Abstract
D-allulose, a rare sugar with emerging potential as a low-calorie sweetener, has garnered attention as an alternative to other commercially available alternative sweeteners, such as sugar alcohols, which often cause severe gastrointestinal discomfort. D-allulose-6-phosphate 3-epimerase (AlsE) is a prokaryotic enzyme that converts D-allulose-6-phosphate into D-fructose-6-phosphate, enabling its use as a carbon source. However, the taxonomic breadth of AlsE across gut bacteria remains poorly understood, hindering insights into the utilization of D-allulose by microbial communities. In this study, we provide experimental evidence showing that Clostridium innocuum is capable of D-allulose metabolism via a homologous AlsE. A bioinformatics search of 85,202 bacterial genomes identified 116 bacterial species with AlsE homologs, suggesting a limited distribution of AlsE in bacteria. Additionally, Escherichia coli contains a copy of alsE, but it does not grow on D-allulose as a sole carbon source unless alsE is heterologously expressed. A metagenomic analysis revealed that 15.8% of 3079 adult healthy human metagenomic samples that we analyzed contained alsE, suggesting a limited prevalence of the enzyme in the gut microbiome. These results suggest that the gut microbiome has limited capacity to metabolize D-allulose via alsE, supporting its use as an alternative sweetener with minimal impact on microbial composition and gastrointestinal symptoms. This finding also enables personalized nutrition, allowing diabetic individuals to assess their gut microbiota for alsE, and manage glycemic response while reducing gastrointestinal distress.
Similar content being viewed by others
Introduction
The obesity epidemic is a serious health issue affecting many countries worldwide1. According to the National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS), 41.9% of U.S. adults aged 20 and older are obese2. As an individual’s amount of adipose tissue increases, so too does their risk for metabolic diseases, including type 2 diabetes3, which is caused by insulin resistance and lack of insulin, resulting in chronic hyperglycemia4. Over 415 million people worldwide suffer from diabetes, over 90% of whom have type 2 diabetes5. In the U.S., 14.8% of adults aged 20 or older are also affected2.
Previous studies have linked increased sugar consumption to the obesity and diabetes epidemic6,7. Further, researchers propose that a high-carbohydrate diet promotes the deposition of calories into fatty tissue, leading to weight gain through increased hunger8. The main culprits of type 2 diabetes are excessive sugar consumption and a sedentary lifestyle5. There is no cure for type 2 diabetes available as of 2024, and much more research is needed on methods to mitigate and prevent diabetes, including decreasing the consumption of fructose, glucose, and sucrose.
One potential strategy to minimize sugar consumption is to use sugar substitutes, such as aspartame, sucralose, erythritol, xylitol, and sorbitol9. These alternative sweeteners tend to taste sweet, but the human body does not metabolize them, thereby reducing the adverse health effects of excess sugar consumption10. These sweeteners may be derived from plant extracts or from chemical synthesis11.
Many of the alternative sweeteners currently approved by the U.S. Food and Drug Administration include sugar alcohols and non-nutritive sweeteners (NNS), both of which have been associated with some side effects on the human gut microbiome. Sugar alcohol consumption can lead to gastrointestinal discomfort and have laxative effects through osmotic pressure and increased gas production through gut bacterial fermentation, resulting in diarrhea and bloating12. Moreover, increased blood erythritol levels have been associated with increased platelet reactivity, resulting in cardiovascular events such as strokes13. On the other hand, regular NNS consumption can lead to functional alteration of gut microbiota composition, resulting in an impaired glycemic response and glucose intolerance14,15. Therefore, it is extremely important to understand the mechanisms of how alternative sweeteners interact with the human gut microbiome. These findings have led to an increasing interest in fructose epimers, sugar molecules that resemble fructose but have altered stereochemistry at one carbon atom16. One example is D-allulose (also known as D-psicose), a rare low-calorie sweetener that is the C-3 epimer of fructose and is found in small amounts in certain fruits16. Previous studies have suggested that D-allulose has a low glycemic index, making it promising for reducing the risk of diabetes17,18,19,20. Due to advances in the industrial process and bacterial engineering methods, D-allulose production is becoming increasingly economically viable21. Thus, D-allulose is a promising way to decrease sucrose and fructose consumption.
Although D-allulose is a promising alternative sweetener, its side effects are poorly understood compared to other types of alternative sweeteners. A significant portion of ingested D-allulose reaches the gut microbiome, as approximately 30% passes through the small intestine unabsorbed and is excreted in feces22,23. While 70% of D-allulose is absorbed via glucose transporter type 5 (GLUT5) in the small intestine, the substantial unabsorbed fraction has the potential to interact with and impact the gut microbial community22,23. Studies in murine models have shown that D-allulose can induce changes in the gut microbiome24,25. Comparatively, Suez et al.26 showed that saccharin, sucralose, and aspartame can induce glucose intolerance through modifications of the gut microbiome composition and function26. Therefore, there is an urgent need to better understand both the potential for D-allulose utilization by gut bacteria and its effects on human gut microbiome composition.
Some bacteria possess the ability to metabolize D-allulose using the enzyme D-allulose-6-phosphate 3-epimerase (AlsE), encoded by the gene alsE27. In E. coli K-12, alsE is in the D-allose operon, which has been well characterized28. First, D-allose is converted into D-allulose 6-phosphate via AlsK and RpiB. Then, AlsE catalyzes the reversible conversion of D-allulose 6-phosphate to D-fructose 6-phosphate29. Environmental and clinical isolates of Klebsiella pneumoniae, an opportunistic pathogen responsible for a significant number of nosocomial bacterial infections30, are capable of metabolizing D-allulose using a homologous AlsE, raising concerns that consuming D-allulose may confer opportunistic pathogens an advantage in colonization31,32. However, there has not been a full systematic annotation of the prevalence and distribution of alsE in the human gut microbiome.
