Abstract
Adenosine-to-inosine (A-to-I) RNA editing is a critical post-transcriptional modification that enhances tumor genome diversity and contributes to cancer progression. In non-small cell lung cancer (NSCLC), while specific A-to-I editing events have been identified, their functional mechanisms and clinical relevance remain poorly understood. Here, through whole-transcriptome analysis of NSCLC specimens, we discovered a hyper-editing event at position c.1746 in the long non-coding RNA SNHG3 (c.1746 A > I), which correlates with advanced metastatic stages and reduced patient survival. Functional studies demonstrated that edited SNHG3 (SNHG3ED) exhibits significantly greater pro-metastatic activity compared to its wild-type counterpart (SNHG3WT). Mechanistically, SNHG3ED shows enhanced binding affinity for the chromatin remodeler SSRP1, triggering SSRP1-mediated replication origin assembly and subsequent upregulation of fatty acid metabolism and ferroptosis-related genes. This molecular rewiring promotes fatty acid oxidation, confers resistance to ferroptosis, and importantly, drives docetaxel (DTX) chemoresistance. In DTX-resistant NSCLC cell lines, patient-derived organoids, and Nude mouse xenograft tumor model, antisense oligonucleotide-based targeting of SNHG3ED effectively restored DTX sensitivity and suppressed tumor growth. Our findings demonstrate that SNHG3 c.1746 A > I editing serves both as a novel prognostic biomarker for NSCLC and as a mechanistically defined therapeutic target to overcome DTX resistance, which offers a potential therapeutic target to improve DTX efficacy.

Similar content being viewed by others
Introduction
Metastasis remains the principal cause of therapeutic failure and mortality among cancer patients, representing approximately 90% of cancer-related fatalities. Despite considerable advancements in elucidating the critical molecular mechanisms governing cancer metastasis, relatively few molecules have been successfully targeted to enhance patient survival outcomes. A potential reason for this limitation is that pivotal regulators of metastasis are frequently associated with aberrant gene expression or mutations. The identification of novel key regulators of metastasis and the innovation of targeted therapeutic strategies aimed at these regulators could substantially improve survival rates among cancer patients.
RNA editing, particularly adenosine-to-inosine (A-to-I) editing, represents over 70% of human RNA editing events and is emerging as a significant cancer hallmark1. This modification, which is recognized as guanine post-transcriptionally, mimics an A-to-G mutation, enhancing transcriptomic and proteomic diversity in cancers2,3,4,5. Dysregulation of A-to-I editing, such as the S367G mutation in AZIN1, promotes various cancers6,7,8,9. Notably, allele-selective drugs targeting the editing site have shown feasibility and promise as a therapeutic strategy for tumors10.
Recent in silico analyses of The Cancer Genome Atlas (TCGA) RNA sequencing data have revealed numerous progression-related A-to-I editing sites across various cancers11,12. However, their effects on cancer progression are largely unknown. We have previously identified a comprehensive set of survival-related A-to-I editing sites specific to non-small cell lung cancer (NSCLC) and developed an A-to-I editing-based model to predict NSCLC prognosis13. Our findings also highlighted false positives in the determination of A-to-I editing sites in prior in silico analyses14, underscoring the need for further research to understand the functional impact of these editing events in NSCLC.
In this study, we analyzed publicly available A-to-I editing data for NSCLC and identified a pro-metastatic editing site in the small nucleolar RNA host gene 3 (SNHG3), which was hyper-edited in NSCLC tissues compared to adjacent normal tissues. This editing event correlates with increased metastasis by modulating fatty acid metabolism and ferroptosis resistance. Targeting this SNHG3 editing site could offer a novel therapeutic strategy for NSCLC.
Results
Identification of A-to-I editing sites related to metastasis and survival in NSCLC
We analyzed public A-to-I editing profiles of TCGA-NSCLC patients from Synapse (https://www.synapse.org/#!Synapse:syn2374375/files/) and clinical data from the TCGA database (https://portal.gdc.cancer.gov/). Among 34,430 editing sites, 275 were linked to lymph node metastasis (P < 0.05, |r | ≥ 0.05; Fig. 1a and Supplementary Data 1-1), and 189 to survival (P < 0.005, |lnHR | > 0.4; Fig. 1b and Supplementary Data 1-2). Three sites were both metastasis- and survival-related (Fig. 1c), with our focus on an A-to-I editing site in SNHG3 for several reasons: the site is situated in the 3’-untranslated region (3’-UTR) of SNHG3, distinguishing it from other sites in gene introns or intergenic regions. SNHG3 is a recognized oncogenic long non-coding RNA (lncRNA) in various cancers, especially NSCLC15. Notably, this editing site is uniquely associated with poor prognosis in NSCLC among 12 cancer types analyzed in the TCGA dataset (Supplementary Fig. 1). Multivariable Cox regression adjusted for age, sex, and tumor purity (ESTIMATE score) confirmed the independent prognostic value (P = 0.032). We designated the selected site as c.1746 A > I due to its location 1746 nt downstream of the transcription start site of SNHG3 (NR_002909.2). After confirming c.1746 A > I as a genuine A-to-I editing event through in vitro and in vivo by direct sequencing (Fig. 1d) and BaseScope analysis (Fig. 1e, f), we proceeded to analyze the editing level of c.1746 A > I in a cohort of 206 NSCLC patients using pyrosequencing (Fig. 1g). The c.1746 A > I exhibited a hyper-editing occurrence in tumor tissues (Fig. 1h), and hyper-editing of this editing displayed a significant positive association with lymph node metastasis and distant metastasis (Fig. 1i, j), respectively, while showing no correlation with tumor size (Supplementary Fig. 2a). Notably, hyper-editing of c.1746 A > I was further linked to poorer survival outcomes in NSCLC patients (Fig. 1k). Multivariable Cox regression adjusted for age and sex further confirmed the independent prognostic value (P = 0.048). Stability testing confirmed consistent editing levels across different tissue pieces (Supplementary Fig. 2b), supporting the conclusion that c.1746 A > I in SNHG3 is over-edited in NSCLC and correlated with metastasis.
a Volcano plot identifying lymph node metastasis-associated A-to-I editing events (|r | ≥ 0.05, P < 0.05), with positively and negatively correlated sites shown in red and light green, respectively. b Survival-associated A-to-I editing events (|lnHR | > 0.4, P < 0.005), where red indicates increased mortality risk and green indicates decreased risk. c Venn diagram and table displaying three overlapping A-to-I editing sites that are significantly associated with both metastasis and survival. d Sanger sequencing chromatograms comparing cDNA (top) and genomic DNA (gDNA, bottom), with the arrow indicating the c.1746 A > I editing site. e, f BASEscope in situ hybridization images showing distinct localization of wild-type SNHG3 (SNHG3WT, green) and edited SNHG3 (SNHG3ED, red) in cultured cells (e; scale bar = 10 μm) and clinical specimens (f; scale bar = 50 μm). g Quantitative analysis of c.1746 A > I editing levels by pyrosequencing. h Comparison of editing frequencies between matched tumor and adjacent normal tissues (paired t-test). Significant differences in editing levels between patients with and without lymph node metastasis (i) or distant metastasis (j) (unpaired t-test with Welch’s correction). k Kaplan-Meier survival curves demonstrating poorer overall survival in patients with high c.1746 A > I editing (log-rank test). Error bars represent mean ± SD (i, j).
ADAR1 Catalyzes the Editing of SNHG3 c.1746 A > I
Upon analyzing the expression data of NSCLC samples from the TCGA database, we observed a significant correlation between ADAR1, rather than ADAR2, and c.1746 A > I editing (Supplementary Fig. 2c, d). This finding was consistent in our cohort (Supplementary Fig. 2e). Furthermore, we confirmed that ADAR1 is responsible for editing c.1746 A > I by demonstrating that silencing ADAR1 (Supplementary Fig. 2f, g) led to reduced editing of c.1746 A > I in NSCLC cells (Supplementary Fig. 2h).
Enhanced pro-metastatic potential of SNHG3 through c.1746 A > I
In silico predictions using LNCediting that analyzed a 51-nt fragment (29-nt upstream and 21-nt downstream of c.1746) showed that c.1746 A > I may lead to structural changes in SNHG3 (Fig. 2a)16. Utilizing lentiviral vectors, wild-type SNHG3 (SNHG3WT) and edited SNHG3 (SNHG3ED, carrying either A or G allele at the c.1746 site) were overexpressed. Assessment of SNHG3 expression levels and c.1746 A > I editing confirmed the successful overexpression of SNHG3 and hyper-editing of SNHG3 in NSCLC cells (Supplementary Fig. 3a, b). Importantly, the analysis indicated that c.1746 A > I did not significantly alter SNHG3 expression levels, consistent with the lack of significant correlation between c.1746 A > I editing and SNHG3 expression in the TCGA dataset (Supplementary Fig. 3c).
a Computational modeling (LNCediting) predicts structural alterations in SNHG3 induced by c.1746 A > I editing. Functional assays of NSCLC cells stably expressing SNHG3WT, SNHG3ED or empty vector (control; Scale bar =300μm) in wound healing (b, n = 5–10), Transwell migration (c, n = 12; Scale bar = 50μm) and Matrigel invasion (d, n = 12; Scale bar = 50μm) assays (two-way ANOVA with Tukey’s test for b; one-way ANOVA with Tukey’s test for c, d). e Longitudinal bioluminescence imaging of orthotopically implanted cells at 2, 8, or 10 weeks (n = 5; Scale bar =1 cm), with metastatic signal quantification (two-way ANOVA). f Ex vivo analysis showing bioluminescent lung metastases (left; Scale bar =1 cm), corresponding H&E sections (middle; Scale bar =100μm), and metastasis frequency (right). g Competitive metastasis assay using 1:1 mixed SNHG3ED/SNHG3WT cells (ratio paired t-test; Scale bar =1 cm). h Sanger sequencing showing the existence of c.1746 A > I in plasma exosomes derived from three NSCLC patients. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. N indicates biological replicates.
