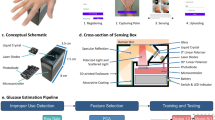
Abstract
Noninvasive blood glucose monitoring offers substantial advantages for patients, but current technologies are often not sufficiently accurate for clinical applications or require personalized calibration. Here we report multiple μ-spatially offset Raman spectroscopy, which captures Raman signals at varying skin depths, and show that it accurately detects blood glucose levels in humans. In 35 individuals with or without type 2 diabetes, we first determine the optimal depth for glucose detection to be at or below the capillary-rich dermal–epidermal junction, where we observe a strong correlation between specific Raman bands and venous plasma glucose concentrations. In a second study, comprising 230 participants, we then improve accuracy of our regression model to reach a mean absolute relative difference of 14.6%, without personalized calibration, whereby 99.4% of calculated glucose values fall into clinically acceptable zones of the consensus error grid (zones A and B). These findings highlight the ability and robustness of multiple μ-spatially offset Raman spectroscopy for noninvasive blood glucose measurement in a clinical setting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
Custom Python code has been applied for data analysis in this work. The code necessary for reanalysing the data presented in this paper is available in Zenodo at https://doi.org/10.5281/zenodo.14605629 (ref. 46).
References
IDF Diabetes Atlas 10th edition (International Diabetes Federation, accessed 22 January 2025); https://diabetesatlas.org/atlas/tenth-edition/
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Guy, R. H. A sweeter life for diabetics? Nat. Med. 1, 1132–1133 (1995).
Heinemann, L. Finger pricking and pain: a never ending story. J. Diabetes Sci. Technol. 2, 919–921 (2008).
Fruhstorfer, H. & Lange, H. Capillary blood sampling: how much pain is necessary? Part 3: pricking the finger can be less painful. Pract. Diabetes. Int. 12, 253–254 (1995).
Lin, R., Brown, F., James, S., Jones, J. & Ekinci, E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet. Med. 38, e14528 (2021).
Xie, X. et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2, 894–906 (2018).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Villena Gonzales, W., Mobashsher, A. & Abbosh, A. The progress of glucose monitoring—a review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 19, 800 (2019).
So, C.-F., Choi, K.-S., Wong, T. K. & Chung, J. W. Recent advances in noninvasive glucose monitoring. Med. Devices 5, 45–52 (2012).
Lin, T. et al. Non-invasive glucose monitoring: a review of challenges and recent advances. Sensors 20, 6925 (2017).
Rao, G. et al. Reverse iontophoresis: noninvasive glucose monitoring in vivo in humans. Pharm. Res. 12, 1869–1873 (1995).
Martín-Mateos, P. et al. In-vivo, non-invasive detection of hyperglycemic states in animal models using mm-wave spectroscopy. Sci. Rep. 6, 34035 (2016).
Sim, J. Y., Ahn, C. G., Jeong, E. J. & Kim, B. K. In vivo microscopic photoacoustic spectroscopy for non-invasive glucose monitoring invulnerable to skin secretion products. Sci. Rep. 8, 1059 (2018).
Uluç, N. et al. Non-invasive measurements of blood glucose levels by time-gating mid-infrared optoacoustic signals. Nat. Metab. 6, 678–686 (2024).
Maruo, K. et al. In vivo noninvasive measurement of blood glucose by near-infrared diffuse-reflectance spectroscopy. Appl. Spectrosc. 57, 1236–1244 (2003).
Enejder, A. M. K. et al. Raman spectroscopy for noninvasive glucose measurements. J. Biomed. Opt. 10, 031114 (2005).
Pleus, S. et al. Proof of concept for a new Raman-based prototype for noninvasive glucose monitoring. J. Diabetes Sci. Technol. 15, 11–18 (2020).
Pors, A. et al. Accurate post-calibration predictions for noninvasive glucose measurements in people using confocal Raman spectroscopy. ACS Sens. 8, 1272–1279 (2023).
Zhang, L. et al. Spectral tracing of deuterium for imaging glucose metabolism. Nat. Biomed. Eng. 3, 402–413 (2019).
Kang, J. W. et al. Direct observation of glucose fingerprint using in vivo Raman spectroscopy. Sci. Adv. 6, 5206–5230 (2020).
Matousek, P. et al. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl. Spectrosc. 59, 393–400 (2005).
