Abstract
Nicotinamide adenine dinucleotide kinase (NADK) catalyses the phosphorylation of NAD+ to produce NAD phosphate, the oxidized form of NADPH, a cofactor that serves a critical role in driving reductive metabolism. Cancer cells co-express two distinct NAD kinases that differ by localization (NADK, cytosol; NADK2, mitochondria). CRISPR screens performed across hundreds of cancer cell lines indicate that both are dispensable for growth in conventional culture media. By contrast, NADK deletion impaired cell growth in human plasma-like medium. Here we trace this conditional NADK dependence to the availability of folic acid. NADPH is the preferred cofactor of dihydrofolate reductase (DHFR), the enzyme that mediates metabolic activation of folic acid. We find that NADK is required for enabling cytosolic NADPH-driven DHFR activity sufficient to maintain folate-dependent nucleotide synthesis under low folic acid conditions. Our results reveal a basis for conditional NADK essentiality and suggest that folate availability determines whether DHFR activity can be sustained by alternative electron donors such as NADH.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data needed to evaluate the findings of this study can be found within the article, extended data or supplementary information. The individual plasmids generated in this study have been deposited in Addgene (identifiers found in Supplementary Table 5). Unique reagents generated in this study are available upon reasonable request from the corresponding author. Identifiers for deposited datasets accessed in this study are found in Supplementary Table 5. Source data are provided with this paper.
Code availability
This paper does not report original code.
References
Rancati, G., Moffat, J., Typas, A. & Pavelka, N. Emerging and evolving concepts in gene essentiality. Nat. Rev. Genet. 19, 34–49 (2017).
Shalem, O. et al. Genome-Scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Hart, T. et al. High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell 163, 1515–1526 (2015).
Wang, T. et al. Identification and characterization of essential genes in the human genome. Science 350, 1096–1101 (2015).
Han, K. et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 580, 136–141 (2020).
Jain, I. H. et al. Genetic screen for cell fitness in high or low oxygen highlights mitochondrial and lipid metabolism. Cell 181, 716–727.e11 (2020).
Michl, J. et al. CRISPR–Cas9 screen identifies oxidative phosphorylation as essential for cancer cell survival at low extracellular pH. Cell Rep. 38, 110493 (2022).
Cantor, J. R. The rise of physiologic media. Trends Cell Biol. 29, 854–861 (2019).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272.e17 (2017).
Rossiter, N. J. et al. CRISPR screens in physiologic medium reveal conditionally essential genes in human cells. Cell Metab. 33, 1248–1263.e9 (2021).
Meyers, R. M. et al. Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nat. Genet. 49, 1779–1784 (2017).
Mary, C. et al. Crystal structure of human NADK2 reveals a dimeric organization and active site occlusion by lysine acetylation. Mol. Cell 82, 3299–3311.e8 (2022).
Du, J., Estrella, M., Solorio-Kirpichyan, K., Jeffrey, P. D. & Korennykh, A. Structure of human NADK2 reveals atypical assembly and regulation of NAD kinases from animal mitochondria. Proc. Natl Acad. Sci. USA 119, e2200923119 (2022).
Lerner, F., Niere, M., Ludwig, A. & Ziegler, M. Structural and functional characterization of human NAD kinase. Biochem. Biophys. Res. Commun. 288, 69–74 (2001).
Ghandi, M. et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 569, 503–508 (2019).
Pollak, N., Niere, M. & Ziegler, M. NAD kinase levels control the NADPH concentration in human cells. J. Biol. Chem. 282, 33562–33571 (2007).
Xiao, W., Wang, R.-S., Handy, D. E. & Loscalzo, J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid. Redox Signal. 28, 251–272 (2018).
Chandel, N. S. NADPH—the forgotten reducing equivalent. Cold Spring Harb. Perspect. Biol. 13, a040550 (2021).
Hosios, A. M. & Vander Heiden, M. G. The redox requirements of proliferating mammalian cells. J. Biol. Chem. 293, 7490–7498 (2018).
Hoxhaj, G. et al. Direct stimulation of NADP+ synthesis through Akt-mediated phosphorylation of NAD kinase. Science 363, 1088–1092 (2019).
