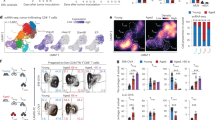
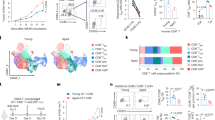
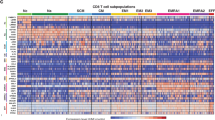
Abstract
Age-related increases in cancer have traditionally been attributed to compromised antitumor immunity of exhausted and dysfunctional CD8⁺ T cells. Here we provide an alternative mechanism: in aging, cancer also progresses with the help of fully functional CD8⁺ T cells. These transcriptionally and epigenetically distinct cells (termed double-positive CD8+ T cells (DP8)) express CD39, CD73, CD101 and CXCR6 on their surface and accumulate during healthy aging in mice, requiring B cells presenting cognate antigens. In aged mice, progressing tumors recruit DP8 cells via the CXCL16–CXCR6 axis to suppress antitumor CD4+ T cells in an ADP/adenosine-dependent manner, and targeting DP8 cell function or recruitment can reverse tumor growth in aged mice. This tumor-promoting mechanism of DP8 cells appears to be conserved in older humans, as we detected DP8-like cells in various tumors, including late-onset breast cancer. We propose that this tumor-promoting role of CD8+ T cells should be considered in the development of therapeutics tailored for older humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings of this study can be found in the supplementary materials or source data files accompanying this paper. RNA-seq and ATAC–seq data are publicly available in the GEO (GSE273565 and GSE295367, respectively). All other data are available from the corresponding author upon reasonable request.
References
Zhang, H., Weyand, C. M. & Goronzy, J. J. Hallmarks of the aging T-cell system. FEBS J. 288, 7123–7142 (2021).
Decman, V. et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J. Immunol. 188, 1933–1941 (2012).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
Terekhova, M. et al. Single-cell atlas of healthy human blood unveils age-related loss of NKG2C+GZMB−CD8+ memory T cells and accumulation of type 2 memory T cells. Immunity 56, 2836–2854 (2023).
Connolly, K. A. et al. A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol. 6, eabg7836 (2021).
Chen, A. C. Y. et al. The aged tumor microenvironment limits T cell control of cancer. Nat. Immunol. 25, 1033–1045 (2024).
Pei, S. et al. Age-related decline in CD8+ tissue resident memory T cells compromises antitumor immunity. Nat. Aging 4, 1828–1844 (2024).
Yao, C. et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat. Immunol. 20, 890–901 (2019).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51, 1043–1058 (2019).
Beltra, J. C. et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841 (2020).
Harding, C., Pompei, F. & Wilson, R. Peak and decline in cancer incidence, mortality, and prevalence at old ages. Cancer 118, 1371–1386 (2012).
Ershler, W. B., Stewart, J. A., Hacker, M. P., Moore, A. L. & Tindle, B. H. B16 murine melanoma and aging: slower growth and longer survival in old mice. J. Natl Cancer Inst. 72, 161–164 (1984).
Pettan-Brewer, C. et al. B16 melanoma tumor growth is delayed in mice in an age-dependent manner. Pathobiol. Aging Age Relat. Dis. https://doi.org/10.3402/pba.v2i0.19182 (2012).
Lee-Chang, C. et al. Aging converts innate B1a cells into potent CD8+ T cell inducers. J. Immunol. 196, 3385–3397 (2016).
Lee-Chang, C. et al. Accumulation of 4-1BBL+ B cells in the elderly induces the generation of granzyme-B+ CD8+ T cells with potential antitumor activity. Blood 191, 4141–4151 (2014).
Galeano Nino, J. L. et al. Cytotoxic T cells swarm by homotypic chemokine signalling. Elife 9, e56554 (2020).
Di Pilato, M. et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 184, 4512–4530 (2021).
Darash-Yahana, M. et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS ONE 4, e6695 (2009).
Kumar, B. V. et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20, 2921–2934 (2017).
Mackay, L. K. et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013).
Wang, X. et al. CD8+ T cells exacerbate AD-like symptoms in mouse model of amyloidosis. Brain Behav. Immun. 122, 444–455 (2024).
Gupta, P. K. et al. CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog. 11, e1005177 (2015).
Fang, F. et al. Expression of CD39 on activated T cells impairs their survival in older individuals. Cell Rep. 14, 1218–1231 (2016).
