Abstract
Insufficient infiltration of cytotoxic T cells into solid tumors remains a critical obstacle in cancer immunotherapy. Despite extensive efforts to comprehend the mechanisms governing this limited infiltration, few studies have focused on the evolution of T cell motility behavior after co-culture. In this study, we combined quantitative cell trajectory analysis, computational modeling, and bulk/single-cell RNA sequencing to systematically characterize the impact of cell interactions. We reveal that in a 2.5D co-culture system with multiple cancer-cell clusters, cancer-specific T cells exhibit increased directional persistence, which facilitates their efficient searching of cancer-cell clusters. Additionally, these T cells form prolonged interactions with cancer cells, which is the most crucial factor for their accumulation on cancer-cell clusters. Furthermore, post-interaction, a cancer-cell subpopulation displays immunosuppressive traits, reducing T cell attractant expression, and undergoing epithelial-to-mesenchymal transition. These findings offer valuable insights into improving immunotherapy efficacy and tackling T cell infiltration challenges in solid tumors.
Similar content being viewed by others
Introduction
Cytotoxic T lymphocytes (CTLs, CD8+) have emerged as a pivotal force in cancer immunotherapy due to their remarkable cytotoxic capabilities1,2. However, challenges persist in optimizing the effectiveness of immunotherapies3,4,5. For instance, cancer cells can exploit mutations to evade immune surveillance, thus diminishing the efficacy of immunotherapy6,7. Previous studies have underscored the impact of extrinsic components, such as tumor-associated macrophages8,9, myeloid-derived suppressor cells10,11, cancer-associated fibroblasts12,13, and the extracellular matrix14,15, in hindering the infiltration of CTLs in solid tumors, thereby reducing patient response to immunotherapy16,17. Despite these findings, few studies have delved into intrinsic factors, such as the motility behaviors and gene expression profiles of CTLs with different tumor-recognition abilities.
These observations highlight the necessity of investigating CTL motility within the tumor microenvironment. While recent studies have emphasized the essential role of collective behaviors of CTLs in tumor killing18,19, several questions remain unanswered. For instance, what strategies do CTLs employ when navigating multi-tumor clusters, balancing between exploration and exploitation20? Additionally, what are the temporal changes in gene expression among CTLs and cancer cells and how do they further affect the behavior and interactions of these cells? Answers to these questions will be crucial for enhancing T-cell engineering and design to address obstacles in cancer immunotherapy21,22.
Technically, it is challenging to characterize cellular behaviors in vivo, particularly due to the necessity of intravital high-resolution real-time imaging techniques and the constraints on imaging duration23,24. To address these challenges, several 3D in vitro co-culture systems, such as spheroids, scaffold-based models, or microfluidics platforms have been developed to investigate the interactions between T cells and tumors18,25,26, as well as T cells and extracellular matrix fibers14,27,28. These systems serve as valuable tools for studying T cell mobility patterns and their interactions within controlled environments. While 3D systems offer a more physiologically relevant environment, they often require more sophisticated imaging equipment and considerable technical expertise29,30. Consequently, 2.5D systems have been developed to study collective cellular behavior30,31, despite certain apparent limitations in fully replicating the physical and biochemical microenvironment of real tissues. Nevertheless, 2.5D systems could provide a viable alternative, requiring more accessible imaging technology and facilitating detailed investigations of cellular behaviors.
In this study, we harnessed a 2.5D in vitro co-culture platform to quantify the influence of cancer-T cell interactions on the spatiotemporal dynamics of CTL motility. Using an integrated approach combining live-cell microscopy, quantitative image analysis, computational modeling, and transcriptomic profiling, we decoded the temporal motility patterns of CTLs in proximity to and atop multiple cancer-cell clusters post interaction. Furthermore, we quantified the impact of these spatiotemporal dynamics on CTL’s cancer-seeking behavior and their accumulation on cancer-cell clusters. In addition, gene expression analysis revealed potential molecular drivers underlying CTL behavioral adaptations and diverse tumor-immune responses. Our research underscores the critical role of cancer-T cell interactions in governing CTL movement patterns, offering valuable insights into the spatial organization of CTLs encircling cancer cell clusters within solid tumors.
Results
A time-lapse pipeline for both motility and gene-expression of CTLs
To investigate the time-dependent interactions between cancer cells and cancer-specific CTLs and their impact on CTL mobility patterns, spatial distribution, as well as gene-expression, we developed a comprehensive pipeline, integrating time-lapse imaging, cell tracking and trajectory analysis, computational modeling based on cell mobility statistics, time-resolved gene expression profiling of both cancer cells and CTLs (Fig. 1A).
A Schematic representation illustrating the integrated workflow for experimental set-up, motility analysis, and gene expression profiling. B Diagrams of the co-culture experiments and representative 2D microscopic images for the WT and OVA groups. Scale bars, 100 µm. C Quantification of the number of recruited CTLs on cancer cells over time for WT or OVA groups, manually counted within a 15 µm surrounding region every 30 min (see Methods). For each time point, the total number of CTLs residing on cancer cells within the imaging field was counted (n = 28 for the OVA groups and n = 9 for the WT groups from one independent experiment, mean ± s.e.m.). D Counts of live cancer cells over time in different regions of the imaging field for the WT and OVA groups (n = 9, mean ± s.e.m.). E Interaction time of CTLs on cancer clusters for B16-OVA and B16-WT groups (n = 217 vs. 225). C–E Statistical significance was determined using the Two-sided Wilcoxon rank-sum test: p < 0.001 (***), p < 0.01 (**), p < 0.05 (*), p > 0.05 (n.s.). For (C), the p values at t = 0 h, 6 h, 12 h, and 18 h were 2.13E-01, 8.26E-02, 4.96E-05 and 3.72E-04, respectively. For (D), the p values at t = 0 h, 6 h, 12 h, and 18 h were 3.32E-02, 5.22E-03, 4.11E-05 and 4.11E-05, respectively. For (E), the p-value is 4.46E-41.
We employed a co-culture model in a 2.5D µ-slide chamber, combining pre-activated OT-1 CD8+ T cells with either OVA-expressing B16 melanoma cells (B16-F10-OVA) or wild-type B16 cells (B16-F10-WT) as controls (Fig. 1A, B, Methods). After embedding the CTLs in a thin collagen matrix overlaying a sub-confluent tumor monolayer (Supplementary Fig. 1A), time-lapse imaging was performed for 24 h at 2-min intervals, allowing continuous observation of cell dynamics within a ~5.27 µm optical section. Our interest lies in understanding the behavior of T cells within such thin layer of collagen-fiber network around cancer-cell clusters (Supplementary Fig. 1A), and the following time-lapse imaging analysis is specifically tailored to track their movement around and on tumor cells within the focal imaging plane.
Qualitatively, under the microscope, the most apparent observation was cancer cells could be recognized by CTLs labeled with the red fluorescence in Fig. 1B (Supplementary Fig. 1B, Supplementary Movie 1 and Supplementary Movie 2). Not surprisingly, the density of CTLs increased more rapidly over time in co-culture with OVA cancer cells than in co-culture with WT cancer cells (Fig. 1C and Supplementary Data 1). In addition, the WT cancer cells demonstrated continued growth in the co-culture with CTLs, whereas the number of OVA cancer cells gradually decreased (Fig. 1D and Supplementary Data 1).
To elucidate the faster accumulation of CTLs shown in Fig. 1C, we first hypothesized that it might result from the faster growth or swifter mobility of CTLs co-culture with OVA cancer cells. However, no significant differences in CTL doubling time or migration velocity were observed in the two co-culture conditions (Supplementary Fig. 1C, Supplementary Fig. 1D and Supplementary Data 1). Alternatively, the accumulation of CTLs could be attributed to their reduced mobility around the OVA cancer cells or a more pronounced chemotactic motion towards OVA cancer cells. Intriguingly, manually tracking CTLs on both types of cancer cells revealed an extended engagement between CTLs and OVA cancer cells (Fig. 1E). Given the constraints of manually handling CTL tracks, in the following analysis, we turned to an automated tracking strategy to investigate the potential chemotaxis behavior of CTLs.
Quantitative motility patterns of antigen-specific T cells
To quantitatively investigate the motility behaviors of CTLs in the co-culture with OVA and WT cancer cells, we focused on characterizing their motility patterns before and after CTLs reached cancer cell clusters. The workflow for time-lapse image processing and data analysis is illustrated in Fig. 2A, B (Methods, Supplementary Fig. 2). A selection criterion was applied to include only T cells exhibiting continuous motion across at least 10 frames, thus guaranteeing the consistency and validity of motion analysis within the imaging plane. Utilizing the automated image analysis pipeline, we obtained a total of 4276 and 5146 CTL tracks from five biological replicates in the OVA and WT groups, respectively. Subsequent analyses were conducted based on these CTL trajectories and the spatial boundaries of cancer-cell clusters (Methods).
A Pipeline for processing time-lapse images and tracking T cells. B Schematic representation of data analysis, including dwell time, mean square displacement, directional bias, directional persistence of CTLs towards cancer cell clusters. C Density of CTLs on cancer clusters (cells/mm2) over time, demonstrating a significant T cell accumulation in OVA groups compared to WT groups (n = 5). Statistical significance was determined using the Two-sided Wilcoxon rank-sum test: p < 0.001 (***), p < 0.01 (**), p < 0.05 (*), p > 0.05 (n.s.). The p-values at 0 h, 6 h, 12 h, and 18 h were 1, 1, 7.94E-03 and 7.94E-03, respectively. D Dwell time distribution of T cells on cancer-cell clusters in Series 1, divided into 6-h intervals. The left panel (0–6 h) shows n = 149 adhesion events for the WT groups and n = 110 events for the OVA groups; the right panel (6–12 h) shows n = 131 events for the WT groups and n = 223 events for the OVA groups. The distribution for 12–18 h is shown in Supplementary Fig. 3D. E Step size distributions of CTLs over 6-hour intervals: Left panel (0–6 h), middle panel (6–12 h), and right panel (12–18 h). The p-values for (E) (left, middle, and right) are 9.9E-23, 3.3E-26 and 1.8E-70, respectively. Data represent results from n = 5 independent experiments. D, E Statistical significance was determined using the Two-sided two-sample Kolmogorov–Smirnov test. KS statistic D and p-value are shown inside the plotting boxes. F Directional bias and G persistence distributions of CTLs movement towards the nearest cancer cell boundary within three time-intervals. Statistical significance was determined using the two-sided unpaired Student’s t test with p < 0.001 (***), p < 0.01 (**), p < 0.05 (*). [a, b) defines the value range where x ≥ a and x < b. C–G Red lines (or bars) represent OVA groups, and cyan lines (or bars) represent WT groups. C, F, G, n = 5 independent experiments, mean±s.e.m. The p-values for (F, G) are provided in Supplementary Data 8.