Luongo, T. S. et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature 588, 174–179 (2020).
Kory, N. et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci. Adv. 6, eabe5310 (2020).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Goodman, R. P., Calvo, S. E. & Mootha, V. K. Spatiotemporal compartmentalization of hepatic NADH and NADPH metabolism. J. Biol. Chem. 293, 7508–7516 (2018).
Tran, D. H. et al. Mitochondrial NADP+ is essential for proline biosynthesis during cell growth. Nat. Metab. 3, 571–585 (2021).
Zhu, J. et al. Mitochondrial NADP(H) generation is essential for proline biosynthesis. Science 372, 968–972 (2021).
Ju, H.-Q., Lin, J.-F., Tian, T., Xie, D. & Xu, R.-H. NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 5, 231 (2020).
Eagle, H. Nutrition needs of mammalian cells in tissue culture. Science 122, 501–504 (1955).
Flickinger, K. M. et al. Conditional lethality profiling reveals anticancer mechanisms of action and drug-nutrient interactions. Sci. Adv. 10, eadq3591 (2024).
McKay, D. L., Perrone, G., Rasmussen, H., Dallal, G. & Blumberg, J. B. Multivitamin/mineral supplementation improves plasma B-vitamin status and homocysteine concentration in healthy older adults consuming a folate-fortified diet. J. Nutr. 130, 3090–3096 (2000).
Fazili, Z., Pfeiffer, C. M. & Zhang, M. Comparison of serum folate species analyzed by LC–MS/MS with total folate measured by microbiologic assay and Bio-Rad radioassay. Clin. Chem. 53, 781–784 (2007).
Pfeiffer, C. M. et al. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2. Brit. J. Nutr. 113, 1965–1977 (2015).
Jacques, P. F., Selhub, J., Bostom, A. G., Wilson, P. W. F. & Rosenberg, I. H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 340, 1449–1454 (1999).
Hou, Z. & Matherly, L. H. Biology of the major facilitative folate transporters SLC19A1 and SLC46A1. Curr. Top. Membr. 73, 175–204 (2014).
Kawai, S., Mori, S., Mukai, T., Hashimoto, W. & Murata, K. Molecular characterization of Escherichia coli NAD kinase. Eur. J. Biochem. 268, 4359–4365 (2001).
Poncet-Montange, G., Assairi, L., Arold, S., Pochet, S. & Labesse, G. NAD kinases use substrate-assisted catalysis for specific recognition of NAD. J. Biol. Chem. 282, 33925–33934 (2007).
Mori, S. et al. NAD-binding mode and the significance of intersubunit contact revealed by the crystal structure of Mycobacterium tuberculosis NAD kinase–NAD complex. Biochem. Biophys. Res. Commun. 327, 500–508 (2005).
Ohashi, K., Kawai, S. & Murata, K. Identification and characterization of a human mitochondrial NAD kinase. Nat. Commun. 3, 1248 (2012).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2016).
Zheng, Y. & Cantley, L. C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 216, 253–266 (2019).
Wilson, P. M., Danenberg, P. V., Johnston, P. G., Lenz, H.-J. & Ladner, R. D. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 11, 282–298 (2014).
Ghergurovich, J. M. et al. Methionine synthase supports tumour tetrahydrofolate pools. Nat. Metab. 3, 1512–1520 (2021).
Técher, H., Koundrioukoff, S., Nicolas, A. & Debatisse, M. The impact of replication stress on replication dynamics and DNA damage in vertebrate cells. Nat. Rev. Genet. 18, 535–550 (2017).
Mullen, N. J. & Singh, P. K. Nucleotide metabolism: a pan-cancer metabolic dependency. Nat. Rev. Cancer 23, 275–294 (2023).
Diehl, F. F. et al. Nucleotide imbalance decouples cell growth from cell proliferation. Nat. Cell Biol. 24, 1252–1264 (2022).
Schmoller, K. M. & Skotheim, J. M. The biosynthetic basis of cell size control. Trends Cell Biol. 25, 793–802 (2015).
