Abstract
Immune cells undergo rapid metabolic reprogramming to fuel effector responses. However, whether the metabolic pathways that supply these functions differ between human and mouse immune cells is poorly understood. Using a comparative metabolomics approach, here we show both conserved and species-distinct metabolite alterations in cytokine-activated primary human and mouse natural killer (NK) cells. Activated human NK cells fail to perform de novo serine synthesis, resulting in broadly impaired effector functions when serine starved ex vivo or during in vivo dietary serine restriction, limiting their antitumour function. In contrast, activated mouse NK cells perform de novo serine synthesis to fuel one-carbon metabolism and proliferation, resulting in increased metabolic flexibility during ex vivo and dietary serine restriction. While NK cells from both species require one-carbon metabolism to proliferate and produce interferon-γ, GCLC-dependent glutathione synthesis tunes cytotoxic versus inflammatory function in human NK cells. Thus, activated NK cell functions display species-specific requirements for serine metabolism, and environmental serine availability dictates activated human NK cell functions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Metabolomics data from naive and IL-2/IL-15-stimulated NK cells are publicly available from the Metabolomics Workbench (ST004027). Publicly available RNA-seq data of cytokine-stimulated mouse and human NK cells were accessed from the Gene Expression Omnibus at GSE140035. All other data are available in the main text and supplementary materials or by request from the authors. Source data are provided with this paper.
References
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Shay, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl Acad. Sci. USA 110, 2946–2951 (2013).
Kowalski, G. M. & Bruce, C. R. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am. J. Physiol. Endocrinol. Metab. 307, E859–E871 (2014).
Perlman, R. L. Mouse models of human disease: an evolutionary perspective. Evol. Med. Public Health https://doi.org/10.1093/emph/eow014 (2016).
Artyomov, M. N. & Van den Bossche, J. Immunometabolism in the single-cell era. Cell Metab. 32, 710–725 (2020).
O’Neill, L. A. J., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Vijayan, V. et al. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide: a divergent role for glycolysis. Redox Biol. https://doi.org/10.1016/j.redox.2019.101147 (2019).
Lanier, L. L. Evolutionary struggles between NK cells and viruses. Nat. Rev. Immunol. 8, 259–268 (2008).
Li, J. H. & O’Sullivan, T. E. Back to the future: spatiotemporal determinants of NK cell antitumor function. Front. Immunol. https://doi.org/10.3389/fimmu.2021.816658 (2022).
Riggan, L., Shah, S. & O’Sullivan, T. E. Arrested development: suppression of NK cell function in the tumor microenvironment. Clin. Transl. Immunol. 10, e1238 (2021).
Keppel, M. P., Saucier, N., Mah, A. Y., Vogel, T. P. & Cooper, M. A. Activation-specific metabolic requirements for NK cell IFN-γ production. J. Immunol. 194, 1954–1962 (2015).
Mah, A. Y. et al. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight https://doi.org/10.1172/jci.insight.95128 (2017).
Marcais, A. et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 15, 749–757 (2014).
Assmann, N. et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 18, 1197–1206 (2017).
Donnelly, R. P. et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J. Immunol. 193, 4477–4484 (2014).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018).
Li, J. H. et al. MEF2C regulates NK cell effector functions through control of lipid metabolism. Nat. Immunol. https://doi.org/10.1038/s41590-024-01811-2 (2024).
Keating, S. E. et al. Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J. Immunol. 196, 2552–2560 (2016).
Wiedemann, G. M. et al. Deconvoluting global cytokine signaling networks in natural killer cells. Nat. Immunol. 22, 627–638 (2021).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017).
Ma, E. H. et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357 (2017).
Sheppard, S. et al. Fatty acid oxidation fuels natural killer cell responses against infection and cancer. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2319254121 (2024).
Pelletier, A. et al. Resting natural killer cell homeostasis relies on tryptophan/NAD+ metabolism and HIF‐1α. EMBO Rep. https://doi.org/10.15252/embr.202256156 (2023).
Duvel, K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 (2010).
Jensen, T. I. et al. Targeted regulation of transcription in primary cells using CRISPRa and CRISPRi. Genome Res. 31, 2120–2130 (2021).