To address this gap, we combined bioinformatic predictions and experimental verification to characterize the distribution of alsE in human gut microbes. Our bioinformatic predictions were validated through growth experiments, culturing bacteria in media with D-allulose as the sole carbon source. Our investigation of multiple representatives of the major gut bacterial clades expanded the known phylogenetic range of D-allulose metabolism from phylum Pseudomonadota to include phylum Bacillota by identifying that Clostridium innocuum 6_1_30 is capable of using D-allulose as a sole carbon source. Through comparative genomics and protein homology searches, we identified a putative AlsE in C. innocuum that is homologous to AlsE in K. pneumoniae (35% identity, e-value 2.89e-41) that has a divergent operon organization compared to the known D-allulose metabolizers. We verified the function of the C. innocuum AlsE in the E. coli Keio Knockout Collection, observing that E. coli deficient in native alsE was able to grow on D-allulose when transformed with C. innocuum alsE. We also found that E. coli, despite encoding alsE, cannot grow on D-allulose as a sole carbon source unless alsE is heterologously expressed. To comprehensively characterize the taxonomic distribution of AlsE, we performed a systematic search across 85,202 bacterial genomes, identifying 116 species encoding putative alsE homologs. The limited distribution of alsE in the gut microbiome supports D-allulose’s promise as an alternative sweetener with minimal impact on both microbial composition and gastrointestinal symptoms, two common drawbacks of current artificial sweeteners. Although our focus is on alsE, it is important to note that there could be alternative undiscovered pathways bacteria can use to metabolize D-allulose that our study did not cover. These findings provide insights into bacterial D-allulose metabolism, supporting its development as an alternative sweetener to help reduce sugar consumption in the context of rising rates of obesity and diabetes.
Results
Investigating the potential for D-allulose utilization by gut microbes
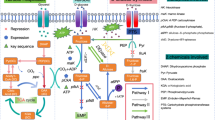
To identify gut bacterial species capable of utilizing D-allulose as a carbon source, we conducted a preliminary identification of species with D-allulose-6-phosphate 3-epimerase (AlsE) homologs by conducting a BLASTp search against 85,202 non-redundant genomes from the Genome Taxonomy Database33,34. We used the experimentally verified Klebsiella pneumoniae AlsE as the query, with a threshold of 50% identity and bitscore greater than 200. There were 272 species that met the threshold, mostly from non-gut bacteria. Some gut bacteria genera with AlsE homologs include Klebsiella, Escherichia, and Clostridium. Interestingly, Clostridium innocuum, a common gut bacterium species, contained an AlsE hit (50.49% identity, 2.30e-77 e-value, 220 bitscore). Given that AlsE catalyzes the reversible conversion from D-allulose 6-phosphate into D-fructose 6-phosphate, C. innocuum could potentially use D-allulose as a carbon source (Fig. 1A).
To experimentally validate our bioinformatic predictions and characterize D-allulose metabolism across diverse gut bacteria, we tested representative strains from major gut bacterial phyla including Bacillota, Bacteroidota, Actinobacteriota, and Pseudomonadota for growth on D-allulose as a sole carbon source (Fig. 2A). Growth was quantified by spectrophotometric measurement at OD600, with significant growth defined as a three-fold increase in OD600 compared to media negative controls. Clostridium innocuum 6_1_30 demonstrated robust growth on D-allulose with a 6:1 ratio in its OD600 measurement compared to the media blank, revealing a previously unknown metabolic capability (Fig. 2B). Notably, Escherichia coli DC10B did not grow on D-allulose despite encoding alsE within its D-allose operon (Fig. 2C), prompting further investigation.
Identification of Clostridium innocuum 6_1_30 as a gut bacteria species that can grow on allulose as a sole carbon source. A Investigation of 7 gut bacteria species (Clostridium innocuum 6_1_30, Bacteroides cellulosilytious - CL02T12C19, Lactobacillus reuteri CF48-3A, Clostridium symbiosum WAL-14163, Escherichia coli DC10B, Bifidobacterium adolescentis L2-32, Ruminococcus gnavus CC55_001C) for growth on allulose as the sole carbon source. Each data point is the average of n = 5 technical replicates from a single biological replicate (n = 4) per species. The error bars represent the standard error across the n = 3 technical replicates. B Growth curve of C. innocuum 6_1_30 on allulose with minimal media. Allulose is the growth curve of C. innocuum when grown on allulose, while Glucose is a positive control of C. innocuum growing on glucose, and Blank refers to C. innocuum grown on blank media as a negative control. C Growth curve of E. coli DC10B (Col02) on allulose with minimal media. The error bars for B and C correspond to the standard error of OD600 on n = 3 technical replicates.
Escherichia coli does not readily utilize D-allulose in vitro despite encoding alsE
Although E. coli encodes alsE within the D-allose operon (alsRBACEK) (Fig. 1B), previous studies have demonstrated that this operon is specifically induced in response to D-allose28. We hypothesized that while E. coli encodes the metabolic machinery for D-allulose utilization; this capability may not be active in the absence of D-allose.
To test this hypothesis, we placed alsE under the control of an IPTG-inducible promoter to enable controlled expression independent of its native regulation. Using the Keio collection, a comprehensive library of single-gene knockout mutants in E. coli BW2511335, we cloned the alsE gene from strain JW2760 into a pCW-lic vector backbone under an inducible tac promoter, creating the pCW-lic-E.coli alsE construct. This plasmid was transformed into the Keio alsE knockout strain, and the transformed bacteria were cultured in M9 minimal media supplemented with D-allulose and IPTG12.
Our results showed that both the Keio alsE knockout and the untransformed E. coli were unable to grow using D-allulose as the sole carbon source. In contrast, the transformed E. coli overexpressing alsE exhibited robust growth (Fig. 3A, B). In addition, we found that wild-type E. coli JW2760 and Keio E. coli transformed with either E. coli or C. innocuum alsE exhibit robust growth on allose, while the native Keio E. coli BW25113 did not (Supplementary Fig. 2). Our findings are consistent with a previous study showing that the alsRBACE operon is induced by allose36.