Firstly, we quantified the behaviors of CTLs when their fluorescent signals overlapped with those of cancer cells, indicating direct physical contact and interactions between CTLs and cancer cells (Methods). A marked increase in CTL accumulation on OVA cancer-cell clusters was observed within 6–12 h post co-culture (Fig. 2C, Supplementary Fig. 3A, B and Supplementary Data 2), consistent with earlier manual counting (Fig. 1C). Notably, the total number of CTLs per unit area remained similar between the two co-culture systems over time (Supplementary Fig. 3B), suggesting that enhanced accumulation of CTLs on OVA cancer-cell clusters is unlikely due to differential CTL proliferation, in agreement with prior observations. Although we occasionally observed CTLs “hopping” onto cancer clusters from other z-planes, quantification revealed that such events were rare—approximately 30 times less frequent than those approaching cancer cells within the thin fiber network layer (Supplementary Fig. 3C).
Furthermore, to evaluate CTL-cancer cell interaction dynamics, we quantified the dwell time of CTLs on cancer-cell clusters (Fig. 2D; Methods). The distribution closely followed a heavy-tailed power-law pattern. Notably, within the first 6 h, dwell time distributions of CTLs were similar between co-cultures with OVA and WT cancer cells (Kolmogorov-Smirnov test, D = 0.15, p = 0.096). However, during 6–18 h of the co-culture, CTLs in the OVA condition exhibited a noticeably longer-tailed distribution (D = 0.25, p = 6.2 × 10−5), with some CTLs remaining on cancer-cell clusters for up to 100 minutes (Fig. 2D, Supplementary Fig. 3D–H and Supplementary Data 2). These findings suggest that once CTLs recognized and interacted with cancer cells, they tended to engage for prolonged periods.
Secondly, to investigate the motility behaviors of CTLs before they reached cancer-cell clusters, we studied the step sizes, mean square displacement (MSD), directional bias and directional persistence of CTLs whose fluorescent signals did not overlap with cancer cells (Methods). We observed that the difference in step-size distribution between the two groups gradually increased over time, with the KS statistic rising from D = 0.05 at 0–6 h to D = 0.07 during 12–18 h, as determined by the two-sample Kolmogorov–Smirnov test (p < 0.001, Fig. 2E). After 12 h, CTLs co-cultured with OVA cancer clusters exhibited shorter step sizes compared to those with WT cancer clusters, particularly within 5 μm of the cancer boundary (D = 0.001, p < 0.001, Supplementary Fig. 4). Moreover, CTLs demonstrated super-diffusive motion, characterized by \({{\rm{MSD}}} \sim {{{\rm{\tau }}}}^{{{\rm{\alpha }}}}\), with the exponent α around 1.3 (Supplementary Fig. 5A). Intriguingly, during the first 6 h of co-culture, no significant difference in α was observed between the two co-cultures (Supplementary Fig. 5A, panel 2). However, in the subsequent 6–12 h (panel 3) and 12–18 h (panel 4), CTLs co-cultured with OVA cancer cells exhibited higher α values compared to those with WT cancer cells, suggesting that CTLs co-cultured with OVA cancer cells may possess a more efficient searching capability32.
To further investigate the motility patterns of CTLs with respect to their distance towards cancer cell clusters, we examined directional bias and directional persistence (Methods, ref. 33). CTLs co-cultured with OVA cancer cells gradually increased their motion bias towards the nearest cancer-cell clusters from the first 6 h to the 9–18 h of the co-culture, particularly within 0–10 μm of the cancer-cell boundary (Fig. 2F, Supplementary Fig. 5B and Supplementary Data 2). By contrast, CTLs co-cultured with WT cancer cells showed a gradual decrease in directional bias, especially within 0–10 μm range. Although CTLs co-cultured with OVA cancer cells only displayed a mild bias (~0.1) toward the nearby cancer clusters within 9–18 h (Fig. 2F and Supplementary Fig. 5B), the difference was significant compared to CTLs co-cultured with WT cancer cells.
For the directional persistence, CTLs co-cultured with OVA cancer cells exhibited considerably stronger persistence compared to those cultured with WT cancer cells, particularly during 3–12 h (Fig. 2G, Supplementary Fig. 5C and Supplementary Data 2). These observations indicate that the interactions between OVA cancer cells and CTLs might enhance the ability of CTLs to maintain the consistent movement direction. It is noteworthy that a consistent decrease in directional persistence over time was observed in both co-culture systems. One possible explanation is that the orientation or density of collagen fibers surrounding cancer cell clusters might affect the directional persistence. However, we did not observe significant differences in fiber orientation around cancer cell clusters between the two co-culture conditions (Supplementary Fig. 6A–C). Therefore, the mechanism underlying such decrease of persistent motion when CTLs approaching cancer-cell clusters is still elusive.
Furthermore, we assessed pairwise correlations among step size, directional persistence, and directional bias across varying distances from the cancer boundary per 6-hour intervals (Supplementary Fig. 7). No significant correlation was found between step size and directional bias. Although mild correlations (~0.2) between directional persistence and bias were observed within 10 μm of cancer clusters, they were negligible. Moreover, step size and directional persistence exhibited similarly weak correlations (~0.25) beyond 10 μm in both co-culture conditions. Overall, these findings suggest that the interplay among these motility parameters is weak and distance-dependent.
Computational studies revealed the key motility patterns governing efficient searching and the accumulation of CTLs on cancer-cell clusters
In Fig. 2, we provided a quantitative and systematic characterization of the 2-dimentional motility patterns of CTLs. To further elucidate the roles of different T-cell motility patterns in their searching and accumulation on cancer-cell clusters, we developed a 2-dimensional agent-based model where four motility characteristics of T cells, i.e., dwell time, directional bias, persistence, and step-size distribution, were varied and their effects on the accumulation of T cells were compared (Fig. 3A; Methods), with a display of simulated trajectories in one simulation in Fig. 3B and Supplementary Movie 3.
A Schematic representation of agent-based models. B An illustration depicting the trajectories of 20 CTLs after moving 100 steps each. C Schematics of the calculation method of mean arrival time of CTLs towards cancer clusters. D Violin plot and statistical significance test of the mean arrival time of CTLs to cancer clusters under different patterns when altering only the differences in the directional persistence or directional bias or step sizes. 100 replicated simulations were performed, with each simulation consisting of 200 cells moving for 800 steps. Statistical significance was determined using the two-sided unpaired Student’s t-test: p < 0.001 (***), p < 0.01 (**), p < 0.05 (*), p > 0.05 (n.s.). The p-values from left to right are 2.97E-05, 1.59E-05 and 4.36E-02, respectively. The accumulation of CTLs on cancer-cell clusters as a function of time when considering different variations: E step size, F directional persistence, G directional bias, and H dwell time. The shaded area represents the 95% confidence interval of the simulated data, while the bold line represents the mean. Statistical significance was determined using the Two-sided Wilcoxon rank-sum test: p < 0.001 (***), p < 0.01 (**), p < 0.05 (*), p > 0.05 (n.s.). For clarity in visualization, data points are displayed with sub-sampling at an interval of 5. For (E), the p-values at t = 100, 200, 400, and 600 were 1.77E-3, 3.31E-2, 0.651 and 0.398, respectively. For (F), the p-values at t = 100, 200, 400, and 600 were 0.315, 0.113, 0.651, and 0.398, respectively. For (G), the p-values at t = 100, 200, 400, and 600 were 2.97E-06, 3.26E-12, 1.16E-08 and 1.04E-06, respectively. For (H), the p-values at t = 100, 200, 400, and 600 were 2.27E-26, 4.98E-25, 1.33E-22 and 2.20E-24, respectively.
Firstly, we explored the impact of motility patterns on the mean arrival time of CTLs towards cancer-cell clusters (Fig. 3C). Specifically, the distance-dependent directional persistence, bias, and step-size distribution of CTLs characterized from the two coculture systems were utilized (Methods). By manipulating one pattern while maintaining the other two patterns consistent with the WT co-culture system, we investigated whether the observed differences in CTL motility patterns between the two co-culture systems could influence the mean arrival time of CTLs. Notably, statistically significant differences were observed when altering the directional persistence or bias, rather than the step-size (Fig. 3D and Supplementary Data 3). Intriguingly, CTLs in the co-culture with OVA cancer cells reached cancer-cell clusters more rapidly, emphasizing the importance of directional persistence and bias in CTLs’ search strategy.
In addition, to ensure the robustness of our findings, we conducted similar analyses with a single cancer-cell cluster in the simulation (Supplementary Fig. 8A, B & Supplementary Movie 4). The observed trends remained consistent when considering only the differences in directional persistence or step-size, while showing inconsistency in directional bias, which can be attributed to the initial distribution of CTLs (Supplementary Fig. 8C). These results suggest that stronger directional persistence could enhance CTLs’ efficiency in searching for cancer-cell clusters.
Subsequently, we investigated the impact of motility patterns on the accumulation of CTLs on cancer-cell clusters. No significant difference in the number of CTLs on cancer-cell clusters was found when varying the step-size (Fig. 3E) or the directional persistence distribution (Fig. 3F), with dwell-time distribution held constant as in the WT co-culture. In contrast, altering directional bias resulted in a modest change in CTL accumulation (Fig. 3G and Supplementary Data 3). Notably, simulations incorporating the dwell-time distribution from the OVA co-culture resulted in a marked increase in CTL accumulation, compared to those using the WT-derived distribution. (Fig. 3H). This effect remained qualitatively robust regardless of cancer-cell clusters configuration (Supplementary Fig. 8D–G). These findings suggest that enhanced adhesion, rather than the motility outside of cancer-cell clusters, plays a dominant role in the accumulation of CTLs on cancer clusters.