Canale, F. P. et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells. Cancer Res. 78, 115–128 (2018).
Vignali, P. D. A. et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nat. Immunol. 24, 267–279 (2023).
Bono, M. R., Fernandez, D., Flores-Santibanez, F., Rosemblatt, M. & Sauma, D. CD73 and CD39 ectonucleotidases in T cell differentiation: beyond immunosuppression. FEBS Lett. 589, 3454–3460 (2015).
Li, X. Y. et al. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov. 9, 1754–1773 (2019).
Tallon de Lara, P. et al. CD39+PD-1+CD8+ T cells mediate metastatic dormancy in breast cancer. Nat. Commun. 12, 769 (2021).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Czesnikiewicz-Guzik, M. et al. T cell subset-specific susceptibility to aging. Clin. Immunol. 127, 107–118 (2008).
Lee, K. A. et al. Characterization of age-associated exhausted CD8+ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell 15, 291–300 (2016).
Mogilenko, D. A. et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved hallmark of inflammaging. Immunity 54, 99–115 (2021).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019).
Fang, F. et al. The cell-surface 5′-nucleotidase CD73 defines a functional T memory cell subset that declines with age. Cell Rep. 37, 109981 (2021).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin µ chain gene. Nature 350, 423–426 (1991).
Bhandarkar, V., Dinter, T. & Spranger, S. Architects of immunity: how dendritic cells shape CD8+ T cell fate in cancer. Sci. Immunol. 10, eadf4726 (2025).
Overwijk, W. W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003).
Shevchenko, I. et al. Enhanced expression of CD39 and CD73 on T cells in the regulation of anti-tumor immune responses. Oncoimmunology 9, 1744946 (2020).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Tsui, C. et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 609, 354–360 (2022).
Jin, Y. et al. Expression of Id3 represses exhaustion of anti-tumor CD8 T cells in liver cancer. Mol. Immunol. 144, 117–126 (2022).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789 (2019).
Park, S. L. et al. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature 565, 366–371 (2019).
Biragyn, A. et al. Tumor-associated embryonic antigen-expressing vaccines that target CCR6 elicit potent CD8+ T cell-mediated protective and therapeutic antitumor immunity. J. Immunol. 179, 1381–1388 (2007).
Antony, P. A. et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol 174, 2591–2601 (2005).
Quezada, S. A. et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207, 637–650 (2010).
Muller, C. E. et al. Polyoxometalates—a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg. Med. Chem. Lett. 16, 5943–5947 (2006).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Ngiow, S. F. et al. A threshold level of intratumor CD8+ T-cell PD1 expression dictates therapeutic response to anti-PD1. Cancer Res. 75, 3800–3811 (2015).
Ciavattone, N. G. et al. Evaluating immunotherapeutic outcomes in triple-negative breast cancer with a cholesterol radiotracer in mice. JCI Insight 9, e175320 (2024).
Chalmin, F. et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36, 362–373 (2012).
Jing, Y. et al. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 70, 777–784 (2009).
Della Bella, S. et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 122, 220–228 (2007).
Rivera, A., Chen, C. C., Ron, N., Dougherty, J. P. & Ron, Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol. 13, 1583–1593 (2001).
von Bergwelt-Baildon, M. S. et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 99, 3319–3325 (2002).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Hellstrom, K. E. & Hellstrom, I. From the Hellstrom paradox toward cancer cure. Prog. Mol. Biol. Transl. Sci. 164, 1–24 (2019).
Olkhanud, P. B. et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 69, 5996–6004 (2009).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Ouyang, J. F., Kamaraj, U. S., Cao, E. Y. & Rackham, O. J. L. ShinyCell: simple and sharable visualization of single-cell gene expression data. Bioinformatics 37, 3374–3376 (2021).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb.) 2, 100141 (2021).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21 29 21–21 29 29 (2015).
Acknowledgements
We are grateful to C. Dunn (NIA) for help in cell sorting. This research was supported by the Intramural Research Program of the NIA, NIH and the Samuel Waxman Cancer Research Foundation grant U01 CA236979.