Zatulovskiy, E. & Skotheim, J. M. On the molecular mechanisms regulating animal cell size homeostasis. Trends Genet. 36, 360–372 (2020).
Matthews, H. K., Bertoli, C. & de Bruin, R. A. M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 23, 74–88 (2022).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016).
Green, A. C. et al. Formate overflow drives toxic folate trapping in MTHFD1 inhibited cancer cells. Nat. Metab. 5, 642–659 (2023).
Kim, J. et al. Formate rescues neural tube defects caused by mutations in Slc25a32. Proc. Natl Acad. Sci. USA 115, 4690–4695 (2018).
Minton, D. R. et al. Serine catabolism by SHMT2 Is required for proper mitochondrial translation initiation and maintenance of formylmethionyl-tRNAs. Mol. Cell 69, 610–621.e5 (2018).
Lee, W. D. et al. Tumor reliance on cytosolic versus mitochondrial one-carbon flux depends on folate availability. Cell Metab. 33, 190–198.e6 (2020).
Ercikan-Abali, E. A. et al. Dihydrofolate reductase protein inhibits its own translation by binding to dihydrofolate reductase mRNA sequences within the coding region. Biochemistry 36, 12317–12322 (1997).
Huang, S. et al. Role of lysine-54 in determining cofactor specificity and binding in human dihydrofolate reductase. Biochemistry 29, 8063–8069 (1990).
Bailey, S. W. & Ayling, J. E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl Acad. Sci. USA 106, 15424–15429 (2009).
Tedeschi, P. M. et al. NAD+ kinase as a therapeutic target in cancer. Clin. Cancer Res. 22, 5189–5195 (2016).
Hsieh, Y.-C. et al. Enhanced degradation of dihydrofolate reductase through Inhibition of NAD kinase by nicotinamide analogs. Mol. Pharmacol. 83, 339–353 (2013).
Oka, S., Titus, A. S., Zablocki, D. & Sadoshima, J. Molecular properties and regulation of NAD+ kinase (NADK). Redox Biol. 59, 102561 (2022).
Rana, T. M. et al. Genome-wide CRISPR screen for essential cell growth mediators in mutant KRAS colorectal cancers. Cancer Res. 77, 6330–6339 (2017).
Tedeschi, P. M. et al. Suppression of cytosolic NADPH pool by thionicotinamide increases oxidative stress and synergizes with chemotherapy. Mol. Pharmacol. 88, 720–727 (2015).
Schild, T. et al. NADK is activated by oncogenic signaling to sustain pancreatic ductal adenocarcinoma. Cell Rep. 35, 109238 (2021).
Ilter, D. et al. NADK-mediated de novo NADP(H) synthesis is a metabolic adaptation essential for breast cancer metastasis. Redox Biol. 61, 102627 (2023).
Sullivan, M. R. et al. Methionine synthase is essential for cancer cell proliferation in physiological folate environments. Nat. Metab. 3, 1500–1511 (2021).
Bertino, J. R., Göker, E., Gorlick, R., Li, W. W. & Banerjee, D. Resistance mechanisms to methotrexate in tumors. Oncologist 1, 223–226 (1996).
Alt, F. W., Kellems, R. E., Bertino, J. R. & Schimke, R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J. Biol. Chem. 253, 1357–1370 (1978).
Chen, L., Ducker, G. S., Lu, W., Teng, X. & Rabinowitz, J. D. An LC–MS chemical derivatization method for the measurement of five different one-carbon states of cellular tetrahydrofolate. Anal. Bioanal. Chem. 409, 5955–5964 (2017).
Kanarek, N. et al. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 559, 632–636 (2018).