Zheng, X. et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat. Immunol. 24, 802–813 (2023).
Gao, X. et al. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep. 22, 3507–3520 (2018).
Mah, A. Y. & Cooper, M. A. Metabolic regulation of natural killer cell IFN-γ production. Crit. Rev. Immunol. 36, 131–147 (2016).
Argüello, R. J. et al. SCENITH: a flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 32, 1063–1075.e7 (2020).
Wu, G., Lupton, J. R., Turner, N. D., Fang, Y.-Z. & Yang, S. Glutathione metabolism and its implications for health. J. Nutr. 134, 489–492 (2004).
Mailloux, R. J., McBride, S. L. & Harper, M.-E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 38, 592–602 (2013).
Sbodio, J. I., Snyder, S. H. & Paul, B. D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 176, 583–593 (2018).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376 (2017).
Maddocks, O. D. K. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2012).
Tajan, M. et al. Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy. Nat. Commun. 12, 366 (2021).
Ma, S., Caligiuri, M. A. & Yu, J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. 43, 833–847 (2022).
Laskowski, T. J., Biederstädt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22, 557–575 (2022).
Romee, R. et al. Cytokine activation induces human memory-like NK cells. Blood https://doi.org/10.1182/blood-2012-04-419283 (2012).
Marin, N. D. et al. Memory-like differentiation enhances NK cell responses to melanoma. Clin. Cancer Res. 27, 4859–4869 (2021).
Sudholz, H., Delconte, R. B. & Huntington, N. D. Interleukin-15 cytokine checkpoints in natural killer cell anti-tumor immunity. Curr. Opin. Immunol. https://doi.org/10.1016/j.coi.2023.102364 (2023).
Rautela, J. & Huntington, N. D. IL-15 signaling in NK cell cancer immunotherapy. Curr. Opin. Immunol. 44, 1–6 (2017).
Choi, C. & Finlay, D. K. Optimising NK cell metabolism to increase the efficacy of cancer immunotherapy. Stem Cell Res. Ther. 12, 320 (2021).
O’Brien, K. L. & Finlay, D. K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 19, 282–290 (2019).
D’Avola, A. et al. PHGDH is required for germinal center formation and is a therapeutic target in MYC-driven lymphoma. J. Clin. Invest. https://doi.org/10.1172/jci153436 (2022).
Shan, X. et al. Serine metabolism orchestrates macrophage polarization by regulating the IGF1–p38 axis. Cell. Mol. Immunol. 19, 1263–1278 (2022).
Rodriguez, A. E. et al. Serine metabolism supports macrophage IL-1β production. Cell Metab. 29, 1003–1011.e4 (2019).
Ma, E. H. et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity 51, 856–870.e5 (2019).
Kern Coquillat, N. et al. Pivotal role of exogenous pyruvate in human natural killer cell metabolism. Nat. Metab. https://doi.org/10.1038/s42255-024-01188-4 (2025).
Bjorkstrom, N. K., Strunz, B. & Ljunggren, H. G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 22, 112–123 (2022).
Maddocks, O. D. K., Labuschagne, C. F., Adams, P. D. & Vousden, K. H. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Mol. Cell 61, 210–221 (2016).
Papalazarou, V. et al. Phenotypic profiling of solute carriers characterizes serine transport in cancer. Nat. Metab. 5, 2148–2168 (2023).
Pranzini, E. et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell Rep. https://doi.org/10.1016/j.celrep.2022.111233 (2022).
Méndez-Lucas, A. et al. Identifying strategies to target the metabolic flexibility of tumours. Nat. Metab. 2, 335–350 (2020).
Choi, B.-H. et al. Lineage-specific silencing of PSAT1 induces serine auxotrophy and sensitivity to dietary serine starvation in luminal breast tumors. Cell Rep. https://doi.org/10.1016/j.celrep.2021.110278 (2022).
Tong, H. et al. Dual impacts of serine/glycine-free diet in enhancing antitumor immunity and promoting evasion via PD-L1 lactylation. Cell Metab. 36, 2493–2510.e2499 (2024).
Poznanski, S. M. et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 33, 1205–1220.e5 (2021).