Author information
Authors and Affiliations
Contributions
M.B. designed and performed the research and collected and analyzed data. K.K., E.R., F.-Z.B. and C.C. performed experiments. B.P., N.G., H.I.-A., Z.D. and I.B. analyzed data and provided critical interpretation. D.S., L.F., R.S., S.S., S.O. and M.G. provided materials and analyzed patient data. A.B. wrote the manuscript and conceived, designed and supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Flow cytometry analysis of naïve T cells from young and aged WT mice.
(a) Representative gating strategy used for FACS staining of T cells. (b) Numbers (#) of CD8+ T cells and (c) numbers (#, left) and frequency (%, right) of CD4+ T cells in various tissues of Y-WT and A-WT mice. (d) Representative dot-plots of IFNγ and TNFα expression (intracellular FACS staining) in LN CD4+ and CD8+ T cells and (e) summary results (frequency, left; and numbers, right) of IFNγ+, GzmB+, IL10+, IL17+, TNFα+ CD4+ and CD8+ cells in indicated tissues of Y-WT and A-WT mice. (f) Heatmap of surface proteins expression in FACS-stained CD4+ and CD8+ T cells using a modified LEGENDScreen™ Mouse PE Kit. (g) Gating strategy used for quantification of exhaustion-associated markers in CD8+ T cells. (h) Frequency of CD8+ T cells expressing exhaustion markers in PeC; and (i and j) representative dot plots for expression of Tox1 in indicated subpopulation of LN CD8+ cells with or without expression of Ly108 and CD69 (i), and CD101 and PD1 (j). In b, c, e and h, shown is mean number or frequency ± SEM, where each symbol is for a single mouse, n = 5-10 (b, c), n = 5 (e), and n = 5-6 (h) mice per group. Experiments were reproduced three times. * P < 0.05**; P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test.
Extended Data Fig. 2 Aging dysregulates T cells in a B-cell dependent manner.
(a) Frequency (upper) and numbers (lower panels) of IFNg+TNFa+ CD4+ and CD8+ cells in spleen of Y- and A- WT and BKO mice. (b) Frequency of splenic CD8+ T cells expressing CD39, Blimp1, ST2, CD103, CD73, KLRG1, TNFR2, and (c) exhaustion markers. (d–f) Representative dot plots of LN CD8+ T cells expressing Ly108 and CD69 (d), CD101 and Tox (e); CD39 and CD73 (f). Summary quantifications (frequency) of dot plots data are shown in the lower panel (d) or on the right (e). (g) Representative dot plot and frequency of LN CD8+ T cells expressing CD39 and CD73 in young (Y) and aged (A) BALB/cByJ mice. (h, i) Representative hierarchical dot plots showing expression of CD62L and CD44 in CD39 and CD73 sub-populations of CD8+ T cells from LN of Y-WT, A-WT, and A-BKO mice (h) and of exhaustion markers in Ly108 and CD69 expressing DP or SP39 cells in LN of A-WT mice (i). Shown are mean numbers or frequency ± SEM, where each symbol is for a single mouse, n = 3-6 mice per group (a–e and g). Experiments were reproduced three times. ** P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test and Kruskal-Wallis test.
Extended Data Fig. 3 B cells induce DP CD8+ T cells by presenting cognate antigens.
Representative contour plot showing that in vitro stimulation with PeC B cells from A-WT (to a lesser extent Y-WT) pulsed with cognate (SIINFEKL), but not irrelevant (gp100) peptide, induce CD39 and CD73 (a) and CD39 and PD1 (b) in CD8+ T cells from young mice. Representative heatmap statistic dot plot (c) and histograms for CD73, CD39, and PD1 surface expression (right panel, d) in OT-I CD8+ T cells are shown together with experimental layout (left panel, d). These experiments were reproduced three times. (e and f), Activation of young OT-1 CD8+ T cells stimulated with DCs (from spleen of Y- or A-WT mice) pulsed with cognate (SIINFEKL) and irrelevant (gp100) peptide. In e, Y and A are for young and aged DCs. Representative histograms for surface expression CD39, CD73, and PD1 shown in OT-1 CD8+ T cells (f). Similar results were also generated using pmel mouse CD8+ T cells stimulated with DCs from spleen or PeC of Y-and A-WT mice, but pulsed with gp10025-32 peptide (not depicted). Shown is mean frequency ± SEM (e), where each symbol is for a single mouse, n = 5-12 mice per group (e). Experiments in a–d were reproduced three times. ** P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test and Kruskal-Wallis test.
