Abstract
Resistance to cyclin-dependent kinase 4/6 (CDK4/CDK6) inhibitors leads to treatment failure and disease progression in women with hormone receptor+HER2− (HR+HER2−) breast cancer (BC). We delineated a hypoxia-sensitive, CCL2-dependent pathway recruiting interleukin-17A (IL-17A)-secreting γδ T cells to mouse HR+HER2− BCs following CDK4/CDK6 inhibition, resulting in repolarization of tumor-associated macrophages (TAMs) toward an immunosuppressive CX3CR1+ phenotype associated with resistance. Increased IL-17A signaling and intratumoral γδ T cell abundance positively correlated with advanced grade and/or reduced survival in two cohorts of individuals with HR+HER2− BC. Circulating γδ T cells and plasma CCL2 levels negatively correlated with progression in an independent series of individuals with HR+HER2− BC receiving CDK4/CDK6 inhibitors. Intratumoral γδ T cells were increased in post- versus pretreatment biopsies from individuals with HR+HER2− BC relapsing on CDK4/CDK6 inhibitors. CX3CR1+ TAMs had negative prognostic impact in women with HR+HER2− BC receiving neoadjuvant PD-1 blockage and radiotherapy. Thus, γδ T cells and CX3XR1+ TAMs may favor resistance to CDK4/CDK6 inhibitors in individuals with HR+HER2− BC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Mouse scRNA-seq and TCR-seq data have been deposited in Gene Expression Omnibus (GEO) under accession number GSE296798. Human scRNA-seq data have been deposited as raw count files in GEO under accession number GSE296805. Human scRNA-seq raw fastq files have not been made publicly available due to privacy concerns but can be made available from the corresponding authors upon request. All requests will be fulfilled within 2 weeks, with data being made available permanently to the requester. Publicly available transcriptional data from the METABRIC dataset39,70 were downloaded from cBioPortal (https://www.cbioportal.org/study/summary?id=brca_metabric) on 18 November 2020. The remaining data supporting the findings of this study are available within the article, Supplementary Information or Source Data files and/or from the corresponding authors upon request. Source data are provided with this paper.
Change history
23 July 2025
A Correction to this paper has been published: https://doi.org/10.1038/s43018-025-01036-8
References
Morrison, L., Loibl, S. & Turner, N. C. The CDK4/6 inhibitor revolution—a game-changing era for breast cancer treatment. Nat. Rev. Clin. Oncol. 21, 89–105 (2024).
Hortobagyi, G. N. et al. A phase III trial of adjuvant ribociclib plus endocrine therapy versus endocrine therapy alone in patients with HR-positive/HER2-negative early breast cancer: final invasive disease-free survival results from the NATALEE trial. Ann. Oncol. 36, 149–157 (2025).
O’Leary, B. et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 8, 1390–1403 (2018).
Wander, S. A. et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 10, 1174–1193 (2020).
Gong, X. et al. Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer Cell 32, 761–776 (2017).
Kudo, R. et al. Long-term breast cancer response to CDK4/6 inhibition defined by TP53-mediated geroconversion. Cancer Cell 42, 1919–1935 (2024).
Petroni, G., Buque, A., Zitvogel, L., Kroemer, G. & Galluzzi, L. Immunomodulation by targeted anticancer agents. Cancer Cell 39, 310–345 (2021).
Petroni, G., Formenti, S. C., Chen-Kiang, S. & Galluzzi, L. Immunomodulation by anticancer cell cycle inhibitors. Nat. Rev. Immunol. 20, 669–679 (2020).
Uzhachenko, R. V. et al. Metabolic modulation by CDK4/6 inhibitor promotes chemokine-mediated recruitment of T cells into mammary tumors. Cell Rep. 35, 108944 (2021).
Schmitt, C. A., Wang, B. & Demaria, M. Senescence and cancer—role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 19, 619–636 (2022).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Peuker, C. A. et al. Treatment with ribociclib shows favourable immunomodulatory effects in patients with hormone receptor-positive breast cancer—findings from the RIBECCA trial. Eur. J. Cancer 162, 45–55 (2022).
Togashi, Y., Shitara, K. & Nishikawa, H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16, 356–371 (2019).
Ali, L. R. et al. PD-1 blockade and CDK4/6 inhibition augment nonoverlapping features of T cell activation in cancer. J. Exp. Med. 220, e20220729 (2023).