Aleemardani, M., Trikić, M. Z., Green, N. H. & Claeyssens, F. The importance of mimicking dermal–epidermal junction for skin tissue engineering: a review. Bioengineering 8, 148 (2021).
Wold, S., Sjöström, M. & Eriksson, L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 58, 109–130 (2001).
Jendrike, N., Baumstark, A., Kamecke, U., Haug, C. & Freckmann, G. ISO 15197: 2013 evaluation of a blood glucose monitoring system’s measurement accuracy. J. Diabetes Sci. Technol. 11, 1275–1276 (2017).
Mosca, S., Conti, C., Stone, N. & Matousek, P. Spatially offset Raman spectroscopy. Nat. Rev. Methods Primers 1, 21 (2021).
Eliasson, C., Macleod, N. A. & Matousek, P. Noninvasive detection of concealed liquid explosives using Raman spectroscopy. Anal. Chem. 79, 8185–8189 (2007).
Conti, C. et al. Advances in Raman spectroscopy for the non-destructive subsurface analysis of artworks: Micro-SORS. J. Cult. Herit. 43, 319–328 (2020).
Ghita, A., Matousek, P. & Stone, N. High sensitivity non-invasive detection of calcifications deep inside biological tissue using transmission Raman spectroscopy. J. Biophotonics 11, jbio.201600260 (2018).
Matousek, P. & Parker, A. W. Bulk Raman analysis of pharmaceutical tablets. Appl. Spectrosc. 60, 1353–1357 (2006).
Vardaki, M. Z. & Kourkoumelis, N. Tissue phantoms for biomedical applications in Raman spectroscopy: a review. Biomed. Eng. Comput. Biol. 11, 1179597220948100 (2020).
Braverman, I. M. The cutaneous microcirculation. J. Investig. Dermatol. Symp. Proc. 5, 3–9 (2000).
Liu, Z. et al. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl Acad. Sci. USA 105, 1410–1415 (2008).
Darvin, M. E. et al. Confocal Raman microscopy combined with optical clearing for identification of inks in multicolored tattooed skin in vivo. Analyst 143, 4990–4999 (2018).
Stone, N., Kendall, C., Shepherd, N., Crow, P. & Barr, H. Near-infrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. J. Raman Spectrosc. 33, 564–573 (2002).
Hastie, T., Tibshirani, R., Friedman, J. H. & Friedman, J. H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction (Springer, 2009).
Leonardo, D. A. M., Luciana, P. A., Fabricio, C. S. G. & Fernando, G. Distinct metabolic profile according to the shape of the oral glucose tolerance test curve is related to whole glucose excursion: a cross-sectional study. BMC Endocr. Disord. 18, 56 (2018).
Di, Z. et al. Spatially offset Raman microspectroscopy of highly scattering tissue: theory and experiment. J. Mod. Opt. 62, 97–101 (2015).
Luo, R., Popp, J. & Bocklitz, T. Deep learning for Raman spectroscopy: a review. Analytica 3, 287–301 (2022).
Soleimaninejad, H., Matroodi, F. & Tavassoli, S. H. Raman spectroscopy of Iranian region calcite using pulsed laser: an approach of fluorescence suppression by time-gating method. J. Spectrosc. 2013, 254964 (2013).
Van Dorpe, P. et al. High optical throughput, high spectral resolution, on-chip Raman spectrometer. In Proc. 26th International Conference on Raman Spectroscopy (ICORS 2018) 03-1079 (ICORS, 2018).
Lintzeri, D. A., Karimian, N., Blume-Peytavi, U. & Kottner, J. Epidermal thickness in healthy humans: a systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 36, 1191–1200 (2022).
Pankrushina, E. A., Kobuzov, A. S., Shchapova, Y. V. & Votyakov, S. L. Analysis of temperature-dependent Raman spectra of minerals: statistical approaches. J. Raman Spectrosc. 51, 1549–1562 (2020).
Schmelzeisen-Redeker, G. et al. Time delay of CGM sensors: relevance, causes, and countermeasures. J. Diabetes Sci. Technol. 9, 1006–1015 (2015).
Basu, A. et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 62, 4083–4087 (2013).