The overexpression of SNHG3, both SNHG3ED and SNHG3WT, promoted tumor progression, enhancing cell proliferation, colony formation, migration, and invasion in vitro, aligning with previous studies (Fig. 2b–d, Supplementary Fig. 3d, e). Notably, SNHG3ED exhibited a specific enhancement in metastatic potential compared to SNHG3WT rather than proliferative capacity in both A549 and PC9 cells. In vivo orthotopic implantation experiments verified that SNHG3ED notably enhanced tumor metastatic ability compared to SNHG3WT, with 50% of SNHG3ED-implanted mice developing contralateral lung metastasis, as opposed to 10% in SNHG3WT-implanted mice (Fig. 2e, f). Additionally, intrapulmonary injection of a 1:1 mixture of SNHG3ED- and SNHG3WT-cells into eight mice demonstrated heightened c.1746 A > I editing levels in three metastatic tumors, compared with matched primary tumors (Fig. 2g). Interestingly, this editing site was identically detected in serum exosomes (Fig. 2h), which play a critical role in facilitating cancer metastasis17. These findings point to c.1746 A > I editing in SNHG3 amplifying its capacity to drive cancer metastasis.
SNHG3ED upregulates genes related to fatty acid metabolism and ferroptosis
To investigate the mechanism underlying SNHG3ED-mediated cancer metastasis, we analyzed the correlation between c.1746 A > I and the NSCLC transcriptome using TCGA data. We identified 1,931 candidate genes associated with c.1746 A > I that exhibited significant correlations (|r | > 0.1, P < 0.05; Fig. 3a; Supplementary Data 2). Among these, 22 genes enriched in ECM-receptor interaction, ferroptosis, and fatty acid metabolism—known to influence cancer metastasis18,19—were further examined (Fig. 3b, c). Subsequent analysis showed upregulation of genes like hyaluronan mediated motility receptor (HMMR), thrombospondin 3 (THBS3), glutathione synthetase (GSS), cystathionine beta-synthase (CBS), and enoyl-CoA hydratase, short chain 1 (ECHS1), carnitine palmitoyltransferase 1B (CPT1B) in SNHG3ED cells compared to SNHG3WT cells at both the mRNA and protein levels (Fig. 3d, e).
a Correlation analysis between c.1746 A > I editing levels and gene expression in TCGA NSCLC samples (Spearman’s correlation, |r | >0.1, P < 0.05). b KEGG pathway enrichment of c.1746 A > I-associated genes. c Correlation network of c.1746 A > I with 22 genes enriched in ECM-receptor interaction, ferroptosis, and fatty acid metabolism (dot size: significance; color: correlation direction). d Quantification of mRNA expression of 22 candidate genes in SNHG3ED vs SNHG3WT cells (n = 3-5, unpaired t-test). e Western blot validation of selected targets (n = 4, unpaired t-test). f Oil Red O staining showing lipid droplet accumulation (n = 10, unpaired t-test; scale bar = 100μm). Metabolite quantification of triglycerides (g), total FFAs (h), and specific medium/long-chain FFAs (i) (n = 6, unpaired t-test). j FAO activity assessed by FAOBlue fluorescence (scale bar = 20μm). Seahorse analysis of OCR (k) and respiratory capacity (l) with Eto (CPT1 inhibitor establishing baseline FAO capacity), Oligo (ATP synthase inhibitor measuring proton leak), FCCP (Uncoupler assessing maximal respiratory capacity), and Rot/AA (Complex I/III inhibitors determining non-mitochondrial respiration) treatment (n = 5–6, one-way ANOVA with Tukey’s multiple comparisons test). m Ferroptosis sensitivity to 10 μM erastin (a ferroptosis inducer) with/without 1 μM ferrostatin-1 (a specific ferroptosis inhibitor) (n = 5, unpaired t-test). Data are mean ± SD. Significance: *P < 0.05, **P < 0.01, ***P < 0.001; ns = not significant. N indicates biological replicates.
SNHG3ED promotes fatty acid oxidation to reduce lipid peroxidation and ferroptosis
We focused on illustrating the effect of c.1746 A > I editing on fatty acid metabolism and ferroptosis. Initial experiments demonstrated that SNHG3ED increased lipid droplet accumulation (Fig. 3f) and triglyceride synthesis (Fig. 3g), concomitant with reduced free fatty acids (FFAs) levels, particularly the saturated fatty acids C16:0 and C18:0 (Fig. 3h, i, Supplementary Fig. 4a), as evidenced by higher triglyceride levels and lower FFAs relative to SNHG3WT. Subsequently, we assessed the influence of SNHG3ED on acyl-carnitine formation, a crucial step mediated by CPT1 in mitochondrial FFA import for β-oxidation. Despite reduced FFAs, SNHG3ED did not consistently alter acyl-carnitine levels compared to SNHG3WT (Supplementary Fig. 4b), suggested potential enhancement of fatty acid oxidation (FAO) ability. Enhanced FAO in SNHG3ED-expressing cells was observed (Fig. 3j) and confirmed by increased oxygen consumption rate (OCR) and maximal respiration, indicative of augmented mitochondrial function (Fig. 3k, l). Treatment with etomoxir (a CPT1 inhibitor) further underscored the dependency of SNHG3ED-expressing cells on FAO for basal respiration. Additionally, SNHG3ED cells showed greater resistance to ferroptosis (Fig. 3m). Thus, SNHG3 c.1746 A > I modifies key aspects of lipid handling and cell survival under oxidative stress in NSCLC.
SNHG3ED-induced FAO activity is enhanced by Palmitic acid
Research has found that the fatty acid, palmitic acid (PA) is present in the pre-metastatic environment of the lungs, promoting metastasis20. We thus examined the impact of PA on c.1746 A > I editing linked to FAO. Cells were treated with palmitate-conjugated bovine serum albumin (BSA) or BSA control. The induction of PA led to substantially higher levels of AcCa (16:0) in SNHG3ED cells compared to SNHG3WT cells (Fig. 4a), and enhanced FAO, reflected in the FAO blue marker (Fig. 4b). Furthermore, while PA significantly increased the OCR in all cells overexpressing SNHG3ED or SNHG3WT (Fig. 4c), it only raised the maximal respiration rates in SNHG3ED cells but not in SNHG3WT cells (Fig. 4d). To further explore the utilization of PA in FAO and tricarboxylic acid (TCA) cycle, a 13C-isotope tracing experiment was undertaken by incubating cells with 13C-labeled PA (Fig. 4e). Significant increases in carbon-labeled Palmitoylcarnitine (Fig. 4f) and Acetyl-CoA (Fig. 4g) were observed in SNHG3ED cells compared to SNHG3WT cells. Additionally, relative to SNHG3WT cells, SNHG3ED cells exhibited enhanced carbon labeling across major TCA cycle metabolites, with significant increases in specific metabolites like M3+ isotopologues of citrate, M2+ isotopologues of succinate, and M2+ isotopologues of acetoacetate in A549 or PC9 cells (Fig. 4h–j, Supplementary Fig. 5a). No notable differences were observed in glycolytic and glutamine metabolites (Supplementary Fig. 5b, c). These findings suggest SNHG3 c.1746 A > I enhances FAO to fuel the TCA cycle.
a Increased palmitoylcarnitine levels in SNHG3ED versus SNHG3WT cells after PA treatment (n = 6, unpaired t-test). b Enhanced FAO activity (FAOBlue fluorescence) following PA stimulation (scale bar=20μm). Seahorse analysis showing differential OCR (c) and respiratory capacity (d) responses to PA and etomoxir (Eto) treatment (n = 4, one-way ANOVA). e Metabolic tracing schematic of 13C-PA through β-oxidation and TCA cycle (red indicates 13C-labeled carbons). Metabolic profiling of Palmitoylcarnitine (f), Acetyl-CoA (g), Citrate (h), Succinate (i), and Acetoacetate (j) in response to 13C-PA treatment (n = 4, unpaired t-test). Data are mean ± SD. Significance: *P < 0.05, **P < 0.01, ***P < 0.001; ns not significant. N indicates biological replicates.