Lontos, K. et al. Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors. J. ImmunoTher. Cancer https://doi.org/10.1136/jitc-2022-006522 (2023).
Ye, L. et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 34, 595–614.e514 (2022).
Ho, P.-C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Handzlik, M. K. et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature 614, 118–124 (2023).
Riggan, L. et al. The transcription factor Fli1 restricts the formation of memory precursor NK cells during viral infection. Nat. Immunol. https://doi.org/10.1038/s41590-022-01150-0 (2022).
Cheng, M. I. et al. The X-linked epigenetic regulator UTX controls NK cell-intrinsic sex differences. Nat. Immunol. https://doi.org/10.1038/s41590-023-01463-8 (2023).
Wang, T. et al. Identification and characterization of essential genes in the human genome. Science 350, 1096–1101 (2015).
Riggan, L. et al. CRISPR-Cas9 ribonucleoprotein-mediated genomic editing in mature primary innate immune cells. Cell Rep. 31, 107651 (2020).
Kluesner, M. G. et al. CRISPR-Cas9 cytidine and adenosine base editing of splice-sites mediates highly-efficient disruption of proteins in primary and immortalized cells. Nat. Commun. 12, 2437 (2021).
Wilhelm, L. P., Voilquin, L., Kobayashi, T., Tomasetto, C. & Alpy, F. in Intracellular Lipid Transport. Methods in Molecular Biology Vol. 1949 (Humana Press, 2019).
Divakaruni, A. S., Paradyse, A., Ferrick, D. A., Murphy, A. N. & Jastroch, M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol. 547, 309–354 (2014).
Perez-Ramirez, C. A. et al. Atlas of fetal metabolism during mid-to-late gestation and diabetic pregnancy. Cell https://doi.org/10.1016/j.cell.2023.11.011 (2023).
Pang, Z. et al. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 52, W398–W406 (2024).
Cordes, T. & Metallo, C. M. Quantifying intermediary metabolism and lipogenesis in cultured mammalian cells using stable isotope tracing and mass spectrometry. Methods Mol. Biol. 1978, 219–241 (2019).
Divakaruni, A. S. et al. Inhibition of the mitochondrial pyruvate carrier protects from excitotoxic neuronal death. J. Cell Biol. 216, 1091–1105 (2017).
Melamud, E., Vastag, L. & Rabinowitz, J. D. Metabolomic analysis and visualization engine for LC−MS data. Anal. Chem. 82, 9818–9826 (2010).
Su, X., Lu, W. & Rabinowitz, J. D. Metabolite spectral accuracy on orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Yazicioglu, Y. F. et al. Asparagine availability controls germinal center B cell homeostasis. Sci. Immunol. https://doi.org/10.1126/sciimmunol.adl4613 (2024).
Acknowledgements
We thank the blood donors, E. Faure and A. Catapang, and the UCLA CFAR Virology Core for providing healthy donor peripheral blood samples. We thank the O’Sullivan, Divakaruni, TeSlaa, Christofk, Bensinger, Covarrubias and Hoffman laboratories for helpful discussion. We thank K. Williams and the UCLA Lipidomics Core for sharing nonpolar metabolite standards. We thank M. Lechner at UCLA for sharing the β2M−/− MC38 cell line. We thank M. Wang and B. Moriarity at the University of Minnesota for sharing the ABE8e adenine base editor plasmid. T.E.O. is supported by the National Institutes of Health (NIH) (R01AI145997 and R01AI174519). J.H.L. is supported by the NIH (T32GM008042, T32GM152342, T32AI007323 and F30AI181449) and the UCLA Molecular Biology Institute Whitcome Fellowship. A.B.B. is supported by the NIH (T32GM136614). C.D.L. and W.R.A. are supported by the NIH (T32GM152342). L.H. is supported by a post-doctoral fellowship from the Belgian American Educational Foundation (BAEF). T.T. is supported by the NIH (P30DK063491) and the Chan Zuckerberg Initiative (CZI 2023-331946). A.S.D. is supported by the NIH (R35GM138003), Agilent Technologies (no. 4814) and the W.M. Keck Foundation (no. 995337). H.R.C. is supported by the NIH (2R01CA215185, 2R01AR070245 and UH2CA286583). The UCLA CFAR Virology Core is supported by the NIH (5P30AI028697).