Bulk RNA sequencing analysis revealed potential molecular mechanisms underlying T-cell motility patterns
To investigate the potential molecular mechanisms underlying T cell motility patterns, we performed both bulk and single-cell RNA sequencing of cancer and T cells sorted from the co-culture systems (Methods). The PCA plot following batch effect correction is presented in Supplementary Fig. 9A. Each point represents a biological replicate collected from multiple microwells across at least two independent experiments (see Methods for details). Note that the two C0A-R1 samples (B16-F10-OVA cells before co-culture, Round 1; hereafter referred to as “R1”) are further away from the C0-R1 (B16-F10-WT cells before co-culture, R1), C0-R2 (B16-F10-WT cells, Round 2; “R2”), and C0A-R2 (B16-F10-OVA cells, R2) samples. This separation may indicate an inconsistency in the sequencing results of the C0A-R1 samples, which are further addressed in the Discussion part.
First, analysis of bulk RNA-seq data of T cells co-cultured with B16-F10-OVA cancer cells revealed that, in addition to classical activation and cytotoxicity-associated cytokines such as interleukin-2, perforin, interferons, and TNFs, chemokines potentially involved in the recruitment and polarization of innate immune cells—such as Xcl1, Ccl1, Ccl3, Ccl4, and Csf2—were also significantly upregulated (Fig. 4A), showing a gradual induction after co-culture initiation (Fig. 4B). Overall, 262 genes were upregulated at 12 h post co-culture (Supplementary Data 4). KEGG and Gene Ontology (GO) enrichment analyses of these genes revealed significant enrichment in cytokine-cytokine receptor interaction and chemokine signaling pathways (Fig. 4C, D), suggesting that antigen-specific CTLs may orchestrate the local immune microenvironment through recruitment of innate immune components.
A Volcano plot of differentially expressed genes between CTLs co-cultured with OVA cancer cells and those co-cultured with WT cancer cells at 12 h after the co-culture (T12A vs T12). Genes that were significantly up and down regulated in T12A samples, i.e., log2(fold change) > 1 and FDR < 0.05, were shown in red and blue, respectively. B Temporal dynamics of selected up-regulated genes in (A). CTLs at 0, 6, 12 h of the co-culture with OVA or WT cancer cells were filtered and sequenced in two replicates. The Z-score of the corresponding expression of the selected genes were calculated and demonstrated. C Results of the KEGG pathway enrichment analysis of differentially expressed genes shown in (A), where the size of the circle corresponds to the number genes in the specific pathway and the color corresponds to FDR values of the enrichment analysis. D Results of the gene ontology (GO) enrichment analysis of differentially expressed genes shown in (A), where different colors correspond to GO terms for biological process (BP), molecular function (MF) or cellular component (CC). E Volcano plot of differentially expressed genes between OVA and WT cancer cells at 12 h after the co-culture with CTLs (C12A vs C12). Genes that were significantly up and down regulated in C12A samples were shown in red and blue, respectively. F Temporal dynamics of selected up-regulated genes in (E). OVA and WT cancer cells at 0, 6, and 12 h of the co-culture with CTLs were filtered and sequenced in two replicates. The Z-score of the corresponding expression of the selected genes were calculated and demonstrated. G Results of the KEGG pathway enrichment analysis of differentially expressed genes shown in (E). H Results of the gene ontology (GO) enrichment analysis of differentially expressed genes shown in (E).
For the gene expression profiles of cancer cells, compared to B16-F10-WT ones, 942 genes were upregulated in B16-F10-OVA cancer cells at 12 h after the co-culture with CTLs (Fig. 4E, Supplementary Data 4). Given the upregulation of interferon-gamma by antigen-specific CTLs, it is not surprising that many of these genes—including Stat3, Ifitm3, and Cdkn1a—are associated with IFN-responsive pathways. To assess whether these differences were intrinsic to the two tumor cell lines or induced by CTL interaction, we performed additional analyses. First, we exposed B16-F10-OVA and B16-F10-WT cells to IFN-γ (50 ng/mL, 24 h) and analyzed their transcriptomes. The two cell lines exhibited highly overlapping gene expression changes (Supplementary Fig. 9B), with no significant differences in pathways related to cell cycle, antigen presentation, immune regulation, or apoptosis (Supplementary Fig. 9C). In addition, EdU incorporation assays showed comparable proliferative activity between the two lines under baseline conditions (Supplementary Fig. 9D). Furthermore, flow cytometry analysis of MHC-I surface expression demonstrated similar levels between the two cell lines at baseline (Supplementary Fig. 9E). Together, these results suggest that the transcriptional divergence observed in co-culture divergence observed in co-culture could be largely driven by antigen-specific CTL interactions, rather than inherent differences in IFN-γ responsiveness or baseline functional states between the tumor cell lines.
Notably, the chemokine genes Cxcl9 and Cxcl10, critical for T cell recruitment, were progressively upregulated in OVA cancer cells following CTL interaction, with expression levels markedly higher than those in the CTLs themselves (Fig. 4F). Moreover, in response to CTL-mediated attack, cancer cells upregulated genes enriched in pathways such as antigen presentation, apoptosis, and TNF signaling, along with GO terms like MHC complex and cell killing (Fig. 4G, H, Supplementary Fig. 9F). Consistently, apoptosis-related genes were significantly elevated in B16-OVA cells after co-culture, compared to baseline (Supplementary Fig. 10), reflecting a strong CTL-induced cytotoxic response.
Furthermore, we noted that pathways of cell adhesion molecules and ECM-receptor interaction are enriched for genes up-regulated in OVA cancer cells (Supplementary Fig. 9G). Notably, several genes related to epithelial-to-mesenchymal transition (EMT) were upregulated, such as Serpine1, Col1a1, Dab2, etc. Using GSEA with human homologs from the 50 MSigDB hallmark gene sets, we observed a strong enrichment of the EMT signature (EMT, NES:1.77, FDR:5.6e-05, Supplementary Fig. 9H). Consistently, EMT-related GO terms were also significantly enriched (Supplementary Data 4). These observations suggest that in response to T-cell attack, cancer cells may tend to metastasize. To evaluate differences in migratory behavior between B16-F10 and B16-F10-OVA cells, we performed a wound healing assay before and after co-culture with CTLs (Supplementary Fig. 11). In the absence of co-culture, both cell lines exhibited comparable migration. Notably, following co-culture, B16-F10-OVA cells displayed significantly enhanced wound closure, indicating an increase in migration capacity. In contrast, B16-F10 cells showed reduced migration. These observations suggest that co-culture with CTLs differentially affects the migratory behavior of the two cell lines, although further studies using dedicated metastasis assays would be required to assess their metastatic potential (Supplementary Fig. 11).
Single-cell RNA sequencing analysis unveiled heterogeneity and resistance mechanisms
By analyzing the bulk RNA-seq data of co-cultured cancer and CTLs, we have revealed essential genes that were differentially expressed in OVA cancer cells and antigen-specific CTLs, respectively. On the other hand, we also observed from the time-lapse images that some cancer cells survived longer and some CTLs remained engaged with cancer cells for extended periods. To further investigate the molecular basis of this heterogeneity within each cell type, we performed single-cell RNA sequencing (scRNA-seq) on the sorted cancer cells and CTLs.
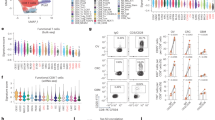
First, we applied the scRNA-seq clustering of all cells at a resolution of 0.5, and then projected the results on a UMAP (Methods), identifying nine sub-clusters of cells (C1–C9, Fig. 5A). Prior to sequencing, CD8+ T cells were separated from tumor cells using Dynabeads (11462D, ThermoFisher), allowing us to categorize the cells into four groups: g-B16-F10, g-CTLs-B16-F10 (CTLs co-cultured with B16-F10), g-B16-F10-OVA, and g-CTLs-B16-F10-OVA (CTLs co-cultured with B16-F10-OVA). These groups are visualized from left to right in the UMAP panels (Fig. 5A). Based on gene signatures, cells in clusters C1 and C2 were categorized as B16-F10 cancer cells after 12 h of co-culture with CTLs, while cells in clusters C3 and C4 were identified as B16-F10-OVA cancer cells after 12 h of co-culture with CTLs. Clusters C5–C9 were assigned as CTLs. It is important to note that, due to the ability of T cells to attach to cancer cells, sorted cell groups may include overlapping clusters. Specifically, CTLs in clusters C5, C6, and C7 were identified within the sorted group g-B16-F10, while CTLs in clusters C5, C6, C7, and C9 were identified within the sorted group g-B16-F10-OVA. Interestingly, a distinct cluster of CTLs (C9) was observed in association with sorted OVA cancer cells but not with WT ones (Fig. 5A).
A UMAP of scRNA-seq data of the sorted cancer and T cells. All cells were combined to perform the clustering, and 9 cell clusters (C1 to C9) were identified after removing ones with low RNA contents or high mitochondrial RNAs (Methods). WTcancer and WTctl (as well as OVAcancer and OVActl) cells were isolated after 12 h of co-culture using Dynabeads positive selection with EpCam and CD8 antibodies, respectively. B Top ten differential expressed genes of the cell clusters shown in (A). The color represents the average expression of cells in the indicated cell cluster. The size of the circle represents the percentage of cells that express the DEG in the indicated cell cluster (Methods). C Merge of non-unique CTL-subclusters and rename of cancer and CTL clusters. OVActl cells belonging to CTL-subclusters that overlapped with the ones in WTctls were merged into one new subcluster named as CD8T-cx. D Gene-expression differences between OVActl cells that belong to CD8T-c2 and CD8T-cx in (C). Genes expressed in more cells (x-axis) from CD8T-c2 and CD8T-cx as well as a higher average expression (y-axis) were shown in red and blue, respectively. E Gene-expression differences between Cancer-c3 and Cancer-c4 cells in (C). Genes expressed in more cells (x-axis) from Cancer-c3 and Cancer-c4 as well as a higher average expression (y-axis) were shown in red and blue, respectively. F Ligand-receptor cellular interactions between CTLs (CD8T-c2) and cancer cells (Cancer-c4 or -c3) in the coculture of OVA cancer cells and CTLs (Methods). Only the interactions with a p value smaller than 0.01 were displayed. The color represents the communication probability as indicated by the color bar.