c.1746 A > I enhances the interaction of SNHG3 with SSRP1
Based on the BaseScope assay results (Fig. 1e) and subcellular localization analysis findings (Fig. 5a), which indicated the nuclear localization of SNHG3, we hypothesized that SNHG3ED enhances the expression of specific genes through transcriptional regulation. To examine this, we conducted RNA pull-down assays followed by mass spectrometry to identify proteins interacting with SNHG3. We identified 348 proteins in A549 and 34 in PC9 with differential binding affinities to SNHG3ED and SNHG3WT (Log2 fold change >1), with 13 proteins appearing in both cell lines (Fig. 5b, Supplementary data 3). Subsequently, we validated interaction with 7 nuclear-localized proteins (Supplementary Fig. 6; data sourced from the Human Protein Atlas project: https://www.proteinatlas.org/). Among these proteins, only SSRP1 co-precipitated with biotin-labeled SNHG3ED or SNHG3WT probes (Fig. 5c). Furthermore, RNA immunoprecipitation (RIP) with an antibody targeting SSRP1 confirmed the interaction between SSRP1 and SNHG3, showing that SNHG3ED exhibits stronger binding to SSRP1 than SNHG3WT (Fig. 5d). Consistently, quantitative colocalization analysis using Immunofluorescence staining revealed significant overlap between SNHG3 and SSRP1 signals (Pearson’s r = 0.72 ± 0.068; Fig. 5e), with prominent colocalization in nucleolar regions. Additionally, in silico analysis using catRAPID omics (http://service.tartaglialab.com/page/catrapid_group) indicated that nucleotides 1500–1900 of SNHG3 may mediate the SNHG3-SSRP1 interaction (Supplementary Fig. 7a) and deletion of these nucleotides in SNHG3 abolished the interaction (Fig. 5f).
a Subcellular localization of SNHG3 by qPCR using NEAT (nuclear) and GAPDH (cytoplasmic) markers. Solid black circles denote cytoplasmic expression levels, while large open circles represent nuclear expression values. b RNA pull-down mass spectrometry showing differential protein binding to SNHG3ED versus SNHG3WT. c Western blot validation of seven candidate nuclear proteins from pull-down assays. d RIP assays confirming enhanced SNHG3ED-SSRP1 binding (n = 3-4, unpaired t-test). e Immunofluorescence showing SNHG3 (red) and SSRP1 (green) nuclear co-localization (scale bar = 10μm), and colocalisation threshold analysis. f SSRP1 binding to full-length versus Δ1500-1900 truncated SNHG3 by RNA pull-down. g qPCR analysis of target genes after SSRP1 knockdown (n = 3, unpaired t-test). Transwell migration (h; Scale bar = 50 μm) and invasion (i; Scale bar = 50μm) assays post-SSRP1 inhibition (n = 3, unpaired t-test). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001; ns = not significant. N indicates biological replicates.
To further elucidate the role of SSRP1 in SNHG3ED’s cancer-promoting effect, we utilized siRNAs to knock down SSRP1 (Supplementary Fig. 7b, c). SSRP1 knockdown downregulated the aforementioned genes related to ECM-receptor interaction, ferroptosis, and fatty acid metabolism (Supplementary Fig. 7d) and abolished the SNHG3ED-induced upregulation (Fig. 5g, Supplementary Fig. 7e). Inhibiting SSRP1 also counteracted or partially offset the enhanced cell migration and invasion induced by SNHG3ED (Fig. 5h, i, Supplementary Fig. 7f, g). TCGA data revealed significant co-expression between SSRP1 and these genes (Supplementary Fig. 7h). Moreover, the c.1746 A > I editing had no impact on SSRP1 expression in NSCLC cells, and there was no substantial correlation between the editing level and SSRP1 expression (Supplementary Fig. 7i, j). In summary, these results highlight the interaction between SNHG3 and SSRP1 in driving pro-metastatic behavior, with the c.1746 A > I editing enhancing this interaction.
Enhancement of SSRP1-dependent transcription elongation by SNHG3ED
Mechanistically, SSRP1 functions to affect nucleosome disassembly and transcription elongation21. To elucidate how the interaction between SNHG3 and SSRP1 affects target gene expression, we monitored transcription elongation using the 4-thiouridine (4sU) combined with 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) transcription elongation assay. Strikingly, SNHG3ED was associated with accelerated transcription elongation rates of GSS, CPT1B, and ECHS1 compared to SNHG3WT, but not CBS (Fig. 6a–d).
4sU-DRB assay showing increased transcriptional elongation rates for GSS (a), ECHS1 (b), and CPT1B (c) in SNHG3ED versus SNHG3WT cells (n = 3, two-way ANOVA). d No significant difference in CBS elongation rates between groups. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. N indicates biological replicates.
Inhibition of SNHG3ED sensitized NSCLC to docetaxel treatment
Drug resistance is a significant factor contributing to cancer metastasis. We investigated whether SNHG3ED affects the sensitivity to anticancer chemotherapeutic agents. By analyzing half maximal inhibitory concentration (IC50) values of various drugs from the Genomics of Drug Sensitivity in Cancer database (GDSC: https://www.cancerrxgene.org/) and SNHG3 expression from the Cancer Cell Line Encyclopedia database (CCLE: https://sites.broadinstitute.org/ccle/), six anti-lung cancer agents were identified: docetaxel (DTX), methotrexate, clofarabine, alvocidib, tivantinib, and BI-2536, whose IC50 values correlated with SNHG3 expression (Fig. 7a). Notably, SNHG3ED cells exhibited increased resistance to DTX and methotrexate (Fig. 7b, Supplementary Fig. 8). DTX was selected for further sensitivity testing due to its established role in advanced-stage lung cancer therapy. We successfully developed a DTX-resistant A549 cell line (A549DR) through a concentration-gradient method. A549DR showed progressively c.1746 A > I editing level and ADAR1 expression (Fig. 7c, d).
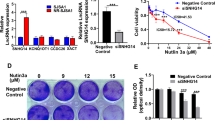
a Drug sensitivity analysis identifies six drugs as candidates correlated with SNHG3 expression (FDR < 10−6). b Increased DTX and methotrexate resistance in SNHG3ED cells (n = 3-5, two-way ANOVA). c Dose-dependent increase in c.1746 A > I editing in A549DR cells (n = 3, one-way ANOVA with Dunnett multiple comparisons test). d Upregulated ADAR1 protein levels in A549DR cells (n = 4, one-way ANOVA). e Therapeutic strategy using LNA-ASOC2 in A549DR cells, xenografts and PDOs. f LNA-ASOC2 restores DTX sensitivity in A549DR cells (n = 4, two-way ANOVA with Tukey’s test). g Bioluminescence imaging of A549DR xenografts post-treatment (n = 5; Scale bar = 1 cm). h Tumor volume reduction with LNA-ASOC2 + DTX combination therapy (n = 5, two-way ANOVA with Tukey’s test). i Representative images of excised xenograft tumors. j LNA-ASOC2 enhances DTX efficacy in patient-derived organoids (n = 6, one-way ANOVA with Tukey’s test). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. N indicates biological replicates.
To evaluate the therapeutic potential of SNHG3ED in NSCLC, we systemically administered an optimized antisense oligonucleotides (ASOs) targeting SNHG3ED in A549DR in vitro and in A549DR cell-derived xenograft (CDX) model in vivo. We designed two ASOs targeting SNHG3WT with thymine (i.e., ASOT) and two ASOs targeting SNHG3ED with cytosine (i.e., ASOC) at the editing site (Supplementary Fig. 9a). The ASOC2 was chosen because all ASOs exerted a similar degradation effect in SNHG3 expression (Supplementary Fig. 9b), but this ASO exhibited a promising precise decline in SNHG3ED with lowest editing level of c.1746 A > I in NSCLC cells (Supplementary Fig. 9c). The ASOC2 was then locked nucleic acid (LNA) modified to improve its stably in vivo. The LNA-ASOC2 was administered to A549DR cells in vitro, A549DR CDX in vivo, and patient-derived organoids (PDOs) from two NSCLC patients ex vivo (Fig. 7e). The LNA-ASOC2 significantly overcame DTX resistance in A549DR cells in a dose-response manner (Fig. 7f). In A549DR CDX, DTX treatment alone did not affect the tumor growth while LNA-ASOC2 alone could suppress the growth and the combination of the ASO and DTX exerted the highest inhibitory effect (Fig. 7g–i). Encouraged by the efficacy of LNA-ASOC2 to overcome DTX resistance in A549DR, and in view of the important clinical implications of such results, we performed LNA-ASOC2 treatment in two PDOs, which has been published previously22. Compared to the DTX treatment group, the addition of LNA-ASOC2 resulted in a significant decrease in the viability of PDOs (Fig. 7j). Furthermore, LNA-ASOC2 alone effectively inhibited PDO growth when compared to the control ASO (ASONC; Supplementary Fig. 9d). Collectively, the above results suggest that SNHG3ED could be a potential target for NSCLC treatment.
Discussion
In this study, our aim was to identify dual-functional A-to-I editing sites that are associated with survival and metastasis. Our findings indicate the following: (1) Hyper-editing of c.1746 A > I in SNHG3 is associated with poor survival and advanced metastatic stages of NSCLC. (2) The c.1746 A > I editing significantly enhances SNHG3 pro-metastatic activity by promoting FAO activity and conferring ferroptosis resistance, particularly under PA stimulation. SNHG3ED exhibits enhanced interaction with SSRP1, regulating proteins involved in FAO and ferroptosis. (3) SNHG3ED contributes to acquired resistance to DTX, and ASO targeting of SNHG3ED could combat DTX resistance. These results strongly suggest that c.1746 A > I editing of SNHG3 promotes NSCLC metastasis by interacting with SSRP1 to coordinate FAO and ferroptosis, and that ASO targeting SNHG3 could enhance DTX treatment efficacy in NSCLC.