Shao, S. Subcutaneous depth-selective spectral imaging with mμSORS enables non-invasive glucose monitoring. Zenodo https://doi.org/10.5281/zenodo.14605629 (2025).
Zakharov, P., Dewarrat, F., Caduff, A. & Talary, M. S. The effect of blood content on the optical and dielectric skin properties. Physiol. Meas. 32, 131–149 (2011).
Acknowledgements
We thank the field workers at Ruijin Hospital and the engineers at Shanghai Photonic View Technology Co., Ltd. for their contribution and the participants for their cooperation in the BESH. The funding by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0508100, Y.Z.), Shanghai Shen-Kang projects (SHDC12024109, L. Zhang and SHDC22022301, W.W.), the Leader Project of the Oriental Talent Program in 2022 (no. 153, Y.Z.). Shanghai Pujiang Program (23PJD059, S.S.), Guangci Innovative Technology Program (KY2023810, C.C.), Guangci Talent Program (RC20240018, C.C.) and Guangci Deep Mind Project of Ruijin Hospital-Shanghai Jiao Tong University School of Medicine are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Y.Z., L. Zhou, G.N., C.C. and W. W. conceived the study and supervised the experiments. C.H., X.Z., M.S. and C.C. built the optical system. L.W., Y.Z., B.T. and J.S. took charge of the recruitment of subjects. L. Zhang, L.W., B.T., Y.C. and H.C. performed the experiments. M.C. collected the OCT data. S.S., Y.S., S.P., C.J., L. Zhou and C.C performed data analysis. Y.Z., L. Zhang, L.W., S.S., L. Zhou and C.C. wrote the paper. G.N., C.C. and W.W. obtained the funding.
Corresponding authors
Ethics declarations
Competing interests
The mμSORS technology presented in this paper is the subject of patents (CN115137298B, CN115120233B and CN214252020U) by Shanghai Photonic View Technology Co., Ltd. C.C. is a shareholder of Shanghai Photonic View Technology Co., Ltd. and the inventor of the patents. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Andreas Birkenfeld, Ioan Notingher, Eric Renard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bilayer sample characterizes depth selectivity of mµSORS.
a, Top view and side view of the Si-tape bilayer sample. b, Raman spectra of the bilayer sample displayed in three dimensions. Shade and box indicate characteristic Raman peaks at 520 cm−1 (Si) and 1041 cm−1 (Scotch tape), respectively. The depth of Si in 15 different phantoms increased from 50 μm to 750 μm with a step of 50 μm. c, Normalized Si (520 cm−1) Raman band intensity varying with its depth for each offset. Error bars indicate mean and standard deviation (SD) over n = 3 measurements. Lines are derived from 9-order polynomial fitting of the measured points.
Extended Data Fig. 2 Acquisition and analysis of OCT scans.
a, The 3D image acquired by OCT (the left hand of D132 as an example). b, The intensity profile along the z (depth) dimension of Subject D132’s left hand (same as a). z = 0 corresponds to the skin surface. Yellow dot indicates the characteristic points that were manually annotated and corresponded to the DEJ depth.
Extended Data Fig. 3 Intensity profiles of four typical thenar OCT images.
I–IV marked samples from a single hand of four typical subjects. The shades indicate the standard deviation (see Methods). Yellow dots indicate the characteristic points that were manually annotated and corresponded to the DEJ depth. Insets: three-dimensional OCT images constructed from a volume of 3 mm (x) * 3 mm (y) * 1.95 mm (z).
Extended Data Fig. 4 Anatomical and spectral characterization of epidermis and dermis of human skin samples.
a, Bright field image of a processed ex-vivo human skin cross-section. A forearm cross-section from one woman of 29 years old were imaged and repeated independently five times with similar results, the zoom-in region of interest was shown. Epidermis is rich in cells, and thus, nucleic acid, while dermis is rich in collagen. The yellow dashed curve indicates the DEJ. Green dots indicate the distribution of glucose molecules, predominantly within the dermis. Scale bar: 250 µm. b, Reference Raman spectra taken from ex-vivo epidermis (black) and dermis (blue) samples of human skin. An ex-vivo fresh upper back tissue from one man of 28 years old was obtained and cut manually to prepare epidermal and dermal samples, five spectra were collected from different region of interest for each sample and the mean spectra were shown as reference spectra. Pink and purple shades indicate characteristic Raman peaks of nucleic acid and collagen.