Su, X., Lu, W. & Rabinowitz, J. D. Metabolite spectral accuracy on orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Acknowledgements
We thank members of the Cantor laboratory for the upkeep of both the LC–MS system and cell sorter and for helpful discussions. We also thank J. Skotheim for helpful comments and discussion, A. Hunger for generating the pLJC2-Blast-Rap2A-3×FLAG plasmid, M. Stefely for figure assets related to the serine tracing schematic and the University of Wisconsin Carbone Cancer Center Flow Cytometry Laboratory (supported by National Institutes of Health (NIH) P30CA014520) for use of its facilities and services. This work was supported by grants from the American Cancer Society (RSG-21-170-01-TBE, to J.R.C.), the Glenn Foundation and American Federation for Aging Research (A22068, to J.A.S.), NIH (R01DK133479, to J.A.S.), the University of Wisconsin–Madison Hatch Grant (WIS04000-1024796, to J.A.S.) and the Juvenile Diabetes Research Foundation (JDRF) (JDRF201309442, to J.A.S.). Fellowship support was provided by the NIH (T32HG002760, to K.S.H.) and the University of Wisconsin–Madison Department of Biochemistry (to K.M.F., K.S.H. and G.M.W.). J.A.S. is a Howard Hughes Medical Institute (HHMI) Freeman Hrabowski Scholar. J.R.C. is a Hartwell Foundation Investigator.
Author information
Authors and Affiliations
Contributions
K.M.F. and J.R.C. initiated the project and designed the research plan. K.M.F. performed most of the experiments, with assistance from K.S.H. and G.R.C. C.M.F. optimized and helped perform cell cycle and cell death experiments. G.M.W. performed the lipidomics with guidance from J.A.S. K.C.F. carried out the cell cycle analysis. K.M.F. and J.R.C. analysed and interpreted the experimental data. J.R.C. wrote the manuscript with assistance from K.M.F. All authors discussed the manuscript. J.R.C. supervised the studies.
Corresponding author
Ethics declarations
Competing interests
J.R.C. is an inventor on an issued patent for HPLM assigned to the Whitehead Institute (Patent no. US11453858). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Jiyeon Kim, Johannes Meiser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
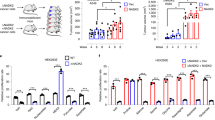
Extended Data Fig. 1 Related to conditional NADK dependence is linked to folic acid availability.
(a) Human cell lines ranked by probability of dependency values for NADK across CRISPR screen data cataloged in the DepMap12. Probability > 0.5 is the reference threshold for essentiality. (b) Immunoblots for expression of NADK and NADK2. RAPTOR served as the loading control in both cases. (c) Reported concentration ranges for folic acid and 5-methyl-THF (5-mTHF) in human plasma32,33,34. (d, e, h) Relative growth of NADK-knockout versus control cells (mean ± s.d., n = 3 biologically independent samples). EV, empty vector. Two-tailed Welch’s t-test comparing the respective mean ± s.d. (bar) versus mean ± s.d. (control cells) between bars. (f) Dependency phenotypes for NADK from conditional essentiality profiling in K562 cells11. (g) Extracellular levels of folic acid following 96-hr culture of NADK-knockout or control cells versus those at inoculation (mean ± s.e.m., n = 3 biologically independent samples). Two-tailed Welch’s t-test comparing the respective mean ± s.d. (bar) versus mean ± s.d. (inoculation) between bars.
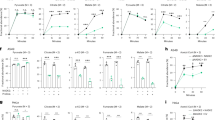
Extended Data Fig. 2 Related to cytosolic NADK activity is essential in low folate conditions.
(a) Amino acid sequence alignment depicting a GGDG motif conserved among NADK proteins across different species. D184 is the putative catalytic aspartate (red) in human NADK. (b) Pseudocolor Coomassie-stained gel imaged using a LI-COR Odyssey FC. 1: M.W. standards; 2: wild-type NADK-3xFLAG; 3: NADK (D184A)-3xFLAG. (c) Immunoblots for expression of NADK and NADK2 in NADK-knockout cells. GAPDH or RAPTOR served as the loading control. (d, f) Relative NADP+ levels in NADK-knockout versus control cells (mean ± s.e.m., n = 3 biologically independent samples). EV, empty vector. MTS, mitochondrial targeting sequence. (e) Cellular NADPH abundances (mean ± s.e.m., n = 3 biologically independent samples). Two-tailed Welch’s t-test. (g) Relative NADP+ levels in NADK2-knockout versus control cells (mean ± s.e.m., n = 3 biologically independent samples). (h) Dependency phenotypes for NADK2 from conditional essentiality profiling in K562 cells11. (i) Defined proline levels in HPLM, RPMI, and DMEM. (j, k) Relative growth of NADK2-knockout versus control cells (mean ± s.d., n = 3 biologically independent samples). (d, f, g, j, k) Two-tailed Welch’s t-test comparing the respective mean ± s.d. (bar) versus mean ± s.d. (control cells) between bars.