The role of A-to-I editing in NSCLC remains largely unexplored. Four independent studies analyzing public and self-generated transcriptomes identified the A-to-I editome for NSCLC, showing elevated A-to-I editing frequencies in NSCLC and associating many A-to-I sites with NSCLC survival or stages11,12,23,24. Despite >1 millions detected editing sites, few have been experimentally confirmed and functionally characterized. For example, the A-to-I editing resulting in the RHOAiso2 R176G mutation activates the RHOA-GTP/p-ROCK1/2 signaling pathway, promoting tumor progression25. Additionally, A-to-I edited miR-411-5p targets the hepatocyte growth factor receptor, enhancing tyrosine kinase inhibitor response in resistant NSCLC cells26. To identify critical A-to-I events driving NSCLC progression, we conducted in silico and empirical studies, revealing the pro-metastatic and theranostic potential of c.1746 A > I editing in SNHG3. Prioritizing the development of this target could provide significant advancements in predicting and inhibiting NSCLC progression.
SNHG3 is a well-established oncogenic long non-coding RNA (lncRNA) known for promoting cancer cell proliferation and metastasis27. While previous studies have shown that SNHG3 is distributed in both the nucleus and cytoplasm, its cancer-promoting mechanism has been predominantly studied in the cytoplasm28,29. We expand on this knowledge by confirming the nuclear localization of SNHG3 and categorizing it as a nuclear-localized transcription regulatory lncRNA that interacts with SSRP1. The nuclear SNHG3 physically binds to SSRP1, a subunit of the Facilitates Chromatin Transcription complex, which is crucial for nucleosome disassembly and transcription elongation30,31. Notably, SNHG3ED exhibits a stronger binding affinity for SSRP1, enhancing SSRP1-mediated transcription elongation, thus increasing gene expressions related to FAO and ferroptosis.
FAO and its key catalytic enzymes ECHS1 and CPT1B were upregulated by the c.1746 A > I editing in SNHG3, consistent with established roles of FAO in lung cancer progression through energy production, metabolic intermediate supply, and therapy resistance32,33,34. The enhanced FAO activity in SNHG3ED cells not only promoted metastasis - particularly to lipid-rich environments like lymph nodes35 - but also conferred ferroptosis resistance through reduced lipid peroxidation36,37. Mechanistically, the simultaneous upregulation of GSS and CBS (established ferroptosis suppressors via glutathione synthesis and transsulfuration pathways) creates a robust antioxidant system that protects SNHG3ED cells from oxidative stress38,39. This dual adaptation explains the heightened FAO response to PA in SNHG3ED cells compared to SNHG3WT, mirroring clinical observations where PA stimulates metastasis-initiating cells in lipid-rich lung environments20,40,41.
Furthermore, our data highlight the therapeutic potential of c.1746 A > I in DTX resistance in NSCLC. By utilizing a LNA-modified ASO design, we constructed LNA-ASOC2 to target c.1746 A > I and found it effectively suppresses tumor cell growth and promotes DTX sensitivity. However, its dual mechanism of action that combines conventional expression inhibition with editing-specific disruption makes it challenging to attribute LNA-ASOC2 anti-tumor efficacy solely to editing-specific inhibition rather than general SNHG3 suppression. Nonetheless, we are convinced that LNA-ASOC2 may provide additional benefits against metastasis-prone cell populations that might be particularly relevant for patients with high c.1746 A > I editing levels in tumors, as the editing event correlates with metastatic progression independent of total SNHG3 expression.
While our findings provide important insights into the functional impact of c.1746 A > I editing in NSCLC progression, several limitations should be acknowledged. First, although our cohort of 206 NSCLC patients provides meaningful clinical correlations, larger multicenter validation is needed to confirm the prognostic value of c.1746 A > I editing. Tumor purity estimation was not performed in this cohort, which may cause confounding effects on these correlations. Second, our mass spectrometry analysis was limited by sample availability, preventing biological replicates that could strengthen the proteomic findings. Third, while this study demonstrates the therapeutic potential of targeting SNHG3 c.1746 A > I editing, we did not perform direct comparative experiments between ASOC2 (editing-targeting) and ASOT1/2 to fully dissect whether the anti-tumor effects are mediated primarily by suppression of the editing event or overall SNHG3 knockdown. Additionally, the in vivo efficacy of LNA-ASOC2 also requires further validation in immunocompetent models to assess potential microenvironmental effects.
In conclusion, our study demonstrates a model in which c.1746 A > I editing enhances pro-metastatic potential and DTX resistance of SNHG3 by interacting with SSRP1 to promote FAO and ferroptosis resistance. This observation may provide insights into the identification of high metastatic risk NSCLC, and inhibiting c.1746 A > I editing of SNHG3 may further improve therapeutic effects.
Methods
Patients and tissue specimens
A total of 206 histopathologically- and stage-confirmed NSCLC patients were analyzed in this study, including a subset that had been previously reported22. The basic characteristics of the subjects are detailed in Supplementary Table 1. Among these patients, 154 were successfully followed up semi-annually via telephone. Overall survival was calculated from the date of initial diagnosis to the date of the last follow-up or death. Notably, there were no significant differences in baseline characteristics and clinical indicators between patients who were successfully followed up and those lost to follow-up, with the exception of smoking status (Supplementary Table 2). Cancerous tissues and adjacent normal tissues were collected intraoperatively and were immediately preserved in RNA stabilization solution before being stored in liquid nitrogen. This study received approval from the institutional review board at Guangzhou Medical University (GMU-20180301), and all patients provided written broad informed consent before participation. All ethical regulations relevant to human research participants were followed.
Cell culture and animal husbandry
The A549 and PC9 cell lines were purchased from the National Collection of Authenticated Cell Cultures (NCACC). All cell lines were cultured in RPMI-1640 medium (GIBCO, Thermo Fisher Scientific, Waltham, MA), supplemented with 10% fetal bovine serum (FBS, GIBCO) and 1% penicillin-streptomycin (GIBCO). The 6–8-week-old female BALB/c nude mice were housed in IVC cages (3-5 mice/cage) with corn cob bedding under controlled conditions (22 ± 1 °C, 50 ± 5% humidity, 12/12 light cycle). Environmental enrichment included paper nesting material and wooden chew sticks. Autoclaved standard chow and acidified water were provided ad libitum. Cages were changed twice weekly by trained staff. We have complied with all relevant ethical regulations for animal use. Animal experiments were approved by the ethics committee of the Guangzhou Medical University (approval number: 2018-004).
DNA, RNA isolation and SNHG3 c.1746 A > I editing test
Genomic DNA (gDNA) were extracted from human lung tissues and cell lines using the DNA Extraction Kit (TIANGEN, China) following the manufacturers’ protocols: tissues were lysed in Buffer GA with Proteinase K (20 mg/mL) at 56 °C for 1-3 hours, followed by RNase A (100 mg/mL) treatment for 5 min at room temperature (RT), column-based purification, and elution in 100 μL AE buffer. Total RNA was isolated using TRIzol Reagent (Thermo Fisher, #15596026) via phase separation (chloroform), isopropanol precipitation, and 75% ethanol washes. Then complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the PrimeScript® Reverse Transcription Kit (Takara Bio Inc.), with oligo (dT) primers (65 °C, 5 min; 42 °C, 60 min; 70 °C, 15 min). Subsequently, PCR amplification was performed using Tag DNA Polymerase (TIANGEN) with the following cycling parameters: initial denaturation at 95 °C for 5 min; 35 cycles of 95 °C for 30 sec, 60 °C for 30 sec, and 72 °C for 45 sec; and a final extension at 72 °C for 10 min. The 25 μL PCR reaction mixture contained 1 × PCR buffer, 2.5 mM dNTPs, 10 μM each primer, 0.5 U Tag DNA Polymerase, and 50 ng cDNA or 100 ng gDNA template. The PCR products were submitted to conventional Sanger sequencing by Shanghai Sangon Biotech Co., Ltd. (China) using their standard protocols.
Pyrosequencing was conducted using the PyroMark Q96 ID System (Qiagen, Germany) following standardized protocols to determine the editing level of the c.1746 A > I editing. Briefly, target regions were amplified via PCR using biotinylated primers to generate single-stranded, biotinylated DNA templates. The PCR products were then immobilized onto streptavidin-coated sepharose beads (PyroMark Q96 Reagents, Qiagen) through 5 min incubation at room temperature with constant agitation. Using the PyroMark Vacuum Prep Workstation, the bead-DNA complexes were captured, washed with 70% ethanol, denatured with 0.2 M NaOH, and neutralized in PyroMark Wash Buffer. Sequencing primers were annealed to the purified templates by heating to 80 °C for 2 min in a PyroMark Q96 Plate. The sequencing reaction was performed automatically using PyroMark Gold Q96 Reagents (Qiagen) according to the manufacturer’s cycling conditions, with nucleotide dispensation orders optimized for each target site. Raw pyrosequencing data were collected and analyzed using PyroMark Q96 ID Software (v2.5.8, Qiagen) to quantify A-to-I editing levels. The pyrosequencing procedures, from PCR product sequencing to final data analysis, were performed by Sangon Biotech under standardized quality control measures, including internal controls for sequencing efficiency and background signal monitoring. Primer sequences used in this study are listed in the Supplementary data 5 (Key Resources Table).
