Blunt-Zero vector. These recombinant constructs were subsequently transformed into E. coli DH5α competent cells, from which multiple independent clones were selected for Sanger sequencing to determine the precise editing profiles across target genes and sgRNA sites.
The sequencing results were analyzed through comparative assessment with wild-type sequences using SnapGene software, enabling the comprehensive characterization of mutation typologies. Source data for editing results in stable transformed poplar are provided in Supplementary Data 7–14.
Off-target analysis
Potential off-target sites were predicted by the online tool CRISPR-GE45. Sequences with 1–5 bp mismatches to the target sites in the 84K (Populus alba x P. tremula var. glandulosa) genome were selected as potential off-target sites. The top three potential target sites with the highest priority were selected. The potential off-targets were amplified by specific primers and used to perform Sanger sequencing directly. Off-target site data were analyzed by comparing sequencing results to wild-type sequences using SnapGene software, similar to the method used for target mutation site analysis. Off-target sites and primers used to amplify target sequences are listed in Supplementary Table 17.
Herbicide resistance evaluation of PagALS variants in vitro and in vivo
The herbicide resistance analysis in this study was structured through a dual methodological approach, encompassing both foliar application experiments and tissue absorption assays, thereby providing complementary perspectives on resistance phenotypes46.
For the foliar application experiments, tribenuron (Qiaochang Modern Agriculture Co., Ltd., Registration No. PD20085533) was selected as the representative sulfonylurea herbicide. The assessment was conducted using wild-type 84K poplar seedlings that had been grown for 3 months after transplanting from tissue culture to soil and maintained under standardized cultivation conditions. Following a comprehensive literature review and preliminary optimization trials, an application concentration of 3000 mg/L tribenuron was established. The herbicide solution was administered via directional spray application (at a 45° angle from the apical region), with each plant receiving 50 mL per application. Treatment was administered at 2-day intervals, with a total of seven applications throughout the experimental period. Plants were maintained under controlled environmental conditions (16 h photoperiod at 28 °C, 8 h dark period at 23 °C).
It is noteworthy that the applied concentration substantially exceeded conventional field application rates (standard forestry seedling spacing of 0.3 × 0.5 m typically receives 150 g/ha, approximating 2 mg per plant), with our experimental regimen delivering 150 mg per application—a concentration significantly higher than agricultural deployment norms, thus ensuring practical relevance and stringent selection pressure.
For the tissue absorption experiments, a complementary methodological approach was employed. The rooting medium (1/2 MS medium supplemented with 30 g/L sucrose, 6 g/L agar, 0.05 mg/L NAA, and 0.05 mg/L IBA, with pH adjusted to 5.8 prior to autoclaving) was augmented with nicosulfuron at a concentration of 0.5 mg/L (Yuanye, Cat# S40988). Stem segments of 2–3 cm length were excised from 6- to 8-week-old tissue culture plantlets of both mutant and wild-type genotypes. For experimental consistency and to optimize regeneration potential, the uppermost stem segments containing apical buds were selected and evaluated separately from lower stem sections, as apical dominance typically influences regenerative capacity in woody plant tissue culture systems. These excised segments, along with their apical buds, were subsequently inserted into the herbicide-supplemented medium at the predetermined sensitive concentration (0.5 mg/L nicosulfuron). Growth dynamics were systematically monitored to evaluate differential herbicide resistance profiles between transgenic lineages and wild-type controls, thereby elucidating the functional efficacy of the introduced genetic modifications.
Statistics and reproducibility
Appropriate biological replication was incorporated into the experimental design to ensure reproducibility. Biological replicates were defined as follows: for stable transformation analysis and off-target analysis with different vectors, multiple independent transgenic lines were selected; for herbicide resistance assessment, multiple independent plant individuals were selected; and for qPCR, independent batches of plant tissue were used. Sample sizes (n) for each analysis are indicated in the corresponding figure legends or table legends.
Statistical analyses were performed using GraphPad Prism 8.0.2 software. Quantitative data were analyzed using a t-test for comparisons between two groups. Statistical significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001. Exact P values are provided in the Supplementary Data 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw high-throughput sequencing data generated in this study are available in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA1262697 and SRA Run accession numbers SRR33565000, SRR33565001, SRR33565002, SRR33565003, SRR33565004. The vector plasmids generated in this study have been deposited with Addgene, accession numbers 239978–239981. Source data for all figures and tables are provided with this paper in the following supplementary files: Source data for Fig. 1b are provided in Supplementary Data 1. Detailed Hi-TOM deep sequencing results, including source data for Tables 1–4 and Supplementary Fig. 2, are provided in Supplementary Data 2–6. Source data for Fig. 4a, b, Tables 5–7, Supplementary Tables 1–4, and Supplementary Tables 9 and 10 are provided in Supplementary Data 7–10. Source data for Figs. 2 and 3, Supplementary Tables 5–8, and Supplementary Tables 11–16 are provided in Supplementary Data 11–14. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
No custom computer code was used for the analysis presented in this study. All analyses were performed using standard software, including Hi-TOM system (web-based platform), Hi-TOM 2.0 analytical platform (web-based platform), SnapGene software (version 4.3.6), CRISPR-GE online platform, the software integrated with the Bio-Rad iQ5 system, and GraphPad Prism (version 8.0.2).