Author information
Authors and Affiliations
Contributions
J.H.L. designed the project, performed and analysed all experiments, and wrote the paper with input from all authors. Q.F., C.D.L., M.L.W., W.R.A. and L.H. performed and analysed experiments. A.B.B. and A.S.D. performed and analysed isotope-labelled glucose tracing and respirometry experiments. J.G.B., N.M., A.K. and H.R.C. performed and analysed targeted LC–MS experiments. E.D.K. and T.T. performed and analysed isotope-labelled serine tracing experiments. T.E.O. conceived and designed the project, supervised experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
T.E.O. is a scientific advisor for Modulus Therapeutics and Xyphos Biosciences, companies that have a financial interest in human NK cell-based therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Clair Gardiner, Maxim Nosenko and Felix Wensveen for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cytokine activation induces conserved and distinct metabolic changes in human and mouse NK cells.
(a) Representative histograms and quantification of forward scatter and side scatter of freshly isolated naïve or 3 day IL-2/15-stimulated human NK cells. (b) Percent of 13C labelling of lactate, pyruvate, and citrate in naïve or 3 day IL-2/15-stimulated human NK cells cultured with [U-13C]-glucose for 24 h and analysed by GC/MS. (c) Representative histograms and quantification of tetramethylrhodamine ethyl ester (TMRE) MFI normalized to MitoTracker Green MFI in naïve or 3 day IL-2/15-stimulated human NK cells. (d) Representative histograms and quantification of MFI of phosphorylated AKTT308, phosphorylated S6S235/236, and phosphorylated AKTS473 of freshly isolated naïve or 3 day IL-2/15-stimulated human NK cells. (e) Quantification of MFI of CD69, KLRG1, and NKG2D of naïve or 3 day IL-2/15-stimulated human NK cells. (f) Schematic showing workflow of LC/MS analysis of NK cell metabolites from naïve or cytokine-stimulated cells. (g) Distribution across metabolic pathways of detected metabolites that were present in all samples. (h) Inertia barplot from PLS-DA analysis indicating number of components needed to capture inertia of sample dataset. (i) Variable Importance in Projection (VIP) scores of top 20 metabolites contributing to predictive model generated by PLS-DA analysis. (j) Heatmap showing log2 fold change of metabolites associated with serine/glycine metabolism pathways in naïve or stimulated NK cells compared to the average metabolite abundance of naïve cells. (k) Gating strategy for human NK cells. Data represent mean ± SEM or individual paired donors. Data are representative of (a, b) n = 4, (c) n = 6, (d) n = 5, (e) n = 7, and (j) n = 3 independent human donors and (j) n = 3 mice. *p < 0.05, **p < 0.01, ***p < 0.001 by (a) two-sided Student’s t-test, (b) two-way ANOVA, or (c-e) two-sided paired t-test. Exact p-values: (a) (left) p = 0.0001, (right) p = 0.0131; (b) p = 0.0045, 0.0089; (c) p = 0.0062; (d) p = 0.0251, 0.0250, 0.0109; (e) p < 0.0001, p = 0.0029, p = 0.0442. Created in BioRender. Li, J. (2025) https://BioRender.com/8kzb774.
Extended Data Fig. 2 Mouse NK cell activation is driven by IL-15.
