Abstract
Sociocultural changes in recent decades have promoted fathers’ involvement in childcare, which is crucial for the brain and behavioral plasticity of offspring. The study elucidates the effect and mechanism of father’s companionship on defensive attack behavior of adult male offspring mice. The study comprises a father companionship group, where offspring cohabited with sire and dam until weaning, and a control group, where offspring cohabited solely with the dam until weaning. The study shows that father’s companionship increases defensive attack behavior of adult offspring. Additionally, the metabolite L-aspartic acid is upregulated in the father’s companionship group compared to the control group in male offspring, and intracerebroventricular micro-injection of L-aspartic acid confirms its impact on defensive attack behavior. C-Fos immunohistochemistry reveals that c-Fos expression in lateral periaqueductal gray (LPAG) is activated. Subsequently, micro-injection of L-aspartic acid into LPAG increases defensive attack behavior. Additionally, 16S rRNA sequencing reveals a higher abundance of Bilophila and a lower abundance of Bifidobacterium in the father companionship group, which correlates with L-aspartic acid levels, suggesting a gut-brain axis mechanism for the effect of father companionship on defensive attack behavior.
Similar content being viewed by others
Introduction
The early life period, from the prenatal stage to the first two years postnatal1, is a critical window for the plasticity of brain and behavior2. Brain development is highly sensitive to environmental stimulation3. Parental stimulation of the immature, still-developing infant brain would be expected to promote the maturation of neural functions, with long-lasting effects on offspring behavior4. While the importance of maternal care on psychological and behavioral development has been well-established, the impact of paternal care on offspring behavior has been less explored5,6. The empirical evidence from 30 countries shows an increasing involvement of fathers in childcare over recent decades7. Recent studies have begun to unveil the importance of father involvement in the nurturing of offspring, highlighting its role in modulating stress responses and the social behavior of offspring8. For instance, a study reported that the absence of a father’s care was associated with increased anxiety levels in offspring9. Early-life exposure to high levels of father’s care is found associated with delayed epigenetic aging10. It is evident that a father’s care has beneficial effects on the child’s development.
Within the domains of human and animal societies, fathers and mothers assume different roles in shaping the behavior of their offspring11. Fathers are more likely to confront challenges and threats, and their behavior can have a profound impact on offspring’s defensive behavior and social adaptability12. Defensive attack behavior is an instinctive behavior that enables individuals to respond swiftly to perceived threats, protecting themselves from harm, and is crucial for survival and environmental adaptation13. The influence of early-life father’s companionship on the defensive attack behavior of offspring is of significant importance, no studies have yet focused on the impact of father’s companionship in early life on the defensive attack behavior of offspring, and the mechanisms underlying this effect remain unclear, necessitating further investigation.
Experimental approaches to the study of the influence of mammalian fathers on offspring have primarily focused on species exhibiting biparental care, such as Peromyscus californicus and Microtus ochrogaster14. However, father care is rare among mammals, observed in only 5–10% of mammals15, which has limited broader investigations into fathers’ effects on offspring development. Notably, previous studies have reported that widely-used laboratory rodents, male ICR strain mice, display sire caregiving behaviors upon exposure to pups16,17. This study uses ICR mice to investigate how sire care shapes offspring’s defensive attack behavior and its underlying mechanisms.
Existing literature has demonstrated that the periaqueductal gray (PAG) plays a central role in mediating defensive attack behavior. PAG is located in the midbrain and is the main structure involved in integrating various sensory inputs related to threat detection and coordinating appropriate defensive and aggressive behavioral outputs18. Different columns within the PAG play distinct roles in modulating defensive behaviors19,20. For instance, the ventrolateral PAG (VLPAG) is primarily involved in defense responses of freezing and immobility18, and the lateral PAG (LPAG) has been found to be critical for prey detection and attack behavior21.
L-aspartic acid is an excitatory amino acid that plays a significant role in neurotransmission22. It has been demonstrated that L-aspartic acid binds to N-methyl-D-aspartate (NMDA) receptors and plays a modulatory role in the excitatory pathway23. Microinjections of L-aspartic acid into the PAG have been shown to evoke defensive behaviors24, supporting its role in modulating defensive responses.
This study investigates the impact of father’s companionship on the defensive attack behavior in offspring mice, with a focus on the neurochemical mechanisms mediated by L-aspartic acid. We hypothesize that father’s companionship upregulates endogenous L-aspartic acid levels, leading to activation of the PAG and consequent modulation of defensive attack behavior. Furthermore, given the emerging recognition of gut microbiota as a critical regulator of neurobehavioral development25, we explore whether father’s companionship induces alterations in gut microbial composition and its correlation with changes in L-aspartic acid levels. This investigation helps to elucidate potential gut-brain axis mechanisms underlying the long-term effects of father’s companionship on offspring defensive behavior.
The study found that early-life father’s companionship increased the defensive attack behavior of adult offspring. In addition, the metabolite L-aspartic acid was upregulated in male offspring in the father’s companionship group compared to the control group, and intracerebroventricular injection of L-aspartic acid confirmed its effect on defensive attack behavior. Furthermore, C-Fos immunohistochemistry showed that c-Fos expression was activated in the LPAG. Following these findings, L-aspartic acid was injected into the LPAG, which increased defensive attack behavior. The study further performed 16S rRNA gene sequencing of the gut microbiota to investigate the microbiota regulated by the father’s companionship and its potential correlations with serum metabolomics. 16S rRNA gene sequencing revealed a higher abundance of Bilophila and a lower abundance of Bifidobacterium in the father’s companionship group, which correlated with L-aspartic acid levels, suggesting a gut-brain axis mechanism for the effect of the father’s companionship on the plasticity of defensive attack behavior.