Yuan, Y. et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur. J. Cancer 154, 11–20 (2021).
Petroni, G. et al. Radiotherapy delivered before CDK4/6 inhibitors mediates superior therapeutic effects in ER+ breast cancer. Clin. Cancer Res. 27, 1855–1863 (2021).
Petroni, G., Cantley, L. C., Santambrogio, L., Formenti, S. C. & Galluzzi, L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat. Rev. Clin. Oncol. 19, 114–131 (2022).
Petroni, G., Buqué, A., Coussens, L. M. & Galluzzi, L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat. Rev. Drug Discov. 21, 440–462 (2022).
De Angelis, C. et al. Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER+ breast cancer. Clin. Cancer Res. 27, 4870–4882 (2021).
Teh, J. L. F. & Aplin, A. E. Arrested developments: CDK4/6 inhibitor resistance and alterations in the tumor immune microenvironment. Clin. Cancer Res. 25, 921–927 (2019).
Buque, A. et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat. Commun. 11, 3819 (2020).
Alamilla-Presuel, J. C., Burgos-Molina, A. M., González-Vidal, A., Sendra-Portero, F. & Ruiz-Gómez, M. J. Factors and molecular mechanisms of radiation resistance in cancer cells. Int. J. Radiat. Biol. 98, 1301–1315 (2022).
Beringer, A. & Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 15, 491–501 (2019).
Gelderblom, H. et al. Long-term outcomes of pexidartinib in tenosynovial giant cell tumors. Cancer 127, 884–893 (2021).
Buque, A. et al. Impact of radiation therapy dose, fractionation, and immunotherapeutic partner in a mouse model of hormone receptor-positive mammary carcinogenesis. J. Natl Cancer Inst. 117, 934–947 (2025).
Buqué, A. & Galluzzi, L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer 4, 599–601 (2018).
Rugo, H. S. et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin. Cancer Res. 24, 2804–2811 (2018).
Petroni, G. & Galluzzi, L. Impact of treatment schedule on the efficacy of cytostatic and immunostimulatory agents. Oncoimmunology 10, 1889101 (2021).
Guo, S., Jiang, X., Mao, B. & Li, Q. X. The design, analysis and application of mouse clinical trials in oncology drug development. BMC Cancer 19, 718 (2019).
Heckler, M. et al. Inhibition of CDK4/6 promotes CD8 T-cell memory formation. Cancer Discov. 11, 2564–2581 (2021).
Teh, J. L. F. et al. Activation of CD8+ T cells contributes to antitumor effects of CDK4/6 inhibitors plus MEK inhibitors. Cancer Immunol. Res. 8, 1114–1121 (2020).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Edwards, S. C. et al. PD-1 and TIM-3 differentially regulate subsets of mouse IL-17A-producing γδ T cells. J. Exp. Med. 220, e20211431 (2023).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Jin, C. et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013 (2019).
Beck, B. H. et al. Dynamics of circulating γδ T cell activity in an immunocompetent mouse model of high-grade glioma. PLoS ONE 10, e0122387 (2015).
O’Brien, R. L. & Born, W. K. Two functionally distinct subsets of IL-17 producing γδ T cells. Immunol. Rev. 298, 10–24 (2020).
Itohara, S. et al. T cell receptor δ gene mutant mice: independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell 72, 337–348 (1993).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
De Giovanni, C. et al. Bioprofiling TS/A murine mammary cancer for a functional precision experimental model. Cancers 11, 1889 (2019).
Comşa, Ş., Cîmpean, A. M. & Raica, M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 35, 3147–3154 (2015).
Ma, R. Y., Black, A. & Qian, B. Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563 (2022).
Nalio Ramos, R. et al. Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell 185, 1189–1207 (2022).
Mantovani, A., Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022).
Galassi, C., Chan, T. A., Vitale, I. & Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 42, 1825–1863 (2024).
Ho, A. Y. et al. PEARL: a phase Ib/II biomarker study of adding radiation therapy to pembrolizumab before neoadjuvant chemotherapy in human epidermal growth factor receptor 2-negative breast cancer. J. Clin. Oncol. 42, 4282–4293 (2024).
Antonelli, L. R. et al. Disparate immunoregulatory potentials for double-negative (CD4−CD8−) αβ and γδ T cells from human patients with cutaneous leishmaniasis. Infect. Immun. 74, 6317–6323 (2006).