(a) PCA analysis of metabolites from naïve, 50 ng/mL IL-15-activated, or 50 ng/mL IL-15 and 200 IU/mL IL-2-activated mouse NK cells. (b) MFI of KLRG1, NKG2D, and CD69 of naïve, IL-15, or IL-2/15-activated mouse NK cells after 3 days activation. (c) Percent IFN-γ+ mouse NK cells stimulated with 50 ng/mL IL-15, 50 ng/mL IL-15 and 200 IU/mL IL-2, or 20 ng/mL IL-15 and 200 IU/mL IL-2. (d) MFI of phosphorylated STAT5 of mouse NK cells stimulated with 50 ng/mL IL-15 or 50 ng/mL IL-15 and 200 IU/mL IL-2. (e) Gating strategy for mouse NK cells. Data represent mean ± SEM of two independent experiments. Data representative of (a) n = 3, (b) n = 7 (naïve), n = 3 (IL-2/15), and n = 4 (IL-15), and (c, d) n = 3 (IL-2/15) and n = 4 (IL-15) independent mice. * p < 0.05, *** p < 0.001, **** p < 0.0001 by (b, c) two-way ANOVA or (d) two-sided Student’s t-test. Exact p-values: (b) (left to right) p = 0.0002, p < 0.0001, p < 0.0001; (c) p = 0.0173 IL-2 200 IU/mL + IL-15 20 ng/mL vs IL-2 200 IU/mL + Il-15 50 ng/mL, p = 0.0002 IL-2 200 IU/mL + IL-15 20 ng/mL vs IL-15 50 ng/mL.
Extended Data Fig. 3 Environmental serine differentially supports human and mouse NK cell functions.
(a) Relative intracellular serine abundance in human and mouse NK cells cultured in Ser+ or Ser- media with IL-2/15 (human) or IL-15 (mouse) for 72 h before metabolite extraction. (b) Representative histograms (left) and quantification (right) of MFI of granzyme B (GzmB) and perforin of Ser+ or Ser- human NK cells after 48 h culture. FMO, fluorescence minus one control. (c) Representative histograms (left) and quantification (right) of percent CD107a+ and MFI of CD107a+ cells of Ser+ or Ser- human NK cells after 48 h culture followed by 4 h stimulation with K562 cells and IL-2/15 in the presence of anti-CD107a antibody, brefeldin A, and monensin. (d) MFI of GzmB and perforin of Ser+ or Ser- mouse NK cells stimulated with anti-Ly49H platebound antibody. (e) Quantification of percent CD107a+ and MFI of CD107a+ cells of Ser+ or Ser- mouse NK cells stimulated with anti-Ly49H platebound antibody. (f) MFI of phosphorylated pAKTT308, pS6S235/236, or pAKTS473 in naïve or D3 IL-15-activated mouse NK cells. Data represent mean ± SEM or individual paired donors across at least two independent experiments. Data are representative of (a) n = 3, (b) n = 5, and (c) n = 7 independent human donors, and (a) n = 4, (d, f) n = 8 and (e) n = 6 independent mice. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by (a, d, e) two-way ANOVA or (b, c, f) two-sided paired t-test. Exact p-values: (a) p = 0.0003 human ser+ vs ser-, p = 0.0021 mouse ser+ vs ser-, p = 0.0014 human ser- vs mouse ser-; (b) p = 0.0087, p = 0.0419; (c) p = 0.0181, p < 0.0001; (d) p = 0.0005; (e) p = 0.0318, p < 0.0001; (f) p = 0.0275, p = 0.0002, p = 0.0286.
Extended Data Fig. 4 De novo serine synthesis is not conserved in NK cells.