Results
Early-life father’s companionship increased the defensive attack behavior of adult offspring
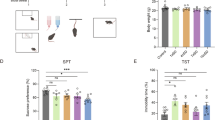
As shown in the experimental design (Fig. 1a). The defensive attack test of the offspring was conducted at 8 weeks post-birth. The effect of father’s companionship on the latency of biting (Fig. 1b), frequency of biting (Fig. 1c), and biting duration (Fig. 1d) were analyzed. The results indicated that father companionship increased the frequency of biting (two-way ANOVA, effects of father companionship: F (1,68) = 4.370, P = 0.040, η2 = 0.060; effect of sex: F (1,68) = 0.493, P = 0.485, η2 = 0.007; interaction effect: F (1, 68) = 1.238, P = 0.270, η2 = 0.018) and biting duration (two-way ANOVA, effects of father companionship: F (1,68) = 5.331, P = 0.024, η2 = 0.073; effect of sex: F (1, 68) = 0.241, P = 0.625, η2 = 0.004; interaction effect: F (1,68) = 0.803, P = 0.374, η2 = 0.012) of male and female offspring mice.
a Experimental design. In the father companionship group, the sire lived with the dam and offspring from conception through weaning. In the control group, only the dam lived with offspring during this period. b The effect of father’s companionship on the latency of biting (two-way ANOVA, effects of father companionship: F (1, 68) = 2.185, P = 0.144, η2 = 0.031; effect of sex: F (1, 68) = 1.766, P = 0.188, η2 = 0.025; interaction effect: F (1, 68) = 0.236, P = 0.629, η2 = 0.003). c Father’s companionship increased the frequency of biting (two-way ANOVA, effects of father companionship: F (1, 68) = 4.370, P = 0.040, η2 = 0.060; effect of sex: F (1, 68) = 0.493, P = 0.485, η2 = 0.007; interaction effect: F (1, 68) = 1.238, P = 0.270, η2 = 0.018). (d) Father’s companionship increased the biting duration (two-way ANOVA, effects of father companionship: F (1, 68) = 5.331, P = 0.024, η2 = 0.073; effect of sex: F (1, 68) = 0.241, P = 0.625, η2 = 0.004; interaction effect: F (1, 68) = 0.803, P = 0.374, η2 = 0.012). n = 17-19 per group. Values are shown as mean ± standard error. FC father companionship, PD pregnancy day, PND postnatal day. * P <0.05.
Early-life father’s companionship elevated L-aspartic acid in male offspring
The LC-MS/MS analysis of serum was conducted to obtain an overview of the metabolic profile of offspring mice. The metabolic profile of male offspring was provided in Fig. 2. Figure 2a shows the expression abundance of quality control for samples. The orthogonal partial least-squares discriminant analysis (OPLS-DA) was used to identify the differences in metabolites between the father’s companionship and the control group among male offspring. According to the OPLS-DA analysis, significant differences in metabolites between the two groups were identified (Fig. 2b). As shown in Fig. 2c, the data with |log2FoldChange | > 0.263 and P < 0.05 were indicative of significant metabolic differences, and a total of 658 differential metabolites were identified, with 325 metabolites significantly up-regulated and 333 metabolites significantly down-regulated (Supplementary Data 1). In addition, the functions of differential metabolites were analyzed using KEGG pathway enrichment and displayed in a bubble chart (Fig. 2d). Larger bubbles correspond to pathways with more differential metabolites. KEGG pathway analysis highlighted enrichment in D-Amino acid metabolism, cysteine and methionine metabolism, and ABC transporters. Notably, L-aspartic acid was involved in D-Amino acid metabolism, cysteine and methionine metabolism, and ABC transporters, and 3-sulfinoalanine was involved in cysteine and methionine metabolism (Supplementary Table 1). Figure 2e shows the metabolites that were up-regulated and down-regulated. The abundance of L-aspartic acid was elevated in the father’s companionship group compared to the control group (t(10) = 4.380, P = 0.001, t test), whereas the abundance of 3-sulfinoalanine was reduced (t(10) = 9.374, P < 0.001, t test) (Fig. 2f–g). The study performed correlation analysis between metabolites and defensive attack behavior (Supplementary Data 2). L-aspartic acid was found positively associated with the frequency of biting (r = 0.706, P = 0.013) (Fig. 2i), and 3-sulfinoalanine was found negatively associated with the frequency of biting (r = –0.617, P = 0.037) (Fig. 2l). In addition, the study analyzed metabolic profiles in female offspring (Supplementary Fig. 1), revealing no significant differences in L-aspartic acid (t(10) = 1.380, p = 0.198, t test) or 3-sulfinoalanine (t(10) = 1.315, p = 0.218, t test) between father companionship and control groups. These findings suggest that the biological mechanisms mediating the effects of father companionship on increased defensive attack behavior may distinct between male and female offspring. This mechanistic divergence likely originates in inherent biological dimorphism between the sexes. Given the profound sexual dimorphism in neurobiological pathways, the present study focuses specifically on elucidating the mechanisms underlying increased defensive attack behavior in male offspring.
a Expression abundance of quality control for samples. b OPLS-DA plot between the father companionship and control group. c Volcano plot showing the differential metabolites between groups. d Bubble diagram of the KEGG enrichment pathway for significantly regulated differential metabolites. e The Lollipop map shows differences in up-regulated and down-regulated metabolites in the father companionship group compared with the control group. f The abundance of L-aspartic acid was elevated in the father’s companionship group compared to the control group (t(10) = 4.380, P = 0.001, t test). n = 6 per group. g The abundance of 3-sulfinoalanine was reduced in the father’s companionship group compared to the control group (t(10) = 9.374, P < 0.001, t test). n = 6 per group. h–j Correlation between the abundance of L-aspartic acid and defensive attack behavior. L-aspartic acid was positively associated with the frequency of biting (r = 0.706, P = 0.013). k–m Correlation between the abundance of 3-sulfinoalanine and defensive attack behavior. 3-sulfinoalanine was negatively associated with the frequency of biting (r = –0.617, P = 0.037). n = 12 (6 in the intervention group and 6 in the control group). FC father companionship, KSAHI Kaposi sarcoma-associated herpesvirus infection. PAC-DAP (2-Phenylacetyl) (2 R)-2,5-diaminopentanoate. * P < 0.05; **P < 0.01; ***P < 0.001.
