Abstract
Tauopathies encompass a range of neurodegenerative disorders, such as Alzheimer’s disease (AD) and frontotemporal lobar degeneration with tau inclusions (FTLD-tau), for which there are currently no successful treatments. Here, we show impaired glycogen metabolism in the brain of a tauopathy Drosophila melanogaster model and people with AD, indicating a link between tauopathies and glycogen metabolism. We demonstrate that the breakdown of neuronal glycogen ameliorates the tauopathy phenotypes in flies and induced pluripotent stem cell (iPSC)-derived neurons from people with FTLD-tau. Glycogen breakdown redirects glucose flux to the pentose phosphate pathway and alleviates oxidative stress. Our findings uncover a critical role for the neuroprotective effects of dietary restriction (DR) by increasing glycogen breakdown. Mechanistically, we show a potential interaction between tau protein and glycogen, suggesting a vicious cycle in which tau binding promotes glycogen accumulation in neurons, which in turn exacerbates tau accumulation which further disrupts cellular homeostasis. Our studies identify impaired glycogen metabolism as a key hallmark for tauopathies and offer a promising therapeutic target in tauopathy and other neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw data for complete MS data sets for proteomic analysis have been uploaded to the MassIVE repository of the Center for Computational Mass Spectrometry at UCSD and can be downloaded using the following link: https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=93387e0de9b7465ab0466d3bd4bb901f
The data are also available at ProteomeXchange with the ID PXD044485. Raw data from RNA sequencing and list of targeted metabolites and their values are available in Figshare using the following link https://figshare.com/s/ad5c367a220c6cca66f8. Source data are provided with this paper.
Code availability
The code for Cox proportional hazard ratio calculation is available in Figshare using the following link: https://figshare.com/s/ad5c367a220c6cca66f8 (ref. 90).
References
Goedert, M., Eisenberg, D. S. & Crowther, R. A. Propagation of tau aggregates and neurodegeneration. Annu Rev. Neurosci. 40, 189–210 (2017).
Kovacs, G. G. Tauopathies. Handb. Clin. Neurol. 145, 355–368 (2017).
Brunden, K. R., Trojanowski, J. Q. & Lee, V. M. Y. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat. Rev. Drug Discov. 8, 783–793 (2009).
Costantini, L. C., Barr, L. J., Vogel, J. L. & Henderson, S. T. Hypometabolism as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 9, S16 (2008).
Johnson, E. C. B. et al. Large-scale proteomic analysis of alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 26, 769 (2020).
Sattler, R. et al. Frontotemporal dementia and glucose metabolism. Front. Neurosci. 16, 812222 (2022).
Blin, J., Horwitz, B., Baron, J. C. & Agid, Y. Does frontal cortex hypometabolism in progressive supranuclear palsy result from subcortical dysfunction? Eur. J. Neurol. 1, 221–228 (1995).
Minhas, P. S. et al. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science 385, eabm6131 (2024).
Gibbs, M. E., Anderson, D. G. & Hertz, L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54, 214–222 (2006).
Mann, D. M. A., Sumpter, P. Q., Davies, C. A. & Yates, P. O. Glycogen accumulations in the cerebral cortex in Alzheimer’s disease. Acta Neuropathol. 73, 181–184 (1987).
Duran, J., Gruart, A., García-Rocha, M., Delgado-García, J. M. & Guinovart, J. J. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum. Mol. Genet 23, 3147–3156 (2014).
Li, C. et al. Decreased glycogenolysis by miR-338-3p promotes regional glycogen accumulation within the spinal cord of amyotrophic lateral sclerosis mice. Front. Mol. Neurosci. 12, 114 (2019).
Cai, Y. et al. Glycogenolysis is crucial for astrocytic glycogen accumulation and brain damage after reperfusion in ischemic stroke. iScience 23, 101136 (2020).
Adeva-Andany, M. M., González-Lucán, M., Donapetry-García, C., Fernández-Fernández, C. & Ameneiros-Rodríguez, E. Glycogen metabolism in humans. BBA Clin. 5, 85–100 (2016).
Brown, A. M. & Ransom, B. R. Astrocyte glycogen and brain energy metabolism. Glia 55, 1263–1271 (2007).
Oe, Y., Baba, O., Ashida, H., Nakamura, K. C. & Hirase, H. Glycogen distribution in the microwave-fixed mouse brain reveals heterogeneous astrocytic patterns. Glia 64, 1532–1545 (2016).