Following the identification of the nine clusters, we conducted differential expression gene (DEGs) analysis and generated a heatmap to visualize transcriptional differences across clusters (Methods and Supplementary Fig. 12A). For cancer cells, WT clusters (C1–C2) and OVA clusters (C3–C4) exhibited distinct expression patterns, consistent with bulk RNA-seq results. For CTLs, clusters C5–C8 showed highly similar profiles, while cluster C9 displayed notable differences, indicating a distinct subpopulation within OVA-CTLs. Consistently, the top 10 signature genes of the subcluster C9 (Fig. 5B) were among the up-regulated genes identified in bulk RNA-seq (Fig. 4A), but exhibited significantly lower expression in the other OVA-CTL sub-clusters. This result suggested that only a subset of OVA-CTLs (mainly C9) contributed significantly to the observed differences from WT-CTLs in bulk analysis. In addition, volcano plot analysis between CTLs in C9 (CD8T-c2 in Fig. 5C) and the merged cluster of C5–C8 (CD8T-cx) revealed that CD8T-c2 cells exhibited elevated expression of chemokine genes (e.g., Xcl1, Ccl1, Ccl3, and Ccl4), adhesion-related genes (e.g., Crtam) and effector genes (e.g., Ifng and Prf1), with a higher proportion of cells expressing these genes (Fig. 5D and Supplementary Data 5). Functional enrichment analysis of CD8T-c2 DEGs highlighted pathways involved in positive regulation of cytokine production, cell killing, and regulation of cell–cell adhesion (Supplementary Fig. 12B), in agreement with bulk RNA-seq analysis.
For cancer cells, scRNA-seq analysis also revealed heterogeneity within OVA-cancer cells, particularly in response to CTLs attack. Notably, the Cancer-c4 subcluster exhibited elevated expression of migration- and metastasis-associated genes, such as Fn134, Tubb335, and Dst36, compared to Cancer-c3 (Supplementary Data 5). Among them, Fn1 was expressed in a 15% higher proportion of Cancer-c4 cells. Although Tubb3 and Dst showed similar percentages of expressing cells between the two clusters, their average expression levels were approximately two-fold higher in Cancer-c4 (Fig. 5E). Conversely, Cancer-c3 cells showed higher expression of T-cell chemokine genes (Cxcl9, Cxcl10) and antigen-presentation-related genes (H2-T23, H2-K1) than those in Cancer-c4. For instance, Cxcl9 exhibited approximately two-fold higher expression in Cancer-c3, despite a similar proportion of cells expressing the gene.
Functional enrichment analysis further supported these transcriptional differences. DEGs in Cancer-c3 were enriched in pathways associated with interferon response, positive regulation of cytokine production, and cytokine-mediated signaling response (Supplementary Fig. 12C). By contrast, DEGs in Cancer-c4 were enriched in pathways were enriched in pathways related to cell adhesion, ameboid cell migration, regulation of cell morphogenesis, etc. (Supplementary Fig. 12D, Supplementary Data 5). Gene set enrichment further confirmed a strong enrichment for EMT-related genes in Cancer-c4 (Supplementary Fig. 12E). These results, together with the wound healing assay (Supplementary Fig. 11) showing enhanced migration in B16-F10-OVA (Cancer-c3/c4) compared to B16-F10 (Cancer-c1/c2), suggest that Cancer-c4 cells may acquire higher metastatic potential and CTL resistance upon interaction.
Finally, to move beyond transcriptional changes and explore the intercellular communication underlying cancer-T cell interactions, we performed CellChat analysis37 analysis using the scRNA-seq data of cancer and T cells at 12 h after the co-culture. Notably, Cancer-c3 cells have several unique interactions with the CTLs (Fig. 5F), such as CD6-ALCAM, H2-K1-CD8A/CD8B1 and H2-T23-CD8A/CD8B1. Some of these interactions have been shown to be important for the activation/expansion/adhesion of T cells38,39 or the antigen-presentation of cancer-cells40,41. Moreover, it was interesting to note that only CTLs in CD8T-cx cluster interacted with OVA cancer cells via CXCL9-CXCR3 and CXCL10-CXCR3, which could be responsible for the motility behavior of antigen-specific T cells toward cancer cells (Supplementary Fig. 12F).
To validate the role of the CXCR3-ligand axis (comprising CXCL9, CXCL10, and CXCL11) in regulating CTL motility, we conducted co-culture experiments of B16-F10-OVA tumor cells and CTLs using the CXCR3 antagonist ACT-660602 (HY-151096, MCE)42. Specifically, 408 nM ACT-660602 was added to the co-culture medium, while the control group was maintained under identical conditions without the antagonist (wi/wo-ACT-OVA). Following ACT-660602 treatment, CTLs in the co-culture system exhibited reduced motility persistence compared to untreated controls (Supplementary Fig. 13A, B) suggesting a critical role for the CXCR3-ligand axis in guiding CTL migration toward tumor targets. Notably, no significant differences were observed in dwell time between the treated and control groups (Supplementary Fig. 13C), indicating that CXCR3 signaling primarily modulates locomotory behavior rather than cell-cell interaction dynamics. Furthermore, a quantitative analysis of the temporal dynamics of tumor cell area and T cell density on tumor-cell clusters revealed that the ACT-660602-treated group exhibited a larger tumor cell area and reduced T cell density on tumor-cell clusters at 18 h post-co-culture compared to the untreated group (Supplementary Fig. 13D–F). These findings suggest that the inhibition of the CXCR3-ligand axis impairs both CTL recruitment efficiency and subsequent effector-mediated tumor elimination.
Discussion
In this work, we combined a 2.5D co-culture system with an automated quantitative analysis pipeline to characterize the motility patterns of cytotoxic T cells co-cultured with cancer cells expressing a specific antigen. We found that antigen-specific CTLs exhibited greater directional persistence outside of cancer-cell clusters and prolonged dwell time on cancer cells compared to non-specific CTLs. In addition, through computational simulations, we showed that increased directional persistence can expedite the first arrival time of CTLs on cancer cells, while longer dwell time can augment the accumulation of CTLs on cancer-cell clusters. These motility patterns of CTLs might facilitate their localization and coordinated assault on cancer cells.
To decipher the potential mechanisms underlying the observed motility patterns of CTLs, we also performed gene expression analysis and founded that both cancer cells and CTLs expressed more chemokines and cytokines after the attack of CTLs. These molecules might increase the directional persistence and bias of CTLs towards cancer-cell clusters. Further knock-out experiments are warranted to quantify the effects of these molecules on CTL motility. Previous studies have shown that CCL3/4 is responsible for attracting CTLs in 3D co-culture systems19. In this work, by blocking the CXCR3-CXCL9/10 signaling, the results suggested that CXCL9/10 could also play a crucial role in the observed enhanced directional bias of CTLs in our 2.5D co-culture system. Note that, in addition to the chemotaxis-driven biased motion, at a distance close to tumor-cell clusters, a higher directional bias of CTLs could also result from CTLs’ direct contact interactions with tumor cells (Supplementary Fig. 14), potentially triggering TCR-mediated changes in motility19,43. Therefore, it would be valuable to investigate this phenomenon further using systematic gene knockout experiments and higher-resolution imaging techniques44,45 in the future.
Furthermore, to understand the extended dwell-time of CTLs on cancer cells, we identified several potential adhesion candidates mediating interactions between cancer cells and antigen-specific CTLs, such as the MHC-I/TCR complex and integrins, based on gene expression data. Notably, TCR activation has been reported to reduce the T cell motility via various mechanisms46,47,48. The quantitative contribution of these molecular pathways on dwell time could be further consolidated using the gene knock-out, RNAi or interaction-blockade experiments in future studies.
In addition to CTLs, our analysis of scRNA-seq data revealed that cancer cells differentiated into two groups following interaction with antigen-specific CTLs. One group exhibited reduced expression of antigen-presenting molecules and chemokines but increased expression of EMT-related and immunosuppressive genes. These observations might help to provide mechanistic insights on the potential immune evasion of cancer cells. Previous studies have suggested that cancer-cell EMT induced by immune attack can suppress the activity of CTLs49,50. In parallel, recent studies on sublethal T cell interactions highlight the possibility that tumor cells can acquire resistance following non-lethal contacts with T cells51,52. Together, detailed mechanistic studies are still required to unveil the detailed biological mechanisms. Additionally, a limitation is that not all biological replicates for bulk RNA-seq were processed in fully independent runs, and the single-cell RNA seq was performed only once. Nevertheless, the consistency of our findings is supported by IFN-γ response comparisons (Supplementary Fig. 9B), DEG overlap analysis (Supplementary Fig. 9F), and cross-validation between bulk and single-cell datasets (Supplementary Data 7). This limitation could be further addressed in future studies involving 3D co-culture systems.
Finally, our investigation focused solely on the interactions between cancer cells and antigen-specific CTLs in the controlled 2.5D co-culture. However, actual tumors comprise various other cell types, such as cancer-associated fibroblasts, endothelial cells, and tumor-associated macrophages. Co-culturing multiple cell types in vitro holds promise for elucidating the effects of paracrine and autocrine interactions on the spatial distribution of antigen-specific CTLs53. In addition, the current computational model remains a simplification of the complex in vivo environment. For instance, it does not take into account cell proliferation, cytotoxic killing, or tumor evolution processes such as metastasis. Moreover, the influence of fiber network distributions—potentially affecting both motility and cell-cell interactions—was also not incorporated. Future models that integrate these factors may offer a more comprehensive view of CTL dynamics within tumor microenvironments. While certain motility behaviors may differ in vivo (Supplementary Figs. 15–16), the pathway-level similarity in molecular responses suggests that the candidate molecules mediating T cell navigation and sustained adhesion present promising targets for T cell engineering, potentially enhancing the success of cancer immunotherapy. Additionally, the procedure to quantify motility patterns of T cells in the 2.5D co-culture system might be useful for studying T-cell motility behavior in 3D co-culture systems.
Methods
Cell samples
B16-F10 murine melanoma cells were kindly provided by the Stem Cell Bank, Chinese Academy of Sciences (see Supplementary Data 6). To label the cancer cells, B16-F10 cells were first transduced with a plasmid encoding the Ca2+ sensor GCaMP6s (pLV[Exp]-Puro-EF1A > GCaMP6s) using lentiviral infection. Subsequently, B16-OVA cells were generated by transducing these cells with a plasmid encoding ovalbumin (pLV[Exp]-Neo-EF1A > {ovalbumin}) via lentiviral infection. The transduced B16 cancer cells were then cultured in DMEM (GIBCO) supplemented with 10% FBS (GIBCO) and 1% penicillin/streptomycin (GIBCO).