(a) Western blot showing PHGDH protein expression in human (above) or mouse (below) NK cells cultured in Ser+ or Ser- media for 48 h before protein extraction. (b) Schematic of [U-13C]-glucose tracing of naïve or 48 h stimulated human and mouse NK cells. (c) Percent 13C labelling of indicated nonessential amino acids in human or mouse NK cells cultured with [U-13C]-glucose for 24 h with cytokine stimulation immediately after isolation. (d) Isotopologue distribution of labelled serine in naïve or D3 IL-15-stimulated mouse NK cells labelled with [U-13C]-glucose in the final 24 h of culture. (e, f) Percent specific lysis of K562 cells (e) and percent IFN-γ+ (f) of human NK cells cultured for 48 h in asparagine-replete (Asn+) or asparagine-free (Asn-) media with IL-2/15 for 48 h before stimulation. (g) Immunoblot showing protein expression of PHGDH and β-actin loading control in nontargeting control (NTC) edited or PHGDHCRISPRa human NK cells 48 h after electroporation. (h) Abundance of 13C-labelled serine in NTC or PHGDHCRISPRa human NK cells. (i) Indel frequency calculated using SYNTHEGO ICE of mouse NK cells edited with sgRosa26 or sgPhgdh cRNP complexes. (j) Relative serine abundance in NTC or PhgdhcRNP mouse NK cells cultured in Ser+ (left) or Ser- (right) media. (k) Relative phosphoserine abundance in NTC or PhgdhcRNP mouse NK cells cultured in Ser- media. Data represent mean ± SEM or individual paired donors across at least two independent experiments. Data are representative of (a) n = 3 independent human donors and mice, (c) n = 5 mice and 4 independent donors, (d) n = 5 mice, (e, f) n = 5 independent human donors, (g) n = 3, and (h) n = 7 donors and (j, k) n = 5 mice per group. *p < 0.05, **p < 0.01, ****p < 0.0001 by (c-f) two-way ANOVA, (h) one-way ANOVA, or (j, k) two-sided Student’s t-test. Exact p-values: (c) p = 0.0084, p = 0.0097; (d) p < 0.0001; (h) p = 0.0037 NTC ser+ vs PHGDHCRISPRa ser-; p = 0.0202 NTC ser- vs PHGDHCRISPRa ser-; (j) p = 0.0295; (k) p = 0.0311. Created in BioRender. Li, J. (2025) https://BioRender.com/v7pq7fo.
Extended Data Fig. 5 Serine-dependent sphingolipid metabolism promotes human NK cell cytotoxicity.
(a) Percent specific lysis of A375 melanoma cells by NTC-edited or PHGDHCRISPRa human NK cells cultured in Ser+ or Ser- media for 48 h followed by tumour cell coculture with IL-2/15 for 16 h at a 2:1 E:T ratio. (b) MFI of LDLR in human NK cells cultured in media containing the indicated serine concentrations for 48 h with IL-2/15. (c, d) MFI of LDLR (c) and pS6 (d) of NTC-edited or PHGDHCRISPRa human NK cells cultured in Ser+ or Ser- media for 48 h. (e) Transcript expression of genes encoding sphingolipid synthesis enzymes in naïve or IL-2/15-stimulated human NK cells. (f) Percent specific lysis of K562 cells by human NK cells cultured for 48 h in Ser+ media, Ser- media with 1% ethanol vehicle control, or Ser- media supplemented with the indicated ceramide species (50 μM). (g) MFI of BODIPY 493/503 of human NK cells cultured for 48 h in Ser+ media, Ser- media with 1% ethanol vehicle control, or Ser- media supplemented with sphingomyelin (d18:1/24:0) (5 μM). (h) Viable cell yield of human NK cells cultured for 48 h in Ser+ media, Ser- media with 1% ethanol vehicle control, or Ser- media supplemented with sphingomyelin (d18:1/24:0) (5 μM). (i) MFI of pS6 in Ser + , Ser-, or Ser- + sphingomyelin (d18:1/24:0) cultured human NK cells. (j, k) Quantification of percent IFN-γ+ and IFN-γ MFI of cytokine-producing cells (j) or MFI of GzmB and perforin (k) of Ser + , Ser-, or Ser- + sphingomyelin (d18:1/24:0) cultured human NK cells stimulated with IL-2/15, K562 cells, and/or IL-12 or IL-18 where indicated. (l) Percent CD107a+ Ser + , Ser-, or Ser- + sphingomyelin (d18:1/24:0) cultured human NK cells stimulated with K562 cells. (m) MFI of filipin III staining of Ser + , Ser-, or Ser- + sphingomyelin (d18:1/24:0) cultured human NK cells treated with or without methyl-β-cyclodextrin. Data represent individual paired donors or mice. Data are representative of (a-d) n = 5, (e-h, l, m) n = 6, (i, j) n = 4, and (k) n = 4-6 independent human donors. *p < 0.05, **p < 0.01, ****p < 0.0001 by (a, c, d, f,-i, k) one-way ANOVA, (b) two-sided paired t-test, or (e, j, l, m) two-way ANOVA. Exact p-values: (a) (left) p = 0.0091, (right) p = 0.0441; (b) p = 0.0135; (c) p = 0.0255; (d) p = 0.0065 NTC ser+ vs NTC ser-, p = 0.0237 NTC ser+ vs PHGDHCRISPRa ser-; (e) (left to right) p = 0.0197, 0.0097, 0.0082, 0.0316, 0.0182; (f) p = 0.0031; (i) p = 0.0418; (j) p < 0.0001; (k) p = 0.0251; (l) p = 0.0439; (m) p = 0.0243, p = 0.0317.