Extended Data Fig. 3 Related to NADK is conditionally required for nucleotide synthesis and supports cell cycle progression in low folate conditions.
(a) Relative levels of various lipid classes in NADK-knockout versus control cells (mean ± s.e.m., n = 3 biologically independent samples). Parenthetical value corresponds to the total number of filtered species that comprised each lipid class for analysis (see Supplementary Table 2). Cer_NS, ceramide non-hydroxyfatty acid-sphingosine. EtherPE, ether-linked phosphatidylethanolamine. PC, phosphatidylcholine. PE, phosphatidylethanolamine. SM, sphingomyelin. PS, phosphatidylserine. Two-tailed Welch’s t-test comparing the respective mean ± s.d. (bar) versus mean ± s.d. (control cells) between bars. (b) (top) Dose-responses of control cells to treatment with hydrogen peroxide (H2O2) (mean ± s.d., n = 3 biologically independent samples). (bottom) Defined concentrations of glutathione in HPLM and RPMI. (c) Relative growth of H2O2-treated NADK-knockout and control cells versus untreated control cells (mean ± s.d., n = 3 biologically independent samples). Source data for control cells are shared from Extended Data Fig. 3b. Two-tailed Welch’s t-test comparing the respective mean ± s.d. (bar) versus mean ± s.d. (untreated control cells) between bars. **P < 0.005. (d) Specific growth rates for control cells (mean ± s.d., n = 3 biologically independent samples). Two-tailed Welch’s t-test. (e) Flow cytometric analysis of NADK-knockout and control cells stained with 7-Aminoactinomycin D (7-AAD), which is excluded from live cells. EV, empty vector. (f) (top) Immunoblot for expression of phosphorylated CHK1 (p-CHK1). ACTN1 served as the loading control. Bottom row, p-CHK1 signal normalized by ACTN1 signal in the respective lane versus lane 1. (bottom) Immunoblot for expression of p-CHK2. GAPDH served as the loading control. Bottom row, p-CHK2 signal normalized by GAPDH signal in the respective lane versus lane 1. (g) Relative levels of IMP and dTMP in NADK-knockout versus control cells at indicated time points during log growth (mean ± s.e.m., n = 3 biologically independent samples). Two-tailed Welch’s t-test versus control cells (above bars) or comparing the respective mean ± s.d. (bar) versus mean ± s.d. (control cells) between bars. *P < 0.05, **P < 0.005. (c, g) Values above brackets indicate fold-change between bars.
Extended Data Fig. 4 Related to NADK deletion alters subcellular contributions to 1 C production and depletes cellular folate pools.
(a) Fractional labeling of dTTP in control cells (mean ± s.d., n = 3 biologically independent samples). Value above bracket indicates difference in M + 1 labeling. Two-tailed Welch’s t-test for comparisons of M + 1 labeling. (b) Immunoblot for expression of SHMT2 in control and SHMT2-knockout cells. RAPTOR served as the loading control. EV, empty vector. (c) Fractional labeling of ATP in SHMT2-knockout cells (mean ± s.d., n = 3 biologically independent samples). Values above bracket indicate differences in M + 1 (bottom bracket) and M + 2 (top bracket) labeling. Only unlabeled ATP could be detected from SHMT2-knockout cells transduced with empty vector (EV). Two-tailed Welch’s t-test for comparisons of M + 1 (bottom) and M + 2 (top) labeling.
Extended Data Fig. 5 Related to NADK facilitates DHFR activity in low folate conditions.