OT-1 CD8+ T cells isolation, and activation
CD8⁺ T cells were isolated from the spleens of female C57BL/6J-OT-I mice (Cyagen Biosciences, Suzhou; Cat# C001198). Single-cell suspensions were prepared using Cell Staining Buffer, and CD8⁺ OT-1 T cells were positively selected using CD8 magnetic beads. The isolated cells were then activated using the Dynabeads™ T-Activator CD3/CD28 kit (GIBCO, Cat#11456D) according to the manufacturer’s protocol. After activation, the beads were removed by magnetic separation and washing to obtain purified activated T cells.
The naive CD8⁺ OT-I T cells were activated using Dynabeads® Mouse T-Activator CD3/CD28 (GIBCO)54 according to the manufacturer’s instructions. Cells were cultured in RPMI 1640 medium supplemented with heat inactivated 10% FBS (GIBCO), 1% penicillin/streptomycin (GIBCO), 2 mM L-glutamine (GIBCO), 50 mM β-mercaptoethanol (GIBCO) and 30 U/ml mouse recombinant IL-2 (Biolegend).
Flow cytometry assay of OT-1 CD8+ T cells
For flow cytometry analysis, the activated OT-1 CD8+ T cells were fixed and stained with fluorophore-conjugated antibodies diluted in flow cytometry staining buffer. After staining, the cells were resuspended in buffer and immediately acquired using a CytoFLEX S flow cytometer (Beckman). Data were analyzed using FlowJo software. (1) Lymphocytes were initially gated based on forward scatter (FSC) and side scatter (SSC) parameters. (2) Single cells were selected within the lymphocyte gate to exclude doublets and cell aggregates. (3) CD3 expression was used to identify the T cell population. (4) The CD3⁺ population was further gated to identify CD8⁺ T cell subsets. Quantitative analysis indicated that CD8⁺ T cells accounted for ~98.8% of the population, confirming the high purity of the isolated T cells (Supplementary Fig. 17).
Co-culture of cancer and T cells
Co-culture experiments were conducted in 2.5D µ-slide chambers (ibidi, 8-well), using pre-activated, OVA-specific CD8⁺ cytotoxic T lymphocytes (OT-1 CTLs) and B16-F10 mouse melanoma cells. Two tumor cell lines were used: B16-OVA (expressing Ovalbumin) and B16-WT (lacking OVA expression) as the control. B16-F10-OVA or B16-F10-WT cells were seeded onto Poly-D-lysine (Sigma) coated µ-Slide wells and incubated overnight. For antigen presentation, B16-OVA cells were pulsed with 1 μM OVA peptide at 37 °C for 1 h, then washed twice with serum-free DMEM. Next, OT-1 CTLs were labeled with anti-CD45-PE (Invitrogen) and mixed with neutralized collagen I solution (Corning) at a final E:T (effector-to-target) ratio of 2:1. The collagen solution was prepared by adjusting the pH to 7.5 using NaOH and mixing with 10× DPBS. The CTL-collagen suspension was added onto the sub-confluent tumor monolayer. After polymerization for 45 min at 37 °C, 400 µL of complete medium was added to each well. The experimental setup was adapted from protocols described in ref. 55.
Live-cell imaging-based cytotoxicity assay
Time-lapse imaging was performed for 24 h at 2-min intervals. Tumor cells were visualized via the GCaMP6s fluorescent reporter, while CTLs were labeled with anti-CD45-PE through immunofluorescence staining. Bright-field and time-lapse fluorescent images were acquired using a NIKON TI2-E system equipped with a 20× objective lens. FITC (excitation: 480 nm; emission: 512–550 nm) and TxRed (excitation: 540 nm; emission: 577–632 nm) channels were used for fluorescence detection. All co-culture experiments were conducted at 37 °C and 5% CO2 within an incubation chamber enclosing both the microscope stage and body.
Sample preparation for bulk and single-cell RNA sequencing
After co-culture for 0 h, 6 h, and 12 h, a collagenase solution was added to the µ-Slide 8 well and incubated for 15 min to release T cells from the collagen gel, followed by trypsin EDTA for 5 min to detach cancer cells. The resulting cell suspension containing cancer cells and T cells was subjected to positive selection using Dynabeads conjugated with EpCam and CD8 antibodies, respectively. Bead-bound cells were then resuspended in release buffer and the bead-free cell suspension was transferred to new tubes. Bulk RNA sequencing was performed on cells from co-culture at 0 h, 6 h, and 12 h, whereas single-cell RNA sequencing was specifically conducted on cells obtained from the 12 h co-culture.
For bulk RNA-seq, samples were derived from multiple biological replicates across at least two independent experiments. In each experiment, approximately 12,000 tumor cells were seeded per well in µ-Slide 8-well plates for cytotoxicity assays. To obtain sufficient material (~500,000 cells per condition per time point), we cultured over 10 plates in parallel for each condition (OVA and WT at 0, 6, and 12 h), referred to as the first batch (“R1”). This procedure was independently repeated to generate a second batch of samples (“R2”), to assess the transcriptional responses of B16-F10 and B16-OVA cell lines to IFN-γ stimulation (C0-R2, C0A-R2, WT-IFN, OVA-IFN). For single-cell RNA-seq, samples were collected from one experiment, using pooled cells from ~40 wells per condition.
EdU incorporation assay
To evaluate potential differences in proliferative activity between the two cancer cell lines, a cell cycle analysis was performed using an EdU incorporation assay. B16-F10 and B16-OVA cells were seeded onto 35-mm glass-bottom dishes and incubated for 24 h. EdU labeling was conducted using the VF555 Click-iT EdU Universal Cell Proliferation Detection Kit (MCE, Cat#HY-K1086), following the manufacturer’s instructions. Fluorescent signals were imaged using a Nikon Ti2-E fluorescence microscope.
MHC class I surface expression Assay
To assess MHC class I surface expression levels in B16-F10 and B16-OVA cell lines, flow cytometry analysis was performed using a PE-conjugated anti-mouse H-2Kd antibody (Biolegend, Cat#116607). Cells were stained according to the manufacturer’s instructions and analyzed on a FongCyte™3 Flow Cytometer. Data acquisition and subsequent analysis were carried out using FlowJo software.
Wound healing assay
B16-F10-OVA cells post-CTL killing (clusters c3 and c4), B16-F10-WT cells post-CTL killing (clusters c1 and c2), and untreated B16-F10-WT and B16-F10-OVA cells (as controls) were seeded in 96-well plates at a density of 3000 cells per well and cultured for 24 h. Once the cells reached confluency, a straight scratch was introduced into the monolayer using a sterile cell scraper. The wells were then washed with PBS to remove debris and replenished with fresh medium. Time-lapse bright-field images of the wound were captured every 10 min over a 24-h period using a NIKON Ti2-E microscope system equipped with a 20 × objective. The wound area was quantified by manually tracing the cell-free regions in sequential images using ImageJ software, and closure rates were calculated as the percentage reduction in wound area over time.
Validation of CXCR3-ligand axis functionality
To investigate CXCR3-ligand axis functionality, we implemented targeted inhibition using ACT-660602 (HY-151096; MCE), a selective CXCR3 antagonist. Specifically, 408 nM ACT-660602 was supplemented into the culture medium of the B16-F10-OVA co-culture system (wi-ACT-OVA), while the control group maintained identical conditions without the antagonist (wo-ACT-OVA). Immune-mediated cellular interactions were continuously tracked through time-lapse imaging following standardized protocols from our live-cell imaging-based cytotoxicity assay.
Mouse experiments
All experiments were conducted in accordance with the ethical guidelines approved by the Animal Research Committee of Guangdong Medical University (Protocol No. GDY2104034). All mice were maintained under SPF (Specific Pathogen-Free) conditions. Six-week-old female NOD-SCID mice were purchased from Cyagen Biosciences (Suzhou) Inc (Cat#C001070). To evaluate the anti-tumor properties of OT-1 CD8 + T cells activated with anti-CD3/anti-CD28 Dynabeads in vivo, melanoma mouse models were established using NOD-SCID mice. A total of 20 mice were injected with either B16-F10-OVA (GCaMP6s-transfected) (n = 10) or B16-F10 (GCaMP6s-transfected) cells (n = 10), with the latter serving as the control group. Cage assignments were not randomized to minimize animal stress from frequent handling; however, all treatments and measurements were performed within consistent daily time windows to reduce potential confounders.
Tumor model establishment
For melanoma model induction, approximately 5 × 105 B16-F10-OVA (GCaMP6s-transfected) (n = 5) or B16-F10 (GCaMP6s-transfected) cells (n = 5) were suspended in 50 µL PBS, mixed with an equal volume of Matrigel (50 µL), and injected subcutaneously into the dorsal aspect of the right hind leg of mice. When tumor volumes reached approximately 100 mm³ (approximately 7 days post-injection), each tumor-bearing mouse received 5 × 106 OT-1 CD8 + T cells via tail vein injection. Body weight, tumor dimensions and general health status were monitored every four days. Humane endpoints included tumor volume >1 cm³, weight loss >20%, ulceration, or distress signs. Mice meeting these criteria were euthanized immediately; others were euthanized 15 days post-injection using approved methods. To ensure objective quantification, tumor volume measurements were performed independently by two trained technicians blinded to group allocation. All raw measurement data were compiled and anonymized by a third researcher uninvolved in experimental procedures. One B16-F10 melanoma-bearing tumors exceeded the ethical maximum tolerated volume (1 cm³) and was excluded from analysis. Statistical analysis was performed using the two-sided unpaired Students t-test (n ≥ 4).
Cell isolation for comparative transcription analysis
To compare T cell gene signatures between in vitro co-culture systems and in vivo tumors, B16-F10-OVA (n = 5) and B16-F10 (n = 5) melanoma-bearing mice were euthanized two days post-T cell administration. Fresh tumor tissues were minced into ~1 mm fragments using sterile scalpels and enzymatically dissociated in digestion medium containing mouse collagenase (1 mg/mL) and hyaluronidase (1000 U/mL) at 37 °C for 45 min. The resulting cell suspension was filtered through Falcon 100 µm cell strainers to remove undigested tissue, washed repeatedly to eliminate dead cells, and resuspended in culture medium (DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, 1% sodium pyruvate, 1% HEPES, 500 µL insulin, and 10 nM estrogen). Cell concentration and viability were quantified using Countess™ Cell Counting Chamber Slides.
