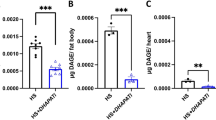
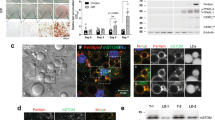
Abstract
Genetic and dietary cues are known drivers of obesity, yet how they converge at the molecular level is incompletely understood. Here we show that PPARγ supports hypertrophic expansion of adipose tissue via transcriptional control of LPCAT3, an endoplasmic reticulum (ER)-resident O-acyltransferase that selectively enriches diet-derived omega-6 polyunsaturated fatty acids (n-6 PUFAs) in the membrane lipidome. In mice fed a high-fat diet, lowering membrane n-6 PUFA levels through genetic or dietary interventions results in aberrant adipose triglyceride (TG) turnover, ectopic fat deposition and insulin resistance. Additionally, we detail a non-canonical adaptive response in ‘lipodystrophic’ Lpcat3–/– adipose tissues that engages a futile lipid cycle to increase metabolic rate and offset lipid overflow to ectopic sites. Live-cell imaging, lipidomics and molecular dynamics simulations reveal that adipocyte LPCAT3 activity enriches n-6 arachidonate in the phosphatidylethanolamine (PE)-dense ER–lipid droplet interface. Functionally, this localized PE remodelling optimizes TG storage by driving the formation of large droplets that exhibit greater resistance to adipose TG lipase activity. These findings highlight the PPARγ–LPCAT3 axis as a mechanistic link between dietary n-6 PUFA intake, adipose expandability and systemic energy balance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data for all figures are provided with the paper. RNA-seq and sNuc-seq datasets generated for this paper are available in the NCBI Gene Expression Omnibus repository under accession code GSE218943. Reads were aligned to the mm10 (GRCm38) mouse reference genome. All source data and input files for MD simulations can be found at Zenodo94. All unique biological materials used are readily available from the authors. Source data are provided with this paper.
References
Sakers, A., De Siqueira, M. K., Seale, P. & Villanueva, C. J. Adipose-tissue plasticity in health and disease. Cell 185, 419–446 (2022).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
Kim, S. M. et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 20, 1049–1058 (2014).
Spalding, K. L. et al. Dynamics of fat cell turnover in humans. Nature 453, 783–787 (2008).
Kubota, N. et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609 (1999).
Medina-Gomez, G. et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 3, e64 (2007).
Lim, K., Haider, A., Adams, C., Sleigh, A. & Savage, D. B. Lipodistrophy: a paradigm for understanding the consequences of ‘overloading’ adipose tissue. Physiol. Rev. 101, 907–993 (2021).
Garg, A. Acquired and inherited lipodystrophies. N. Engl. J. Med. 350, 1220–1234 (2004).
Slawik, M. & Vidal-Puig, A. J. Adipose tissue expandability and the metabolic syndrome. Genes Nutr. 2, 41–45 (2007).
Bluher, M. Metabolically healthy obesity. Endocr. Rev. https://doi.org/10.1210/endrev/bnaa004 (2020).
Lotta, L. A. et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 49, 17–26 (2017).
Suzuki, K. et al. Genetic drivers of heterogeneity in type 2 diabetes pathophysiology. Nature 627, 347–357 (2024).
Hall, K. D. et al. The energy balance model of obesity: beyond calories in, calories out. Am. J. Clin. Nutr. 115, 1243–1254 (2022).
Ludwig, D. S. et al. The carbohydrate–insulin model: a physiological perspective on the obesity pandemic. Am. J. Clin. Nutr. https://doi.org/10.1093/ajcn/nqab270 (2021).
Lands, B. A critique of paradoxes in current advice on dietary lipids. Prog. Lipid Res. 47, 77–106 (2008).
Ludwig, D. S., Willett, W. C., Volek, J. S. & Neuhouser, M. L. Dietary fat: From foe to friend? Science 362, 764–770 (2018).
Savage, D. B. Mouse models of inherited lipodystrophy. Dis. Model. Mech. 2, 554–562 (2009).
Speakman, J. R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. (Lond.) 43, 1491–1492 (2019).