References
Molla, K. A., Sretenovic, S., Bansal, K. C. & Qi, Y. Precise plant genome editing using base editors and prime editors. Nat. Plants 7, 1166–1187 (2021).
Li, J. et al. Plant base editing and prime editing: the current status and future perspectives. J. Integr. Plant Biol. 65, 444–467 (2023).
Chen, Y. Y. et al. CRISPR/Cas9-mediated base-editing system efficiently generates gain-of-function mutations in Arabidopsis. Sci. China Life Sci. 60, 520–523 (2017).
Zhang, R. et al. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nat. Plants 5, 480–485 (2019).
Glaus, A. et al. Repairing a deleterious domestication variant in a floral regulator gene of tomato by base editing. Nat. Genet. 57, 231–241 (2025).
Li, Y. M. et al. Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize. Crop J. 8, 449–456 (2020).
Hu, L. et al. Precision genome engineering through cytidine base editing in rapeseed (Brassica napus. L). Front. Genome Ed.2, 605768 (2020).
Malabarba, J. et al. New strategies to overcome present CRISPR/Cas9 limitations in apple and pear: efficient dechimerization and base editing. Int. J. Mol. Sci. 22, 319 (2021).
Li, G., Sretenovic, S., Eisenstein, E., Coleman, G. & Qi, Y. P. Highly efficient C-to-T and A-to-G base editing in a Populus hybrid. Plant Biotechnol. J. 19, 1086–1088 (2021).
Yao, T. et al. CRISPR/Cas9-based gene activation and base editing in Populus. Hortic. Res. 10, uhad085 (2023).
Rallapalli, K. & Komor, A. The design and application of DNA-editing enzymes as base editors. Annu. Rev. Biochem. 92, 43–79 (2023).
Wu, Y. et al. Increasing cytosine base editing scope and efficiency with engineered Cas9-PmCDA1 fusions and the modified sgRNA in rice. Front. Genet. 10, 379 (2019).
Ren, Q. R. et al. Improved plant cytosine base editors with high editing activity, purity, and specificity. Plant Biotechnol. J. 19, 2052–2068 (2021).
Huang, J. et al. Discovery of deaminase functions by structure-based protein clustering. Cell 186, 3182 (2023).
Fan, T. et al. High performance TadA-8e derived cytosine and dual base editors with undetectable off-target effects in plants. Nat. Commun. 15, 5103–5103 (2024).
Komor, A. C. et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T: a base editors with higher efficiency and product purity. Sci. Adv. 3, eaao4774 (2017).
Zhang, S. Z., Feng, S. J., Jiang, W., Huang, X. X. & Chen, J. P. Construction and optimization of a base editor based on the MS2 system. Anim. Models Exp. Med. 2, 185–190 (2019).
Zhang, X. et al. Increasing the efficiency and targeting range of cytidine base editors through fusion of a single-stranded DNA-binding protein domain. Nat. Cell Biol. 22, 740 (2020).
Wei, C. J. et al. Expanding the editing window of cytidine base editors with the Rad51 DNA-binding domain in rice. Front. Plant Sci. 13, 865848 (2022).
Develtere, W. et al. Continual improvement of CRISPR-induced multiplex mutagenesis in Arabidopsis. Plant J. 119, 1158–1172 (2024).
Gaillochet, C. et al. Systematic optimization of Cas12a base editors in wheat and maize using the ITER platform. Genome Biol. 24, 6 (2023).
Wagner, R. G., Little, K. M., Richardson, B. & McNabb, K. The role of vegetation management for enhancing productivity of the world’s forests. Forestry 79, 57–79 (2006).
Wagner, R. G., Newton, M., Cole, E. C., Miller, J. H. & Shiver, B. D. The role of herbicides for enhancing forest productivity and conserving land for biodiversity in North America. Wildl. Soc. Bull. 32, 1028–1041 (2004).
Briggs, E. L., Greene, D. U., Clabo, D. C. & Gandhi, K. J. K. Herbicides have variable effects on understory plant and insect communities in Southern United States working forests. J. For. 122, 285–301 (2024).
Jin, M., Chen, L., Deng, X. W. & Tang, X. Development of herbicide resistance genes and their application in rice. Crop J. 10, 26–35 (2022).