OT-1 CD8 + T cells were positively selected from the suspension using CD8+ antibody-conjugated Dynabeads. After magnetic separation, bead-bound cells were incubated with release buffer, and bead-free CD8 + T cells were collected for bulk RNA sequencing. Concurrently, GCaMP6s-expressing B16-F10-OVA and B16-F10 tumor cells were isolated via fluorescence-activated cell sorting (FACS) and processed for bulk RNA sequencing.
Manual analysis of CTL recruitment, division time and interaction time
For measurement of effector cell recruitment (Fig. 1C), the detected regions of cancer cells were expanded by adding a belt area with a constant diameter of 15 μm (“dilation circle”), and CTLs within the dilated region were manually counted. Division time and interaction time of OT-1 CTLs were analyzed by Fiji56,57 (Supplementary Fig. 1C and Fig. 1E). Migration velocity was processed using Imaris software (RRID:SCR_007370) (Supplementary Fig. 1D).
Quantification of CTL trajectories
CTLs were identified using the TxRed fluorescence channel and their trajectories were generated utilizing Trackmate 758, an open-source Fiji plugin. First, the T cell spots were detected using the LOG (Laplacian of Gaussian) detector. The estimated object diameter was set to 14 μm and the quality threshold was set to 0.18. Subsequently, track segments were established using the LAP tracker, with the maximal distance allowed for frame-to-frame linking set to 25 μm. Additionally, only tracks with a duration of at least 10 frames were selected for further analysis. Finally, the resulting track table and spot table were obtained. The former provides information about the trajectories of the CTLs over time while the latter contains the coordinates of all T-cell centroids.
Identification of cancer-cell clusters
Cancer-cell clusters were imaged by splitting the FITC fluorescence channel. To extract the main tumor region, basic image processing was conducted using Fiji. Tumor cell positions were updated frame-by-frame to ensure that their dynamic changes were accurately incorporated into the analysis. This encompassed threshold adjustment, Gaussian blur, and background subtraction. Subsequently, the preprocessed tumor images underwent further processing using the MATLAB Image Processing Toolbox and customized algorithms. These operations included morphological closing, hole filling, edge smoothing, and the removal of small impurities. Cancer-cell clusters were defined as connected regions with an area exceeding 400 pixels. These operations were then applied to stacks of tumor images, generating a time sequence of binarized cancer-cell clusters data.
Quantification of the density of CTLs
To quantify the accumulation of CTLs on cancer-cell clusters over time (Fig. 2C), the density of CTLs on cancer-cell clusters was calculated by dividing the number of CTLs present on cancer-cell clusters by the area of tumor regions, measured in cells/mm2. The positions of CTLs obtained from Trackmate tracking relative to cancer-cell clusters were determined by assessing whether the centroids of CTLs overlapped with tumor regions.
The density of CTLs around the cancer clusters (cells/mm2, Supplementary Fig. 3A) was calculated by dividing the number of T cells within each dilation belt (enlarged to a diameter of 5 μm) by the area of the dilation belt, which expanded every 5 μm from the boundary of the cancer clusters, covering a total range of 0–45 μm. Additionally, the density of T cells on cancer clusters was also included. A 3D representation of CTL density around cancer clusters was displayed for 6 h, 9 h and 12 h, respectively.
The total density of CTLs within the imaging field (Supplementary Fig. 3B) was quantified by dividing the total number of CTLs in the imaging field by the total area of the field, also measured in cells/mm2.
Dwell-time distribution of CTLs on cancer-cell clusters
To quantify the duration of CTL interactions with OVA cancer cell and WT cancer cell, the dwell-time distribution of CTLs was assessed (Fig. 2D, Supplementary Fig. 3D–H). Firstly, a dwelling event was defined as the occurrence when the centroid of CTL was within a minimal distance of less than 5 μm from the boundary of cancer-cell clusters. This criterion was established based on radius of the CTL is approximately 5–6 μm. The dwell-time was then calculated by determining the number of consecutive frames in which CTL was present on cancer-cell clusters. In cases where a CTL attached to the tumor cell clusters multiple times within a track, each attachment was considered as a separate dependent adhesion event.
To compare the dwell time distributions between CTLs cocultured with OVA cancer cell and those with WT cancer cell within different stages, the adhesive time distributions during three specific time periods were statistically calculated: 0–6 h, 6–12 h, and 12–18 h.
Directional bias and persistence analysis
To explore the motility patterns of CTLs with respect to their distance towards cancer cell clusters in the two co-culture systems, we conducted a study utilizing the concepts of directional bias and persistence as introduced by Weavers et al.33. Directional bias, denoted by \(\alpha\), represents the angle between the instantaneous velocity of CTLs and the direction from CTLs to the nearest point on the cancer-cell cluster, reflecting the ability of cells to move in the direction of an attractant source. Additionally, directional persistence, denoted by \(\beta\), measures the angle between CTL velocities in two consecutive steps, indicating the ability of cells to maintain their movement direction. The bias angle distributions range from 0° to 180° whereas the persistence angle distributions range from −180° to 180°. The cosine function was applied to the angles to normalize the values between −1 and 1, where a higher value indicates a greater bias or persistence.
For each CTL spot, the distance to the nearest pixel of the cancer cluster boundary was calculated. The trajectory segments of CTLs located outside the cancer clusters were exclusively selected for analyzing directional bias and persistence. These segments were then grouped based on their distances from the nearest cancer-cluster boundary, with each group defined by 5 μm intervals. Subsequently, the averaged bias or persistence was computed for each distance-interval. To compare the behavioral dynamics of CTLs, the average bias and persistence distributions for CTLs cocultured with OVA cancer cells and those with WT cancer cells were calculated over three specific time-periods: 0–6 h, 6–12 h, and 12–18 h, respectively (Fig. 2F, G). Additionally, directional bias and persistence analyses were conducted per 3-h intervals, as shown in the supplementary information (Supplementary Fig. 5B, C).
Mean square displacement of CTLs
To investigate the motility rules and compared the rules in different time intervals, the mean square displacement was calculated to characterize the diffusive behavior for CTLs. The calculation was performed using the following equation59:
Here, \({l}_{i,i+n}\) represents the Euclidean distance of a CTL from frame \(i\) to frame \(i+n\); N represents overall time periods for calculation; n represents the time lag.
The MSD can also be described using a power-law form60:
The trajectory segments of CTLs before reaching the cancer cell clusters were extracted. Then, the logarithm of the MSD as a function of the time was fitted, and the linear portions of the curve were identified (Supplementary Fig. 5A). To ensure credibility and accuracy of the calculations, only the first 10 frames of continuous segments in the tracks were selected for the fitting process.
Quantification of collagen fibers
To investigate potential variations in collagen fiber distribution and arrangement between WT and OVA cancer cell environments, collagen fiber orientation were analyzed around cancer clusters in two co-culture systems at 0 h and 6 h, respectively. For the analysis of fiber orientation, CT-fire software61 was utilized to extract and calculate the directional alignment angles between collagen fibers and cancer boundaries (Supplementary Fig. 6A–C).
Correlation test among directional bias, directional persistence and step size
To investigate whether there exists correlation among directional bias, directional persistence and step size, Spearman correlation tests were performed by per 5 μm within 0–6 h, 6–12 h, and 12–18 h, respectively (Supplementary Fig. 7).
Agent-based models
To further investigate the impact of observed differences in T-cell motility patterns on their searching efficiency and the accumulation on cancer-cell clusters, we constructed 2D agent-based models that incorporated four key features of T cells: dwell time, directional bias, directional persistence and step size. In addition, computational simulations offer the flexibility to manipulate the initial distributions of CTLs and cancer clusters, which is challenging to control under experimental conditions. To address this, we developed two models: a single-cancer-cluster model (Supplementary Fig. 8) and a multi-cancer-cluster model (Fig. 3), allowing us to explore the potential effects of the initial distribution of CTLs and cancer clusters.
The multiple-cancer-cluster model is based on the following assumptions:
-
1)
The model is implemented on a 2D square region with dimensions of 590 μm × 590 μm, matching the experimental scale. The model includes CTLs and cancer-cell clusters. To simplify the modeling process, the models disregard the mobility of cancer cells, as well as the proliferation or death of both CTLs and cancer cells, and the weak correlation among step size, directional bias and persistence.
-
2)
To simplify the model, the initial distribution of cancer-cell clusters is identical to Series 1 in the experiments. Initially, 200 CTLs are randomly dispersed outside cancer-cell clusters within the defined region. The simulation utilized parameters derived from Series 1 in the WT and OVA experimental systems, including step size, directional bias, directional persistence as a function of distances towards cancer-cell clusters, and dwell time distributions within 6–12 h, as significant differences were observed in the directional bias, persistence, and dwell time distribution of CTLs during this period (Fig. 2D–G). The step size, directional bias and persistence distributions are updated with a resolution of 5 μm distance from the nearest cancer-cell boundary.
-
3)
When T cells are outside of cancer-cell clusters, they undergo a distance-dependent persistent or bias walk.
If we specifically focus on the influence of the differences in the distribution of directional persistence, we can determine the position of T cells at any given time by following the equations below.
$${T}_{x}^{t+1}={T}_{x}^{0}+\mathop{\sum }_{i=0}^{t+1}{d}_{i}\cos \left({\sum }_{0}^{i}{{{\rm{\beta }}}}_{i}\right)$$$${T}_{y}^{t+1}={T}_{y}^{0}+{\sum }_{i=0}^{t+1}{d}_{i}\sin \left({\sum }_{0}^{i}{{{\rm{\beta }}}}_{i}\right)$$\({T}_{x}^{t}\) and \({T}_{y}^{t}\) represents the coordinates of a CTL at time t. \({\beta }_{0}\) represents the angle between the initial motion vector and the positive x-axis, which is randomly set. \({d}_{t}\) represents the step size from t to t + 1, sampled from the step-size distributions. Here, we developed an algorithm to realize the sampling process from a known distribution. \({\beta }_{t}\) represents the turning angles from t to t + 1, sampled from persistence distributions based on the distance towards cancer-cell clusters.