Intracerebroventricular injection of L-aspartic acid increased defensive attack behavior in adult male mice
The study performed intracerebroventricular injection of L-aspartic acid and 3-sulfinoalanine to ascertain their impact on defensive attack behavior. Male mice administered 0.2 μmol/μL L-aspartic acid at a volume of 3 μL displayed significantly longer biting durations than those in the saline control group (one-way ANOVA, F (2, 21) = 4.069, P = 0.032; Fisher’s LSD post-test, p = 0.011) (Fig. 3d). In contrast, intracerebroventricular injections of 3-sulfinoalanine had no significant effect on defensive attack behavior (Fig. 3f–h).
a The experimental design of intracerebroventricular injection of L-aspartic acid and defensive attack behavior test. b–d Latency of biting (one-way ANOVA, F (2, 21) = 1.250, P = 0.307), frequency of biting (one-way ANOVA, F (2, 21) = 2.275, P = 0.127), and biting duration (one-way ANOVA, F (2, 21) = 4.069, P = 0.032) after intracerebroventricular injection of L-aspartic acid. Mice treated with 0.2 μmol/μL L-aspartic acid for 3 μL exhibited longer biting duration compared to the saline control group (Fisher’s LSD post-test, p = 0.011). n = 8 per group. e The experimental design of intracerebroventricular injection of 3-sulfinoalanine and defensive attack behavior test. f–h Latency of biting (t (14) = 0.238, P = 0.815, t test), Frequency of biting (t (14) = 0.646, P = 0.529, t test), and biting duration (t (14) = 0.226, P = 0.825, t test) after intracerebroventricular injection of 3-sulfinoalanine. n = 8 per group. Values are shown as mean ± standard error. * P < 0.05.
Micro-injection of L-aspartic acid in LPAG increased defensive attack behavior in adult male mice
Previous studies showed that PAG mediated defensive attack behavior. We analyzed the c-fos expression among dorsomedial PAG, dorsolateral PAG, LPAG, and VLPAG, to investigate the potential brain regions underlying the effects of the father’s companionship. The defensive attack test was applied to the male offspring mice, and brain samples were collected for the assessment of c-Fos expression 90 min following the behavior test. The c-Fos immunohistochemistry showed that the c-Fos expression in LPAG was significantly higher in the father’s companionship group compared with the control group (t (16) = 2.366, P = 0.031, t test) (Fig. 4d).
a–e c-Fos expression in the LPAG was significantly higher in the father’s companionship group than in the control group (t (16) = 2.366, P = 0.031, t test), whereas no significant difference was observed in the DMPAG (t (16) = 0.346, P = 0.734, t test), DLPAG (t (16) = 0.809, P = 0.430, t test), and VLPAG (t (16) = 0.759, P = 0.459, t test); (3 mice per group were analyzed with 3 sections per mouse). f The experimental design of injection of L-aspartic acid in LPAG and defensive attack behavior test. g–i Latency of biting (one-way ANOVA, F (2, 23) = 0.164, P = 0.850), frequency of biting (one-way ANOVA, F (2, 23) = 1.042, P = 0.369), and biting duration (one-way ANOVA, F (2, 23) = 3.988, P = 0.033) after injection of L-aspartic acid in LPAG. Mice treated with 1 μmol/μL L-aspartic acid for 0.2 μL exhibited longer biting duration compared to the saline control group (Fisher’s LSD post-test, P = 0.010). n = 8–9 per group. j The experimental design of intraperitoneal injection of L-aspartic acid and defensive attack behavior test. k–m The latency of biting (one-way ANOVA, F (3, 33) = 1.582, P = 0.212), frequency of biting (one-way ANOVA, F (3, 33) = 1.266, P = 0.302), and biting duration (one-way ANOVA, F (3, 33) = 4.176, P = 0.013) after intraperitoneal injection of L-aspartic acid. Mice treated with 100 mg/kg (Fisher’s LSD post-test, P = 0.045) or 200 mg/kg (Fisher’s LSD post-test, P = 0.025) L-aspartic acid exhibited longer biting duration compared to the saline control group. n = 8–10 per group. Values are shown as mean ± standard error. DLPAG dorsolateral periaqueductal gray, DMPAG Dorsomedial periaqueductal gray. FC father companionship, LPAG lateral periaqueductal gray, VLPAG ventrolateral periaqueductal gray. PND, postnatal day. Scale bar, 200 µm. * P < 0.05.
The serum metabolomic profile of male offspring indicated that father’s companionship up-regulated L-aspartic acid, and the L-aspartic acid was positively associated with the defensive attack behavior. The study further explored whether the L-aspartic acid in LPAG mediates the increased defensive attack behavior. The male mice received administration of L-aspartic acid in LPAG (Fig. 4f), and the defensive attack test was performed 20 min post-injection. The results indicated that the administration of L-aspartic acid (1 μmol/μL for 0.2 μL) in LPAG significantly increased the biting duration when compared to the control group that received saline (one-way ANOVA, F (2, 23) = 3.988, P = 0.033; Fisher’s LSD post-test, P = 0.010) (Fig. 4i).
Systemic L-aspartic acid treatment mimics the effects of father’s companionship on defensive attack behavior in adult male mice
The study further investigated the effect of systemic L-aspartic acid treatment on the defensive attack behavior to mimic the effects of father’s companionship. The intraperitoneal injection of L-aspartic acid was performed on adult male mice and the defensive attack test was conducted (Fig. 4j). The results indicated that the injection of L-aspartic acid with 100 mg/kg or 200 mg/kg significantly increased the biting duration when compared to the control group that received a saline injection (one-way ANOVA, F (3, 33) = 4.176, P = 0.013) (Fig. 4m).