Extended Data Fig. 4 Phenotype of CD8 + TILs in mice with AT3 and B16 tumors.
(a) Representative spectrum plots of CD39+CD73+ CD8+ T cells in dLNs of Y- and A- C57BL/6J mice with B16 and AT3 tumors and (b) frequency of CD39+CD73+ CD8+ T cells in dLNs of BALB/cByJ mice with 4T1.2 and EMT6 tumors. (c–f) Comparative contour plots for co-expression of selected surface markers in DP8 and SP39 cells in dLNs (c and d) and TILs (e and f) of Y- (upper) and A- (lower panels) mice with AT3 (c, e) and B16 (d, f) tumors. Red and Blue arrows and numbers are for expression of indicated markers in CD39+CD73+ (DP8) and CD39+ CD73− (SP39) cells, respectively. (g and h) DP8 cells acquire exhausted phenotype in TME. Sorted LN and splenic DP8 cells from Y-WT mice were labeled with eFluor450 and i.v. injected into A-WT mice with 12-day old AT3 tumor (h-i, Schema of the experiment (Image was created using BioRender.com) and gating strategy for cells used, h-ii), Representative dot plots showing the expression of indicated markers in the host DP8 and SP39 cells in LNs of tumor-free (LN naïve) and tumor-bearing A-WT mice (ndLN, dLN) and the tumor tissue (TIL, g). (Shown is mean number or frequency ± SEM (b), where each symbol is for a single mouse, n = 5-12 (b) mice per group. Experiments were reproduced three times. ** P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test and Kruskal-Wallis test.
Extended Data Fig. 5 Phenotype of CD8 + TILs in mice with tumors.
(a–d) TIL DP8 cells only acquire exhausted phenotype at the late stage of tumor. (a) AT3 tumor growth (mm2) in Y- and A-WT mice; red squares indicate the days when tumors were processed for TIL phenotyping; (b) representative contour plots (left) and quantification (right panel) showing expression of exhaustion markers in DP8 cells in dLNs and TILs at different timepoints of tumor growth. (c) Frequency of DP8 cells in different memory subsets and (d) with CD3 or CD8 cells in dLNs and TILs of Y- and A- C57BL/6J mice at different time points of AT3 tumor growth. (e) unlike TIL CD8 + T cells, A-AT3 tumor LN CD8 T cells upregulate expression of effector molecules compared to naïve mouse LN CD8+ T cells. (f-i) Growth of i.p. challenged ID8-RFP ovarian cancer is increased in PeC of aged C57BL/6J mice (f) together with CD8+ T cells (g) and DP8 cells (h, numbers; and i, frequency). Shown is mean number or frequency ± SEM (f–i), where each symbol is for a single mouse, n = 5-6 (e), n = 10-11 (f, g), and n = 2-6 (h-i) mice per group. Experiments were reproduced three times. ** P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test and Kruskal-Wallis test.
Extended Data Fig. 6 Transcriptome (a-h) and epigenetic (i-j) profiles of CD8+ T cells in mice with AT3 and B16 tumor.
scRNA-seq analysis of CD8+ T cells from tumor tissues of young and aged mice with AT3 and B16 tumors (n = 3 mice per group; Y-AT3, A-AT3, Y-B16, and A-B16). (a) Volcano plots showing up and downregulated DEGs in CD8+ T cell subclusters of A-AT3 compared to Y-AT3 and (b) heatmaps showing significantly upregulated genes in each cluster, by group. Venn-diagram of up (left) and downregulated (right) DEGs in CD8+ T cells (c) and DP8 cells (f); Pathway analysis of up (left) or downregulated (right) DEGs in A-AT3 versus Y-AT3 (d) and A-B16 and Y-B16 (e). Representative heat maps of group specific significant DEGs of DP8 cells (g); and Violin plots showing dysfunctional (upper) and cytotoxic (lower panel) gene scores in SP39 cells (j). (i) FACS-sort purity result of CD8+ T cells isolated for ATAC-seq analysis. Circles are the cells isolated, such as LN CD8+ T cells from Y- and A-WT mice, DP8 cells from dLN and TIL of A-AT3 mice, and SP39 cells from TIL of Y-B16 mice. (i) Heatmap illustrating the average ATAC-seq peak intensity (top) of the indicated genes. Rows represent averaged z-scores. All ATAC-seq data are representative of two or three biologically independent samples.