A, B Verification of the Escherichia coli alsE functionality. Keio ΔalsE::E. coli alsE is the growth curve of the transformed Keio E. coli containing the wild-type E. coli alsE with IPTG to induce ectopic expression, and without IPTG, resulting in no gene expression. pCW-lic-E. coli_alsE is the growth curve of the transformed pCW-lic vector, containing the wild-type E. coli alsE with IPTG to induce ectopic expression, and without IPTG, resulting in reduced gene expression. C, D Verification of the Clostridium Innocuum 6_1_30 alsE functionality. Keio ΔalsE::C. Innocuum alsE is the growth curve of the transformed Keio E. coli containing the C. innocuum alsE with IPTG to induce ectopic expression, and without IPTG, resulting in reduced gene expression. pCW-lic_C.Inn_alsE is the growth curve of the transformed pCW-lic vector, containing the C. innocuum alsE with IPTG to induce ectopic expression, and without IPTG, resulting in reduced gene expression. The error bars correspond to the standard error of OD600 on n = 3 technical replicates.
Limited distribution of alsE across E. coli strains
We then investigated the presence and absence of alsE across E. coli genomes, using a previously published pangenome consisting of 1324 E. coli genomes37. alsE was present in 598 out of 1324 E. coli genomes (45%), suggesting that alsE is a strain-specific gene and may not be present in every individual’s gut microbiome, despite the prevalence of E. coli exceeding 90% among humans38.
Identification of alsE in Clostridium innocuum
Given that Clostridium innocuum 6_1_30 grew on D-allulose as a sole carbon source, we investigated the genomic origins of its D-allulose metabolism. Based on our prior preliminary search results, we hypothesized that a homologous alsE was responsible for D-allulose metabolism in C. innocuum rather than a novel pathway. To conduct a comprehensive search for AlsE homologs in C. innocuum, we used the Klebsiella pneumoniae MGH 78578 AlsE (NCBI accession: GCA_000016305.1) as the query to search the C. innocuum genome. BLASTp revealed two AlsE homologs in C. innocuum, referred to as ci04257 and ci04568 (ci04257: 50.49% identity, 2.09e-76 e-value; ci04568: 35% identity, 2.89e-41 e-value). We examined the gene neighborhood of the two alsE candidates. The neighborhood of ci04257 consisted mainly of genes encoding hypothetical proteins. On the other hand, ci04568 was adjacent to phosphotransferase systems (PTS), which could potentially perform the phosphorylation and import step of D-allulose utilization (Fig. 1B). In addition, the neighboring genes are annotated with sugar metabolism functions, such as fructose bisphosphate aldolase. Therefore, we hypothesized that ci04568 encodes an enzyme that possibly performs a similar function to AlsE. Interestingly, the putative alsE gene neighborhood in C. innocuum is completely divergent from the alsE gene neighborhood in other species known to metabolize D-allulose, such as Klebsiella pneumoniae (Fig. 1B)31. Of note, this putative alsE was a core gene present in all 283/283 available C. innocuum genomes on NCBI, with all of them containing a nearly identical, if not identical, homolog to alsE in 6_1_30.
We then sought to functionally validate the candidate alsE in C. innocuum by cloning the gene into a pCW-lic vector backbone under an inducible tac promoter, resulting in the pCW-lic_C.inn_alsE construct to heterologously express C. innocuum’s alsE in E. coli (Supplementary Fig. 1). The plasmid was then transformed into the Keio collection E. coli alsE knockout. The transformed bacteria were subsequently inoculated in D-allulose-supplemented M9 and induced alsE’s expression using IPTG12. The Keio alsE knockout demonstrated no growth on D-allulose, while complementation of the C. innocuum gene into the knockout restored function, resulting in growth on D-allulose (Fig. 3C, D).
Few gut bacterial species encode AlsE
Once we experimentally verified the function of the C. innocuum and E. coli AlsE, we used ProkFunFind39, a bioinformatics pipeline, to systematically search for AlsE in bacteria. We used the experimentally verified AlsE protein sequences from K. pneumoniae MGH 78578, C. innocuum 6_1_30, and E. coli K-12 as queries to search the 85,202 non-redundant prokaryotic genomes from the Genome Taxonomy Database (GTDB) for species that contained homologs to AlsE. We used a more stringent filtering criterion compared to the preliminary search, filtering hits based on a 30% identity threshold and a maximum e-value of 1e-100. Our search revealed 116 putative bacterial species with AlsE (Supplementary Table 2, Fig. 4). The vast majority of these species were from the phylum Pseudomonadota (103/116), 10 were from the phylum Bacillota, and 3 were from Fusobacteriota. Out of those 116 species, only 35 are known to be part of the animal gut microbiota. Some known members of the human gut microbiome with AlsE include Klebsiella oxytoca, Enterobacter cloacae, and Serratia marcescens. Other species with AlsE that are not gut-associated are primarily isolated from plants and soil, including Klebsiella planticola40, Rahnella aquatilis41, and members of the Kosakonia genus42,43. Of note, all species that failed to grow on D-allulose in our initial investigation (Bacteroides cellulosilytious, Lactobacillus reuteri, Clostridium symbiosum, Bifidobacterium adolescentis, Ruminococcus gnavus) lacked alsE homologs, except for Escherichia coli, as previously discussed.
Species tree showing the taxonomic distribution of AlsE in microbial genomes from GTDB, colored by order. The species tree was generated by pruning the GTDB species tree using the Gotree prune command59.
Presence of alsE in the healthy adult gut microbiome
To investigate the prevalence of alsE in the human gut microbiome, we examined the presence and absence of alsE in 3079 healthy adult human gut microbiomes that passed quality checks (Supplementary Table 3). We built a reference database using both experimentally characterized and bioinformatically discovered alsE, with thresholds of 30% identity and 1e−100 e-value. We then aligned healthy adult human stool metagenomic reads downloaded from SRA to our alsE reference database and normalized the alignment counts into counts per million. To strike a balance between spurious hits and sensitivity, we considered any metagenomes with at least 1 count per million to contain alsE. 488 out of 3079 metagenomes met our threshold for alsE presence, approximately 15.8%.