Dong, H., Huang, Y. & Wang K. The development of herbicide resistance crop plants using CRISPR/Cas9-mediated gene editing. Genes 12, 912 (2021).
Qiu, D. et al. The genome of Populus alba x Populus tremula var. glandulosa clone 84K. DNA Res. 26, 423–431 (2019).
Shi, T. et al. High-quality genome assembly enables prediction of allele-specific gene expression in hybrid poplar. Plant Physiol. 195, 652–670 (2024).
Cardi, T. et al. CRISPR/Cas-mediated plant genome editing outstanding challenges a decade after implementation. Trends Plant Sci. 28, 1144–1165 (2023).
Xu, R. et al. Genome editing with type II-C CRISPR-Cas9 systems from Neisseria meningitidis in rice. Plant Biotechnol. J. 20, 350–359 (2022).
Tan, J., Zhang, F., Karcher, D. & Bock R. Engineering of high-precision base editors for site-specific single nucleotide replacement. Nat. Commun. 10, 2019 (2019).
Yang, L. et al. Engineering APOBEC3A deaminase for highly accurate and efficient base editing. Nat. Chem. Biol. 20, 1176–1187 (2024).
Jia, K. et al. Phage peptides mediate precision base editing with focused targeting window. Nat. Commun. 13, 1662 (2022).
Bai, M. et al. Expressing a human RNA demethylase as an assister improves gene-editing efficiency in plants. Mol. Plant 17, 363–366 (2024).
Wang, W. et al. Application of nicotinamide to culture medium improves the efficiency of genome editing in hexaploid wheat. Int. J. Mol. Sci. 24, 4416 (2023).
Shi, L. et al. Creation of herbicide-resistance in allotetraploid peanut using CRISPR/Cas9-meditated cytosine base-editing. Plant Biotechnol. J. 21, 1923–1925 (2023).
Zhang, R. et al. Generating broad-spectrum tolerance to ALS-inhibiting herbicides in rice by base editing. Sci. China Life Sci. 64, 1624–1633 (2021).
Wu, J. et al. Engineering herbicide-resistant oilseed rape by CRISPR/Cas9-mediated cytosine base-editing. Plant Biotechnol. J. 18, 1857–1859 (2020).
Hoengenaert, L., Van Doorsselaere, J., Vanholme, R. & Boerjan, W. Microparticle-mediated CRISPR DNA delivery for genome editing in poplar. Front. Plant Sci. 14, 1286663 (2023).
Hoengenaert, L., Anders, C., Van Doorsselaere, J., Vanholme, R. & Boerjan, W. Transgene-free genome editing in poplar. New Phytol. 247, 224–232 (2025).
Luo, J. et al. Cytosine base editors (CBEs) for inducing targeted DNA base editing in Nicotiana benthamiana. BMC Plant Biol. 23, 305 (2023).
Song, J., Lu, S., Chen, Z., Lourenco, R. & Chiang, V. Genetic transformation of Populus trichocarpa genotype Nisqually-1: a functional genomic tool for woody plants. Plant Cell Physiol. 47, 1582–1589 (2006).
Liu, Q. et al. Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci. China Life Sci. 62, 1–7 (2019).
Sun, T., Liu, Q., Chen, X., Hu, F. & Wang, K. Hi-TOM 2.0: an improved platform for high-throughput mutation detection. Sci. China Life Sci. 67, 1532–1534 (2024).
Xie, X. et al. CRISPR-GE: a convenient software toolkit for CRISPR-based genome editing. Mol. Plant 10, 1246–1249 (2017).
Brasileiro, A. C. M., Tourneur, C., Leple, J.-C., Combes, V. & Jouanin, L. Expression of the mutantArabidopsis thaliana acetolactate synthase gene confers chlorsulfuron resistance to transgenic poplar plants. Transgenic Res. 1, 133–141 (1992).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFD2200101).
Author information
Authors and Affiliations
Contributions
Hai Lu designed the experiments; Han Liu, Mengyu Zhang, and Leiqian Sun performed the experiments; Yu Peng, Yu Sun, Yawei Fan, Hui Li, and Di Liu performed data analysis; Han Liu, Di Liu, and Hai Lu wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The plant materials utilized in this study were sourced from Beijing Forestry University and processed in compliance with all relevant institutional protocols and national regulations. All genetic modification procedures strictly adhered to current biosafety guidelines.
Peer review
Peer review information
Communications Biology thanks Shahnoush Nayeri and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, H., Zhang, M., Sun, L. et al. Synergistic optimization enhancing the precision and efficiency of cytosine base editors in poplar. Commun Biol 8, 904 (2025). https://doi.org/10.1038/s42003-025-08308-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08308-0
This article is cited by
-
Transgene-free genome editing in citrus and poplar trees using positive and negative selection markers
Plant Cell Reports (2025)