Early-life father’s companionship changed the gut microbiota composition of adult male offspring
Given that previous research has established the role of gut microbiota in modulating psychosocial behaviors, the study further conducted 16S rRNA gene sequencing to investigate the gut microbiota influenced by the father’s companionship and to explore its potential associations with serum metabolomics. The gut microbial diversity was assessed, encompassing both α-diversity and β-diversity measures. The ACE index, Chao1 index, Shannon index, and PD-whole-tree index indicate that father companionship and control groups did not show the difference in α-diversity among male offspring (Fig. 5a). The analysis of the beta diversity based on the principal coordinates analysis (PCoA) and the Bray-Curtis dissimilarity revealed the difference in β-diversity among male offspring (Fig. 5b), suggesting that father’s companionship modulates gut microbiota composition. The average relative abundance of the gut microbiome at the genus levels in the father companionship group and the control group were analyzed (Fig. 5c). The relative abundances of Bacteroides and Lachnoclostridium were increased in the father companionship group compared to the control group, while Muribaculaceae was decreased among male offspring. Meanwhile, Linear discriminant analysis (LDA) effect size (LEfSe) analysis for taxonomic composition gut microbiota was shown in Fig. 5d. The LEfSe analysis showed that Bilophila was the predominant bacteria in the father’s companionship group, and Bifidobacterium was the predominant bacteria in the control group. The correlation analysis between gut microbiota and defensive attack behavior was performed among male offspring (Supplementary Data 3), the results showed that the relative abundance of Bilophila was found positively associated with the frequency of biting (r = 0.757, P = 0.006) (Fig. 5f), and the relative abundance of Bifidobacterium was found negatively associated with the frequency of biting (r = −0.690, P = 0.016) (Fig. 5i). In addition, the gut microbiota of female offspring showed that the relative abundance of Bilophila was higher in the father companionship group compared with the control group (t(10) = 3.549, p = 0.005, t test). There were no between-group differences in the relative abundance of Bifidobacterium among female offspring mice (t(10) = 1.563, p = 0.149, t test) (Supplementary Fig. 2).
a The ACE index, Chao1 index, Shannon index, and PD-whole-tree index indicate that father’s companionship and control groups did not show differences in α-diversity. b PCoA plot indicated the difference in β-diversity among male mice. c Relative abundance of gut microbial community at the genus level. d Linear discriminant analysis (LDA) effect size (LEfSe) analysis for taxonomic composition gut microbiota. e–g Correlation between the relative abundance of Bilophila and defensive attack behavior. The relative abundance of Bilophila was positively associated with the frequency of biting (r = 0.757, P = 0.006). h–j Correlation between the relative abundance of Bifidobacterium and defensive attack behavior. The relative abundance of Bifidobacterium was negatively associated with the frequency of biting (r = -0.690, P = 0.016). FC Father companionship.
The study further analyzed the correlations between the gut microbiota and serum metabolites among male offspring, the results showed that the relative abundance of Bilophila was positively associated with L-aspartic acid, while the relative abundance of Bifidobacterium was negatively associated with L-aspartic acid (Fig. 6a, b).
a Heatmap showing the correlation of serum metabolomics and gut bacteria. b Network showing correlation of serum metabolomics and gut bacteria. The relative abundance of Bilophila was positively associated with L-aspartic acid, while the relative abundance of Bifidobacterium was negatively associated with L-aspartic acid. SSANYPA, (2S)-2-[(2S)-2-Aminopropanoyl]-naphthalen-2-ylamino] pentanedioic acid; ACAD,1-[(5-Amino-5-carboxypentyl) amino]-1-deoxyfructose.
Discussion
In recent years, shifts in human sociocultural dynamics have resulted in a growing number of fathers engaging in direct childcare roles. This development has sparked a renewed interest in exploring the mechanisms and impacts of paternal involvement in childcare7. The behavioral, neural, and molecular implications of father’s companionship on offspring are becoming more evident and are a subject of growing research attention. The current study used the ICR mice model to explore the neural and molecular basis of the father’s companionship on offspring behavior.
Defensive behaviors are adaptive responses that are crucial for survival when confronted with threats13. This study shows that early-life father’s companionship increases the defensive attack behavior of offspring. This aligns with the growing body of literature highlighting the importance of early-life experiences in shaping behavioral and neurological outcomes26.
The observation that L-aspartic acid upregulates in male but not female offspring in response to father companionship, despite both sexes exhibiting increased defensive attack behavior, suggests that the underlying biological mechanisms mediating this effect may be distinct between male and female offspring. This mechanistic difference likely arises from inherent biological dimorphisms between the sexes27. Extensive evidence documents neurobiological distinctions between female and male mice. For instance, a study comparing inflammatory and behavioral responses to chronic stress revealed different immune processes mediating stress-induced depression-like behaviors and cognitive impairments in females versus males, despite comparable behavioral outcomes28. Another study on sex-dependent mechanisms of pain hypersensitivity found that a neural circuit from the medial preoptic area to the VLPAG mediates pain hypersensitivity in male mice but not in female mice29. Considering the sexual dimorphisms, it is plausible that different mechanisms mediate the effect of father companionship on defensive attack behavior in male and female offspring. The present study focuses specifically on elucidating the neurobiological mechanisms underlying increased defensive attack behavior in male offspring.
The study found that c-Fos expression in LPAG is significantly higher in the father’s companionship group compared to the control group, suggesting that LPAG may play a crucial role in mediating the impact of the father’s companionship on the defensive attack behavior of male offspring. This finding align with established evidence that LPAG activation drives attack responses21.
L-aspartic acid was found positively associated with defensive attack behavior. These findings suggest that peripherally modulated L-aspartic acid levels may influence neural circuits regulating defensive attack behavior30. Currently, the permeability of L-aspartic acid across the blood-brain barrier remains to be fully elucidated. Previous studies showed that the neutral amino acid transporter alanine-serine-cysteine transporter 2 mediates L-aspartic acid translocation across the blood-brain barrier31,32. Furthermore, elevated plasma L-aspartic acid levels were shown to increase its brain concentrations by 30-60% in the previous study33. In addition, it has been demonstrated that L-aspartic acid binds to NMDA receptors and serves as a neuromodulator30. L-aspartic acid may enhance NMDA receptor-mediated excitatory neurotransmission in the LPAG, potentially increasing defensive attack behavior34. The increase in defensive attack behaviors following both LPAG microinjection and intraperitoneal administration of L-aspartic acid supports its role as a neurochemical modulator of LPAG activity.