We then investigated the prevalence of alsE in a metatranscriptomics cohort (HPFS, n = 675) to assess whether alsE is expressed in vivo44. We used a stringent criterion so that almost no mismatches are allowed (See methods). We found that 7/675 samples had at least 1 read mapped to our alsE reference, indicating that the gene is induced in vivo on rare occasions. However, we acknowledge that metatranscriptomics data can be incomplete and noisy due to high variability in microbial gene expression and the instability of bacterial RNA45,46.
Delineation of AlsE from Pentose-5-Phosphate 3-Epimerase
In order to elucidate the evolutionary origins of AlsE, we used a combination of phylogenetic analyses, ancestral state reconstruction, and sequence conservation. Using eggNOG-mapper (v6.0), we determined that AlsE belonged to the orthologous group COG0036 (pentose-5-phosphate 3-epimerase). We constructed a phylogenetic tree using the top 1323 homologs of the 3 experimentally verified AlsE protein sequences against all COG0036 sequences. Based on branch lengths and the presence of the experimentally verified AlsE, we identified a putative AlsE clade that contains 515 sequences. We were able to identify conserved amino acid changes in the putative AlsE node from the ancestral node (Fig. 5A), such as from G52 to S52, V134 to Y134, L142 to T142, and an N147 to D147 (Fig. 5B). Based on the high entropy in the alignment at these positions, these are conserved changes and may differentiate AlsE from other pentose-5-phosphate 3-epimerases.
A Gene tree constructed from putative AlsE sequences and related enzymes annotated as pentose-5-phosphate-3-epimerase (COG0036), showing a possible delineation of the AlsE clade and the location of Klebsiella pneumoniae, Clostridium innocuum, and Escherichia coli AlsE. B Diagram showing the change and conservation (entropy) of residues in the putative AlsE clade, as well as the GRASP predicted ancestral states of N129 (AlsE clade) and N91 (putative ancestral node). Residues with a predicted conserved change from the ancestral state are labeled with a star. C D-allulose docked to the AlphaFold2-predicted structure of C. innocuum 6_1_30 AlsE, colored by amino acid conservation via Consurf.
To determine putative catalytic residues, we performed a structural alignment of the AlphaFold2-predicted structure of C. innocuum AlsE to the crystal structure of E. coli AlsE (pdb: 3CT7). Based on the active site residues reported by Chan et al.29 in E. coli AlsE, we determined that the putative active site residues in both Clostridium innocuum and Klebsiella pneumoniae are similar based on the structural alignment (Fig. 5C)29. In C. innocuum, these putative residues are His 32, Asp 35, His 66, and Asp 175, which align to His 34, Asp 36, His 67, and Asp 176 in E. coli, respectively. Therefore, despite being distant homologs, C. innocuum likely shares similar catalytic residues to E. coli AlsE.
Discussion
Many widely used commercial alternative sweeteners, such as sugar alcohols, are associated with significant gastrointestinal discomfort12. This discomfort arises from the malabsorption of these sweeteners, leading to osmotic diarrhea, and from fermentation by gut microbes, which produce gas12. Consequently, there is an urgent need to identify alternative sweeteners, such as D-allulose, that do not cause gastrointestinal symptoms.
The presence of gut bacteria that can potentially metabolize D-allulose via D-allulose-6-phosphate 3-epimerase (AlsE) has significant implications for its use as a commercial alternative sweetener. Prior to our study, while D-allulose metabolism had been identified in some human gut bacteria, there had not been a systematic analysis of the presence, abundance, and distribution of enzymes involved in D-allulose metabolism across gut bacterial species - a knowledge gap that limited our understanding of how gut bacteria utilize this sweetener. In our study, we demonstrated that Clostridium innocuum can metabolize D-allulose through a homologous AlsE by examining its growth on D-allulose media. These findings shed light on the role of the gut microbiome in D-allulose metabolism.
During the initial investigation for gut microbial species capable of growing on D-allulose as a sole carbon source, C. innocuum 6_1_30 grew on D-allulose as a sole carbon source, while E. coli was unable to grow despite encoding alsE in its genome, which was intriguing. Past studies have shown that despite E. coli having alsE in its genome, its expression was too weak to support the production of D-allulose from D-fructose without genetic modifications21,47. This is consistent with our findings that the E. coli only grew on D-allulose when alsE was heterologously expressed, verified via the insertion of the respective alsE genes into the Keio alsE knockout mutant, which resulted in E. coli gaining the ability to use D-allulose as a sole carbon source. Interestingly, previous studies show that ribose and xylose act as regulators of the E. coli alsRBACE operon, suggesting that additional sugars can trigger the transcription of alsE36. Given these factors, further studies are necessary in order to characterize the transcriptional regulation of the alsRBACE in the gut.
Our findings show that AlsE protein homologs are only present in a few gut bacterial species. Out of 85,202 bacterial genomes from the GTDB, only 116 bacterial species were annotated to contain AlsE homologs. Our finding that E. coli cannot grow on D-allulose without heterologously expressing alsE suggests that some of these 116 species may not be able to metabolize D-allulose effectively. In addition, only 35 of these species are known to be present in animal gut microbiomes. These data suggest that D-allulose utilization might be restricted to a small number of species within the human gut microbiome. This finding is in alignment with the scarcity of D-allulose in nature. D-allulose has only been found in small quantities in a few plant species, such as Itea virginica and wheat48,49. Moreover, several of the bacterial species with putative alsE were primarily isolated from plants such as wheat or maize, including Klebsiella planticola40, Rahnella aquatilis41, and members of the Kosakonia genus42,43. We speculate that D-allulose metabolism may confer a metabolic advantage for these bacteria that live in plant-associated habitats, where exposure to D-allulose is more likely. Alternatively, alsE may have evolved primarily to confer D-allose metabolism, with D-allulose metabolism being incidental.