BASEScope
BASEScope probes were custom-designed in collaboration with Advanced Cell Diagnostics (ACD, Newark, CA). BASEScope assays were performed according to the manufacturer’s guidelines (ACD). Briefly, cell suspensions or tissue sections were placed on slides and incubated in hydrogen peroxide for 10 minutes at RT, followed by treatment with protease for 30 minutes at 40 °C, washed with distilled water between each treatment. Prior to use, probes were pre-heated at 40 °C in a water bath or incubator for 10 minutes, then cooled to RT. BASEScope probes were applied for 2 hours at 40 °C in a hybridization oven, followed by incubation with a sequence of amplification reagents: AMP1 (30 minutes at 40 °C), AMP2 (30 minutes at 40 °C), AMP3 (15 minutes at 40 °C), AMP4 (30 minutes at 40 °C), AMP5 (30 minutes at 40 °C), AMP6 (15 minutes at 40 °C), AMP7 (30 minutes at 40 °C), AMP8 (30 minutes at 40 °C), and TSA Plus Fluorescent Dye (10 minutes at RT), with wash buffer rinses between each step. The slides were subsequently dried at 60 °C for 15 minutes and mounted using VectaMount solution. Images were acquired with a Zeiss LSM 800 Confocal Laser Scanning Microscope.
Real-time PCR and Western blotting analysis
Total RNA and protein were extracted from human lung cell lines using TRIzol reagent (Thermo Fisher Scientific) and RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific), respectively, according to the manufacturers’ protocols. Real-time PCR was performed with the RealStar Green Power Mixture (GenStar) on a 7500 Fast Real-Time PCR System (Applied Biosystems). Each sample was analyzed at least three biological replicates and normalized to the endogenous reference control gene β-actin or GAPDH. Western blotting was conducted following established protocols. Protein concentrations were quantified using the BCA Protein Assay Kit (Beyotime, Shanghai, China). 10-30 μg of protein were separated by polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride (PVDF) membrane. Primary antibodies were incubated overnight at 4 °C in western primary antibody dilution buffer (Biosharp, Anhui, China), followed by incubation with HRP-conjugated secondary antibodies in QuickBlockTM secondary antibody dilution buffer (Beyotime, Shanghai, China) for 1 hour. Protein bands were visualized using a chemiluminescence detection kit (Amersham Biosciences). All antibody information including antibody solutions, was provided in the Supplementary data 5 (Key Resources Table). Densitometry was performed with Fiji software according to standard procedure.
Vector construction, siRNA synthesis and cell transfection
To generate stable cell lines that overexpress SNHG3, the full-length SNHG3WT (NR_002909.2 with c.1746 A) and SNHG3ED (c.1746 G) sequences were chemically synthesized and inserted into the pLVX-mCMV-ZsGreen-PGK-Puro vector. Lentivirus was produced by co-transfecting human embryonic kidney 293 T cells with either SNHG3WT, SNHG3ED, or an empty vector, using the Lenti-PacTM HIV (Genecopoeia, Rockville, MD). The harvested viral particles were subsequently utilized to infect A549 and PC9 cell lines, and the efficiency of transfection was assessed via real-time PCR.
To knock down the expression of ADAR1 and SSRP1, three specific siRNAs targeting each gene were synthesized by RiboBio Co., Ltd (Guangzhou, China). The transfection of the mixed siRNAs was carried out using the Lipofectamine 2000 Kit (Invitrogen) according to the manufacturer’s instructions. The efficiency of transfection was evaluated through both real-time PCR and western blotting to confirm the reduction in target gene expression.
In vitro cell behavior assays
To assess cell proliferation, approximately 1–2 × 103 cells were seeded in 100 μl of culture media in 96-well plates, and cell viability was evaluated using the Cell Counting Kit-8 (Beyotime) according to the manufacturer’s instructions. For the oncogenicity assay, 200 cells were plated in 2 ml of culture media in 6-well plates and incubated for 7–14 days. After incubation, the cells were stained with crystal violet and subsequently counted. For migration and invasion assays, approximately 2 × 105 cells were seeded in low-serum medium (0.5% FBS) in Corning Transwell insert chambers (Corning, New York), either with or without BD BioCoat Matrigel Invasion Chamber (BD Biosciences, Bergen, NJ). The lower chambers contained complete growth medium with 10% FBS as chemoattractant. Cells that migrated through the membrane were then fixed, stained, and counted under a light microscope. For the scratch assay, approximately 1-2 × 104 cells were plated in 100 μl of culture media containing 1% FBS in 96-well plates. Wound closure was then monitored over time using the IncuCyte Live-Cell Analysis System to assess cell migration under these low-proliferation conditions.
Orthotopic implantation and subcutaneous injection mouse models
To generate a lung cancer orthotopic xenograft model, SNHG3WT or SNHG3ED cells with the firefly luciferase gene were transplanted into the right lung of 6–8-week-old female BALB/c nude mice (5 × 106 cells per mouse). Prior to injection, the mice were anesthetized with isoflurane and their limbs were secured to a surgical board. A 1 cm incision was made between the third and fourth ribs on the left thoracic side using sterilized scissors. Blunt dissection with forceps exposed the intercostal space, and a 1 ml insulin syringe was used to inject the prepared cell suspension into the lung. The incision was then closed with sterile sutures, and the site was disinfected with iodine solution. The mice were placed in a left lateral recumbency for 5–10 minutes to prevent fluid displacement during recovery. Mice were imaged using the IVIS Lumina III (Xenogen Corporation, Hopkinton, MA), and bioluminescent flux was quantified for both primary tumors and metastases.
To establish a subcutaneous transplant xenograft model for therapeutic assays, 6–8-week-old female BALB/c nude mice were utilized. Briefly, 5 × 106 A549DR cells were injected subcutaneously into the dorsal flank of the mice. Tumor volume was calculated using the formula: 0.5 × length2 × width. Upon completion of the treatment cycle, tumors were surgically excised and weighed.
Hematoxylin and eosin staining
The xenograft tumors were embedded in paraffin and sectioned to a thickness of 5–10 μm. Hematoxylin and eosin (H&E) staining was performed according to established protocols, which included deparaffinization; rehydration; staining with hematoxylin; washing; staining with eosin; dehydration, clearing; and mounting of the tissue sections for subsequent microscopic examination.
Oil red O staining
Cells were fixed in 4% formaldehyde for 15 minutes and then incubated in 60% isopropanol for 15-30 seconds. The cells were stained with 0.2% Oil Red O in 60% isopropanol for 30 minutes, followed by washing with water and counterstaining with hematoxylin for 30 seconds. Lipid droplet quantification was performed using Image-Pro Plus 6.0 software (Media Cybernetics) on images captured with a standard light microscope.
Lipids analysis and metabolic profiling
Triglyceride and total FFA levels in cell cultures were measured using the Triglyceride-GloTM kit (Promega, Madison, WI) and a human FFA ELISA Kit (Ruidahenghui, China), respectively, following the manufacturer’s instructions. Medium- and long-chain FFAs and acylcarnitines were quantified using high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (HPLC-MS/MS) at BioMiao Biological Technology (Beijing, China) Co., Ltd. Approximately 5 × 106 cells were collected and lysed in ice-cold methanol. Metabolites were quantified on a Shimadzu LC-20ADXR (Shimadzu, Japan) connected to a Sciex 5500+ mass spectrometer (AB Sciex, Singapore). Medium- and long-chain FFAs were separated on a Thermo Hypersil GOLD column after injecting 3 μL of the metabolite extract, using 2 mM ammonium acetate in water as the weak eluent and acetonitrile as the strong eluent. The chromatographic gradient transitioned from 83% weak elution to 5% over 27 minutes at a flow rate of 0.4 mL/min. Acylcarnitines were separated on the same column after injecting 2 μL of the metabolite extract, applying 0.1% formic acid in water as the weak eluent and 0.1% formic acid in acetonitrile as the strong eluent, with a gradient from 98% to 2% over 13 minutes. Moreover, Palmitoylcarnitine was quantified after a 48-hour treatment with PA (50 mM) in serum-free DMEM supplemented with 0.5 mM Glucose, 1 mM Glutamine, 1% FBS, and 0.5 mM L-carnitine. It was separated chromatographically on an ACE Excel PFP C18 column after injecting 5 μL of the metabolite extract under the aforementioned elution conditions described above. The metabolites were ionized using a Turbo VTM heated electrospray ionization source and detected in selected reaction monitoring mode, with optimized parameters including positive ion spray voltage of 5.5 kV, negative ion spray voltage of −4.5 kV, curtain gas at 35 psi, and heater temperature at 550 °C.
FAO activity detection
FAO activity was measured using the FAOblue assay (Funakoshi, Tokyo, Japan) as described by Uchinomiya et al.42, and the Seahorse XF Long Chain FAO Stress Test and Palmitate-BSA FAO Stress Test, following the manufacturers’ instructions. For the FAOblue assay, FAOblue was added to PBS-washed cells, which were then incubated at 37 °C for over 30 minutes. After incubation, the cells were washed and observed for blue fluorescence under live conditions. Images were acquired using a Zeiss LSM 800 Confocal Laser Scanning Microscope. For the Seahorse XF Long Chain FAO Stress Test, cells were seeded on XFe24 plates (5 × 104 cells/well) and cultured for 24–48 hours. Cells were then incubated with assay medium at 37 °C in a non-CO2 incubator for 60 minutes, followed by the long chain FAO stress test using 4 µM Etomoxir, 1.5 µM Oligomycin, 0.5 µM fluorocarbonyl cyanide phenylhydrazone (FCCP), and 0.5 µM Rotenone/Antimycin A in sensor cartridges. The OCR was measured using the Seahorse XFe24 analyzer (Agilent, Santa Clara, CA). For the Seahorse XF Palmitate-BSA FAO Stress Test, cells were cultured in substrate-limited growth media for 24 hours, followed by incubation with assay medium at 37 °C in a non-CO2 incubator for 60 minutes. Subsequently, OCR was measured in the presence or absence of XF Palmitate-BSA. After the assay, cell numbers were counted by automated cell counters (Thermo Fisher Scientific).