Martinez, C. et al. Functional double-negative T cells in the periphery express T cell receptor Vβ gene products that cause deletion of single-positive T cells. Eur. J. Immunol. 23, 250–254 (1993).
Le Naour, A., Rossary, A. & Vasson, M. P. EO771, is it a well-characterized cell line for mouse mammary cancer model? Limit and uncertainty. Cancer Med. 9, 8074–8085 (2020).
Lin, E. Y. et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 (2003).
Attalla, S., Taifour, T., Bui, T. & Muller, W. Insights from transgenic mouse models of PyMT-induced breast cancer: recapitulating human breast cancer progression in vivo. Oncogene 40, 475–491 (2021).
Im, S. A. et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 381, 307–316 (2019).
Turner, N. C. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 379, 1926–1936 (2018).
Slamon, D. J. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 382, 514–524 (2020).
Rodriguez-Ruiz, M. E., Vitale, I., Harrington, K. J., Melero, I. & Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21, 120–134 (2020).
McCartney, A. et al. Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front. Oncol. 9, 666 (2019).
Mills, K. H. G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 23, 38–54 (2023).
Rugo, H. S. et al. Abemaciclib in combination with pembrolizumab for HR+, HER2− metastatic breast cancer: phase 1b study. NPJ Breast Cancer 8, 118 (2022).
Park, J. H. et al. Tumor hypoxia represses γδ T cell-mediated antitumor immunity against brain tumors. Nat. Immunol. 22, 336–346 (2021).
de Vries, N. L. et al. γδ T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature 613, 743–750 (2023).
Wu, Y. et al. An innate-like Vδ1+ γδ T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci. Transl. Med. 11, eaax9364 (2019).
Craven, K. E., Gokmen-Polar, Y. & Badve, S. S. CIBERSORT analysis of TCGA and METABRIC identifies subgroups with better outcomes in triple negative breast cancer. Sci. Rep. 11, 4691 (2021).
Janssen, A. et al. γδ T-cell receptors derived from breast cancer-infiltrating T lymphocytes mediate antitumor reactivity. Cancer Immunol. Res. 8, 530–543 (2020).
Daley, D. et al. γδ T cells support pancreatic oncogenesis by restraining αβ T cell activation. Cell 166, 1485–1499 (2016).
Serre, K. & Silva-Santos, B. Molecular mechanisms of differentiation of murine pro-inflammatory γδ T cell subsets. Front. Immunol. 4, 431 (2013).
Cano, C. E. et al. BTN2A1, an immune checkpoint targeting Vγ9Vδ2 T cell cytotoxicity against malignant cells. Cell Rep. 36, 109359 (2021).
Cassetta, L. & Pollard, J. W. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 17, 887–904 (2018).
Weiss, S. A. et al. A phase I study of APX005M and cabiralizumab with or without nivolumab in patients with melanoma, kidney cancer, or non-small cell lung cancer resistant to anti-PD-1/PD-L1. Clin. Cancer Res. 27, 4757–4767 (2021).
Foster, C. C. et al. Phase I study of stereotactic body radiotherapy plus nivolumab and urelumab or cabiralizumab in advanced solid tumors. Clin. Cancer Res. 27, 5510–5518 (2021).
Rueda, O. M. et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 567, 399–404 (2019).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194 (2013).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 24, 2206–2223 (2013).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 144, 545–563 (2020).
Gradishar, W. J. et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 18, 452–478 (2020).
Cardoso, F. et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 29, 1634–1657 (2018).
Gállego Pérez-Larraya, J. et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med. 386, 2471–2481 (2022).
Abengozar-Muela, M. et al. Diverse immune environments in human lung tuberculosis granulomas assessed by quantitative multiplexed immunofluorescence. Mod. Pathol. 33, 2507–2519 (2020).
Shiao, S. L. et al. Single-cell and spatial profiling identify three response trajectories to pembrolizumab and radiation therapy in triple negative breast cancer. Cancer Cell 42, 70–84 (2024).
Klapp, V. et al. Cellular senescence in the response of HR+ breast cancer to radiotherapy and CDK4/6 inhibitors. J. Transl. Med. 21, 110 (2023).