(a) Immunoblots for expression of DHFR and NADK in control and DHFR-knockout cells. RAPTOR served as the loading control. (b) (left) Relative growth of NADK-knockout and MTX-treated control cells versus vehicle-treated control cells (mean ± s.d., n = 3 biologically independent samples). Two-tailed Welch’s t-test comparing the respective mean ± s.d. (bar) versus mean ± s.d. (vehicle-treated control cells) between bars. (right) MTX is a potent DHFR inhibitor. MTX, methotrexate. (c) Relative levels of tetrahydrofolate (THF), 10-formyl-THF (10-fTHF), and 5-methyl-THF (5-mTHF) in MTX- versus vehicle-treated control cells (mean ± s.e.m., n = 3 biologically independent samples). Two-tailed Welch’s t-test versus vehicle-treated control cells. (d) Schematic for a method to evaluate DHFR activity by measuring THF production from reactions containing recombinant DHFR and different combinations of folic acid, DHF, and NAD(P)H. (e) Pseudocolor Coomassie-stained gel imaged using a LIC-OR Odyssey FC. 1: M.W. standards; 2: DHFR-3xFLAG. (f) Schematic for a method to evaluate MTHFD1 (CD) activity by measuring 5,10-meTHF production from reactions containing recombinant MTHFD1, 10-formyl-THF, and NAD(P)H. (g) Pseudocolor Coomassie-stained gel imaged using a LIC-OR Odyssey FC. 1: M.W. standards; 2: MTHFD1-3xFLAG. (h) 5,10-meTHF levels measured from reactions that contained recombinant MTHFD1 with indicated substrate combinations (mean ± s.d., n = 3 independent reactions). Value above brackets indicates fold-change between bars. Two-tailed Welch’s t-test. (i) Dependency phenotypes for NADK from conditional essentiality profiling of the indicated cell lines using a focused sgRNA library11.
Extended Data Fig. 6 Related to TN can form a drug-nutrient interaction with folic acid.
(a) Dose-responses of control cells treated with thionicotinamide (TN) (mean ± s.d., n = 3 biologically independent samples). (b, e) Relative levels of (b) NADP+ (e) and NAD+ in TN-treated control and NADK-knockout versus untreated control cells (mean ± s.e.m., n = 3 biologically independent samples). Two-tailed Welch’s t-test versus control cells (above bars) or comparing the respective mean ± s.d. (bar) versus mean ± s.d. (control cells) between bars. *P < 0.05, **P < 0.005. EV, empty vector. (c) Relative growth of TN-treated control and NADK2-knockout versus untreated control cells (mean ± s.d., n = 3 biologically independent samples). Two-tailed Welch’s t-test comparing the respective mean ± s.d. (bar) versus mean ± s.d. (control cells) between bars. (d) Mass-to-charge ratios (m/z) for various products of TN metabolism via the NAD+ salvage pathway based on either addition (+H) or removal (-H) of a proton adduct. Only peaks corresponding to NADPS could not be detected in either ionization mode in TN-treated K562 cells. NMNS, TN mononucleotide. NADS, thio-NAD. NADPS, thio-NADP. NADPSH, thio-NADPH.
Supplementary information
Supplementary Table 1
Synthetic media construction.
Supplementary Table 2
Datasets related to lipidomics.
Supplementary Table 3
Datasets related to cell cycle distributions.
Supplementary Table 4
Datasets related to cellular metabolomics.
Supplementary Table 5
Reagents and resources.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Figs. 1, 2 and 5
Uncropped immunoblots.
Source Data Fig. 3
Gating strategy for cell cycle analysis.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Figs. 1–5
Uncropped immunoblots and Coomassie-stained gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Flickinger, K.M., Mellado Fritz, C.A., Huggler, K.S. et al. Cytosolic NADK is conditionally essential for folate-dependent nucleotide synthesis. Nat Metab 7, 1150–1167 (2025). https://doi.org/10.1038/s42255-025-01272-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01272-3
This article is cited by
-
SLC46A1 deficiency-mediated folate restriction suppresses colorectal cancer progression through epigenetic-transcriptional reprogramming
Cell Death & Disease (2026)
-
NAD kinase essentiality in cancer: in real life, it is all about folates
Nature Metabolism (2025)