Similarly, if we focus solely on the role of directional bias, we can determine the position of CTLs using the following equations. The motion of CTLs depends on searching for the nearest cancer-cluster point at any given time, with the coordinates of denoted as \(\left({C}_{x}^{t},{C}_{y}^{t}\right)\).
$$\left\langle {x}_{t},{y}_{t}\right\rangle =\langle {C}_{x}^{t}-{T}_{x}^{t},{C}_{y}^{t}-{T}_{y}^{t}\rangle$$$${\alpha }_{{x}_{t}}={\cos }^{-1}\frac{{x}_{t}}{{||}\langle {x}_{t},{y}_{t}\rangle {||}}$$$${T}_{x}^{t+1}={T}_{x}^{t}+{d}_{i}\cos ({\alpha }_{t}-{\alpha }_{{x}_{t}})$$$${T}_{y}^{t+1}={T}_{y}^{t}+{d}_{i}\sin ({\alpha }_{t}-{\alpha }_{{x}_{t}})$$\({\alpha }_{{x}_{t}}\) represents the angle between the motion vector and the positive x-axis at time t. \({\alpha }_{t}\) represents bias angle from t to t + 1 sampled from bias distributions with respect to the distance towards cancer-cell clusters.
-
4)
Once T cells reach cancer-cell clusters, they can remain for a duration time t sampled from the dwell-time distributions. Each CTL underwent 800 computational simulation steps.
-
5)
Then, the effects of the four patterns within two systems were explored individually by altering the directional bias distribution or directional persistence or step-size distribution, while maintaining consistency in the other two patterns (Table 1). In terms of searching efficiency, we computed the mean arrival time for all CTLs to initially reach cancer clusters in each simulation, which is depicted as a data point in the violin plot generated using the Violinplot function (https://github.com/bastibe/Violinplot-Matlab) illustrated in Fig. 3D.
-
6)
In addition, we examined the number of T cells accumulating on cancer-cell clusters over time to explore whether the motion outside of cancer-cell or adhesion could affect the accumulation of CTLs on cancer cell clusters. Each simulation was repeated 100 times.
Table 1 Computational modeling of the effects of the four motility patterns
Bulk RNA-seq data processing
The raw sequencing reads were trimmed to remove sequences with low quality and short read lengths using Fastp (version 0.20.0). Subsequently, HISAT2 was employed to map the high-quality reads onto the mouse reference genome mm10. The read count matrix corresponding to the genes and samples was generated using featureCounts. Next, the count matrix was subjected to the R package DESeq2 to identify differentially expressed genes with a log2(fold change) cutoff of 1 and a false discovery rate (FDR) of 0.05 (Fig. 4A, E). Additionally, we transformed the count matrix into a TPM matrix to compare the special genes expression levels among different samples (Supplementary Fig. 10A) and R package ggplot2 and pheatmap were used to visualize (Fig. 4B, F). GO term enrichment analysis (MF, molecular function, CC, cellular component, BP, biological process) and KEGG pathway analysis were performed using the R package clusterProfiler62 based on the up-regulated and down-regulated genes, with significance determined by an FDR threshold of less than 0.05 (Fig. 4C, D, G, H). The batch effect between samples from the Round 1 and Round 2 count matrices was corrected using R package sva. Principal component analysis (PCA) was performed using the prcomp function and visualize the first two principal components with the ggplot2 package (Supplementary Fig. 9A). Gene set variation analysis (GSVA) was conducted for specific gene set using the R package GSVA (Supplementary Fig. 9C). Gene set enrichment analysis (GSEA) was conducted for GO, KEGG and HALLMARK gene sets using the R package GSEA (Supplementary Fig. 9H).
ScRNA-seq data processing
Raw gene expression matrices were generated for each sample using the Cell Ranger (v6.1.2) pipeline coupled with the mouse reference genome version mm10-2020-A. The output filtered gene expression matrices were analyzed using R software (version 4.1.2) with the Seurat package (version 4.1.1)63. A custom R script was used to combine the expression data and metadata from all libraries corresponding to a single batch. The expression data matrix was loaded into a Seurat object along with the library metadata for downstream processing. The percentage of mitochondrial transcripts for each cell (percent.mt) was calculated and added as metadata to the Seurat object. Cells were further filtered before dimensionality reduction (nFeature_RNA > 200 & percent.mt<10%). Expression values were then scaled to 10,000 transcripts per cell and log-transformed (NormalizeData function, normalization.method = “LogNormalize”, scale.factor = 10000). We calculated variable features using the FindVariableFeatures function with selection.method = “vst” and nfeatures = 2000. Effects of variables (percent.mt) was estimated and regressed out using the ScaleData function (vars.to.regress = “percent.mt”), and the scaled and centered residuals were used for dimensionality reduction and clustering.
Dimensionality reduction
To reduce the dimensionality of the dataset, the RunPCA function was conducted with default parameters on linear-transformation scaled data generated by the ScaleData function. Next, the ElbowPlot and DimHeatmap functions were used to identify proper dimensions of the dataset (first 11 PCs).
Cell clustering and cluster identification
We first performed the FindNeighbors function before clustering, which takes as input the previously defined dimensionality of the dataset (first 11 PCs). Clustering was performed at varying resolution values using the FindClusters function, and we chose a value of 0.7 for the resolution parameter for the initial stage of clustering. After non-linear dimensional reduction and projection of all cells into two-dimensional space by UMAP, clusters were assigned preliminary identities based on the expression of combinations of known marker genes for major cell classes and types.
Sub-clustering and secondary clustering
We filtered out low quality clusters and mixed cell clusters (C1, C6, C9, C16, C17). Sub-Clustering was performed on the two major cell types (CD8 T Cells and Melanoma) data subset to resolve additional cell types and subtypes. Secondary Clustering was performed using a standard workflow similar to before but with different dimensionality (first 12 PCs) and a value of 0.5 for the resolution parameter. We filtered out three clusters (C9, C10, C11), renamed all clusters from C1 to C9 (as shown in Fig. 5A) and then performed other downstream analyses.
Differential expression genes (DEGs) identification and functional enrichment
Differential gene expression testing was performed using the FindAllMarkers function in Seurat with the parameter only.pos = TRUE. DEGs were filtered using a minimum log2(fold change) of 0.5, a minimum expression percentage value of 0.3 and a maximal p value of 0.05. Dot plots showing the relative average expression of top 10 DEGs in subclusters were visualized (Fig. 5B). All DEGs’ relative average expression in subclusters was visualized using the DoHeatmap function in Seurat (Supplementary Fig. 12A). GO (BP) enrichment analysis for the functions of the DEGs was conducted using the clusterProfiler62 (version 4.2.2) R package (Supplementary Fig. 12B–D).
DEGs analysis between two groups
A Wilcoxon rank-sum test for differential gene expression comparing CD8T-c2 (C9) versus CD8T-cx (merged clusters of C5 to C8, Fig. 5C) in OVActl was performed using the FindAllMarkers function (min.cells.group = 0, min.pct = 0, logfc.threshold = -Inf) in Seurat (Fig. 5D). Differential gene expression comparing Melanoma_c3_Cxcl9 versus Melanoma_c4_Rhox5 (cell type of secondary-clustered dataset) was also performed with the same function and parameters (Fig. 5E). Percentage difference (Δ Percent of Cells) and log2(fold change) are shown in a volcano plot. Genes highlighted in red or blue have adjusted p < 0.05.
Cell-cell communication analysis (CellChat)
We used CellChat37 (v1.4.0) to infer cell-cell communication based on prior ligand-receptor interaction databases. We separately loaded the normalized counts data of WT group (WTcancer, WTctl) and OVA group (OVAcancer, OVActl) extracted from Seurat object into CellChat and followed the workflow recommended in CellChat to infer and visualize cell-cell communication network. The database of known receptor-ligand pairs was applied to assess cell-cell communication in the selected target clusters (CD8T-c2, CD8T-cx, Melanoma_c3_Cxcl9, Melanoma_c4_Rhox5) of OVA group (Fig. 5F and Supplementary Fig. 12F). Interactions were trimmed based on significant sites with p < 0.01.
Statistics and reproducibility
Statistical analysis of quantitative motility patterns and computational modeling were conducted using MATLAB. Results are presented as mean ± s.e.m., with individual data points shown, unless otherwise stated. The statistical test methods and their corresponding p-values are listed in Supplementary Data 8. For all relevant figures, p-value annotations are as follows: ns: p ≥ 0.05; *: 0.01 < p < 0.05; **: 0.001 < p ≤ 0.01; ***: p ≤ 0.001, unless otherwise indicated. To ensure reproducibility, at least five parallel experiments were conducted, and statistical analyses were performed on the resulting data (Fig. 2, Supplementary Figs. 3–5). Computational simulations were repeated 100 times. For clarity in visualization, data points are shown with a sub-sampling interval of 5 (Fig. 3D–H, Supplementary Fig. 8D–G). To enhance visualization clarity, the curves were smoothed using a smoothing parameter of 0.05 (Figs. 2C, 3D–H, Supplementary Figs. 3B, C, 8D–G). Violin plots display the distribution quartiles, with the white dot indicating the median and individual points indicating raw data (Fig. 3D, Supplementary Fig. 8C). For bulk RNA-seq, samples were derived from multiple biological replicates across at least two independent experiments. For single-cell RNA-seq, pooled cells from ~40 wells per condition were used from a single experiment. All group sizes (n) refer to biological replicates and are indicated in the figure legends.
Data availability
Bulk RNA-seq and single-cell RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus (GEO), under the accession numbers GSE264477, GSE293493, GSE293495 and GSE264478. The key resources table is provided in Supplementary Data 6. Source data are included with this paper (Supplementary Data 1–8) and are also available on Zenodo64,65,66, accessible via https://doi.org/10.5281/zenodo.15819660 and https://doi.org/10.5281/zenodo.15820394. Time-lapse microscopy data are archived at https://doi.org/10.5281/zenodo.15331125. The plasmids pLV[Exp]-Neo-EF1A > {ovalbumin} and pLV[Exp]-Puro-EF1A > GCaMP6s were custom-synthesized by VectorBuilder (IDs: VB220407-1389ztf and VB900129-1022eyu, respectively). These plasmids are available from the corresponding author upon reasonable request. All experimental protocols are described in the Methods section. Protocols were not deposited in a public repository.