Acknowledgements
We are indebted to S. Chen (Weill Cornell Medical College) for the kind gift of an ELISA kit for human CCL2 quantification and to A. Y. Ho (Duke University Medical Center) for coleading with S.L.S. the clinical study NCT03366844 and providing constructive input on the paper. G.P. and L.G. are grateful to the William Guy Forbeck Research Foundation (Carbondale, CO) for fostering the dissemination of knowledge, promoting scientific collaboration and supporting the training of the next generation of leaders in cancer research. This work has been partially sponsored by the 2019 Laura Ziskin Prize in Translational Research (ZP-6177, S.C.F. and H.L.M.) from Stand Up to Cancer and by a Breakthrough Level 2 grant from the US Department of Defense (DoD) Breast Cancer Research Program (BCRP; BC210945, L.G. and S.R.V.K.). The clinical study NCT03366844 was sponsored by the Breast Cancer Research Foundation. C.G. is supported by the American Italian Cancer Foundation (223565-01). F.F. was supported by the Austrian Science Fund (T 974-B30) and by the Oesterreichische Nationalbank (18496). The laboratory of M.E.R.-R. is supported by the Fundación CRIS, Programa Talento Clínico 2020 (PR_TCL_2020-03), the Cancer Research UK (C18915/A29362), FCAECC and AIRC under the Accelerator Award Programme, the RTC2019-006860-1 supported by MCIN/AEI/10.13039/501100011033 and the Instituto de Salud Carlos III through project PI20/00434. B.S. is supported by a grant from the Deutsche Krebshilfe, Integrate project. L.d.l.C.-M. is supported by a grant from the Andalusian Public Foundation Progress and Health (PI-0502-2014 FPS-2014). E.G.-M. is supported by ‘Calasparra se Mueve’, a research funding initiative inspired by women from Calasparra (Murcia), Spain. L.G. is/has also been supported (as a principal investigator unless otherwise indicated) by one R01 grant from the NIH/NCI (CA271915), one Breakthrough Level 2 grant from the US DoD BCRP (BC180476P1), a grant from the STARR Cancer Consortium (I16-0064), a Transformative Breast Cancer Consortium Grant from the US DoD BCRP (W81XWH2120034, S.C.F.), a U54 grant from NIH/NCI (CA274291, principal investigators: J. Deasy, S.C.F., R. Weichselbaum), the 2019 Laura Ziskin Prize in Translational Research (ZP-6177, S.C.F.) from Stand Up to Cancer, a Mantle Cell Lymphoma Research Initiative (MCL-RI, principal investigator: S. Chen-Kiang) grant from the Leukemia and Lymphoma Society, a Rapid Response Grant from the Functional Genomics Initiative, startup funds from the Department of Radiation Oncology at Weill Cornell Medicine, industrial collaborations with Lytix Biopharma, Promontory and Onxeo and by donations from Promontory, the Luke Heller TECPR2 Foundation, Sotio, Lytix Biopharma, Onxeo, Ricerchiamo and Noxopharm. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
L.G., S.C.F., S.R.V.K. and H.L.M. conceived the project and provided senior supervision to the study. G.P. and C.G. performed preclinical experiments as well as analysis on circulating CCL2 in participants, with support from A.B., N.B., T.Y., A. Sato, M.B.-V. and G.C., under supervision from L.G. K.H.G., H.-H.C. and A. Shah performed and analyzed the RNA-seq experiments under supervision from S.R.V.K. C.J.-C. and I.S.-M. performed and analyzed multicolor flow cytometry experiments on human blood samples under supervision from V.S.-M. and L.d.l.C.-M. or E.G.-M., F.A.d.l.P. and M.E.R.-R., respectively. A.K. analyzed public human datasets under supervision from F.F. and Z.T. C.M. and C.W. performed immunofluorescence microscopy and immunohistochemistry experiments on human tissue samples, respectively, under supervision from B.S. C.E.d.A. performed multispectral immunofluorescence on human tissue samples under supervision from M.E.R.-R. B.N.-R. retrieved deidentified clinical data on human samples under supervision from M.E.R.-R. E.N.M. retrieved deidentified clinical data on human samples under supervision from F.A.d.l.P. and E.G.-M. A.M.S. performed hypoxia assays under supervision from J.M.S. B.R. and X.K.Z. provided statistical support to the project. R.K.B. and S.L.S. ran clinical study NCT03366844 and provided samples for scRNA-seq data. G.P., C.G. and L.G. wrote the paper with critical input from all authors. G.P. and C.G. prepared figures under supervision from L.G. All authors approved the submitted version of the article.