Extended Data Fig. 5 mμSORS spectra of 10 groups partitioned by equal binning of VPG levels.
a, Average spectra in the range of 800–1,500 cm−1 for each of the 10 VPG-spectra groups (Fig. 2e) at different offsets in the preliminary BESH of 35 subjects. The black arrow indicates the phenylalanine Raman peak at 1001 cm−1, while the red box indicates the characteristic glucose Raman peak at 1,125 cm−1. b, Normalized Raman spectra (glucose Raman band divided by phenylalanine Raman band) of different offsets averaged over each of the 10 VPG-spectra groups (Fig. 2f), zoomed in around the glucose Raman peak at 1125 cm−1.
Extended Data Fig. 6 Blood glucose prediction results for 78 subjects with type 2 diabetes (D001-D078) in all 230 subjects of expanded BESHs.
Extended Data Fig. 7 Blood glucose prediction results for 78 subjects with type 2 diabetes (D079-D156) in all 230 subjects of expanded BESHs.
Extended Data Fig. 8 Blood glucose prediction results for 48 subjects with type 2 diabetes (D157-D200) and 30 subjects without diabetes (N001-N030) in all 230 subjects of expanded BESHs.
Extended Data Fig. 9 PLS model training for independent test.
a, Consensus error grid (CEG) of the predictions obtained from the PLS regression model on the training set (n = 4,618). b, Model performance metrics plotted against reference glucose concentration in the training set. Orange: MARD. Magenta: CEG: A. Red: CEG: A + B. Cyan shade: histogram of reference glucose concentration (VPG).
Extended Data Fig. 10 Optimizing the time delay in the PLS model for glucose prediction.
a, The VPG data of a typical subject with diabetes during the 5-h OGTT in the preliminary BESH. Measured VPGs (dots) were fitted with polynomials and the backtracked VPG with a certain time lag (stars) were used as reference in PLS models. b, RMSE between the model predictions and the reference VPGs, varying with the time lag from −25 to 0 min. The optimized time lag was at −16 min. The models were trained and tested with spectra from offset 3 in the preliminary BESH. Model predictions were generated using leave-one-subject-out cross-validation scheme (Fig. 2h). c, Counterpart of b for offset 2 and 3 as well as the concatenation (offset 2–3) of these two offsets in the expanded BESHs of 230 subjects. The optimal time lag was at −13 min for both two offsets and the concatenation. Model predictions were generated using subject-wise tenfold cross-validation scheme (Fig. 3d). d, Counterpart of the black curve in c in the training set comprised of 200 subjects in the expanded BESHs (Fig. 4a). The optimal time lag was at −13 min.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Figs. 1–5 and Extended Data Figs. 1–10
Fig. 1: Spectra intensities, OCT results. Fig. 2: Participant data in the BESH, VPG values, spectra intensities. Fig. 3: Participant data in the BESH, PLS model predictions and performance. Fig. 4: VPG in BESH, PLS model predictions and performance. Fig. 5: VPG in BESH, PLS model predictions and performance. Extended Data Fig. 1: Raman spectra and intensities. Extended Data Fig. 2: OCT profile. Extended Data Fig. 3: OCT profiles. Extended Data Fig. 4: Spectra. Extended Data Fig. 5: Spectra. Extended Data Figs. 6–8: VPG values in the BESH, PLS model predictions and performance. Extended Data Fig. 9: PLS model predictions and performance on the training set. Extended Data Fig. 10: PLS model performance with varying time lag to calculate the backtracked VPG concentrations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Zhang, L., Wang, L. et al. Subcutaneous depth-selective spectral imaging with mμSORS enables noninvasive glucose monitoring. Nat Metab 7, 421–433 (2025). https://doi.org/10.1038/s42255-025-01217-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01217-w
This article is cited by
-
Robust spectral sensor for standoff biometric detection
Nature Sensors (2026)
-
A future without needles: non-invasive glucose measurements in patients with diabetes
Nature Metabolism (2025)
-
Scalable miniature on-chip Fourier transform spectrometer for Raman spectroscopy
Light: Science & Applications (2025)
-
Calibration and performance of a Raman-based device for non-invasive glucose monitoring in type 2 diabetes
Scientific Reports (2025)