Code availability
The custom code used in this study is available on Zenodo at https://doi.org/10.5281/zenodo.15819660 and https://doi.org/10.5281/zenodo.15820394.
References
Philip, M. & Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Raskov, H., Orhan, A., Christensen, J. P. & Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 124, 359–367 (2021).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020).
Zhu, Y., Zhu, X., Tang, C., Guan, X. & Zhang, W. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochim. et. Biophys. Acta (BBA) - Rev. Cancer 1876, 188593 (2021).
Restifo, N. P., Smyth, M. J. & Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 16, 121–126 (2016).
Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35, S185–S198 (2015).
Corthay, A. Does the immune system naturally protect against cancer?. Front. Immunol. 5, 197 (2014).
DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019).
Peranzoni, E. et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proc. Natl. Acad. Sci. USA 115, E4041–E4050 (2018).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Lesokhin, A. M. et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 72, 876–886 (2012).
Jenkins, L. et al. Cancer-associated fibroblasts suppress CD8+ T-cell infiltration and confer resistance to immune-checkpoint blockade. Cancer Res. 82, 2904–2917 (2022).
Freeman, P. & Mielgo, A. Cancer-associated fibroblast mediated inhibition of CD8+ Cytotoxic T cell accumulation in tumours: mechanisms and therapeutic opportunities. Cancers 12, 2687 (2020).
Pruitt, H. C. et al. Collagen fiber structure guides 3D motility of cytotoxic T lymphocytes. Matrix Biol. 85-86, 147–159 (2020).
Kaur, A. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9, 64–81 (2019).
Verma, N. K. et al. Obstacles for T-lymphocytes in the tumour microenvironment: therapeutic challenges, advances and opportunities beyond immune checkpoint. EBioMedicine 83, 104216 (2022).
Anderson, K. G., Stromnes, I. M. & Greenberg, P. D. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell 31, 311–325 (2017).
Ronteix, G. et al. High resolution microfluidic assay and probabilistic modeling reveal cooperation between T cells in tumor killing. Nat. Commun. 13, 3111 (2022).
Galeano Niño, J. L. et al. Cytotoxic T cells swarm by homotypic chemokine signalling. eLife 9, e56554 (2020).
Krummel, M., Bartumeus, F. & Gérard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 16, 193–201 (2016).
Ruocco, M. G. et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J. Clin. Investig. 122, 3718–3730 (2012).
You, R. et al. Active surveillance characterizes human intratumoral T cell exhaustion. J. Clin. Investig. 131, e144353 (2021).
Pittet, M. J. & Weissleder, R. Intravital imaging. Cell 147, 983–991 (2011).
Jacquemin, G. et al. Longitudinal high-resolution imaging through a flexible intravital imaging window. Sci. Adv. 7, eabg7663 (2021).
Dekkers, J. F. et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 41, 60–69 (2023).
Cattaneo, C. M. et al. Tumor organoid-T-cell coculture systems. Nat. Protoc. 15, 15–39 (2020).
Sadjadi, Z., Zhao, R., Hoth, M., Qu, B. & Rieger, H. Migration of Cytotoxic T Lymphocytes in 3D Collagen Matrices. Biophys. J. 119, 2141–2152 (2020).
Strating, E. et al. Co-cultures of colon cancer cells and cancer-associated fibroblasts recapitulate the aggressive features of mesenchymal-like colon cancer. Front. Immunol. 14, 1053920 (2023).
Abuwatfa, W. H., Pitt, W. G. & Husseini, G. A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 31, 7 (2024).
Jubelin, C. et al. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 12, 155 (2022).
Pebworth, M. P., Cismas, S. A. & Asuri, P. A novel 2.5D culture platform to investigate the role of stiffness gradients on adhesion-independent cell migration. PLoS ONE 9, e110453 (2014).
Chen, K. & Qin, K.-R. Random walks of a cell with correlated speed and persistence influenced by the extracellular topography. Front. Phys. 9, 719293 (2021).
Weavers, H. et al. Systems analysis of the dynamic inflammatory response to tissue damage reveals spatiotemporal properties of the wound attractant gradient. Curr. Biol. 26, 1975–1989 (2016).
Soikkeli, J. et al. Metastatic outgrowth encompasses COL-I, FN1, and POSTN up-regulation and assembly to fibrillar networks regulating cell adhesion, migration, and growth. Am. J. Pathol. 177, 387–403 (2010).
Katsetos, C. D., Herman, M. M. & Mork, S. J. C. lassI. I. I. beta-tubulin in human development and cancer. Cell Motil. Cytoskeleton 55, 77–96 (2003).
Herold-Mende, C., Kartenbeck, J., Tomakidi, P. & Bosch, F. X. Metastatic growth of squamous cell carcinomas is correlated with upregulation and redistribution of hemidesmosomal components. Cell Tissue Res. 306, 399–408 (2001).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Nair, P., Melarkode, R., Rajkumar, D. & Montero, E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin. Exp. Immunol. 162, 116–130 (2010).
Zimmerman, A. W. et al. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 107, 3212–3220 (2006).
Jaeger, A. M. et al. Deciphering the immunopeptidome in vivo reveals new tumour antigens. Nature 607, 149–155 (2022).
Ohtsuka, M., Inoko, H., Kulski, J. K. & Yoshimura, S. Major histocompatibility complex (Mhc) class Ib gene duplications, organization and expression patterns in mouse strain C57BL/6. BMC Genom. 9, 178 (2008).
Meyer, E. A. et al. Discovery and in vivo evaluation of ACT-660602: a potent and selective antagonist of the chemokine receptor CXCR3 for autoimmune diseases. J. Med. Chem. 65, 11513–11532 (2022).
Pathni, A. et al. Cytotoxic T Lymphocyte activation signals modulate cytoskeletal dynamics and mechanical force generation. Front. Immunol. 13, 779888 (2022).
Baldwin, J. G. et al. Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy. Cell 187, 6614–6630.e6621 (2024).
Brewer, G. Nanotubes power up T cells. Nat. Rev. Cancer 25, 5–5 (2025).
Cernuda-Morollon, E., Millan, J., Shipman, M., Marelli-Berg, F. M. & Ridley, A. J. Rac activation by the T-cell receptor inhibits T cell migration. PLoS ONE 5, e12393 (2010).
Billadeau, D. D., Nolz, J. C. & Gomez, T. S. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 7, 131–143 (2007).
Dustin, M. L. & Long, E. O. Cytotoxic immunological synapses. Immunol. Rev. 235, 24–34 (2010).
Ye, L. Y. et al. Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 76, 818–830 (2016).
Chen, L. et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 5, 5241 (2014).
McKenzie, B., Khazen, R. & Valitutti, S. Greek fire, poison arrows, and scorpion bombs: how tumor cells defend against the siege weapons of cytotoxic T lymphocytes. Front. Immunol. 13, 894306 (2022).
Weigelin, B. & Friedl, P. T cell-mediated additive cytotoxicity - death by multiple bullets. Trends Cancer 8, 980–987 (2022).
Yuan, J., Li, X. & Yu, S. Cancer organoid co-culture model system: novel approach to guide precision medicine. Front. Immunol. 13, 1061388 (2022).
Santos-Zas, I. et al. Cytotoxic CD8(+) T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat. Commun. 12, 1483 (2021).
Weigelin, B. et al. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun. 12, 5217 (2021).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Ershov, D. et al. TrackMate 7: integrating state-of-the-art segmentation algorithms into tracking pipelines. Nat. Methods 19, 829–832 (2022).
Wang, Y., Jeong, Y., Jhiang, S. M., Yu, L. & Menq, C.-H. Quantitative characterization of cell behaviors through cell cycle progression via automated cell tracking. PLoS ONE 9, e98762 (2014).
Oliveira, F. A., Ferreira, R. M. S., Lapas, L. C. & Vainstein, M. H. Anomalous diffusion: a basic mechanism for the evolution of inhomogeneous systems. Front. Phys. 7, 18 (2019).
Bredfeldt, J. S. et al. Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J. Biomed. Opt. 19, 16007 (2014).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Stuart, T. et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902 e1821 (2019).
Li, X., Jin, T., & Wang, L. Codes for simulation part and other figs [Data set]. Zenodo. https://doi.org/10.5281/zenodo.15819660 (2025).
Li, X., Jin, T., & Wang, L. Deciphering antigen-specific T cell navigation tactics and cancer immune evasion in co-cultures [Data set]. Zenodo. https://doi.org/10.5281/zenodo.15820394 (2025).
Li, X. Codes and images for fig2 [Data set]. Zenodo. https://doi.org/10.5281/zenodo.15331125 (2025).
Acknowledgements
This work was supported by the Key-Area Research and Development Program of Guangdong Province (2022B0303030001, 21202107221900001), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0480000), the National Key R&D Program of China (2021YFA0911100), the National Natural Science Foundation of China (32170672, 12274308, 32000886, 12004271, T2221001), the Guangdong Basic and Applied Basic Research Foundation (2021A1515012461, 2019A1515110186), Shenzhen Science and Technology Program (ZDSYS20220606100606013) and the Guangdong Grants (2021QN02Y554).
Author information
Authors and Affiliations
Contributions
W.H. and XF.L. conceived and designed the study. T.J. and L.W. conducted all experiments. T.J. carried out manual analysis. XY.L. performed quantitative analysis and agent-based modeling. XF.L. and XY.L. performed RNA sequencing analysis. XY.L. and XF.L. wrote the manuscript with input from all authors. XF.L., W.H., and M.L. supervised the study. During the revision process, S.W. conducted additional analyses of the bulk RNA-seq data, and Y.L., T.J., and XY.L. validated the accuracy of CTL trajectory tracking. All authors contributed to manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were conducted in accordance with the ethical guidelines approved by the Animal Research Committee of Guangdong Medical University (Protocol No. GDY2104034). We have complied with all relevant ethical regulations for animal use. This study did not involve human participants or data from identifiable individuals. Considerations of inclusion, diversity, and global research equity were not applicable in this study.
Peer review
Peer review information
Communications Biology thanks Liqiang Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joanna Hester, Dario Ummarino and Mengtan Xing. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Jin, T., Wang, L. et al. Deciphering antigen-specific T cell navigation tactics and cancer immune evasion in co-cultures. Commun Biol 8, 1140 (2025). https://doi.org/10.1038/s42003-025-08568-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08568-w