Notably, the limited presence of alsE in gut microbiome species suggests that D-allulose may serve as a valuable alternative to common sugar substitutes, which are known to cause gastrointestinal discomfort and alter microbiome composition. Previous studies have reported that D-allulose can be consumed in relatively high doses, up to 0.5 g/kg body weight, without causing significant gastrointestinal issues50. Thus, the limited metabolism of D-allulose by gut bacteria, combined with its low impact on gastrointestinal function, suggests that it may offer a promising solution for individuals seeking low-calorie sweeteners without adverse digestive effects. Of note, approximately 15.8% of human metagenomes analyzed contained alsE, suggesting that individual gut microbiomes may respond differently to D-allulose consumption. Diabetic individuals looking to cut their glucose consumption may benefit from individual microbiome testing to choose the alternative sweetener that is less likely to be utilized by their gut microbiome.
The presence of alsE as a core gene in Clostridium innocuum is interesting, given the rarity of D-allulose in its natural habitat. We speculate that alsE confers alternative functions, given that past studies show it can act on a variety of sugar substrates51. Although our study suggests there may be concerns of C. innocuum and Klebsiella pneumoniae blooms in the presence of allulose, a 12-week randomized, double-blind, placebo-controlled study demonstrated that D-allulose consumption at 15 g/day does not significantly affect gut microbiota diversity or pathogenic bacteria levels, even in individuals with C. innocuum52. This suggests that C. innocuum proliferation may be constrained by the complexity of the gut environment, where microbial competition, the availability of alternative electron acceptors, and environmental constraints may limit its growth despite the presence of D-allulose. Given the limited number of human-associated microbes capable of utilizing D-allulose, prolonged human consumption could possibly create a selective pressure favoring novel microbial populations, including opportunistic pathogens. While no evidence currently supports the emergence of novel D-allulose-metabolizing pathogens, longitudinal microbiome studies could provide insight into whether sustained consumption of D-allulose alters microbial community composition.
This study has a few limitations. Our study focuses on AlsE as an enzyme responsible for D-allulose metabolism, though we recognize the possibility of alternative mechanisms of D-allulose metabolism. To our knowledge, AlsE is the only currently known enzyme implicated in D-allulose metabolism in bacteria. However, there may be alternative mechanisms of bacterial D-allulose metabolism that are undiscovered, given the limited number of studies on the subject28,31. Due to this possibility of unknown alternative mechanisms, we cannot be certain of D-allulose’s impact on gut microbiome composition at large. Another limitation that warrants future investigation is the effect of D-allulose in more complex systems, such as in animal models or in fecal samples. Some studies have examined the effects of D-allulose in mouse models, finding overall positive effects, including increased endurance53, alteration of the gut microbiome leading to an improvement in high-fat diet-induced obesity24, and improvement of insulin resistance54. Lastly, future mutational analysis of AlsE’s catalytic residues could provide further insight into their functional roles and confirm their catalytic importance.
In conclusion, we shed light on the taxonomic distribution of AlsE in the gut microbiota. We discovered that Clostridium innocuum is capable of growing on D-allulose as a sole carbon source. In addition, while Escherichia coli has alsE, it cannot grow on D-allulose without heterologously expressing alsE, suggesting that many of these bacteria do not necessarily grow on D-allulose as a sole carbon source. A relatively small fraction of gut microbes are capable of utilizing D-allulose, making it a promising alternative to commercially available sugar substitutes, such as sugar alcohols.
Methods
Identification of D-allulose-6-phosphate 3-epimerases in the GTDB genomes
All representative genomes from the Genome Taxonomy Database (GTDB) (release r207) were downloaded, and protein sequences for each genome were predicted using Prokka (version 1.14.6)34,55. The Escherichia coli K-12, Klebsiella pneumoniae MGH78578, and Clostridium innocuum 6_1_30 D-allulose-6-phosphate 3-epimerase protein sequences were searched against 85,202 reference genomes using the ProkFunFind pipeline (v0.1.0)39. The hits were filtered based on an 1e−100 e-value and 30% identity thresholds, resulting in putative 126 AlsE amino acid sequences from 116 nonredundant genomes.
Phylogenetic analyses
Sequences from the GTDB assigned to COG0036 were identified using eggNOG-mapper (version 2.1.3)56. A BLASTp search was conducted (version 2.15.0+) using the identified D-allulose 6-phosphate 3-epimerases as queries against these identified sequences, setting a limit to the top 1305 hits. Sequence alignment was performed using Clustal Omega (version 1.2.4)57,58. Columns that have more than 97% gaps were removed to enhance alignment quality using Goalign (version 0.3.7)59. Phylogenetic analysis was carried out using IQ-TREE (version 2.1.2) with default parameters and model selection60. The reliability of the phylogenetic trees was evaluated using 1000 ultrafast bootstrap replicates. Trees were visualized using the Interactive Tree Of Life (iTOL)61.
Ancestral sequence reconstruction was performed on the AlsE tree using GRASP (version 04-May-2023), with default parameters62. We then manually inspected the tree to delineate AlsE from other Pentose-5-phosphate 3-epimerases. We calculated the entropy of the alignments using Goalign via the compute pssm function (v.0.3.7)59. The figures were created using the Python package logomaker (v0.08)63.
Growth of anaerobic bacteria
Bacterial strains were acquired from the NIH Biodefense and Emerging Infections Research Resources Repository (BEI). Each strain was inoculated from a glycerol stock and grown under anaerobic conditions over a 24-h period at 37 °C in an anaerobic chamber (Coy Laboratory Products) in Brain-Heart Infusion (BHI) broth (Research Products International, B11000) supplemented with glucose. 25 µL of the culture was inoculated into 4 mL of minimal media (M9) supplemented with 10 mg/mL carbon source (glucose or D-allulose)12. The end-point absorbance at 600 nm was measured with a Spectramax M5 plate reader, with end-point bacterial growth calculated using a ratio to the blank, with a ratio of 3 indicating significant growth.