Animal studies have demonstrated that the gut microbiota plays a pivotal role in mediating psychosocial behaviors35, such as anxiety, cognitive performance, and social communication. Our results align with previous research indicating that gut microbiota may be a mediator of the effects of father’s companionship on the behavior of offspring. The present study reveals a significant upregulation of Bilophila in the father’s companionship group compared to the control group, with a positive association between the relative abundance of Bilophila and defensive attack behavior. A previous study showed that the relative abundances of Bilophila were significantly reduced in the gut microbiota of autistic subjects compared to that of the neurotypical subjects36. Bilophila was also found linked with a reduced risk of intracranial aneurysm37. Our study also revealed that the relative abundance of Bifidobacterium was reduced in the father’s companionship group as compared to the control group, and this reduction was negatively correlated with defensive attack behavior. Consistent with our findings, a Netherlands study reported that a higher abundance of Bifidobacterium was associated with lower aggressive behavior38. In addition, the microbiota and metabolomic correlation analysis revealed a positive association between L-aspartic acid levels and Bilophila, and a negative correlation between L-aspartic acid and Bifidobacterium, These findings indicate that Bilophila and Bifidobacterium may regulate L-aspartic acid levels and influence defensive attack behavior. However, the underlying mechanism connecting microbiota and L-aspartic acid levels requires further investigation. Generally, the gut microbiota is increasingly recognized for its role in modulating neurophysiology and neurobehavior development25. The specific mechanisms behind the gut microbiome-brain axis warrant further exploration, and understanding these interactions could potentially be used to improve offspring outcomes39.
There are limitations in this study that could be addressed in future research. The study did not assess the correlation between specific sire care behaviors and offspring defensive attack behavior. Existing literature demonstrates that sire can influence offspring development through direct caregiving behaviors. For instance, studies have shown that sire pup retrieval behavior significantly affects the development of aggressive behavior in offspring14. Additionally, sire may indirectly influence offspring behavior by modulating dam-offspring interactions40. Research has found that sire presence enhances physical contact between offspring and both parents, leading to accelerated physical and behavioral development41. Future studies are suggested to systematically analyze in which way sire influences the behavior of offspring. In addition, the current study focuses on the mechanisms underlying the effect of father’s companionship on the increased defensive attack behavior in male offspring, but the mechanisms in female offspring remain to be explored. Future research is needed to elucidate the specific mechanisms by which father companionship influences defensive attack behavior in female offspring.
In conclusion, this study elucidated the neurobiological mechanism underlying the effect of early-life father’s companionship on the increased defensive attack behavior of adult male offspring. We have identified a pivotal role for LPAG and L-aspartic acid in the modulation of defensive attack behavior of male offspring. These results highlight the significant impact of the father’s companionship during the early-life stages on the behavioral development of offspring. The study paves the way for further research into the complex interplay between father’s companionship, neurophysiology, and offspring behavior, offering valuable insights into the biological mechanisms that shape defensive strategies in offspring.
Methods
Experimental animals
Adult ICR mice were purchased from Beijing HFK Bioscience Co., Ltd. and housed in a controlled environment. Temperature was controlled between 20 and 25 °C, relative humidity between 50 and 55%, and a 12-h light/dark cycle. Food and water were always available to the mice.
Experimental setup
Six experiments were conducted in this study (Supplementary Fig. 3). Experiment 1 established the father companionship and control model to investigate the effects of father companionship on the defensive attack behavior of offspring. Then, the offspring were sacrificed for serum metabolomics and gut microbiota analyses. Experiment 2 examined the effect of intracerebroventricular injection of L-aspartic acid on defensive attack behavior. Experiment 3 assessed the effect of intracerebroventricular injection of 3-sulfinoalanine on defensive attack behavior. Experiment 4 utilized c-Fos immunohistochemistry to analyze neuronal activation in the PAG. Building on the findings from the above experiments. Experiment 5 and Experiment 6 performed LPAG injection of L-aspartic acid and intraperitoneal administration of L-aspartic acid, respectively, to confirm the effects of L-aspartic acid on defensive attack behavior.
Father companionship and control model
The mice were housed at a ratio of one male to two females per cage. After the female mice were pregnant, one pregnant female from each cage was randomly moved to a new cage, while the remaining female and male mice stayed in the original cage. There are two groups in the study, namely the father’s companionship group and the control group. In the father’s companionship group, the sire and dam lived together, and the offspring from these pairs were classified as the intervention group9. The sire and dam lived with their offspring until weaning, which occurs 21 days after birth10. In the control group, one pregnant dam was housed per cage, and the offspring from the dam were classified as the control group. Only the dam lived with the offspring from conception to the weaning in the control group. The protocol was approved by the Animal Care and Use Committee of Hebei Medical University. All animal experiments were conducted in accordance with the ARRIVE guidelines. No adverse events were observed. Upon completion of the experiments, mice were humanely euthanized. We have complied with all relevant ethical regulations for animal use.
Defensive attack test
The defensive attack test of the offspring was conducted at 8 weeks post-birth. The behavior test was conducted during the dark cycle (9:00–16:00). The mice were transferred to the test room and were habituated to the room conditions for 1 h before the defensive attack test was started. The test was performed using a plastic dummy snake designed with a head-like alligator clip to deliver mechanical stimulation42. The tails of the mice were clamped by the alligator clip for 60 s to simulate persistent noxious mechanical stimulation. The defensive attack responses of the mice to the dummy snake in the enclosed arena (25 cm × 25 cm × 30 cm) were analyzed, including the latency to biting, frequency of biting, and biting duration43. The mice were chosen randomly from each group, and two observers blind to the experimental groups quantified the latency to biting, frequency of biting, and biting duration during the 60-s stimulation period using digital timers. Following each trial, the enclosed arena was disinfected with 75% alcohol to eliminate any residual odors left by the previous subject.