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span—from yeast to humans. Science 328, 321–326 (2010).
Mair, W. & Dillin, A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 77, 727–754 (2008).
Masoro, E. J. & Austad, S. N. The evolution of the antiaging action of dietary restriction: a hypothesis. J. Gerontol. A Biol. Sci. Med Sci. 51, B387–B391 (1996).
Solon-Biet, S. M. et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 (2014).
Mattson, M. P., Duan, W., Lee, J. & Guo, Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech. Ageing Dev. 122, 757–778 (2001).
Graff, J. et al. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J. Neurosci. 33, 8951–8960 (2013).
Kerr, F. et al. Dietary restriction delays aging, but not neuronal dysfunction, in Drosophila models of Alzheimer’s disease. Neurobiol. Aging 32, 1977–1989 (2011).
Van Cauwenberghe, C., Vandendriessche, C., Libert, C. & Vandenbroucke, R. E. Caloric restriction: beneficial effects on brain aging and Alzheimer’s disease. Mamm. Genome 27, 300–319 (2016).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2021 23, 56–73 (2021).
Wittmann, C. W. et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293, 711–714 (2001).
Luis, N. M. et al. Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan under dietary restriction. Cell Rep. 17, 1207–1216 (2016).
Akagi, K. et al. Dietary restriction improves intestinal cellular fitness to enhance gut barrier function and lifespan in D. melanogaster. PLoS Genet. 14, e1007777 (2018).
Chatterjee, S., Sang, T. K., Lawless, G. M. & Jackson, G. R. Dissociation of tau toxicity and phosphorylation: role of GSK-3β, MARK and Cdk5 in a Drosophila model. Hum. Mol. Genet 18, 164–177 (2009).
Gendron, C. M., Chakraborty, T. S., Duran, C., Dono, T. & Pletcher, S. D. Ring neurons in the Drosophila central complex act as a rheostat for sensory modulation of aging. PLoS Biol. 21, e3002149 (2023).
Gillet, L. C. et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics 11, O111.016717 (2012).
Collins, B. C. et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 8, 291 (2017).
Jackson, G. R. et al. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34, 509–519 (2002).
Oliveira, A. C., Santos, M., Pinho, M. & Lopes, C. S. String/Cdc25 phosphatase is a suppressor of Tau-associated neurodegeneration. Dis. Model Mech. 16, dmm049693 (2023).
Zelhof, A. C. et al. The brachyceran de novo gene PIP82, a phosphorylation target of aPKC, is essential for proper formation and maintenance of the rhabdomeric photoreceptor apical domain in Drosophila. PLoS Genet. 16, e1008890 (2020).
Koushika, S. P., Lisbin, M. J. & White, K. ELAV, a Drosophila neuron-specific protein, mediates the generation of an alternatively spliced neural protein isoform. Curr. Biol. 6, 1634–1641 (1996).
Zirin, J., Nieuwenhuis, J. & Perrimon, N. Role of autophagy in glycogen breakdown and its relevance to chloroquine myopathy. PLoS Biol. 11, e1001708 (2013).
Zhang, Y. et al. Decoding the molecular mechanism of selective autophagy of glycogen mediated by autophagy receptor STBD1. Proc. Natl Acad. Sci. USA 121, e2402817121 (2024).
Kanungo, S., Wells, K., Tribett, T. & El-Gharbawy, A. Glycogen metabolism and glycogen storage disorders. Ann. Transl Med. 6, 474 (2018).
Fernie, A. R., Carrari, F. & Sweetlove, L. J. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 7, 254–261 (2004).
Ge, T. et al. The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol. 11, 507678 (2020).
Dinuzzo, M., Mangia, S., Maraviglia, B. & Giove, F. Regulatory mechanisms for glycogenolysis and K+ uptake in brain astrocytes. Neurochem. Int. 63, 458–464 (2013).
Jiang, G. & Zhang, B. B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 284, E671–E678 (2003).
Petersen, M. C., Vatner, D. F. & Shulman, G. I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 (2017).
Hao, Y. et al. Activation of PKA via asymmetric allosteric coupling of structurally conserved cyclic nucleotide binding domains. Nat. Commun. 10, 1–12 (2019).
Yamada, T. et al. The role of glycogen in development and adult fitness in Drosophila. Development 146, dev176149 (2019).