Absorbance assay
The transformed Keio ΔalsE::C. Innocuum alsE & Keio ΔalsE::E. coli alsE constructs, and the untransformed native Keio E. coli BW25113 were shaken in Luria-Bertani (LB) supplemented with 100 µg/mL carbenicillin (GoldBio, C-103-25) and 50 µg/mL kanamycin (Bio Basic, KB0286) overnight at 37 °C. 25 µL of the overnight culture was inoculated in 2 mL triplicates of minimal media (M9) supplemented with 100 µM Isopropyl β- d-1-thiogalactopyranoside (IPTG, GoldBio, I2481C25), 100 µg/mL carbenicillin, 50 µg/mL kanamycin, 10 mg/mL Glucose (Sigma-Aldrich, 310042), 5 mg/mL Allose (Chem-Impex, 27965), and 10 mg/mL D-allulose12 (Chem-Impex, 32353). For kinetic measurements, 250 µL of each triplicate was aliquoted into a 96-well acrylic, clear bottom plate (Celltreat, 229592), sealed with a Breathe Easy membrane (Electron Microscopy Sciences, 70536-10), and incubated at 37 °C for 48–70 h, depending on the strain observed. The average absorbance at 600 nm was measured with a Spectramax M5 plate reader.
pCW-lic_C.inn_alsE & pCW-lic-E.coli_alsE constructs
In order to achieve ectopic expression of alsE from Clostridium innocuum 6_1_30 and E. coli JW276035 in the knockout mutants, the alsE gene was amplified and cloned into the pCW-lic vector backbone (Addgene plasmid # 26098; http://n2t.net/addgene:26098; RRID:Addgene_26098). Genomic DNA from C. innocuum and E. coli JW2760 was utilized in a polymerase chain reaction (PCR) using Phusion High-Fidelity DNA Polymerase (NEB, M0530S) with the specific primers listed in Supplementary Table 1. A Monarch PCR & DNA Cleanup Kit (NEB, T1030S) was used to purify the amplified product. The pCW-lic vector backbone was digested with restriction enzymes NdeI (NEB, R0111S) and HindIII-HF (NEB, R3104S), followed by purification with a Monarch PCR & DNA Cleanup Kit. A Gibson assembly was completed using Gibson Assembly Master Mix (NEB, E2611S) in accordance with the manufacturer’s instructions. The resulting constructs were stored at −20 °C until needed for use.
Keio-pCW construct
The alsE gene was amplified and cloned into the pCW-lic vector backbone under a tac promoter and transformed into the Keio collection alsE knockout as detailed above, with the same primers outlined in Supplementary Table 1. For the control, an empty pCW-lic vector was cloned into the Keio alsE knockout.
Chemical competency
The Keio collection alsE knockout was made competent using the Mix & Go! E. coli Transformation Kit and Buffer Set (Zymo, T3001) in accordance with the manufacturer’s protocol and stored at −80 °C until needed for use.
Transformation
Both constructs were independently transformed into the chemically competent Keio collection alsE knockout in accordance with the manufacturer’s protocol (Zymo, T3001). The resulting transformed cells were plated on LB agar plates supplemented with 100 µg/µL of carbenicillin. Successful transformation was validated via Oxford Nanopore sequencing by Plasmidsaurus.
Structural prediction and molecular docking
The structure for the Clostridium innocuum 6_1_30 AlsE was predicted using AlphaFold2 (v2.3.0)64. Binding pockets were predicted using fpocket (v4.0) with default parameters65. The pockets were compared to the homologous Escherichia coli AlsE (3CT7) to identify putative substrate binding regions and catalytic residues29. The structure for D-allulose (PubChem compound identifier: 50909805) was docked onto the predicted AlsE structure using AutoDock Vina (v4.2)66,67. The docking simulation was performed within 15 Å × 15 Å × 15 Å cubes centered on the center points of the chosen fpocket substrate binding pocket, with exhaustiveness set to 32. Docking results were visualized using PyMOL68. We used Foldseek to identify the top structural homolog69. The predicted AlsE protein structure was aligned with the E. coli 3CT7, and the putative catalytic residues were identified based on the previous work by Chan et al.29, using TM-Align29,70. Protein sequence conservation of AlsE was visualized using ConSurf based on the putative AlsE clade71,72.
Profiling of alsE presence in the gut
To build the reference database, we used alsE identified by ProkFunFind, which were filtered based on a threshold of e-value 1e−100 and percent identity 30%, resulting in a total of 126 sequences. We downloaded a collection of adult healthy metagenomic biosamples that passed basic quality control (n = 3410) from SRA, and then trimmed adapters with Trim-Galore with default settings (https://github.com/FelixKrueger/TrimGalore). The reads were then mapped to a human reference (assembly T2T-CHM13v2.0) to identify potential contaminants and removed them using Samtools (v1.16)73. We removed any samples with less than a million reads after curation, resulting in 3079 samples, and then aligned the remaining reads to the alsE reference database using bowtie2 (v2.4.1)74. The number of reads mapped to the alsE reference was summarized by normalizing the number of reads in the sample and then multiplying by one million to obtain counts per million (cpm). If a biosample had multiple SRRs, we concatenated the read counts and total reads across all SRRs per sample before calculating cpm. We considered samples with at least 1 cpm as containing alsE, to account for spurious alignments. For metatranscriptomics data, we mapped reads from the HPFS cohort (n = 675) to the alsE database with the parameter --min_score(‘C,0,0’) to enforce stringent alignment criteria.
Statistics and reproducibility
No statistical method was used to predetermine sample size. No data were excluded from the analysis. The experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. All genomic data analyzed in this study are available through the GTDB. Accession numbers can be found in the Supplementary Data file. The SRR accession numbers of all metagenomic samples are listed in the Supplementary Data and are available on the Sequence Read Archive (SRA) at NCBI. The pCW-lic vector backbone is available on Addgene (Addgene plasmid # 26098; http://n2t.net/addgene:26098; RRID:Addgene_26098). Source data for Figs. 2, 3, and S1 are provided with this paper (Supplementary Data 1–3).