Serum LC-MS metabolomics analysis
In terms of sample preparation for serum metabolomics analysis, a volume of 100 μL of each sample was transferred to a 1.5 mL Eppendorf tube. Subsequently, 300 μL of an ice-cold mixture of methanol and acetonitrile (2:1, v/v) was added to each sample, followed by vortex mixing for 1 min. The samples were then subjected to ultrasonic extraction in an ice-water bath for 10 min and subsequently stored at –20 °C for 30 min. The extracts were centrifuged at 4 °C at 13,000 rpm for 20 min. The supernatants (150 μL) from each tube were collected using crystal syringes, filtered through 0.22 μm microfilters, and transferred to LC vials. Quality control samples were prepared by pooling aliquots from all samples to create a composite sample.
The study utilized an ACQUITY UPLC I-Class plus system from Waters Corporation (Milford, USA), coupled with a Q-Exactive mass spectrometer featuring a heated electrospray ionization (ESI) source, provided by Thermo Fisher Scientific (Waltham, MA, USA). This setup was employed for metabolic profiling under both positive and negative ESI modes. An ACQUITY UPLC HSS T3 column (1.8 μm, 2.1 × 100 mm) was used for chromatographic separation in both ionization modes. The mobile phase was a binary gradient elution system comprising solvent A (water with 0.1% formic acid, v/v) and solvent B (acetonitrile with 0.1% formic acid, v/v). The gradient program was as follows: 0.01 min, 5% B; 2 min, 5% B; 4 min, 30% B; 8 min, 50% B; 10 min, 80% B; 14 min, 100% B; 15 min, 100% B; 15.1 min, 5% B and 16 min, 5% B. The flow rate was set at 0.35 mL/min, and the column temperature was 45 °C. Samples were maintained at 10 °C throughout the analysis. The injection volume was 3 μL.
16S rRNA gene sequencing
Immediately following collection, cecal contents were frozen and stored at –80 °C. Bacterial DNA was extracted from these samples using the DNeasy PowerSoil kit (Qiagen, Hilden, Germany). The concentration and integrity of the extracted DNA were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively. PCR amplification targeting the V3-V4 hypervariable regions of the bacterial 16S rRNA gene was conducted in a 25 μL reaction volume with universal primer pairs: 343 F (5′-TACGGRAGGCAGCAG-3′) and 798 R (5′-AGGGTATCTAATCCT-3′).
The reverse primer contained a sample barcode and both primers related to an Illumina sequencing adapter. The Amplicon quality was visualized using gel electrophoresis. The quality of the Amplicons was assessed through gel electrophoresis. The PCR-generated products underwent purification using Agencourt AMPure XP beads supplied by Beckman Coulter Co., USA, and their quantities were determined with the Qubit dsDNA assay kit. The concentrations were subsequently adjusted for sequencing. The sequencing process was carried out on an Illumina NovaSeq6000 platform, which involved two paired-end read cycles of 250 bases each (Illumina Inc., San Diego, CA).
Raw sequencing data were in FASTQ format. Paired-end reads were preprocessed using Cutadapt software to identify and cut off adapters. Following trimming, paired-end reads were filtering low-quality sequences, denoised, merged and detect and cut off the chimera reads using DADA2 with the default parameters of QIIME2. Ultimately, the software generated representative reads and an ASV abundance table. The representative read for each ASV was selected using the QIIME 2 package. All representative reads were annotated and blasted against the Silva database Version 138 using the q2-feature-classifier with default settings.
Immunohistochemistry
The defensive attack test was applied to the male offspring mice, and brain samples were collected for the assessment of c-Fos expression 90 min following the behavior test. Male offspring were perfused with saline followed by 4% paraformaldehyde solution. The brains were extracted and immersed in 4% paraformaldehyde for post-fixation for 16 h at 4 °C. Subsequently, the brains were immersed in 30% sucrose solution and dehydrated for 2 days at 4 °C. Thereafter, the brains were sectioned coronally into slices of 30 μm in thickness using a freezing microtome.
For c-Fos immunostaining, the slices were first rinsed three times with PBS44,45, followed by a blocking step with a solution containing 0.3% Triton X-100 and 3% donkey serum in PBS for 50 min at room temperature. Afterward, the slices were rinsed three more times with PBS. The primary c-Fos antibody incubation (rabbit mAb at a 1:1000 dilution, Cell Signaling Technology, 2250) was carried out at 4 °C for 16 h. Subsequently, the slices were washed four times with PBS before being incubated with the secondary antibody (anti-rabbit IgG at a 1:1000 dilution, Cell Signaling Technology, 4414) for 1.5 h at room temperature. After four additional washes with PBS, the sections were incubated with DAPI for 7 min. Finally, the sections were washed three times with PBS before being mounted on slides and coverslipped. The images were obtained using a Pannoramic SCAN digital scanner (3D Histech Ltd., Budapest, Hungary). Digitalized images were analyzed using the Pannoramic Slide Viewer software (3D Histech Ltd., Budapest, Hungary). To quantify the density of c-Fos-positive cells within the PAG, we analyzed three coronal sections per mouse across three mice per experimental group. Positive cells were counted manually by an investigator blind to the experimental conditions. The density of positive cells of a section was calculated as the number of positive cells divided by the area of targeted brain regions. The cell density was then statistically compared across groups to evaluate any significant differences in c-Fos expression46.
Surgery and drug micro-injection
For intracerebroventricular drug micro-injection and LPAG micro-injection, eight-week-old male ICR mice were anesthetized with 1% sodium pentobarbital (100 mg/kg, Sigma, St. Louis, MO, USA). The head of mice was then fixed in a stereotaxic frame (Reward; Shenzhen, China) where a hole was drilled at stereotaxic coordinates (ventricle: AP -0.7 mm; ML –1 mm from bregma; LPAG: AP –4.72 mm; ML –0.55 mm from bregma) on the surface of the skull. The cannula was grasped by a clamp and descended until its tip contacted the designated areas in the brain (ventricle: AP –0.7 mm; ML –1 mm; DV –1.7 mm from bregma; LPAG: AP –4.72 mm; ML –0.55 mm; DV –2.5 mm from bregma). Subsequently, dental acrylic cement and two small anchor screws were utilized to fix the cannula. The mice were then returned to their cages to recuperate for 1 week, before undergoing micro-injection of medication.