Blasbalg, T. L., Hibbeln, J. R., Ramsden, C. E., Majchrzak, S. F. & Rawlings, R. R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 93, 950–962 (2011).
Ailhaud, G. et al. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 45, 203–236 (2006).
Abe, I. et al. Lipolysis-derived linoleic acid drives beige fat progenitor cell proliferation. Dev. Cell 57, 2623–2637.e8 (2022).
Hilgendorf, K. I. et al. Omega-3 fatty acids activate ciliary FFAR4 to control adipogenesis. Cell 179, 1289–1305.e21 (2019).
Inazumi, T. et al. Prostaglandin E2–EP4 axis promotes lipolysis and fibrosis in adipose tissue leading to ectopic fat deposition and insulin resistance. Cell Rep. 33, 108265 (2020).
Oh, D. Y. et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010).
Forman, B. M. et al. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83, 803–812 (1995).
Pietilainen, K. H. et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol. 9, e1000623 (2011).
Grzybek, M. et al. Comprehensive and quantitative analysis of white and brown adipose tissue by shotgun lipidomics. Mol. Metab. 22, 12–20 (2019).
Hishikawa, D. et al. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl Acad. Sci. USA 105, 2830–2835 (2008).
Hashidate-Yoshida, T. et al. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife https://doi.org/10.7554/eLife.06328 (2015).
Rong, X. et al. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife https://doi.org/10.7554/eLife.06557 (2015).
Demeure, O. et al. Regulation of LPCAT3 by LXR. Gene 470, 7–11 (2011).
He, M. et al. Inhibiting phosphatidylcholine remodeling in adipose tissue increases insulin sensitivity. Diabetes 72, 1547–1559 (2023).
Sarvari, A. K. et al. Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 33, 437–453.e5 (2021).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Sun, K., Kusminski, C. M. & Scherer, P. E. Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 (2011).
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017).
Rubio-Cabezas, O. et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol. Med. 1, 280–287 (2009).
Reed, A. et al. LPCAT3 inhibitors remodel the polyunsaturated phospholipid content of human cells and protect from ferroptosis. ACS Chem. Biol. 17, 1607–1618 (2022).
Ben M’barek, K. et al. ER membrane phospholipids and surface tension control cellular lipid droplet formation. Dev. Cell 41, 591–604.e7 (2017).
Choudhary, V. et al. Architecture of lipid droplets in endoplasmic reticulum is determined by phospholipid intrinsic curvature. Curr. Biol. 28, 915–926.e9 (2018).
Gao, M., Huang, X., Song, B. L. & Yang, H. The biogenesis of lipid droplets: lipids take center stage. Prog. Lipid Res. 75, 100989 (2019).
Bacle, A., Gautier, R., Jackson, C. L., Fuchs, P. F. J. & Vanni, S. Interdigitation between triglycerides and lipids modulates surface properties of lipid droplets. Biophys. J. 112, 1417–1430 (2017).
Prevost, C. et al. Mechanism and determinants of amphipathic helix-containing protein targeting to lipid droplets. Dev. Cell 44, 73–86.e4 (2018).
Caillon, L. et al. Triacylglycerols sequester monotopic membrane proteins to lipid droplets. Nat. Commun. 11, 3944 (2020).
Lands, W. E. Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 231, 883–888 (1958).
Farvid, M. S. et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation 130, 1568–1578 (2014).
Wu, J. H. Y. et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 5, 965–974 (2017).
DeLong, C. J., Shen, Y. J., Thomas, M. J. & Cui, Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 274, 29683–29688 (1999).
Horl, G. et al. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J. Biol. Chem. 286, 17338–17350 (2011).
Thiam, A. R., Farese, R. V. Jr. & Walther, T. C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 14, 775–786 (2013).
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019).
Zadoorian, A., Du, X. & Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 19, 443–459 (2023).
Harayama, T. & Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 19, 281–296 (2018).
Vanni, S., Hirose, H., Barelli, H., Antonny, B. & Gautier, R. A sub-nanometre view of how membrane curvature and composition modulate lipid packing and protein recruitment. Nat. Commun. 5, 4916 (2014).