Karch, C. M. et al. Resource a comprehensive resource for induced pluripotent stem cells from patients with primary tauopathies. Stem Cell Rep. 13, 939–955 (2019).
Wang, C. et al. Scalable production of iPSC-derived human neurons to identify tau-lowering compounds by high-content screening. Stem Cell Rep. 9, 1221–1233 (2017).
Zhu, Y. et al. Single-cell analysis for glycogen localization and metabolism in cultured astrocytes. Cell Mol. Neurobiol. 40, 801–812 (2020).
Marr, L. et al. Mechanism of glycogen synthase inactivation and interaction with glycogenin. Nat. Commun. 13, 1–14 (2022).
Nakamura, M. et al. Pathological progression induced by the frontotemporal dementia-associated R406W tau mutation in patient-derived iPSCs. Stem Cell Rep. 13, 684–699 (2019).
Tracy, T. E. et al. Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration. Cell 185, 712 (2022).
Groen, A. C., Coughlin, M. & Mitchison, T. J. Microtubule assembly in meiotic extract requires glycogen. Mol. Biol. Cell 22, 3139–3151 (2011).
Puri, R., Jain, N. & Ganesh, S. Increased glucose concentration results in reduced proteasomal activity and the formation of glycogen positive aggresomal structures. FEBS J. 278, 3688–3698 (2011).
Wu, J. et al. A conserved domain for glycogen binding in protein phosphatase-1 targeting subunits. FEBS Lett. 439, 185–191 (1998).
Bhat, N. R. & Thirumangalakudi, L. Increased tau phosphorylation and impaired brain insulin/IGF signaling in mice fed a high fat/high cholesterol diet. J. Alzheimers Dis. 36, 781–789 (2013).
Faraco, G. et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 574, 686–690 (2019).
Liang, Z. et al. Long-term high-fat diet consumption induces cognitive decline accompanied by tau hyper-phosphorylation and microglial activation in aging. Nutrients 15, 250 (2023).
Wilson, K. A. et al. GWAS for lifespan and decline in climbing ability in flies upon dietary restriction reveal decima as a mediator of insulin-like peptide production. Curr. Biol. 30, 2749–2760 (2020).
Katewa, S. D. et al. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 16, 97–103 (2012).
Hodge, B. A. et al. Dietary restriction and the transcription factor clock delay eye aging to extend lifespan in Drosophila melanogaster. Nat. Commun. 13, 1–15 (2022).
Pandey, M. et al. Mir-125-chinmo pathway regulates dietary restriction-dependent enhancement of lifespan in Drosophila. eLife 10, e62621 (2021).
Wilson, K. A. et al. Evaluating the beneficial effects of dietary restrictions: a framework for precision nutrigeroscience. Cell Metab. 33, 2142–2173 (2021).
Perlegos, A. E. et al. TDP-43 impairs sleep in Drosophila through Ataxin-2–dependent metabolic disturbance. Sci. Adv. 10, eadj4457 (2024).
Wander, C. M. et al. Corpora amylacea are associated with tau burden and cognitive status in Alzheimer’s disease. Acta Neuropathol. Commun. 10, 110 (2022).
Dallmeier, J. D. et al. Corpora amylacea negatively correlate with hippocampal tau pathology in Alzheimer’s disease. Front. Neurosci. 18, 1286924 (2024).
Puri, R., Suzuki, T., Yamakawa, K. & Ganesh, S. Hyperphosphorylation and aggregation of tau in laforin-deficient mice, an animal model for Lafora disease. J. Biol. Chem. 284, 22657–22663 (2009).
Machado-Salas, J. et al. Ontogeny of lafora bodies and neurocytoskeleton changes in laforin-deficient mice. Exp. Neurol. 236, 131 (2012).
Favaro, E. et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 16, 751–764 (2012).
Zhang, H. et al. TCR activation directly stimulates PYGB-dependent glycogenolysis to fuel the early recall response in CD8+ memory T cells. Mol. Cell 82, 3077–3088.e6 (2022).
Dias-Santagata, D., Fulga, T. A., Duttaroy, A. & Feany, M. B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Invest. 117, 236–245 (2007).
Ton, A. M. M. et al. Oxidative stress and dementia in Alzheimer’s patients: effects of synbiotic supplementation. Oxid. Med. Cell Longev. 2020, 2638703 (2020).
Sun, R. C. et al. Brain glycogen serves as a critical glucosamine cache required for protein glycosylation. Cell Metab. 33, 1404–1417 (2021).