In the intracerebroventricular micro-injection with 3-sulfinoalanine (Aladdin, Shanghai, China), the solution was prepared by dissolving 3-sulfinoalanine in saline and adjusting the pH to 7 with NaOH. The mice were randomly assigned to two groups: one receiving 3-sulfinoalanine at 0.2 μmol/μL, and the other receiving saline. Each group was injected with a volume of 5 μL47. The defensive attack test was conducted 20 min after the injection.
For intracerebroventricular micro-injection with L-aspartic acid (Aladdin, Shanghai, China), L-aspartic acid was dissolved in saline, and the pH was adjusted to 7 using NaOH solution. The male mice were randomly assigned to three groups: one receiving L-aspartic acid at a concentration of 0.2 μmol/μL, another at a concentration of 0.1 μmol/μL, and a control group administered with saline. Each group was injected with 3 μL. The defensive attack test was performed 20 min post-injection.
For LPAG L-aspartic acid micro-injection, the male mice were randomly assigned to three groups: one group received L-aspartic acid at a concentration of 1 μmol/μL with a volume of 0.2 μL, another at 0.5 μmol/μL with a volume of 0.2 μL, and a control group was injected with 0.2 μL of saline. The defensive attack test was performed 20 min post-injection.
Intraperitoneal administration
For intraperitoneal administration of L-aspartic acid, male ICR mice were randomly assigned to four groups that received L-aspartic acid at a dosage of 200 mg/kg, 100 mg/kg, 50 mg/kg, and a control group with saline. Each subject received a 0.4 ml volume of the respective solution. The defensive attack test was performed 30 min post-injection48.
Statistics and reproducibility
Data analysis and statistical graphing were performed using SPSS 26.0 and GraphPad Prism 8 software. Descriptive analysis was presented as means ± standard error. One-way analysis of variance (ANOVA), two-way ANOVA, or t test were used for comparisons between groups as appropriate. The correlation analysis was performed using Spearman correlation. A two-tailed test was used for all statistical tests. The difference was considered statistically significant when p < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in the paper and the supplementary data.
References
Sly, P., Blake, T. & Islam, Z. Impact of prenatal and early life environmental exposures on normal human development. Paediatr. Respir. Rev. 40, 10–14 (2021).
Ahmad, S. et al. Multifaceted atlases of the human brain in its infancy. Nat. Methods 20, 55–64 (2023).
StGeorge, J. M., Wroe, J. K. & Cashin, M. E. The concept and measurement of fathers’ stimulating play: a review. Attach Hum. Dev. 20, 634–658 (2018).
Condon, E. M., Dettmer, A., Baker, E., McFaul, C. & Stover, C. S. Early life adversity and males: Biology, behavior, and implications for fathers’ parenting. Neurosci. Biobehav Rev. 135, 104531 (2022).
Savage, L. -É, Tarabulsy, G. M., Pearson, J., Collin-Vézina, D. & Gagné, L.-M. Maternal history of childhood maltreatment and later parenting behavior: A meta-analysis. Dev. Psychopathol. 31, 9–21 (2019).
Tegegne, A. M., Habitu, Y. A., Ferede, Y. A. & Fentie, E. A. Completion of maternal and child health continuum of care and associated factors in West Gondar Zone, North West Ethiopia, 2023: a community based cross sectional study. BMC Pregnancy Childbirth 24, 734 (2024).
Feldman, R., Braun, K. & Champagne, F. A. The neural mechanisms and consequences of paternal caregiving. Nat. Rev. Neurosci. 20, 205–224 (2019).
Puglisi, N., Rattaz, V., Favez, N. & Tissot, H. Father involvement and emotion regulation during early childhood: a systematic review. BMC Psychol. 12, 675 (2024).
Ferreyra, E. et al. Biparental care in C57BL/6J mice: Effects on adolescent behavior and alcohol consumption. Psychopharmacol. (Berl.) 237, 1841–1850 (2020).
Danoff, J. S. et al. Father’s care uniquely influences male neurodevelopment. Proc. Natl. Acad. Sci. USA 120, e2308798120 (2023).
Langenhof, M. R. & Komdeur, J. Why and how the early-life environment affects development of coping behaviours. Behav. Ecol. Sociobiol. 72, 34 (2018).
Abraham, E. & Feldman, R. The neurobiology of human allomaternal care; implications for fathering, coparenting, and children’s social development. Physiol. Behav. 193, 25–34 (2018).
Tseng, Y.-T., Schaefke, B., Wei, P. & Wang, L. Defensive responses: behaviour, the brain and the body. Nat. Rev. Neurosci. 24, 655–671 (2023).
Frazier, C. R. M., Trainor, B. C., Cravens, C. J., Whitney, T. K. & Marler, C. A. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Hormones Behav. 50, 699–707 (2006).
Saltzman, W. & Ziegler, T. E. Functional significance of hormonal changes in mammalian fathers. J. Neuroendocrinol. 26, 685–696 (2014).
Cai, W. et al. Involvement of the dopamine system in paternal behavior induced by repeated pup exposure in virgin male ICR mice. Behav. Brain Res. 415, 113519 (2021).
Liang, M. et al. Pairmate-dependent pup retrieval as parental behavior in male mice. Front. Neurosci. 8, 186 (2014).
Zhang, H. et al. The contribution of periaqueductal gray in the regulation of physiological and pathological behaviors. Front. Neurosci. 18, 1380171 (2024).
Zhao, H. et al. Control of defensive behavior by the nucleus of Darkschewitsch GABAergic neurons. Natl. Sci. Rev. 11, nwae082 (2024).
Wang, H. et al. Direct auditory cortical input to the lateral periaqueductal gray controls sound-driven defensive behavior. PLoS Biol. 17, e3000417 (2019).