Shindou, H. et al. Docosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cells. J. Biol. Chem. 292, 12054–12064 (2017).
Iizuka-Hishikawa, Y. et al. Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J. Biol. Chem. 292, 12065–12076 (2017).
Morigny, P., Houssier, M., Mouisel, E. & Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie 125, 259–266 (2016).
Zimmermann, R. et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004).
Kershaw, E. E. et al. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 55, 148–157 (2006).
Tontonoz, P. & Spiegelman, B. M. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77, 289–312 (2008).
Guan, H. P. et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med. 8, 1122–1128 (2002).
Gandotra, S. et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 364, 740–748 (2011).
Chao, L. et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest. 106, 1221–1228 (2000).
Kim, J. K. et al. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes 52, 1311–1318 (2003).
Liu, L. et al. Adipose-specific knockout of Seipin/Bscl2 results in progressive lipodystrophy. Diabetes 63, 2320–2331 (2014).
Qian, K. et al. CLSTN3β enforces adipocyte multilocularity to facilitate lipid utilization. Nature 613, 160–168 (2023).
Senault, C., Hlusko, M. T. & Portet, R. Effects of diet and cold acclimation on lipid composition of rat interscapular brown adipose tissue. Ann. Nutr. Aliment. 29, 67–77 (1975).
Reitman, M. L. Metabolic lessons from genetically lean mice. Annu. Rev. Nutr. 22, 459–482 (2002).
Deol, P. et al. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PLoS ONE 10, e0132672 (2015).
Gong, J. et al. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195, 953–963 (2011).
Nishino, N. et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118, 2808–2821 (2008).
Zhou, L. et al. Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nat. Commun. 6, 5949 (2015).
Mina, A. I. et al. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab. 28, 656–666.e1 (2018).
Huynh, F. K., Green, M. F., Koves, T. R. & Hirschey, M. D. Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol. 542, 391–405 (2014).
Herman, M. A. et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484, 333–338 (2012).
Cui, L., Mirza, A. H., Zhang, S., Liang, B. & Liu, P. Lipid droplets and mitochondria are anchored during brown adipocyte differentiation. Protein Cell 10, 921–926 (2019).
Hsieh, W. Y., Williams, K. J., Su, B. & Bensinger, S. J. Profiling of mouse macrophage lipidome using direct infusion shotgun mass spectrometry. STAR Protoc. 2, 100235 (2021).
Su, B. et al. A DMS shotgun lipidomics workflow application to facilitate high-throughput, comprehensive lipidomics. J. Am. Soc. Mass. Spectrom. 32, 2655–2663 (2021).
Patel, R. et al. ATGL is a biosynthetic enzyme for fatty acid esters of hydroxy fatty acids. Nature 606, 968–975 (2022).
Gruber, A. et al. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J. Biol. Chem. 285, 12289–12298 (2010).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Jo, S., Lim, J. B., Klauda, J. B. & Im, W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 97, 50–58 (2009).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010).
Campomanes, P., Prabhu, J., Zoni, V. & Vanni, S. Recharging your fats: CHARMM36 parameters for neutral lipids triacylglycerol and diacylglycerol. Biophys. Rep. (N Y) https://doi.org/10.1016/j.bpr.2021.100034 (2021).
Gautier, R. et al. PackMem: a versatile tool to compute and visualize interfacial packing defects in lipid bilayers. Biophys. J. 115, 436–444 (2018).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Shinoda, W., DeVane, R. & Klein, M. L. Zwitterionic lipid assemblies: molecular dynamics studies of monolayers, bilayers, and vesicles using a new coarse grain force field. J. Phys. Chem. B 114, 6836–6849 (2010).
Jimenez-Rojo, N. et al. Conserved functions of ether lipids and sphingolipids in the early secretory pathway. Curr. Biol. 30, 3775–3787.e7 (2020).
Tschöp, M. H. et al. A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 (2011).
Hu, S. et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell. Metab. 28, 415–431.e4 (2018).
Tol, M. J. et al. Dietary control of peripheral adipose storage capacity through membrane lipid remodelling. Zenodo https://doi.org/10.5281/zenodo.15241070 (2025).