Extended Data Fig. 7 Representative expression profiles in subclusters of TIL CD8+ cells.
(a) scRNA-seq UMAP of indicated transcripts in the 4 subclusters of TIL CD8+ T cells from A-AT3 mice; (b) representative histogram shows that CXCR6 is preferentially expressed and increased on DP8 cells but decreased in SP39 cells; (c) Surface CXCR6 expression (frequency) is upregulated in DP8 cells in PB (blood) and LNs by middle age of WT mice in a B-cell dependent manner; (d) representative histograms of surface markers in DP8 cells from A-AT3, Y-AT3, A-B16 and Y-B16 mice Shown is mean number or frequency ± SEM (c), where each symbol is for a single mouse, n = 3-5 (d) mice per group. Experiments were reproduced three times. ** P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test and Kruskal-Wallis test.
Extended Data Fig. 8 Immunosuppressive CXCR6+ DP8 cells infiltrate AT3 tumor.
(a) CD103+CD8+ T cells decrease in TILs of A-AT3 mice. (b) Tumor weight (g, at day 35 post tumor challenge) in A-At3 mice treated with Abs to deplete CD4+ or CD8+ T cells or control IgG; (c) CXCL16 shRNA knockdown in the AT3 cells decrease tumor growth (left panel) and production of CXCL16 (ELISA quantification of CM of indicated cells, right panel); (d) Representative dot plot showing presence of CD4+ and CD8+ T cells in TILs of A-AT3 mice; (e and f) CD3+ T cells (eFluor450 labelled and FACS-sorted from spleen of young mice) were stimulated with anti-CD3/28 beads in the presence or absence of sorted splenic DP8 and CD73+CD39− CD8+ T cells from Y-WT and A-WT (e) or spleen of Y-AT3 and A-WT (f) mice. Shown is frequency of proliferated CD4+ T cells after 5 days co-culture. (g and h) DP8 cells suppress target CD4+ T cells using CD39. The same suppression assay as in e was performed in the presence or absence of 10 µM POM1 (inhibitor of CD39 activity; (h) POM1 does not reduce viability of target CD3+ T cells. Shown is frequency ± SEM (a, b,c, e-i); (i) While adoptively transferred CD8+ T cells from Y-WT reduces AT3 tumor in A-Rag KO mice, A-WT CD8+ T cells failed to do so. Injection diagrams were created in BioRender.com and numbers represent the days of injection. Each symbol is for a single mouse, n = 7-8 (a), n = 3-7 (b), n = 6-9 (c), n = 3-6 (e–g) and n = 6-7 (i) mice per group. Experiments were reproduced three times. * P < 0.05; ** P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test and Kruskal-Wallis test.
Extended Data Fig. 9 DP8-like cells are present in humans.
Representative gating strategy of exhaustion markers expressed on DP and SP38 human CD8+ cells (a) and frequency of exhausted-like SP39 in CD8+ T cells from human PBMCs activated for 12 days with anti-CD3/28 Ab-beads (b). (c) Normalized enrichment score (NES) of GSEA for top 300 genes from human breast cancer TIL CD8+ T cells (GSE110686) in A-AT3 DP8 versus Y-AT3 DP8 and A-AT3 DP8 versus A-B16 DP8. (d) Summary abstract showing that healthy aging increases CXCR6+ DP8 cells via B cells presenting cognate antigens, which presumably occurs in LNs. Upon recruitment into the tumor tissue expressing CXCL16, CXCR6+ DP8 cells upregulate exhausted-like phenotype and importantly, promote tumor growth via inhibiting antitumor effector cells at least in part by hydrolyzing eATP and possibly generating immunosuppressive molecules. Shown is frequency ± SEM (b), where each symbol is for a single donor, n = 6-8 people per group. Experiments were reproduced three times. ** P < 0.01; *** P < 0.001 in unpaired Mann-Whitney test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Legends.
Supplementary Tables 1–6
Combined supplementary tables.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Bodogai, M., Park, B., Braikia, FZ. et al. A distinct population of CD8+ T cells expressing CD39 and CD73 accumulates with age and supports cancer progression. Nat Aging 5, 2055–2069 (2025). https://doi.org/10.1038/s43587-025-00966-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-025-00966-3
This article is cited by
-
Respiratory virus immune response in the aged host
Immunity & Ageing (2026)