13C tracer experiments and metabolite levels
For metabolic flux experiments, [13C16]-palmitate tracing was conducted using ultra-performance liquid chromatography coupled to triple quadruple mass spectrometer (UPLC-TQ-MS) at Metabo-Profile Biotechnology (Shanghai, China) Co., Ltd. Cells (1 × 107) were incubated in growth medium with 2.5 μM [13C16]-PA for 24 h. Then cells were washed with PBS and collected at −80 °C. Before processing, cell samples were thawed on an ice bath to minimize degradation. Each cell sample tube was supplemented with 400 µL of 80% methanol solution, followed by sonication (JY92-IIN, NingBo Scientz Biotechnology Co., Ltd.). The samples were then centrifuged at 18,000 × g for 15 minutes at 4 °C. The supernatants were collected and concentrated via centrifugation. The dried extracts were reconstituted in 100 µL of 80% methanol solution for UPLC-TQ-MS. Metabolic flux analysis was detected on an UPLC-tandem triple quadrupole mass spectrometer (ACQUITY-I UPLC/Xevo TQS, Waters, USA).
Cytosolic/nuclear fractions test
Cytosolic and nuclear fractions were collected using PARISTM kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Briefly, 5 × 106 cells were washed twice with ice-cold PBS and lysed in Cell Fractionation Buffer containing RNase inhibitor (1:100 dilution). After 10 min incubation on ice, lysates were centrifuged at 500×g for 5 min at 4 °C to pellet nuclei. The supernatant (cytosolic fraction) was transferred to a fresh tube and further cleared by centrifugation at 12,000 × g for 10 min. The nuclear pellet was washed twice with Cell Fractionation Buffer, then lysed in Nuclear Extraction Buffer by vortexing every 5 min for 30 min on ice. Then lyse nuclear pellet and cytoplasmic fraction were submitted to total RNA isolation. The expression of SNHG3, NEAT and GAPDH were determined by real-time PCR.
RNA pull-down and mass spectrometry
Full-length biotin-labeled sequences of SNHG3WT (wild-type sequence with A at position 1746), SNHG3ED (edited sequence with G at position 1746), and truncated SNHG3 (ΔSNHG3: nucleotide deletions at positions 1500–1900) probes were synthesized in vitro, and RNA pull-down assays were performed using the BersinBioTM RNA pull-down kit (BersinBio, Guangzhou, China) in accordance with the manufacturer’s instructions. Briefly, 2 × 107 cells were sonicated in 1.7 mL of cell lysis buffer containing 17 µL of protease inhibitor. The cell lysate was then centrifuged to collect the supernatant, which was incubated with agarose beads and subsequently with streptavidin magnetic beads coated with the probes. The beads were washed five times with ice-cold NT2 buffer and eluted using protein elution buffer. The eluted proteins were analyzed by mass spectrometry or western blotting.
Each cell line was analyzed once by mass spectrometry by a commercial service provider (BersinBio). The protein extraction procedure began with thawing cell samples, followed by the addition of an appropriate volume of lysis buffer containing 8 M urea and 1% protease inhibitor. The samples were then subjected to ultrasonication for cell disruption. For tryptic digestion, proteins were first reduced with 5 mM dithiothreitol (DTT) at 56 °C for 30 min, followed by alkylation with 11 mM iodoacetamide (IAA) in the dark at room temperature for 15 min. The urea concentration was diluted below 2 M using triethylammonium bicarbonate (TEAB). Trypsin was added at a 1:50 (enzyme-to-protein ratio, w/w) for overnight digestion, followed by a second digestion step at a 1:100 ratio (w/w) for an additional 4 hours. For liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, the digested peptides were dissolved in mobile phase A (0.1% formic acid and 2% acetonitrile in water) and separated using an EASY-nLC 1000 ultra-high-performance liquid chromatography system. The gradient was set as follows: 4–16% mobile phase B (0.1% formic acid and 90% acetonitrile in water) over 0–38 min, 16–30% B over 38–52 min, 30–80% B over 52–56 min, and 80% B for 56–60 min, with a constant flow rate of 500 nL/min. The separated peptides were ionized using a NanoSpray Ion Source (NSI) and analyzed on a Thermo ScientificTM Orbitrap FusionTM LumosTM mass spectrometer. The ion source voltage was set to 2.2 kV, and both precursor and fragment ions were detected in the Orbitrap at high resolution. Full-scan MS spectra (350–1600 m/z) were acquired at a resolution of 70,000, while MS/MS scans were performed at 17,500 resolution. Data-dependent acquisition (DDA) was employed, selecting the top 10 most intense precursor ions for higher-energy collisional dissociation (HCD) fragmentation at 28% normalized collision energy. Key parameters included an automatic gain control (AGC) target of 5E4, an intensity threshold of 2E4 ions/s, a maximum injection time of 100 ms, and a dynamic exclusion duration of 30 s to prevent redundant scans. The resulting MS/MS data were processed using Proteome Discoverer (v2.4.1.15) against the Mus_musculus_10090_SP_20190513 database (17,022 entries), supplemented with a decoy database for false discovery rate (FDR) estimation and a common contaminants database. Trypsin/P was specified as the enzyme, allowing up to two missed cleavages. The mass tolerances for precursor ions were set to 20 ppm (first search) and 5 ppm (main search), while fragment ion tolerance was 20 ppm. Carbamidomethylation of cysteine was set as a fixed modification, with variable modifications including methionine oxidation, N-terminal acetylation, and deamidation of glutamine and asparagine. Protein and peptide-spectrum match (PSM) identifications were filtered at a 1% FDR threshold. The raw proteomics data were deposited into the PRIDE (PXD067139).
RIP
RIP was performed using the BersinBioTM RIP Kit (BersinBio) according to the manufacturer’s instructions. Briefly, 1 × 10⁷ cells were washed with ice-cold PBS and lysed in RIP Lysis Buffer supplemented with RNase inhibitor (1:100, Thermo Fisher) and protease inhibitor cocktail (Roche) for 10 min on ice. After centrifugation (12,000 × g, 10 min, 4 °C), supernatants were pre-cleared with Protein A/G Magnetic Beads for 1 hr to reduce nonspecific binding. For immunoprecipitation, 5 μg of anti-SSRP1 antibody or control IgG was conjugated to fresh magnetic beads in RIP Wash Buffer (30 min rotation, RT), then incubated with 500 μg of pre-cleared lysate overnight at 4 °C with gentle rotation. The beads were then washed six times. Immunoprecipitated RNAs were extracted using a phenol-chloroform-isoamyl alcohol mixture and subsequently analyzed by real-time PCR, using primers targeting SNHG3, with input and IgG controls for normalization.
Immunofluorescence staining
To determine the co-localization of SNHG3 with SSRP1, A549 or PC9 cells were fixed, permeabilized, and blocked, followed by an overnight incubation at 4 °C with SSRP1 antibody. After fixation, the cells were rinsed with DPBS and then incubated at RT with AlexaFluor 488-conjugated goat anti-mouse IgG. Subsequently, the cells were washed three times with DPBS. RNA fluorescence in situ hybridization (FISH) was performed using the RiboTM FISH Kit (Guangzhou, China) according to the manufacturer’s instructions. Cells were fixed, permeabilized, and pre-hybridized for 30 min, after which hybridization with SNHG3 probes, designed and synthesized by RiboTM Company, was conducted at 37 °C overnight in the dark. Following hybridization, the cells were washed in wash buffer at 42 °C, rinsed with DPBS, and then incubated with DAPI for 10 min. Images were captured using a laser confocal microscope. Colocalization quantification was performed using the Coloc 2 plugin in Fiji, with Pearson’s correlation coefficient calculated43.
Exosomal RNA isolation
The exosomal RNA used in this study was derived from our prior study44. The serum exosomes were isolated using the SBI ExoQuick exosome precipitation solution (System Biosciences, Palo Alto, CA), which specifically includes a lipoprotein depletion step in its protocol. Briefly, 500 μL of serum pre-cleared by centrifugation (1500 × g for 10 min) was mixed with ExoQuick solution (1:4 ratio), incubated at 4 °C for 2 h, and centrifuged (1500 g, 30 min). The pellet was resuspended in 100 μL PBS. These exosomes had been thoroughly validated through detection of specific exosomal positive markers (CD63, CD81), negative marker (CANX), nanoparticle tracking analysis (NTA) demonstrating the expected size distribution (50-150 nm), and transmission electron microscopy (TEM) confirming typical vesicular morphology, as described previously44. NTA was performed by diluting 100 µL of exosomes in PBS and loading them into a ZetaView system (Particle Metrix, Germany) with a 488 nm laser. Instrument settings included a shutter level of 70, and PBS blanks confirmed minimal background particle interference. Sample videos (43 sec duration) were captured and processed using ZetaView software (v8.04.02 SP2) for size and concentration analysis. For TEM imaging, a 15 µL aliquot of exosomes was applied to a 300-mesh copper grid and allowed to adsorb for 60 seconds. Excess liquid was carefully blotted using filter paper, followed by negative staining with 2% uranyl acetate for one minute. After removing residual stain, the grid was air-dried under an incandescent lamp for approximately 10 minutes. Samples were then visualized using a Tecnai G2 Spirit TEM (FEI, USA) operating at 80 kV.