Laakso, M. et al. The Metabolic Syndrome in Men study: a resource for studies of metabolic and cardiovascular diseases. J. Lipid Res. 58, 481–493 (2017).
Acknowledgements
This article is dedicated to the memory of T. C. P. M. Kemper, a beloved friend and enduring source of inspiration. We are grateful to J. Sandhu, C. Priest and B. Clifford for technical assistance. We thank all current and former members of the Tontonoz and Tarling–Vallim labs for valuable discussions and for sharing reagents. PET–CT was performed at the Crump Institute Preclinical Imaging Technology Center, with the assistance of S. Xu and M. Tamboline. Oxylipin analysis was performed at the RIKEN Center for Integrative Medical Sciences with the assistance of M. Honda. TMT labelling was performed at the Pasarow Mass Spectrometry laboratory with the assistance of W. Cohn and J. P. Whitelegge. RNA-seq and sNuc-seq were performed at the Technology Center for Genomics & Bioinformatics. This work was supported by a postdoctoral fellowship from the American Diabetes Association (1-19-PDF-039 to M.J.T.); the Japan Society for the Promotion of Science abroad and the Osamu Hayaishi Memorial Scholarship for Study Abroad (to Y.S.); the National Institutes of Health (R01DK129276 to P.T.; HL139725 to S.G.Y.); the Leducq Foundation (19CVD04 to P.T. and S.G.Y.); the Swiss National Science Foundation (grants 310030_219264 to S.V.); the European Research Council under European Union’s Horizon 2020 Research and Innovation Programme (grant agreement no. 803952, to S.V.) and grants of the Swiss National Supercomputing Centre (CSCS) under project ID s1131 and s11876.
Author information
Authors and Affiliations
Contributions
M.J.T., Y.S., S.V. and P.T. conceived the project. M.J.T., Y.S., A.H.B., J.S., L.C., M.C.-G., P.H., K.J.W., M.A., D.A.F. and S.V. were responsible for the methodology. A.H.B. and J.S. contributed equally to the computational analyses. K.J.W., B.S., D.P.P. and D.A.F. performed lipidomic analyses. M.J.T., Y.S., A.H.B., J.S., L.C., K.J.W., M.A., D.P.P., D.A.F. and S.V. conducted formal analyses. M.J.T., Y.S., A.H.B., J.S., L.C., A.F., K.Q., J.P.K., S.D.L., Y.G., X.X., J.G., J.J.M., T.A.W., K.J.W., D.P.P. and M.A. performed the investigation. M.C.-G., P.H., C.P., A.J.L., K.J.W., B.S., A.R., N.M., M.A., B.F.C., S.G.Y., D.A.F., R.Z., S.V. and P.T. provided resources. M.J.T., Y.S., A.H.B., J.S., K.J.W., M.A., S.V. and P.T. curated the data. M.J.T., Y.S. and P.T. wrote the manuscript. S.V. and P.T. supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Revati Dewal, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Lpcat3 is an evolutionarily conserved PPARγ target gene.
(a) qPCR analysis of Lpcat1-4 mRNA levels in eWAT and iWAT of 12-week-old NCD-fed wild-type mice (n = 5, 5), and (b) in iWAT from wild-type mice fed low-fat diet (LFD) with or without rosiglitazone (Rosi; 50 mg kg–1) for 14 days (n = 7, 7). (c) Immunoblot analysis of LPCAT3 protein levels in iWAT lysates from (b). Tubulin served as a sample processing control. (d) Immunoblot analysis of LPCAT3 protein levels and known adipogenic markers during the course of 10T1/2 adipocyte differentiation. Calnexin served as a loading control for LPCAT3. (e) Sequence alignment of conserved PPAR and LXR response elements in the Lpcat3 promoter across multiple species. The PAM sequence of sgRNAs targeting Lpcat3-PPRE and LXRE are underlined in red. (f) ChIP-qPCR analysis of PPARγ (or normal rabbit IgG) occupancy at Lpcat3, Plin1, Fabp4, Agpat2, or Aqp7 PPRE elements in wild-type and ΔPPRE 10T1/2 adipocytes (n = 3/group; 100–mm plate per replicate from 3 independent experiments). A region of the 36b4 promoter served as a negative control. (g) PCR analysis of a 700-bp genomic segment flanking the PPRE/LXRE sites in the Lpcat3 promoter region of WT, ΔPPRE, and ΔLXRE clonally-derived cell lines, revealing no major deletions introduced by CRISPR/Cas9 gene editing. Primer anneal sites in the Lpcat3 promoter are shown in orange, indels in green (insertions) or red (deletions). (h) Immunoblot analysis of LPCAT3 protein levels in WT, ΔPPRE, and ΔLXRE 10T1/2 adipocytes differentiated for 4 days. Calnexin served as a loading control for LPCAT3. Data are presented as mean ± SEM. ***P < 0.001 by Welch’s t-tests with FDR correction using the Benjamini, Krieger, and Yekutieli procedure (b); or one-way ANOVA with Tukey’s multiple comparisons test (f).