References
Phelps, N. H. et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024).
Stierman, B. et al. National Health and Nutrition Examination Survey 2017 - March 2020 Pre-pandemic Data Files - Development of Files and Prevalence Estimates for Selected Health Outcomes. https://stacks.cdc.gov/view/cdc/106273 (2021) https://doi.org/10.15620/cdc:106273.
Klein, S., Gastaldelli, A., Yki-Järvinen, H. & Scherer, P. E. Why does obesity cause diabetes?. Cell Metab. 34, 11–20 (2022).
Mejia, E. & Pearlman, M. Natural alternative sweeteners and diabetes management. Curr. Diab Rep. 19, 142 (2019).
Chatterjee, S., Khunti, K. & Davies, M. J. Type 2 diabetes. Lancet 389, 2239–2251 (2017).
Ludwig, D. S., Peterson, K. E. & Gortmaker, S. L. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet Lond. Engl. 357, 505–508 (2001).
Bray, G. A., Nielsen, S. J. & Popkin, B. M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 79, 537–543 (2004).
Ludwig, D. S. & Ebbeling, C. B. The Carbohydrate-insulin model of obesity: beyond “Calories In, Calories Out”. JAMA Intern. Med. 178, 1098–1103 (2018).
Wee, M., Tan, V. & Forde, C. A comparison of psychophysical dose-response behaviour across 16 sweeteners. Nutrients 10, 1632 (2018).
Chattopadhyay, S., Raychaudhuri, U. & Chakraborty, R. Artificial sweeteners – a review. J. Food Sci. Technol. 51, 611–621 (2014).
Leśniewicz, A., Wełna, M., Szymczycha-Madeja, A. & Pohl, P. The identity and mineral composition of natural, plant-derived and artificial sweeteners. Molecules 28, 6618 (2023).
Hattori, K. et al. Gut microbiota prevents sugar alcohol-induced Diarrhea. Nutrients 13, 2029 (2021).
Witkowski, M. et al. The artificial sweetener erythritol and cardiovascular event risk. Nat. Med. 29, 710–718 (2023).
Oku, T. & Nakamura, S. Digestion, absorption, fermentation, and metabolism of functional sugar substitutes and their available energy. Pure Appl. Chem. 74, 1253–1261 (2002).
Suez, J. et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 185, 3307–3328.e19 (2022).
Noronha, J. C. et al. The effect of small doses of fructose and its epimers on glycemic control: a systematic review and meta-analysis of controlled feeding trials. Nutrients 10, 1805 (2018).
Matsuo, T. & Izumori, K. Effects of dietary D -Psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 70, 2081–2085 (2006).
Franchi, F. et al. Effects of D-allulose on glucose tolerance and insulin response to a standard oral sucrose load: results of a prospective, randomized, crossover study. BMJ Open Diab Res. Care 9, e001939 (2021).
Teysseire, F. et al. Metabolic effects and safety aspects of acute d-allulose and erythritol administration in healthy subjects. Nutrients 15, 458 (2023).
Braunstein, C. R. et al. A double-blind, randomized controlled, acute feeding equivalence trial of small, catalytic doses of fructose and allulose on postprandial blood glucose metabolism in healthy participants: the Fructose and Allulose Catalytic Effects (FACE) Trial. Nutrients 10, 750 (2018).
Taylor, J. E. et al. Awakening the natural capability of psicose production in Escherichia coli. Npj Sci. Food 7, 54 (2023).
Iida, T. et al. Failure of d-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metabolism 59, 206–214 (2010).
Kishida, K. et al. D-Allulose is a substrate of glucose transporter type 5 (GLUT5) in the small intestine. Food Chem. 277, 604–608 (2019).
Han, Y. et al. Alteration of microbiome profile by D-Allulose in amelioration of high-fat-diet-induced obesity in mice. Nutrients 12, 352 (2020).
Han, Y., Yoon, J. & Choi, M.-S. Tracing the anti-inflammatory mechanism/triggers of d-allulose: a profile study of microbiome composition and mRNA expression in diet-induced obese mice. Mol. Nutr. Food Res. 64, 1900982 (2020).
Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
Xia, Y. et al. Research advances of D-allulose: An overview of physiological functions, enzymatic biotransformation technologies, and production processes. Foods 10, 2186 (2021).
Kim, C., Song, S. & Park, C. The D-allose operon of Escherichia coli K-12. J. Bacteriol. 179, 7631–7637 (1997).
Chan, K. K., Fedorov, A. A., Fedorov, E. V., Almo, S. C. & Gerlt, J. A. Structural basis for substrate specificity in phosphate binding (β/α)8-Barrels: d-Allulose 6-Phosphate 3-Epimerase from Escherichia coli K-12. Biochemistry 47, 9608–9617 (2008).
Podschun, R. & Ullmann, U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 11, 589–603 (1998).
Blin, C., Passet, V., Touchon, M., Rocha, E. P. C. & Brisse, S. Metabolic diversity of the emerging pathogenic lineages of Klebsiella pneumoniae. Environ. Microbiol. 19, 1881–1898 (2017).
Martin, R. M. et al. Identification of pathogenicity-associated Loci in Klebsiella pneumoniae from Hospitalized Patients. mSystems 3, e00015–e00018 (2018).
McGinnis, S. & Madden, T. L. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 32, W20 (2004).
Parks, D. H. et al. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank-normalized and complete genome-based taxonomy. Nucleic Acids Res. 50, D785–D794 (2022).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, (2006).
Poulsen, T. S., Chang, Y.-Y. & Hove-Jensen, B. d-Allose Catabolism ofEscherichia coli: involvement of alsI and Regulation of als Regulon Expression by Allose and Ribose. J. Bacteriol. 181, 7126–7130 (1999).