Weimer, S. et al. d-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat. Commun. 5, 3563 (2014).
Brushia, R. J. & Walsh, D. A. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 4, D618–D641 (1999).
Zhuo, Y. et al. Inhibition of phosphodiesterase-4 reverses the cognitive dysfunction and oxidative stress induced by Aβ25-35 in rats. Metab. Brain Dis. 31, 779–791 (2016).
Giblin, A. et al. Neuronal polyunsaturated fatty acids are protective in ALS/FTD. Nat. Neurosci. 28, 737–747 (2025).
Ju, Y., Li, S., Kong, X. & Zhao, Q. Exploring fatty acid metabolism in Alzheimer’s disease: the key role of CPT1A. Sci. Rep. 14, 31483 (2024).
Cook, K. R., Parks, A. L., Jacobus, L. M., Kaufman, T. C. & Matthews, K. A. New research resources at the Bloomington Drosophila Stock Center. Fly 4, 88–91 (2010).
Zid, B. M. et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139, 149–160 (2009).
Katewa, S. D. et al. Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in Drosophila. Cell Metab. 23, 143–154 (2016).
Iyer, J. et al. Quantitative assessment of eye phenotypes for functional genetic studies using Drosophila melanogaster. G3 6, 1427–1437 (2016).
Sohn, P. D. et al. Pathogenic tau impairs axon initial segment plasticity and excitability homeostasis. Neuron 104, 458–470.e5 (2019).
Meyer, J. G. & Schilling, B. Clinical applications of quantitative proteomics using targeted and untargeted data-independent acquisition techniques. Expert Rev. Proteom. 14, 419–429 (2017).
Schilling, B., Gibson, B. W. & Hunter, C. L. Generation of high-quality SWATH® acquisition data for label-free quantitative proteomics studies using TripleTOF® mass spectrometers. Methods Mol. Biol. 1550, 223 (2017).
Kurup, K. et al. Calorie restriction prevents age-related changes in the intestinal microbiota. Aging 13, 6298–6320 (2021).
Meador, J. P., Bettcher, L. F., Ellenberger, M. C. & Senn, T. D. Metabolomic profiling for juvenile Chinook salmon exposed to contaminants of emerging concern. Sci. Total Environ. 747, 141097 (2020).
Xia, J., Psychogios, N., Young, N. & Wishart, D. S. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 37, W652 (2009).
Babicki, S. et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 44, W147 (2016).
Bar, S. et al. Neuronal glycogen breakdown mitigates tauopathy via pentose-phosphate-pathway-mediated oxidative stress reduction. figshare https://figshare.com/s/ad5c367a220c6cca66f8 (2025).
Acknowledgements
We would like to thank the members of the Kapahi, Ellerby, Schilling and Tracy labs for their comments throughout the paper and experiments. S.B. is supported by Larry L. Hillblom Foundation fellowship 2019-A-026-FEL. K. A. W. was supported by NIH grant T32AG000266-23 and the CatalystX award from Alex and Bob Griswold. T.A.U.H. was supported by NIH and NIA award F31AG062112, NIH/NIA training grant T32AG000266-24. This work was supported by the NIH and American Federation of Aging Research grants, NIH grants R01AG038688, R21AG054121, AG045835 and RO1-R01AG071995 and the Larry L. Hillblom Foundation to P.K. T.E.T. is supported by NIH grant R01AG070193, L.M.E. is supported by R01AG061879 and P01AG066591. We acknowledge the support of instrumentation for the TripleTOF 6600 from the NIH shared instrumentation grant 1S10 OD016281 (Buck Institute) and a Hevolution Foundation grant to the Buck Institute for Research on Aging (HF-PART-23-1422047). We thank the lab of S. Pletcher, University of Michigan Medical School, for sharing 3XEGS. We thank the Northwest Metabolomics Research Center, University of Washington, for the metabolomics analysis. We thank C. Karch for providing iPSC and isogenic control lines. Support to generate the iPSC was provided by Knight Alzheimer Disease Research Center at Washington University (NIH P30 AG066444, P01 AG03991, and P01 AG026276), NIH AG046374 (C.M.K.), and the Rainwater Charitable Organization. Schematic diagrams were generated with BioRender.com (https://biorender.com/582r1x5, https://biorender.com/xn3s24o, https://biorender.com/f73h247). We thank M. B. Feany and Scott D. Pletcher for fly strains. S.B. personally acknowledges G. T. Meyerhof, M. Dhara, P. Singh, V. Tanwar, L. Gann, W. Gutierrez, M. Gupta, V. P. Narayan, K. R. Kaneshiro, M. M. Shanmugam, G. Chawla, D. Sellegounder, K. Patel, L. Enriquez, E. Morazan, I. Emma and A. K. Cayton for their support.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.B. and P.K. Methodology: S.B., E.B.D., B.S., N.T.S., T.E.T., L.M.E. and P.K. Investigation: S.B., K.A.W., T.A.U.H., S.A., E.B.D., J.B.B., S.S., A.H., E.M.C., J.N.B., J.H.C., G.K., F.S. and A.S. Visualization: S.B., S.A. and E.B.D. Funding acquisition: S.B., K.A.W., T.A.U.H., B.S., T.E.T., L.M.E. and P.K. Project administration: P.K. Supervision: P.K. Writing—original draft: S.B. and P.K. Writing—review and editing: S.B., K.A.W., T.A.U.H., S.A., B.S., E.B.D., J.B.B., G.K., T.E.T., L.M.E. and P.K.