Extended Data Fig. 2 Lpcat3AKO mice display no metabolic phenotype under standard laboratory conditions.
(a) Graphical illustration of in vivo Lpcat3AKO strategy. (b) qPCR analysis of Lpcat3 mRNA levels in fat depots and insulin target-tissues of 12-week-old NCD-fed control and Lpcat3AKO mice (n = 4, 4). (c) Lipidomic analysis of PC/PE or TG-FA species in the inguinal adipocyte fraction isolated from of 22-week-old NCD-fed control and Lpcat3AKO mice (n = 5, 5). (d) Body weight (BW) gain curves and composition of 22-week-old NCD-fed control and Lpcat3AKO mice (n = 30, 34). (e) Wet weights of the indicated tissues in NCD-fed control and Lpcat3AKO mice (n = 23, 25). (f) H&E stainings of iBAT and iWAT depots (scale bar, 100 µm). (g) qPCR analysis of iWAT of NCD-fed control and Lpcat3AKO mice (n = 7, 7). (h) 14C-oleate uptake in insulin-target tissues of 18-week-old NCD-fed control and Lpcat3AKO mice (n = 5, 7). Radioactivity (counts per min, CPM) was calculated in the extracted lipids from whole-organs 4 h post-gavage with 14C-Triolein and normalized for g/tissue. (i) Ex vivo lipolysis in dissected iWAT explants from 18-week-old NCD-fed control and Lpcat3AKO mice (n = 4, 4). Secreted glycerol was measured under basal and stimulated (2 µM isoproterenol, ISO) conditions. (j) Ex vivo β-oxidation in crude lysates prepared from iBAT, iWAT, and livers of 18-week-old NCD-fed control and Lpcat3AKO mice (n = 5, 5), assessed by conversion of 14C-palmitic acid to 14CO2. (k) Plasma TGs and cholesterol in 22-week-old NCD-fed control and Lpcat3AKO mice (n = 13, 7). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Welch’s t-tests with Holm-Sidak’s correction on CLR-transformed values of each lipid class (c); or Welch’s t-tests with FDR correction using the Benjamini, Krieger, and Yekutieli procedure (b,g).
Extended Data Fig. 3 Lpcat3AKO does not affect adipocyte and whole-body glucose homeostasis.
(a) Immunoblot analysis of phospho-Akt/PKB and rpS6 in iWAT of 12-week-old NCD-fed control and Lpcat3AKO mice after i.p. injection with insulin (1 U kg–1). Total Akt/PKB and rpS6 served as sample processing controls. (b) Coronal, sagittal, and transverse views of 12-week-old NCD-fed control and Lpcat3AKO mice by positron emission tomography–computed tomography (PET–CT) imaging 1 h post-injection with 18F-FDG and insulin (1 U kg–1). (c) 18F-FDG uptake (× 106 pCi g–1 tissue) in the indicated insulin-target tissues (n = 4, 4). (d) glucose tolerance tests (GTT; 1.5 g kg–1) and (e) insulin tolerance tests (ITT; 0.75 U kg–1) were performed on 18-week-old NCD-fed control and Lpcat3AKO mice (n = 10, 9). (f) Respiratory exchange ratios (RER) of 18-week-old NCD-fed control and Lpcat3AKO mice were monitored over a period of 48 h in Oxymax metabolic cages (n = 16, 15). Data are presented as mean ± SEM.