4sU/DRB transcription elongation assay
The assay was performed with minor modifications based on previous work45. Briefly, cells were incubated with DRB (100 µM) for 2.5 hour to halt transcription, followed by treatment with 4sU (200 μM) to label newly synthesized RNA. Total RNA was extracted at 0, 4, and 8 min post-4sU treatment using the miRNeasy kit (QIAGEN, Hilden, Germany). RNA samples (50–120 μg) were biotinylated with EZ-Link Biotin-HPDP (Thermo Scientific Pierce) in 0.2 mg/ml dimethylformamide and a biotin labeling buffer for 2 h at 25 °C. Biotinylated RNA was purified, precipitated, washed, dried, and dissolved in 100 μl RNase-free water, and then was pulled down with 50 μl Dynabeads MyOne Streptavidin T1 beads (Thermo Fisher Scientific) in wash buffer for 15 min at 25 °C. The beads were separated and washed, and 4sU-RNA was eluted with 100 μl of 100 mM Dithiothreitol in RNase-free water, followed by purification using RNeasy MinElute Spin columns (QIAGEN). The purified 4sU-RNA was quantified using the RiboGreen Assay (Thermo Fisher Scientific) for subsequent real-time PCR analysis.
Lung cancer organoid generation
Two NSCLC patient-derived organoids (PDOs) were established from tumor surgical resections of two lung cancer patients using the AccuroidTM lung cancer organoid kit, according to the manual (ACCURATE International, Guangzhou, China) as described previously22. Under sterile conditions, lung cancer tissue samples were collected and rinsed with physiological saline before preservation in Tissue Storage Solution at 4 °C. The tissue was placed in a culture dish and carefully minced into 2-4 mm³ fragments using sterile surgical scissors. The fragments underwent 5-10 washes with Organoid Wash Buffer to remove debris. After transferring to a 1.5 mL tube, the tissue was further minced and then digested in 6 mL Tissue Dissociation Solution at 37 °C. The digestion process was monitored microscopically every 5 minutes until appropriate cell clusters (10–100 μm) appeared, typically within 40 minutes. The digested suspension was filtered through a 100 μm strainer and centrifuged at 300 × g for 5 minutes. Following red blood cell lysis when necessary, the cell pellet was resuspended in pre-thawed GFR Basement Membrane Matrix at a 1:10 ratio. The cell-matrix mixture was plated as 20–60 μL droplets in a 24-well plate and allowed to solidify at 37 °C for 15 minutes. Human Lung Cancer Organoid Growth Medium (500 μL) was carefully added to each well, and cultures were maintained at 37 °C with 5% CO₂, with regular medium changes every 2-4 days.
Generation of DTX-resistant A549 cells
A549 cells were treated with increasing concentrations of DTX (0.6, 1.2, 4.8, 9.6, 19.2 nM). At each concentration, the cells were exposed to the drug for a period of 24 hours. Following this treatment, the medium was replaced with fresh, drug-free medium. The cells were subsequently cultured for one week to ensure recovery and adaptation before being subjected to the next concentration in the gradient.
Locked nucleic acid antisense oligonucleotides synthesis and treatment
Two ASOs targeting SNHG3WT with thymine (i.e., ASOT) and two ASOs targeting SNHG3ED with cytosine (i.e., ASOC) at the editing site were designed and synthesized by RiboBio Co., Ltd. A scrambled ASO was purchased from the same company and utilized as a negative control (ASONC). The ASOC2 was further LNA modified at the first and last three nucleotides. The LNA-ASOs were transfected into the A549DR at a concentration of 25 or 50 nM using GP-transfect-Mate (GenePharma, China). After 24 hours, the culture medium was replaced with fresh medium containing varying concentrations of DTX for 72 h. Cell viability was then test using the CCK8 assay. Additionally, following a three-day co-incubation of the ASO with PDOs, the culture medium was replaced either with fresh medium containing 50 nM DTX or without the drug, while maintaining the ASO at the same concentration. After an additional three-day incubation period, cell viability was assessed using the Cell Counting-Lite 3D Luminescent Cell Viability Assay (Vazyme, Nanjing, China), according to the manufacturer’s instructions. Briefly, organoids were digested and seeded into 96-well plates, and then left at RT for 30 minutes. An equal volume of CellCounting-Lite 3D was added, followed by vigorous shaking for 5 minutes to ensure complete cell lysis. The plates were incubated at 25 °C for 25 minutes, after which fluorescence intensity was measured. ASO treatment of the xenograft tumor was initiated after the tumor volume reached 0.5 cm3. Mice were injected with 6 nmol LNA-ASOC2 or control-ASO in the absence or presence of DTX (2 mg/kg) through intratumoral injection, every 3 days for up to 28 days without any delivery system.
Bioinformatics analysis
We identified clinically relevant A-to-I editing events by integrating multi-omics data from The Cancer Genome Atlas (TCGA). First, clinical records for NSCLC patients, including demographic information and survival outcomes, were obtained from the TCGA Data Portal (https://portal.gdc.cancer.gov/). Corresponding A-to-I RNA editing profiles were acquired from the Synapse database (https://www.synapse.org/#!Synapse:syn2374375/files/), which contains genome-wide editing levels derived from TCGA RNA-seq data. Tumor purity was quantified using ESTIMATE algorithm-derived scores obtained through the ESTIMATE web portal (https://bioinformatics.mdanderson.org/estimate/index.html)46. Through systematic matching of unique TCGA barcodes, we performed an exact inner join operation to integrate these datasets while implementing stringent quality control measures, including editing site coverage validation and cross-examination of unmatched samples. To assess prognostic significance, we performed Cox proportional hazards regression for each editing site. Metastasis-associated editing events were identified by comparing the correlation between editing levels of each site and nude metastasis stages, using the Spearman rank correlation test. All analyses were conducted in R 4.3.3 with stringent quality control, and reproducible code has been deposited in the Zenodo repository (https://doi.org/10.5281/zenodo.15621261).
TCGA RNA-seq data were employed to analyze the correlations between gene expression levels and c.1746 A > I editing levels, as well as the correlations among target genes. The transcriptomic data for all lung cancer patients available through TCGA were systematically retrieved from the Genomic Data Commons (GDC) Data Portal (https://portal.gdc.cancer.gov/). The dataset was obtained by first filtering for primary lung cancer cases (TCGA-LUAD and TCGA-LUSC projects), then selecting all available RNA sequencing data under the “Transcriptome Profiling” category with “Gene Expression Quantification” as the data type. Specifically, we acquired the HTSeq normalized count data (workflow type: HTSeq - Counts) to ensure consistency across samples. The complete dataset was downloaded programmatically using the GDC Data Transfer Tool with an authenticated manifest file approach. Metadata files in JSON format were simultaneously obtained to maintain sample annotations and clinical correlations. All data handling and preprocessing were performed in accordance with TCGA’s data use policies and ethical guidelines for secondary analysis of de-identified patient data.
To investigate potential associations between SNHG3 editing and drug sensitivity, we integrated pharmacological and molecular data by retrieving IC50 values of anti-cancer agents from the GDSC (https://www.cancerrxgene.org/) and corresponding SNHG3 expression profiles from the CCLE, with data merging performed through exact matching of cell line names. Correlation patterns between IC50 values and SNHG3 expression levels across cell lines, using the Spearman rank correlation test.
Statistics and Reproducibility
Statistical analysis was performed using GraphPad Prism (v9.0) or R statistical programming language (v4.3.3). The source data behind the graphs in the paper are provided as Supplementary Data 4. The sample sizes were determined based on power calculations for survival analysis, using hazard ratios (HR) derived from TCGA data as effect size estimates. Assuming 80% power at a 5% significance level (two-sided), the minimum required sample size was calculated to detect the expected HR difference between groups using standard survival analysis sample size formulas. The numbers of biological replicates, repetitions of the experiments, and statistical significance are listed in the figure legends. The linear regression and Cox regression were used to analyze the associations of A-to-I editing profiles with lymph node metastasis and overall survival, respectively. The unpaired t-test with Welch’s correction or the paired t-test was used to test differences in two groups. The one-way ANOVA was used to test differences in more than two groups with one independent factor, and the two-way ANOVA was applied for analyzing differences in two or more groups with two independent variables. Tukey multiple comparisons test was used for pairwise comparison between groups. The association analysis between two numerical variables, such as editing levels and gene expression levels, was conducted using Spearman’s rank correlation test. Two-sided P < 0.05 was considered to be statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files). The TCGA lung cancer RNA editing data is derived from the public database (https://www.synapse.org/#!Synapse:syn2374375/files/). The TCGA lung cancer gene expression profile data came from the TCGA database (https://portal.gdc.cancer.gov/). The raw proteomics data were deposited into the PRIDE (PXD067139). The raw data that support the findings of this study are available in Supplementary Data 4. The uncropped and unedited WB and unprocessed scratch images are shown in Supplementary Fig. 10. All other data are available from the corresponding author on reasonable request.