Corresponding author
Ethics declarations
Competing interests
P.K. is a founder and a member of the scientific advisory board at Juvify Bio. The other authors have no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Thomas Preat and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
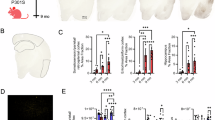
Extended Data Fig. 1 Impact of dietary restriction on lifespan and ROS production in tauopathy flies.
a, Lifespan of control flies (W1118 crossed with tauR406W) shows DR-mediated extension (p = 2.8164e-99) (204 and 192 flies were studied for AL and DR diets respectively). b, Lifespan of control flies (W1118 crossed with tauWT) shows DR-mediated extension (p = 9.80751e-51). For lifespan 203 and 151 flies were studied for AL and DR diets respectively. c, Mean lifespan of controls, tauR406W, and tauWT of 3 independent experiments show lifespan extension by DR. TauWT and tauR406W flies have the shortest lifespan (**p < 0.0001) than controls (n > 100 for each group). d, Lifespan of tauR406W flies shorter on both AL (p = 6.48275e-38) and DR (p = 6.03135e-57) diets than control flies (elav-Gal4 crossed with mito-GFP). For lifespan 156, 171 172 and 169 flies were studied for tauR406W AL, tauR406W DR, mito-GFP AL and mito-GFP DR were studied respectively. e, Lifespan of tauS11A is shorter (p = 1.64475e-19) than control flies (elav-Gal4 crossed with W1118) and shows DR-mediated extension (p = 3.40185e-24). For lifespan 138, 136, 96 and 85 flies were studied for control AL, control DR, tauS11A AL and tauS11A DR respectively. f, bar plot represents lethality during developmental phase in tauR406W flies and control elav-gal4 crossed with W1118. Each dot represents separate vials of embryo generated from 2:1 female: male cross. g, Lifespan of tauR406W driven by 3X Elav-Gene switch induced by RU486 is reduced (p = 9.09456e-09) than control and shows DR mediated extension (p = 4.02488e-28). For lifespan 188, 166, 185 and 168 flies were studied for the group of AL RU-, AL RU + , DR RU- and DR RU+ respectively. ‘RU-‘ and ‘RU + ‘represent vehicles control or RU486 added in food to drive the construct. h, Images of the whole-mount brain with DCFDA staining of control and tauR406W on AL and DR. i, Quantification shows increased (p < 0.0001) ROS stained by DCFDA in tauR406W flies’ brain which is rescued (p < 0.002) by DR. For ROS staining 11, 10, 14 and 12 fly brains were studied for AL RU-, AL RU + DR RU- and DR RU+ groups respectively. An asterisk (*) indicates a significant difference between experimental groups and controls, with the level of significance denoted by the number of asterisks p < 0.05 for *, p < 0.01 for **, p < 0.001 for *** and p < 0.0001 for **** by log-rank test (a, b, d, e and g) or by oneway ANOVA with Tukey’s multiple comparison test (c and i) or by two-tailed Student’s t-test (f). Data in bar graphs are presented as mean ± s.e.m.