Extended Data Fig. 4 Metabolic consequences of membrane n-6 PUFA deficiency in WAT during DIO.
(a) GS–MS analysis of dietary fatty acid profiles and presented as n-6/n-3 PUFA ratio, FA species (% of total), or FA class (sfa:mufa:pufa; n = 3, 3, 3). (b) Body weight (BW) gain curves of 18-week-old control and Lpcat3AKO mice fed either HFD (n = 42, 40), HFDsfa (n = 39, 38), or HFDn-3 (n = 40, 37) for 10 weeks. (c) Average daily food intake of singly-housed control and Lpcat3AKO mice fed the indicated HFDs, recorded during the last 5 weeks of HFD-feeding (n = 5–6/group). (d) EchoMRI analysis of lean mass in control and Lpcat3AKO mice fed HFD (n = 42, 40), HFDsfa (n = 39, 38), or HFDn-3 (n = 40, 37) for 10 weeks. (e) PUFA-derived oxylipin species were analysed in the inguinal adipocyte fraction from control and Lpcat3AKO mice fed the indicated HFDs for 10 weeks (n = 4/group). (f-i) Plasma (f) TG, (g) cholesterol, (h) ALT, and (i) AST levels in control and Lpcat3AKO mice fed the indicated HFDs for 10 weeks (n = 10–13/group). (j) VLDL secretion in control and Lpcat3AKO mice fed HFD (n = 5, 5), HFDsfa (n = 6, 6), or HFDn-3 (n = 6, 7) for 6 weeks. Animals were fasted for 12 h before injection with the LPL inhibitor poloxamer-407 (1 g kg–1; i.p.). Plasma TGs were measured in blood collected retro-orbitally at the indicated times post-injection. (k) Ex vivo hepatic β-oxidation rates in control and Lpcat3AKO mice fed the indicated HFDs for 6 weeks, as assessed by the conversion of 14C-palmitic acid (PA) to 14CO2 (n = 3–4/group). Data are presented as mean ± SEM. *P < 0.05, ***P < 0.001 by two-way RM ANOVA with Sidak’s multiple comparisons test (b); ***P < 0.001 vs. the HFD-fed control group; #P < 0.05, vs. HFDsfa; same genotype, by two-way ANOVA with Tukey’s multiple comparisons test (d); or **P < 0.01, ***P < 0.001 by Welch’s t-tests with FDR correction using the Benjamini, Krieger, and Yekutieli procedure (f,h,i).
Extended Data Fig. 5 Lipolytic dysregulation in Lpcat3AKO mice during DIO.
(a) 14C-oleate uptake in insulin-target tissues from 13-week-old control and Lpcat3AKO mice fed HFD for 5 weeks (n = 7, 8). Radioactivity (counts per min, CPM) was calculated in the extracted lipids from whole-organs 4 h post-gavage with 14C-Triolein and normalized for g/tissue. (b) Ex vivo β-oxidation in crude lysates prepared from iWAT, iBAT, or liver lysates of 13-week-old control and Lpcat3AKO mice fed HFD for 5 weeks, as assessed by the conversion of 14C-palmitic acid (PA) to 14CO2 (n = 4, 4). (c) Ex vivo lipolysis in freshly dissected iWAT explants from 13-week-old control and Lpcat3AKO mice fed HFD for 5 weeks (n = 3, 3). Secreted glycerol was determined under basal and stimulated (2 µM isoproterenol, ISO) lipolytic states. (d) 14C-oleate uptake in insulin-target tissues from 18-week-old control and Lpcat3AKO mice fed HFD for 10 weeks (n = 9, 8). (e,f) 14C-oleate uptake and turnover in (e) iBAT and (f) livers from 18-week-old control and Lpcat3AKO mice fed HFD for 10 weeks. Radioactivity (counts per min, CPM) was calculated in extracted lipids from whole-organs 4 h (n = 9, 8); 4 days (n = 6, 6); and 20 days (n = 7, 7) post-gavage with 14C-Triolein and normalized for g/tissue. (g) UMAP projection of 28,414 sequenced iWAT nuclei or split by genotype (13,466 and 14,948 nuclei for 18-week-old control vs. Lpcat3AKO mice fed HFD for 10 weeks). (h) Violin plots (clusters as columns, genes as rows) of cluster-specific markers. (i) Heatmap of normalized expression values of pan-adipogenic markers in inguinal adipocytes and the SVC fraction. Alternatively activated macrophages (AAMs), lipid-scavenging macrophages (LSMs), adipose stem and progenitor cells (ASPCs). Data are presented as mean ± SEM. ***P < 0.001 by Welch’s t-tests with FDR correction using the Benjamini, Krieger, and Yekutieli procedure (b-d).