Tantoso, E. et al. To kill or to be killed: pangenome analysis of Escherichia coli strains reveals a tailocin specific for pandemic ST131. BMC Biol. 20, 146 (2022).
Tenaillon, O., Skurnik, D., Picard, B. & Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 8, 207–217 (2010).
Dufault-Thompson, K. & Jiang, X. Annotating microbial functions with ProkFunFind. mSystems 9, e00036-24.
Bagley, S. T., Seidler, R. J. & Brenner, D. J. Klebsiella planticola sp. nov.: A new species of enterobacteriaceae found primarily in nonclinical environments. Curr. Microbiol. 6, 105–109 (1981).
Berge, O. et al. Rahnella aquatilis, a nitrogen-fixing enteric bacterium associated with the rhizosphere of wheat and maize. Can. J. Microbiol. 37, 195–203 (2011).
Berger, B. et al. Successful formulation and application of plant growth-promoting Kosakonia radicincitans in Maize Cultivation. BioMed. Res. Int. 2018, 6439481 (2018).
Berger, B., Baldermann, S. & Ruppel, S. The plant growth-promoting bacterium Kosakonia radicincitans improves fruit yield and quality of Solanum lycopersicum. J. Sci. Food Agric. 97, 4865–4871 (2017).
Mehta, R. S. et al. Stability of the human faecal microbiome in a cohort of adult men. Nat. Microbiol. 3, 347–355 (2018).
Tan, L. et al. Analysis of bacterial transcriptome and epitranscriptome using nanopore direct RNA sequencing. Nucleic Acids Res. 52, 8746–8762 (2024).
Franzosa, E. A. et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl. Acad. Sci. 111, (2014).
Guo, Q. et al. Metabolically engineered Escherichia coli for conversion of D-Fructose to D-Allulose via Phosphorylation-Dephosphorylation. Front. Bioeng. Biotechnol. 10, 947469 (2022).
Miller, B. S. & Swain, T. Chromatographic analyses of the free amino-acids, organic acids and sugars in wheat plant extracts. J. Sci. Food Agric. 11, 344–348 (1960).
Ayers, B. J. et al. Iteamine, the first alkaloid isolated from Itea virginica L. inflorescence. Phytochemistry 100, 126–131 (2014).
Han, Y. et al. Gastrointestinal tolerance of D-Allulose in healthy and young adults. a non-randomized controlled trial. Nutrients 10, 2010 (2018).
Patel, S. N., Kaushal, G. & Singh, S. P. A Novel d-Allulose 3-Epimerase gene from the Metagenome of a thermal aquatic habitat and d-Allulose production by Bacillus subtilis Whole-Cell Catalysis. Appl. Environ. Microbiol. 86, e02605–e02619 (2020).
Park, H. et al. Impact of D-allulose consumption on Enteric pathogens in human gut Microbiota: A randomized controlled trial study. J. Funct. Foods 122, 106555 (2024).
Liu, B. et al. D-Allulose improves endurance and recovery from exhaustion in male C57BL/6J Mice. Nutrients 14, 404 (2022).
Bae, H. R. et al. D-Allulose ameliorates dysregulated macrophage function and mitochondrial NADH homeostasis, mitigating obesity-induced insulin resistance. Nutrients 15, 4218 (2023).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Cantalapiedra, C. P., Hernández-Plaza, A., Letunic, I., Bork, P. & Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 38, 5825–5829 (2021).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinforma. 10, 421 (2009).
Lemoine, F. & Gascuel, O. Gotree/Goalign: toolkit and Go API to facilitate the development of phylogenetic workflows. NAR Genomics Bioinforma. 3, lqab075 (2021).
Minh, B. Q. et al. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Letunic, I. & Bork, P. Interactive Tree of Life (iTOL) v6: recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 52, W78–W82 (2024).
Leslie, R., O’Donnell, C. J. & Johnson, A. D. GRASP: analysis of genotype-phenotype results from 1390 genome-wide association studies and corresponding open access database. Bioinforma. Oxf. Engl. 30, i185–i194 (2014).
Tareen, A. & Kinney, J. B. Logomaker: beautiful sequence logos in Python. Bioinformatics 36, 2272–2274 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Le Guilloux, V., Schmidtke, P. & Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinforma. 10, 168 (2009).
Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: New Docking methods, expanded force field, and Python Bindings. J. Chem. Inf. Model. 61, 3891–3898 (2021).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 31, 455–461 (2010).
PyMOL. [(accessed on 22 July 2024)]. Available online: http://www.pymol.org/pymol.
van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. 42, 243–246 (2024).
Zhang, Y. & Skolnick, J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Ben Chorin, A. et al. ConSurf-DB: An accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Sci. Publ. Protein Soc. 29, 258–267 (2020).
Li, H. et al. The sequence alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Acknowledgements
This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov) and the UMIACS cluster at the University of Maryland’s Center for Bioinformatics and Computational Biology (https://www.umiacs.umd.edu/). pCW-LIC was a gift from Cheryl Arrowsmith (Addgene plasmid # 26098; http://n2t.net/addgene:26098; RRID:Addgene_26098) B.H. is supported by startup funding from the University of Maryland and NIH grant 1R35GM155208-01. A.J. and X.J. are supported by the Intramural Research Program of the NIH, National Library of Medicine.
Author information
Authors and Affiliations
Contributions
B.H. and X.J. conceptualized and supervised the project. All authors performed the experiments and analyzed the data. G.M.N., A.J., C.R. and M.G. wrote the original draft of the manuscript. All authors reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Harald Carlsen and Sara Di Rienzi for their contribution to the peer review of this work. Primary Handling Editors: Silvio Waschina and Tobias Goris. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Minabou Ndjite, G., Jiang, A.K., Ravel, C.T. et al. Gut microbial utilization of the alternative sweetener, D-allulose, via AlsE. Commun Biol 8, 970 (2025). https://doi.org/10.1038/s42003-025-08391-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08391-3