Extended Data Fig. 2 Impact of dietary restriction on lifespan and neurodegeneration in tauopathy flies.
a, Western blot using anti tau antibody in both control and tauR406W flies in AL and DR diets (n = 3 biological replicates each with 25 fly head). b, Densiometric analysis of western blot (n = 3 biological replicates each with 25 fly head). c, Table shows the result of Cox Proportional hazard ratio test. The coefficient (coef) is that term’s beta value, with values > 0 meaning that term increases the hazard (risk of death), and values of <0 decreasing the hazard (risk of death). The term exp(coef) is that term’s hazard ratio (HR), with the following breakdown: HR = 1: No effect, HR < 1: Reduction in the hazard, HR > 1: Increase in Hazard. The term se(coef) is the standard error for that term. The z term gives the Wald statistic value. It corresponds to the ratio of each regression coefficient to its standard error (z = coef/se(coef)). The Wald statistics evaluate whether the coefficient is statistically different from 0. The final term, Pr(>|z | ), is that row’s p-value. The rest of the table gives the confidence intervals for each variable. d, Apoptotic cell death detected by TUNEL staining in tauR406W fly whole brain shows increased TUNEL staining in tauR406W (at least 33 fly brain were studied per group). An asterisk (*) indicates a significant difference between experimental groups and controls, with the level of significance denoted by the number of asterisks p < 0.05 for *, p < 0.01 for **, p < 0.001 for *** and p < 0.0001 for **** by oneway ANOVA with Tukey’s multiple comparison test (b). Data in bar graphs are presented as mean ± s.e.m.
Extended Data Fig. 3 Diet dependent changes in glycogen metabolism in flies overexpressing wildtype tau.
a, Venn diagram of the number of proteins upregulated in tauwt (red circle) as well as in control on AL diets (blue circle) (p < 0.0001). Dots represent enriched pathways of overlapping proteins, including fatty acid and glycogen metabolism. b, Venn diagram shows numbers of proteins downregulated in tauwt (red circle) and control on AL diets (blue circle) (p < 0.0001). Dots represent enriched pathways of overlapping proteins, including oxidative phosphorylation and glutathione metabolism. c, Venn diagram shows common upregulated protein between tauwt(red) and tauR406W(blue) (p = 0.001). d, Venn diagram shows common downregulated protein between tauwt(red) and tauR406W(blue) (p < 0.0001). e, Relative mRNA expression of GlyP in tauR406W flies and controls under AL and DR diets shows upregulation of GlyP in tauR406W on DR diets compared to control AL (p = 0.0262) and control DR (p = 0.0199) (n = 6 groups, each with 25 fly heads). f, Relative mRNA expression of AGBE in tauR406W flies and controls under AL and DR diets shows upregulation of AGBE in tauR406W on DR diets compared to control DR (p < 0.0001) or tauR406W AL (p = 0.0001) (n = 6 groups, each with 25 fly heads). g, Relative mRNA expression of GlyS in tauR406W flies and controls under AL and DR diets shows upregulation (*p = 0.111 and **p = 0.0011) of GlyS in tauR406W on DR diet (n = 6 groups each with 25 fly heads). h, Relative mRNA expression of pgm in tauR406W flies and control under AL and DR diets shows upregulation (p < 0.0001) of pgm in tauR406W on AL diet (n = 6 groups each with 25 fly head). i, Relative mRNA expression of AGBE, GlyP and pgm in RNAi flies and GlyP overexpressing flies (***p < 0.0001, **p = 0.0029, ***p < 0.0001 and **p = 0.0066 respectively). The experiments were performed using 3-4 groups each with 25 fly heads. j, Western blot shows tau expression in GlyPS15A; tauR406W, and GlyPWT (n = 25 fly heads for each tissue lysate). k, Densitometric analysis of tau expression is not changed between GlyPS15A; tauR406W and GlyPWT; tauR406W. l, GlyP enzyme activity between GlyPS15A; tauR406W (kinase dead mutant control) control and GlyPWT; tauR406W shows significant (p = 0.0002) increased activity in GlyPWT (data were collected using 320 fly heads in 4 biological replicates and two technical replicates for each group). m, Lifespan of GlyPWT; tauR406W (n = 93 flies) and GlyPS15A; tauR406W (n = 95 flies) shows no significant difference. n, Quantification of glycogen shows significant (p = 0.0058) accumulation of glycogen in the head of elav-Gal4; tauWT in compared to the control elav-Gal4xW1118 (n = 4 groups, 25 fly head in each). o, Western blot shows expression of autophagic marker Atg8(LC3) and Ref(2)P/(p62) in GlyPS15A; tauR406W, and GlyPWT (n = 25 fly heads for each tissue lysate). Samples were derive from the same experiment and that blots were processed in parallel. p, Densiometric analysis of ATG8 expression in GlyPS15A; tauR406W, and GlyPWT (n = 3) shows reduced (p = 0.0108) expression of ATG8. q, Densiometric analysis of Ref(2)P expression in GlyPS15A; tauR406W, and GlyPWT (n = 3). An asterisk (*) indicates a significant difference between experimental groups and controls, with the level of significance denoted by the number of asterisks p < 0.05 for *, p < 0.01 for **, p < 0.001 for *** and p < 0.0001 for **** by Fisher’s exact (a-d) by one-way ANOVA with Tukey’s multiple comparison test(e-h) by two tailed Student’s t-test (i, k, l, n, p and q). Data in bar graphs are presented as mean ± s.e.m.