Extended Data Fig. 6 The Lpcat3AKO iWAT molecular signature is enriched for lipid cycling.
(a) Gene ontology (GO) terms and circos plots of upregulated genes in iWAT of 18-week-old control and Lpcat3AKO mice fed HFD for 10 weeks. GO terms were determined on the top 250 Nnat or Kcnc2 co-expressing iWAT gene transcripts. (b) Violin plots of representative markers for UCP1-dependent and -independent futile cycles.
Extended Data Fig. 7 The non-canonical adaptive Lpcat3AKO response to DIO is WAT-selective.
(a-c) qPCR analysis of pan-adipogenic, DNL, lipid cycling, thermogenic, and ER stress-associated gene expression in (a) iWAT, (b) eWAT, and (c) iBAT of 18-week-old control and Lpcat3AKO mice fed HFD for 10 weeks (n = 6, 6; ND, not detected). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Welch’s t-tests with FDR correction using the Benjamini, Krieger, and Yekutieli procedure (a-c).
Extended Data Fig. 8 Dietary n-6 PUFA restriction or Cidec–/– mirrors Lpcat3AKO adaptation to DIO.
(a) Heatmap of the top 70 differentially expressed genes (35 up or down) in iWAT of 18-week-old control and Lpcat3AKO mice fed the indicated HFDs for 10 weeks (n = 4/group). (b,c) GSEA analyses of the top 500 upregulated genes in (b) iWAT of HFDn-3 or HFDsfa-fed control mice vs. HFD-fed controls or (c) in eWAT of ob/ob Cidec–/– mice vs. ob/ob controls75. The ranked list metric is the product of log2 (fold-change)* –log10 (adjusted P value) from the differentially expressed genes in iWAT from HFD-fed Lpcat3AKO mice vs. controls (NES). (d) Correlation matrix depicting the association between Lpcat3AKO markers and clinical traits in METSIM95. Node colour and size reflect the correlation directionality and p value. Correlations were analysed by midweight bicorrelation coefficient or corrected p value using the R package WGCNA. (e) Body weight (BW) gain curves and (f) composition of 28-week-old control and Lpcat3AKO female mice fed HFD for 20 weeks (n = 14, 13). (g) Wet weight of the indicated tissues in HFD-fed control and Lpcat3AKO female mice (n = 14, 13). (h) Liver wet weights (% of BW; n = 14, 13). Data are presented as mean ± SEM. ***P < 0.001 by two-way RM ANOVA with Sidak’s multiple comparisons test (e); Welch’s t-tests with FDR correction using the Benjamini, Krieger, and Yekutieli procedure (f,g); or Welch’s t-test (h).
Extended Data Fig. 9 Characterization of Lpcat3-null 10T1/2 adipocytes.
(a) TIDE analysis (left) and Western blot analysis of LPCAT3 protein levels (right) validating Lpcat3-KO in 10T1/2 clonal cell lines transfected with sgRNAs targeting either exon 1 (L3_E1) or exon 3 (L3_E3). Calnexin served as a loading control (b) qPCR analysis of adipogenic markers in Lpcat3KO adipocytes expressing LPCAT3WT or LPCAT3H374A and differentiated for 6 days (n = 4, 4). (c) Shotgun lipidomic analysis of a wild-type 10T1/2 clonal cell line differentiated in the presence of 0.1% DMSO (veh ctrl) or the LPCAT3 inhibitor (R)-HTS-3 (10 µM) for 5 days. (d) Shotgun lipidomic analysis, (e) TG content (n = 3 wells/group), and (f) Oil red-O staining of Lpcat3KO adipocytes reconstituted with GFP Ctrl or the indicated GFP-tagged LPCAT3 fusion proteins (day 7 of differentiation). Data are presented as mean ± SEM. ***P < 0.001 by Welch’s t-tests with FDR correction using the Benjamini, Krieger, and Yekutieli procedure (b); or one-way ANOVA with Tukey’s multiple comparisons test (e).