Code availability
All analytical R code used in this study is deposited in the Zenodo repository (https://doi.org/10.5281/zenodo.15621261).
References
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Peng, X. et al. A-to-I RNA editing contributes to proteomic diversity in cancer. Cancer cell 33, 817–828 e817 (2018).
Paz-Yaacov, N. et al. Elevated RNA editing activity is a major contributor to transcriptomic diversity in tumors. Cell Rep. 13, 267–276 (2015).
Cui, L. et al. RNA modifications: importance in immune cell biology and related diseases. Signal Transduct. Target. Ther. 7, 334 (2022).
Qi, L., Chan, T. H., Tenen, D. G. & Chen, L. RNA editome imbalance in hepatocellular carcinoma. Cancer Res. 74, 1301–1306 (2014).
Qin, Y. R. et al. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Cancer Res. 74, 840–851 (2014).
Okugawa, Y. et al. Enhanced AZIN1 RNA editing and overexpression of its regulatory enzyme ADAR1 are important prognostic biomarkers in gastric cancer. J. Transl. Med. 16, 366 (2018).
Takeda, S. et al. Activation of AZIN1 RNA editing is a novel mechanism that promotes invasive potential of cancer-associated fibroblasts in colorectal cancer. Cancer Lett. 444, 127–135 (2019).
Nakamura, K., Shigeyasu, K., Okamoto, K., Matsuoka, H. & Masuyama, H. ADAR1 and AZIN1 RNA editing function as an oncogene and contributes to immortalization in endometrial cancer. Gynecol. Oncol. 166, 326–333 (2022).
Tay, D. J. T. et al. Targeting RNA editing of antizyme inhibitor 1: A potential oligonucleotide-based antisense therapy for cancer. Mol. Ther.: J. Am. Soc. Gene Ther. 29, 3258–3273 (2021).
Wang, Y. et al. Systematic characterization of A-to-I RNA editing hotspots in microRNAs across human cancers. Genome Res. 27, 1112–1125 (2017).
Han, L. et al. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell 28, 515–528 (2015).
Liu, L. et al. A nomogram based on A-to-I RNA editing predicting overall survival of patients with lung squamous carcinoma. BMC Cancer 22, 715 (2022).
Wan, J. et al. Development and validation of a Four Adenosine-to-Inosine RNA editing site-relevant prognostic signature for assessing survival in breast cancer patients. Front. Oncol. 12, 861439 (2022).
Zimta, A. A. et al. An emerging class of long non-coding rna with oncogenic role arises from the snoRNA host genes. Front. Oncol. 10, 389 (2020).
Gong, J. et al. LNCediting: a database for functional effects of RNA editing in lncRNAs. Nucleic acids Res. 45, D79–D84 (2017).
Guo, Y. et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 18, 39 (2019).
Li, D. & Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 5, 108 (2020).
Stevens, L. E. et al. Extracellular Matrix Receptor expression in subtypes of lung adenocarcinoma potentiates outgrowth of micrometastases. Cancer Res. 77, 1905–1917 (2017).
Altea-Manzano, P. et al. A palmitate-rich metastatic niche enables metastasis growth via p65 acetylation resulting in pro-metastatic NF-kappaB signaling. Nat. Cancer 4, 344–364 (2023).
Orphanides, G., Wu, W. H., Lane, W. S., Hampsey, M. & Reinberg, D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature 400, 284–288 (1999).
Lu, L. et al. EIF4a3-regulated circRABL2B regulates cell stemness and drug sensitivity of lung cancer via YBX1-dependent downregulation of MUC5AC expression. Int. J. Biol. Sci. 19, 2725–2739 (2023).
Peng, L. et al. Characterization of RNA editome in primary and metastatic lung adenocarcinomas. Oncotarget 8, 11517–11529 (2017).
Wang, C. et al. Identification of A-to-I RNA editing profiles and their clinical relevance in lung adenocarcinoma. Sci. China Life Sci. 65, 19–32 (2022).
Chen, K. J. et al. Somatic A-to-I RNA-edited RHOA isoform 2 specific-R176G mutation promotes tumor progression in lung adenocarcinoma. Mol. Carcinog. 62, 348–359 (2023).
Romano, G. et al. A-to-I edited miR-411-5p targets MET and promotes TKI response in NSCLC-resistant cells. Oncogene 42, 1597–1606 (2023).
Xu, B. et al. LncRNA SNHG3, a potential oncogene in human cancers. Cancer Cell Int. 20, 536 (2020).
Li, Y., Gao, L., Zhang, C. & Meng, J. LncRNA SNHG3 Promotes Proliferation and Metastasis of Non-Small-Cell Lung Cancer Cells Through miR-515-5p/SUMO2 Axis. Technol. Cancer Res. Treat. 20, 15330338211019376 (2021).
Liu, L., Ni, J. & He, X. Upregulation of the long noncoding RNA SNHG3 promotes lung Adenocarcinoma proliferation. Dis. markers 2018, 5736716 (2018).
Falbo, L. et al. SSRP1-mediated histone H1 eviction promotes replication origin assembly and accelerated development. Nat. Commun. 11, 1345 (2020).
Shen, J. et al. Histone chaperone FACT complex mediates oxidative stress response to promote liver cancer progression. Gut 69, 329–342 (2020).
Padanad, M. S. et al. Fatty acid oxidation mediated by Acyl-CoA Synthetase long chain 3 is required for mutant KRAS Lung Tumorigenesis. Cell Rep. 16, 1614–1628 (2016).
Jeon, S. M., Chandel, N. S. & Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 (2012).
Li, S., Zhu, X., Wang, L. & Zheng, Z. The role of radiation dose-dependent enhancement of fatty acid oxidation in radiation surviving/resistant lung cancer cells. J. Clin. Oncol. 38, e21724 (2020).
Li, M., Xian, H. C., Tang, Y. J., Liang, X. H. & Tang, Y. L. Fatty acid oxidation: driver of lymph node metastasis. Cancer Cell Int. 21, 339 (2021).
Li, L. et al. Transcription factor KLF16 activates MAGT1 to regulate the tumorigenesis and progression of breast cancer. Int. J. Mol. Med. 50, 115 (2022).
Miess, H. et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 37, 5435–5450 (2018).
Liu, J. et al. ATF3-CBS signaling axis coordinates ferroptosis and tumorigenesis in colorectal cancer. Redox Biol. 71, 103118 (2024).
Zhu, H. et al. Gss deficiency causes age-related fertility impairment via ROS-triggered ferroptosis in the testes of mice. Cell Death Dis. 14, 845 (2023).
Zhang, Y., Li, S., Li, F., Lv, C. & Yang, Q. K. High-fat diet impairs ferroptosis and promotes cancer invasiveness via downregulating tumor suppressor ACSL4 in lung adenocarcinoma. Biol. Direct 16, 10 (2021).
Zhang, X. et al. Palmitic acid promotes lung metastasis of melanomas via the TLR4/TRIF-Peli1-pNF-kappaB Pathway. Metabolites 12, 1132 (2022).
Uchinomiya, S. et al. Fluorescence detection of metabolic activity of the fatty acid beta oxidation pathway in living cells. Chem. Commun. 56, 3023–3026 (2020).
Wang Y. et al. Ligand- and voltage-gated Ca(2+) channels differentially regulate the mode of vesicular neuropeptide release in mammalian sensory neurons. Sci. Signal.10, (2022).
Xian, J. et al. Identification of three circular RNA Cargoes in serum exosomes as diagnostic biomarkers of non-small-cell lung cancer in the Chinese population. J. Mol. Diagn. 22, 1096–1108 (2020).
Fuchs, G. et al. Simultaneous measurement of genome-wide transcription elongation speeds and rates of RNA polymerase II transition into active elongation with 4sUDRB-seq. Nat. Protoc. 10, 605–618 (2015).
Yoshihara, K. et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 4, 2612 (2013).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82373120, 81871876), Guangdong Natural Science Foundation (2024A1515013256), Tertiary Education Scientific research project of Guangzhou Municipal Education Bureau (202235407) and Key Research Fund for colleges and universities of Guangdong Education Department (2023ZDZX2049). We extend our gratitude to Li Liu, Minyan Li, Qinfeng Zheng, and Peirong Lu for their contributions in cell culture and preparation.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.Y. and Z.L.Z.; Methodology, Y.B.D.; Investigation, S.Z.C., A.M.Z., R.Y.T., S.M.S. and B.B.W.; Writing - Original Draft, L.Y. and S.Z.C.; Writing - Review & Editing, Y.B.D. and Z.L.Z.; Funding Acquisition, L.Y.; Resources, W.J.W., J.J.X., Z.Q.Y., B.Q. R. and J.C.L.; Supervision, J.C.L. and L.Y.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Preethi H. Gunaratne, Mithun Mitra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Bibekanand Mallick and David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Zhuo, A., Tang, R. et al. A-to-I edited SNHG3 promotes non-small cell lung cancer metastasis by promoting fatty acid oxidation and resisting ferroptosis. Commun Biol 8, 1333 (2025). https://doi.org/10.1038/s42003-025-08776-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08776-4