Extended Data Fig. 4 Metabolomic and transcriptomic changes upon over expression of GlyP in tauopathy fly heads.
a, Pathway analysis of metabolites shows amino acids metabolism, urea cycle, and PPP are the few most common pathways. in GlyPWT; tauR406W compared to GlyPS15A; tauR406W (3 replicates, each with 30 fly heads, were used). b, Quantification of reduced and oxidized glutathione ratio shows significantly (p = 0.0383) increased reduced glutathione in GlyPWT; tauR406W than GlyPS15A; tauR406W (175 flies head for 7 biological replicates were used). c, Volcano plot shows 546 transcripts (red) are upregulated and 473 transcripts (green) are downregulated (3 replicates each with 30 fly heads were used to isolate RNA). d, Pathway analysis of altered RNA shows oxidative phosphorylation, glycolysis or gluconeogenesis, ECM- receptor interaction and TCA cycle are the most common. e, TUNEL staining of GlyPWT; tauR406W with 6-AN treatment shows GlyPWT mediated reversal of apoptotic death of brains abrogate by 6-AN treatment (at least 21 fly brain were analyzed per group). An asterisk (*) indicates a significant difference between experimental groups and controls, with the level of significance denoted by the number of asterisks* for p < 0.05 by two-tailed Student’s t-test (b). Data in bar graphs are presented as mean ± s.e.m.
Extended Data Fig. 5 Neuroprotective effects of 8-Br cAMP in tauopathy flies.
a, Western blot of PKA-C1 and actin of brain tissue lysate of control and tauR406W on AL and DR (n = 3 each tissue lysate with 25 fly heads, experiment was repeated three times independently and observed similar results). b, Normalized densitometric analysis of western blot shows decreased (p = 0.0022) abundance of PKA-C1 of tauR406W on AL. c, Survival curve shows lifespan extension (p = 3.73621e-07) of tauR406W on AL diet with 8-Br-cAMP treatment. For lifespan assay 92 to 97 flies were studied. d, Lifespan of tauR406W on DR diet with 8-Br-cAMP shows no further DR-mediated extension. For lifespan assay 92 to 97 flies were studied. e, TUNEL staining shows cAMP treatment rescues apoptotic cells in tauR406W fly brain (at least 25 fly brain were stained for TUNEL). f, Images of the whole-mount brain with DCFDA staining of control and tauR406W on AL and DR. g, Quantification shows increased (p < 0.0001) ROS stained by DCFDA in tauR406W flies’ brain which is rescued (p < 0.0001) by DR. For ROS staining 23, 24, 25 and 25 fly brains were used for control AL, control DR, tauR406W AL and tauR406W DR. h, GlyP activity in GlyP mutant flies shows reduction compared to wild type (data were collected using 320 fly heads in 4 biological replicates). An asterisk (*) indicates a significant difference between experimental groups and controls, with the level of significance denoted by the number of asterisks p < 0.05 for *, p < 0.01 for **, p < 0.001 for *** and p < 0.0001 for **** by log-rank test (c, d) or by oneway ANOVA with Tukey’s multiple comparison test (b, g), or by two-tailed Student’s t-test (h). Data in bar graphs are presented as mean ± s.e.m.