Extended Data Fig. 10 LPCAT3 activity does not affect gross membrane lipid-packing indices.
(a) Shotgun lipidomic analysis of PC composition in ER or LD–ER-enriched fractions from Lpcat3KO adipocytes expressing Flag-LPCAT3WT or Flag-LPCAT3H374A and differentiated for 6 days. Data are presented as the distribution of PC species (% of total PC) within each sample. (b) Schematic depiction of the trilayer model system containing a bulk oil phase flanked by phospholipid monolayers, solvated with water (left), and top/side views of trilayers exhibiting LPDs that expose the oil core to the solvent (right). (c) Deep/shallow LPDs in model bilayers and LD-like trilayer systems (± 3–5% surface tension, ST). Each replicate corresponds to a block-averaged LPD value derived from individual MD simulation trajectories (n = 3/group). WT and Lpcat3KO compositions reflect complex (left) and simplified (right) lipidomic approximations (Supplementary Table 6). (d,e) Immunoblot analysis of Lpcat3KO adipocytes expressing Flag-LPCAT3WT or Flag-LPCAT3H374A and treated with (d) ± isoproterenol (ISO, 10 µM), or (e) ± insulin (100 nM) for 15 min. Actin and Calnexin served as sample processing controls. (f) Tandem mass tag (TMT) labelling on 8,000g LDs isolated from Lpcat3KO adipocytes expressing Flag-LPCAT3WT or Flag-LPCAT3H374A (day 6 of differentiation). TMT data were overlaid with Uniprot mouse proteome for annotated LD localization. (g) Top/side snapshots from CG–MD simulations of an ER–LD neck system. Bilayer/monolayers consist of 50% PUFA (cyan) and 50% MUFA (white) phospholipids, with a triolein (TO; orange) core. (h) Electron microscopy analysis of Lpcat3KO adipocytes expressing Flag-LPCAT3WT or Flag-LPCAT3H374A (day 3 of differentiation). (i) Lipolysis in Lpcat3KO and Lpcat3/AtglDKO adipocytes expressing Flag-LPCAT3WT or Flag-LPCAT3H374A. Secreted glycerol was measured after ISO (10 µM) treatment for 2 h, normalized to intracellular TG stores (n = 3 wells/group). Where indicated, cells were differentiated in the presence of 0.1% DMSO (veh ctrl) or Atglistatin (ATGLi; 40 µM) for 6 days. Data are presented as mean ± SEM. Protein abundance ratios were compared across groups using Student’s t-test (P < 0.01) while controlling for false-discovery rate with Benjamini-Hochberg procedure (f); or ***P < 0.001 by two-way ANOVA with Tukey’s multiple comparisons test (i).
Supplementary information
Supplementary Information
Supplementary Data Fig. 1.
Supplementary Tables 1–8
Supplementary Tables 1–8.
Source data
Source Data Figs. 1 and 5–7
Figs. 1 and 5–7 uncropped images.
Source Data Figs. 1–3 and 5–7
Graph data for Figs. 1–3 and 5–7.
Source Data Extended Data Figs. 1, 3, 9 and 10
Source Data Extended Data Figs. 1, 3, 9 and 10 uncropped images.
Source Data Extended Data Figs. 1–5 and 7–10
Graph data for Extended Figs. 1–5 and 7–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tol, M.J., Shimanaka, Y., Bedard, A.H. et al. Dietary control of peripheral adipose storage capacity through membrane lipid remodelling. Nat Metab 7, 1424–1442 (2025). https://doi.org/10.1038/s42255-025-01320-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01320-y