Extended Data Fig. 6 Environmental serine supports human NK cell mitochondrial metabolism and SGOC.
(a) Oxygen consumption rate (OCR) of Ser+ or Ser- human NK cells. Cells were subjected to sequential treatment with oligomycin (O), FCCP (F), or rotenone with antimycin A (R + A). (b) Quantification of basal, ATP linked, maximal, and spare OCR in Ser+ or Ser- human NK cells. (c) Extracellular acidification rate (ECAR) of Ser+ or Ser- human NK cells. (d) Basal ECAR of Ser+ or Ser- human NK cells. (e) Schematic of glucose-derived carbon flux through glycolysis and TCA cycle. (f) Percent of 13C-labelled metabolites in Ser+ or Ser- human NK cells after 48 h IL-2/15 stimulation. (g) Percent of 13C-labelled lactate or citrate labelled after 13C glucose tracing in Ser+ or Ser- NTC or PHGDHCRISPRa human NK cells. (h) Percent of pool labelled by [2,3,3-2H]-serine-derived 2H of indicated metabolites in human NK cells cultured with [2,3,3-2H]-serine for 24 h with IL-2/15 stimulation immediately after isolation (24 h) or stimulated with IL-2/15 for 48 h before media was washed and changed to [2,3,3-2H]-serine media for 24 h labelling (72 h). (i, j) Percent specific lysis of K562 (i) and GzmB and perforin MFI (j) of human NK cells treated with SHIN1. (k, l) Percent specific lysis of MC38 (k) and GzmB and perforin MFI (l) of mouse NK cells treated with SHIN1. (m, n) MFI of BODIPY 493/503 (m) and metabolic dependencies measured using SCENITH (n) of human and mouse NK cells treated with SHIN1. Data represent mean ± SEM or individual paired donors across at least two independent experiments. Data are representative of (a-d, i, j) n = 6, (f, h) n = 3, (g) n = 8, (m) n = 5, and (n) n = 7 independent human donors and (k-n) n = 6 mice. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by (a, c, i, k, n) two-way ANOVA, (b, f, h, j, l, m) two-sided paired t-test, or (g) one-way ANOVA. Exact p-values: (a) p = 0.0016, p < 0.0001; (b) p = 0.0010, 0.0007, 0.0057, 0.0181; (c) p < 0.0001; (d) p = 0.0080; (f) p = 0.0071, 0.0015, 0.0164, 0.0017; (g) p = 0.0002, p < 0.0001; (h) p = 0.0146, 0.0239, 0.0209; (i) p = 0.0284; (j) p = 0.0001, 0.0173; (k) p = 0.0459; (l) p = 0.0172, 0.0010.
Extended Data Fig. 7 Glutathione synthesis modulates human NK cell effector functions through mitochondrial metabolism.