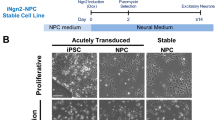
Extended Data Fig. 6 Changes in glycogen metabolism in AD patient brains and iPSC-derived tauopathy neurons.
a, Plot showing correlation between protein abundance of tau (MTBR) and PYGB (n = 980). b, Plot showing correlation between protein abundance of tau (MTBR) and PGM (n = 980) c, Images show glycogen staining in iPSC-derived tauR406W and isogenic control (Iso-tauR406R) neurons by 2-NBDG transduced with GlyP lentivirus. d, Quantification shows glycogen staining increase (p < 0.0001) in control virus-infected tauR406W than isogenic control infected with control lentivirus, and the glycogen staining is reduced (p < 0.0001) in tauR406W cells with PYGB overexpression. e, TOM20 staining of iPSC-generated neurons shows reduced (p < 0.0001) mitochondrial density in tauR406W than isogenic control cells. f, Quantification of TOM20 staining shows reduced (p < 0.0001) mitochondrial density in tauR406W than isogenic control cells. g, TOM20 staining in PYGB overexpressing tauR406W cells show reduced mitochondrial staining in control lentivirus transduction while mitochondrial density is rescued by PYGB containing lentivirus. h, Glycogen staining with 2-NBDG in iPSC-generated tauV337M neurons and its isogenic control. i, Quantification shows increased (p < 0.0001) glycogen staining in tauV337M cells. j, Quantification of glycogen in cell lysate of tauV337M shows significantly increased (p = 0.0008) quantity than isogenic control. k, Quantification of mean fluorescence intensity of glycogenin significantly increases (p = 0.0018) in tauV337M compared to isogenic control. l, Glycogen staining in tauV337M cells and its isogenic control. m, Quantification shows significant increase (p = 0.0007) of glycogen staining in tauV337M neurons while PYGB overexpression reduces (p < 0.0001) glycogen staining in tauV337M cells compared to tauV337M treated with control lentivirus. Each dot represents an image field from n = 3 coverslips per condition for d, f, i, k and m. An asterisk (*) indicates a significant difference between experimental groups and controls, with the level of significance denoted by the number of asterisks p < 0.05 for *, p < 0.01 for **, p < 0.001 for *** and p < 0.0001 for **** by two tailed Student’s t-Test (f, i, j and k) or by two way ANOVA (d and m). Data in bar graphs are presented as mean ± s.e.m.
Extended Data Fig. 7 Tau and glycogen interaction in iPSC-derived tauopathy neurons.
a, c, Co-localization study between tau and glycogenin 1 and across the line intensity plot for tauwt neurons. b, d, Co-localization study between tau and glycogenin 1 and across the line intensity plot for tauR406W neurons. e, Western blot of elute sediment from glycogen co-sedimentation assay with either tauWT or tauP301S using anti tau antibody showed tau sedimented with glycogen f, Western blot of elute sediment from glycogen co-sedimentation assay with BSA showed BSA did not sediment with glycogen. Co-sedimentation assay was repeated 3 times with similar results.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Supplementary Data 1
Proteomic analysis of tauR406W and tauWT on both AL and DR diets, along with a list of significantly altered proteins and GO term analysis.
Supplementary Data 2
Metabolomic analysis of GlyPS15A;tauR406W vs GlyPWT; tauR406W fly heads.
Supplementary Data 3
Transcriptomics analysis of GlyPS15A;tauR406W vs GlyPWT; tauR406W fly heads.
Supplementary Data 4
Human orthologue of common proteins altered in tau fly and people with AD.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Extended Data Fig. 1/Table 1
Statistical source data for Extended Fig. 1.
Source Data Extended Data Fig. 2/Table 2
Statistical source data for Extended Fig. 2.
Source Data Extended Data Fig. 3/Table 3
Statistical source data for Extended Fig. 3.
Source Data Extended Data Fig. 4/Table 4
Statistical source data for Extended Fig. 4.
Source Data Extended Data Fig. 5/Table 5
Statistical source data for Extended Fig. 5.
Source Data Extended Data Fig. 6/Table 6
Statistical source data for Extended Fig. 6.
Source Data Extended Data Fig. 7/Table 7
Statistical source data for Extended Fig. 7.
Source Data Extended Data Figs. 2, 3, 5 and 7
Uncropped western blots of all western blot figure.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bar, S., Wilson, K.A., Hilsabeck, T.A.U. et al. Neuronal glycogen breakdown mitigates tauopathy via pentose-phosphate-pathway-mediated oxidative stress reduction. Nat Metab 7, 1375–1391 (2025). https://doi.org/10.1038/s42255-025-01314-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01314-w