Corresponding authors
Ethics declarations
Competing interests
F.F. has been holding research contracts with iOnctura. M.E.R.-R. reports receiving research funding from Roche and Bioncotech. She also has received speaker’s bureau honoraria from Bristol Myers Squibb (BMS) and Roche. H.L.M. is/has been holding research contracts with BMS, MedImmune, LLC/AstraZeneca, BTG and Merck and has received consulting/advisory honoraria from Amgen, BMS, Celgene, Eli Lilly, Genentech/Roche, Immunomedics, Merck, OBI Pharma, Pfizer, Puma, Spectrum Pharmaceuticals, Syndax Pharmaceuticals, Peregrine, Calithera, Daiichi-Sankyo, Seattle Genetics, AstraZeneca, Gilead, Crown Bioscience and TapImmune. S.C.F. is/has been holding research contracts with Merck, Varian, BMS, Celldex, Regeneron, Eisai and Eli Lilly and has received consulting/advisory honoraria from Bayer, BMS, Varian, Elekta, Regeneron, Eisai, AstraZeneca, MedImmune, Merck US, EMD Serono, Accuray, Boehringer Ingelheim, Roche, Genentech, AstraZeneca, View Ray and Nanobiotix. S.R.V.K. is a founder and consultant at Transomic Technologies and Faeth Therapeutics. L.G. is/has been holding research contracts with Lytix Biopharma, Promontory and Onxeo, has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Imvax, Sotio, Promontory, Noxopharm, EduCom and the Luke Heller TECPR2 Foundation and holds Promontory stock options. All other authors have no conflicts to declare.
Peer review
Peer review information
Nature Cancer thanks Cynthia Ma, Marcos Malumbres and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Palbociclib plus tamoxifen activity against M/D-driven mammary carcinomas is not modulated by CD4+ and CD8+ T cell depletion.
a-f, C57BL/6 J mice bearing M/D-driven tumors were randomly allocated to receive no treatment (control), tamoxifen (T), oral palbociclib (P), focal radiation therapy (RT) followed by (→) T, RT→P (a-c), P + T ± α-CD4 and α-CD8 antibodies (d), or RT→P + T ± an α-PD-1 antibody (e, f). Individual growth curves for primary tumors shown as tumor area relative to the area at the start of treatment (A/A0) (a, e), cumulative relative tumor area (d), survival curves (b, f), and pie charts with drug responses classified at d20 (c), are reported. Pie charts show the percentage of dead mice, objective response (OR; A/A0 ≤ 0.756), stable disease (SD; 0.756 < A/A0 ≤ 1.440), and progressive disease (PD; A/A0 > 1.440), according to RECIST-based criteria. Number of mice, risk ratio (RR) with 95% confidence interval and P values are shown. g, Bubble heatmap of immune cell cluster-defining genes by scRNAseq from M/D-driven tumors treated as reported in Fig. 1f. Data show the mean expression of genes in identified cell clusters and the fraction of cells expressing the gene of interest in 40,853 CD45+ cells (pooled from 27 tumors, with 3 biological replicates/treatment group obtained from independent mice). h, Gini index of TCR γ and δ chain diversity in M/D-driven tumors left untreated (n = 5) or treated with P + T (n = 7) and harvested at endpoint. Box-plots show means ± interquartile range; whiskers extend to 1.5-fold interquartile ranges. P values were determined by linear mixed effects model followed by simultaneous tests of general linear hypotheses (a, d, e), two-sided Gehan-Breslow-Wilcoxon test (b, f), and two-sided Wilcoxon test (h). Part of the results shown in (b) and (f) are also depicted in Fig. 1b, e (control and RT→P + T), and Figs. 2d, 3f, 4i (control).
Extended Data Fig. 2 Transcriptional signature of γδ T cells recruited to M/D-driven mammary carcinomas upon CDK4/CDK6 inhibition.
a, UMAP color-coded for expression of genes associated with the IL17A-producing γδ T phenotype. Data were obtained from 8,395 T cells (pooled from 27 tumors, with 3 biological replicates/treatment group obtained from independent mice) as reported in Fig. 1f, g. b, Correlations between circulating levels of IL17A and IL1β (left) or IL23 (right) in mice bearing M/D-driven tumors treated with palbociclib (P) plus tamoxifen (T) on d0-d5 and assessed at d6 (n = 7). P (by two-sided Spearman’s rank correlation test) and R (Spearman’s rank correlation coefficients) values are reported. c, Serum levels of IL17A in wild-type (WT; n = 11) and Tcrd−/− (n = 4) mice bearing M/D-driven tumor assessed at d0 (before treatment) and after treatment with oral palbociclib (P) plus tamoxifen (T) at d6. Relevant and significant (P < 0.05) statistical differences (by Wilcoxon matched-pairs signed-rank test) are shown.