(a) Transcript expression of glutathione synthesis enzymes in human and mouse NK cells. (b) MFI of ThiolTracker Violet in SHIN1-treated human or mouse NK cells. (c) MFI of ThiolTracker Violet in Rosa26cRNP or PhgdhcRNP edited mouse NK cells cultured for 3 d in Ser+ or Ser- media with IL-15 after editing. (d) Viable cell yield of control edited or GCLC KO human NK cells 6 d post-electroporation. (e) Viable cell yield of control edited or GCLCCRISPRa human NK cells 4 d post-electroporation. (f) MFI of pS6 in control edited or GCLCCRISPRa human NK cells. (g) MFI of GzmB and perforin in K562-stimulated control edited or GCLC KO human NK cells. (h) Quantification of percent CD107a+ and CD107a MFI of degranulating cells of control edited or GCLC KO human NK cells stimulated for 4 h with K562 cells and IL-2/15. (i) MFI of pS6 in control edited or GCLC KO human NK cells. (j, k) OCR (j) and ECAR (k) of control edited or GCLC KO human NK cells. (l) Basal ECAR of control edited or GCLC KO human NK cells. (m) Basal, ATP linked, and maximal OCR of control edited or GCLCCRISPRa human NK cells. (n, o) ECAR of control edited or GCLCCRISPRa human NK cells. (p) MFI of MitoTracker Green FM in control edited or GCLCCRISPRa human NK cells. Data represent mean ± SEM or individual paired donors across at least two independent experiments. Data are representative of (a, g, h, j-l, p) n = 6, (d) n = 8, (e) n = 16, (f) n = 11, (i) n = 8, and (m-o) n = 7 independent human donors, and (a) n = 3, (b) n = 6, and (c) n = 3 independent mice. *p < 0.05, **p < 0.01, ***p < 0.001 by (a, c, h, j, k, n) two-way ANOVA or (b, d-i, l, m, o, p) two-sided paired t-test. Exact p-values: (a) p = 0.0495 human, p = 0.0037, 0.0012, 0.0186 mouse; (b) p = 0.0417, 0.0015; (d) p = 0.0009; (e) p = (g) p = 0.0023, 0.0001; (h) p = 0.0025; (i) p = 0.0214; (l) p = 0.0184.
Extended Data Fig. 8 Dietary serine restriction impairs human NK cell function.
(a) Relative serine abundance in serum of control diet or serine/glycine-free diet-fed C57/BL6 mice after 10 days on diet. (b) Number of NK cells per spleen of control or serine-restricted mice. (c) Distribution across maturation subsets of splenic NK cells from control or serine-restricted mice. (d, e) Percent specific lysis of MC38 tumour cells (d) and percent IFN-γ+ (e) splenic NK cells from control or serine-restricted mice, coloured by mouse sex. (f) MFI of indicated phosphoproteins in splenic NK cells from control or serine-restricted mice. (g) Relative serine abundance in serum of control diet or serine/glycine-free diet-fed NOG-hIL-15 mice after 10 days on diet. (h) Quantification of NK cell subset distribution of adoptively transferred human NK cells in control or serine/glycine-free diet-fed host mice 3 d post transfer. (i) Human NK cells per 100 μL of blood in control or serine/glycine-free diet-fed host mice 3 d post transfer. (j-l) Percent IFN-γ+ (j), percent CD107a+ (k), and proportion of tumour-conjugated NK cells (l) of adoptively transferred NTC-edited or PHGDHCRISPRa human NK cells from peripheral blood of control diet-fed host mice on D3 post-adoptive transfer. (m, n) Individual tumour curves for control or serine/glycine-restricted mice transferred with NTC NK cells (m) or NTC versus PHGDHCRISPRa human NK cells. Data represent mean ± SEM or paired mice receiving same human donor NK cells. Data are representative of (a) n = 5, (b-m) n = 8-9, and (n) n = 4 independent mice per group, with sex deaggregated data represented as (b, c, e, f) n = 5 male and 4 female mice per diet and (d) n = 5 male mice per diet, n = 4 female mice for control diet, and n = 3 female mice for ser/gly- diet. *p < 0.05, **p < 0.01, ****p < 0.0001 by (a, b, d, g) two-sided Student’s t-test, (c, e, f, h, i) two-way ANOVA, (i) one-way ANOVA, and (j-l) two-sided paired t-test. Exact p-values: (a) p = 0.0046; (b) p = 0.0097; (e) p < 0.0001; (g) p = 0.0044; (j) p = 0.0338.
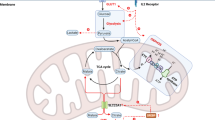
Extended Data Fig. 9 Human and mouse NK cells display species-specific utilization of serine.
Summary schematic of serine contributions to NK cell metabolic reprogramming and effector functions.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blots for all figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J.H., Feng, Q., Ball, A.B. et al. Species-specific serine metabolism differentially controls natural killer cell functions. Nat Metab 7, 1905–1923 (2025). https://doi.org/10.1038/s42255-025-01348-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01348-0
This article is cited by
-
NMR metabolomics in genetically engineered mouse models
Biophysical Reviews (2025)