Yu, H. et al. Periaqueductal gray neurons encode the sequential motor program in hunting behavior of mice. Nat. Commun. 12, 6523 (2021).
Cavallero, A., Marte, A. & Fedele, E. l-Aspartate as an amino acid neurotransmitter: mechanisms of the depolarization-induced release from cerebrocortical synaptosomes. J. Neurochemistry 110, 924–934 (2009).
Nadler, J. V. Aspartate release and signalling in the hippocampus. Neurochem Res. 36, 668–676 (2011).
Bandler, R., Depaulis, A. & Vergnes, M. Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav. Brain Res 15, 107–119 (1985).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol 19, 55–71 (2021).
Volkow, N. D. et al. The HEALthy Brain and Child Development Study (HBCD): NIH collaboration to understand the impacts of prenatal and early life experiences on brain development. Dev. Cogn. Neurosci. 69, 101423 (2024).
Galea, L. A. M., Choleris, E., Albert, A. Y. K., McCarthy, M. M. & Sohrabji, F. The promises and pitfalls of sex difference research. Front Neuroendocrinol. 56, 100817 (2020).
Medina-Rodriguez, E. M., et al. Comparison of inflammatory and behavioral responses to chronic stress in female and male mice. Brain Behav. Immun. 106, 180–197 (2022).
Zhang, M. et al. A neural circuit for sex-dependent conditioned pain hypersensitivity in mice. Nat. Commun. 16, 3639 (2025).
Holeček, M. Aspartic acid in health and disease. Nutrients 15, 4023 (2023).
Oppedisano, F., Pochini, L., Galluccio, M. & Indiveri, C. The glutamine/amino acid transporter (ASCT2) reconstituted in liposomes: transport mechanism, regulation by ATP and characterization of the glutamine/glutamate antiport. Biochim Biophys. Acta 1768, 291–298 (2007).
Tetsuka, K., Takanaga, H., Ohtsuki, S., Hosoya, K. & Terasaki, T. The l-isomer-selective transport of aspartic acid is mediated by ASCT2 at the blood-brain barrier. J. Neurochem 87, 891–901 (2003).
Toth, E. & Lajtha, A. Elevation of cerebral levels of nonessential amino acids in vivo by administration of large doses. Neurochem Res. 6, 1309–1317 (1981).
Bandler, R., Depaulis, A. & Vergnes, M. Indentification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav. Brain Res. 15, 107–119 (1985).
Abavisani, M., Faraji, N., Ebadpour, N., Kesharwani, P. & Sahebkar, A. Beyond digestion: Exploring how the gut microbiota modulates human social behaviors. Neuroscience 565, 52–62 (2024).
Strati, F. et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5, 24 (2017).
Zhang, H., Jiang, X., Li, A. & Wang, X. Causal associations between gut microbiota and cerebrovascular diseases. World Neurosurg. 183, e587–e597 (2024).
Voulgari-Kokota, A., Falcao Salles, J. & Schoemaker, R. G. Aggression shapes the gut microbiome; a study in rats. PLoS One 19, e0312423 (2024).
Shoubridge, A. P. et al. The gut microbiome and mental health: advances in research and emerging priorities. Mol. Psychiatry 27, 1908–1919 (2022).
Braun, K. & Champagne, F. A. Paternal influences on offspring development: behavioural and epigenetic pathways. J. Neuroendocrinol. 26, 697–706 (2014).
Piovanotti, M. R. A. & Vieira, M. L. Presence of the father and parental experience have differentiated effects on pup development in Mongolian gerbils (Meriones unguiculatus). Behav. Process. 66, 107–117 (2004).
Xie, Z. et al. Mechanically evoked defensive attack is controlled by GABAergic neurons in the anterior hypothalamic nucleus. Nat. Neurosci. 25, 72–85 (2022).
Huang, B. et al. Postweaning intermittent sleep deprivation enhances defensive attack in adult female mice via the microbiota-gut-brain axis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 130, 110915 (2024).
Li, X. et al. Neuronal TCF7L2 in Lateral Habenula Is Involved in Stress-Induced Depression. Int J. Mol. Sci. 25, 12404 (2024).
Shi, D.-D. et al. Predictable maternal separation confers adult stress resilience via the medial prefrontal cortex oxytocin signaling pathway in rats. Mol. Psychiatry 26, 7296–7307 (2021).
Sun, Y.-X. et al. The causal involvement of the BDNF-TrkB pathway in dentate gyrus in early-life stress-induced cognitive deficits in male mice. Transl. Psychiatry 13, 173 (2023).
Ferko, A. P. Cysteine sulfinic acid can enhance the central depressant effect of ethanol in mice. Pharm. Biochem. Behav. 39, 653–657 (1991).
Onat, F., Toker, F., Aslan, N., Oktay, S. & Berkman, K. Antinociceptive effect of D-aspartic acid in mice. Pharm. Biochem Behav. 51, 715–719 (1995).
Acknowledgements
This study was funded by the Postdoctoral Foundation of Hebei Medical University, the Hebei Province Central Leading Local Science and Technology Development Fund Project (246Z7746G), the National Natural Science Foundation of China (82171536), the open project of Hebei Key Laboratory of Forensic Medicine (JYFY-23ZR004), the Science Research Project of Hebei Education Department (BJ2025219), Beijing-Tianjin-Hebei Basic Research Cooperation Special Project (H2023206902, 23JCZXJC00130, J230014), the Medical Science Research Project of Hebei (20250178).
Author information
Authors and Affiliations
Contributions
H.S. designed the study. Y.L. wrote the manuscript. Y.L., Y.L., B.L., X.Y., X.L., X.L., X.L., X.Y., and Y.Z. performed the experiments and analyzed the data. Haishui Shi and Li Song revised the manuscript. The final version of the manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Evren Eraslan and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Liu, Y., Yin, X. et al. Effect and mechanism of father’s companionship on defensive attack behavior of adult male offspring mice. Commun Biol 8, 1106 (2025). https://doi.org/10.1038/s42003-025-08531-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08531-9