Extended Data Fig. 3 Impact of CDK4/6 inhibition and hypoxia on CCL2 secretion.
a, CCL2 secretion from TS/A cells exposed to palbociclib (P), tamoxifen (T) or their combination, for 72 h (n = 8 from two independent experiments for control, n = 5 from two independent experiments for T, n = 6 from two independent experiments for P and P + T). Box-plots overlaid by individual data points show medians ± interquartile range; whiskers indicate minima and maxima. Relevant and significant (P < 0.05) statistical differences (by linear mixed-effects regression followed by simultaneous tests of general linear hypotheses) are shown. b, Workflow followed for the quantification of pimonidazole positive area in M/D-driven tumor tissues. Tiled z-stack images were analyzed with Image J. Nuclei and pimonidazole staining images were individually processed with z-projection (sum all slices) and followed by adjusting the threshold in the Otsu method. Regions of interest (ROIs) were selected by auto-detection or manually based on nuclear staining and applied to the pimonidazole staining image to measure pimonidazole-positive area. For representative images and quantification of pimonidazole-positive area, see Fig. 3i.
Extended Data Fig. 4 CX3CR1+ macrophages accumulate in M/D-driven mammary carcinomas exposed to CDK4/6 inhibitors.
a, Volcano plot of differentially expressed genes (DEGs) in myeloid cells from palbociclib (P) plus tamoxifen (T)-treated (n = 3) versus untreated (n = 3) M/D-driven tumors. DEGs highlighted in red are discussed in the manuscript and have [log2(FC)] ≥ 0.5 and adjusted (Adj) P value < 0.001. b, UMAP visualization of 31,723 myeloid cells (pooled from 27 tumors, with 3 biological replicates/treatment group obtained from independent mice, as in Fig. 1f) in 3 main phenotypes, as indicated by the color-coded legend (DC, dendritic cell). c, Bubble heatmap of myeloid cell cluster-defining genes by scRNAseq from M/D-driven tumors treated as reported in Fig. 1f. Data show the mean expression of genes in identified cell clusters and the fraction of cells expressing the gene of interest in 31,723 myeloid cells (pooled from 27 tumors, with 3 biological replicates/treatment group obtained from independent mice). d, Density of F4/80+CX3CR1+ cells infiltrating M/D-driven tumors established in Tcrd−/− mice treated with P + T (n = 7) or established in wild-type mice and left untreated (n = 7), treated with P + T alone (n = 8) or treated with P + T in combination with an α-IL17A (n = 7) or an α-TCRγ/δ (n = 7) antibody. All tumors were harvested at endpoint. e, CD80 and MHCII median fluorescence intensity (MFI) of F4/80+CX3CR1+ cells infiltrating M/D-driven tumors treated with P + T (n = 5), P + T+α-IL17A (n = 5), or left untreated (n = 8), harvested at endpoint and subjected to ex-vivo stimulation with LPS/IFNγ. In (d) and (e) box-plots overlaid by individual data points show medians ± interquartile range; whiskers indicate minima and maxima. Significant (P < 0.05) statistical differences (by Kruskal-Wallis with uncorrected Dunn’s test) are shown.
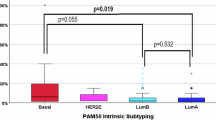
Extended Data Fig. 5 Impact of γδ T cells on human ER+ and ER- breast cancer.
a-c, Disease specific (DSS, a) and overall survival (OS, b, c) of ER− (a, c) and ER+ (b) METABRIC BC patients stratified by median-single-sample score of γδ T cell and IL17 signatures in high and low. Number of patients and P values (determined by Log-rank test) are reported. d-e, Representative images and quantification of CD8 (d) and CD68 (e) expression by immunohistochemistry in diagnostic HR+HER2− BC biopsies of indicated grade (grade 1: n = 16; grade 2: n = 55; grade 3: n = 13). Box-plots overlaid by individual data points show medians ± interquartile range; whiskers indicate minima and maxima. Number of patients are indicated. Only significant (P < 0.05) statistical differences (by Kruskal-Wallis with uncorrected Dunn’s test) are shown. Scale bars, 50 μm.
Extended Data Fig. 6 Impact of TAMs on human HR+ breast cancer.
a, Mean distance of PD-L1+/PD-L1− macrophages and tumor cells to the nearest γδ T cell in diagnostic biopsies of HR+HER2− BC (n = 26) assessed by multispectral imaging. Box-plots overlaid by individual data points show medians ± interquartile range; whiskers indicate minima and maxima. Relevant and significant (P < 0.05) statistical differences (by two-tailed paired t-test) are shown. Scale bar, 100 μm b, UMAPs for macrophage clusters by scRNAseq, color-coded for the indicated cell type, from longitudinal HR+HER2− BC diagnostic biopsies of responder and non-responder patients receiving preoperative pembrolizumab (α-PD-1) followed by (→) RT plus α-PD-1. Data are pooled from 36 biopsies obtained from 12 patients (3 each: at baseline, after PD-1 and at surgery).
Extended Data Fig. 7 Levels of circulating and intratumoral CD8+PD-1+ and TREG cells in HR+ BC patients.
a, Progression-free survival (PFS) curves for patients with HR+ BC treated with palbociclib (P) plus hormonotherapy (HT) stratified based on the median abundance of circulating CD4−CD8− T cells (at baseline) in high ( ≥ median; n = 27) and low ( < median; n = 25). Data from patients from cohort #3 (n = 29) and cohort #4 (n = 23) were pooled. P value determined by Log-rank test is reported. b, Correlations between the number of circulating IL7R+ γδ T cells and CD4+FOXP3+CD25+ regulatory T (TREG) cells or CD8+PD-1+ T cells in longitudinal blood samples (n = 60 samples in total) harvested from n = 24 HR+ BC patients at baseline, after 3 months of treatment and at relapse, as in Fig. 5h, left panel. P (by two-sided Spearman’s rank correlation test) and R (Spearman’s rank correlation coefficients) values are reported. c, Ulcerative colitis tissue section stained for the simultaneous detection of TCRγ/δ, CD8, CD4, FOXP3, PD-1, pan-CK and nucleus (DAPI) used as positive control for the staining of HR+ tumor specimens. Scale bar, 100 μm. For representative images of HR+ breast tumors and quantification of γδ T cells see Fig. 5i. d, Quantification of CD8+PD-1+ T cells, CD4+ T cells, and CD4+FOXP3+ TREG cells in paired HR+ tumor biopsies collected at baseline (n = 8) and after disease progression on P plus HT (n = 8). P values (by two-tailed paired t-test) were all non-significant.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Tables 1–4.
Source data
Source Data Fig. 1
Numerical source data.
Source Data Fig. 2
Numerical source data.
Source Data Fig. 3
Numerical source data.
Source Data Fig. 3
Unprocessed western blots relative to Fig. 3c.
Source Data Fig. 4
Numerical source data.
Source Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 1
Numerical source data.
Source Data Extended Data Fig. 2
Numerical source data.
Source Data Extended Data Fig. 3
Numerical source data.
Source Data Extended Data Fig. 4
Numerical source data.
Source Data Extended Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 6
Numerical source data.
Source Data Extended Data Fig. 7
Numerical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petroni, G., Galassi, C., Gouin, K.H. et al. IL-17A-secreting γδ T cells promote resistance to CDK4/CDK6 inhibitors in HR+HER2− breast cancer via CX3CR1+ macrophages. Nat Cancer 6, 1656–1675 (2025). https://doi.org/10.1038/s43018-025-01007-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-01007-z
This article is cited by
-
Cytokine signaling and resistance to CDK4/6 inhibitors in HR+HER2− breast cancer
npj Precision Oncology (2025)
-
γδ T cells and resistance to CDK4/6 inhibitors in breast cancer
Cell Death & Disease (2025)
-
Radiation therapy as a biological modifier of the breast cancer immune microenvironment
npj Breast Cancer (2025)
-
TIM-3 and γδ T cells: new players in breast cancer dissemination
The EMBO Journal (2025)
-
Alternate actions of CDK4/6 inhibitors beyond cell cycle blockade: unexplored roles in therapy resistance
Cancer and Metastasis Reviews (2025)