Abstract
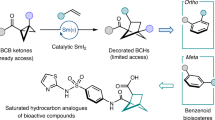
The high fraction of sp3-hybridized carbon atom (Fsp3) character of cyclobutane derivatives renders them as highly promising bioisosteres for otherwise typically flat arenes. Here, to address the current needs in medicinal chemistry for Fsp3-rich molecules, we disclose a distinct strategy that exploits the merger of C–C scission in bicyclo[1.1.0]butanes (BCBs) with ruthenium-catalysed remote C−H functionalization of heteroarenes, affording densely substituted cyclobutanes in a chemo-controlled manner. This approach enabled the rapid and efficient synthesis of versatile tri- and tetrasubstituted cyclobutanes by coupling a wide range of mono- or disubstituted BCBs with heteroarenes and alkyl halides under mild reaction conditions, featuring ample substrate scope. The C–C/C–H functionalization was ensured by a multifunctional ruthenium(II) catalyst that enabled ruthenacycle-mediated halogen-atom transfer (Ru-XAT), as well as the selective functionalization of BCBs by strain release. Experimental and computational mechanistic studies unravelled a multi-catalysis manifold, while the C–H/C–C functionalization strategy allowed for telescoping late-stage modification.

Similar content being viewed by others
Main
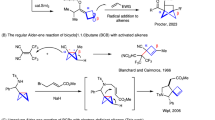
In the quest of ‘escaping from flatland’1,2,3,4,5,6,7,8, highly functionalized cyclobutanes with unique puckered linear geometry have received increasing attention in medicinal chemistry. The fraction of sp3-hybridized carbon atoms (Fsp3) was identified as a key descriptor of drug likeness, and the increased Fsp3 in cyclobutanes renders them as a privileged motif for isosteres in drug design9. The incorporation of cyclobutane scaffolds often enhances the physicochemical and pharmacokinetic properties of drug molecules. In contrast to usually flat arene rings, polysubstituted cyclobutanes with high Fsp3 can provide, among others, improved solubility due to their nonplanar substituent vectors. Indeed, several pharmaceuticals featuring 1,3-bifunctional cyclobutanes have been clinically tested, such as PF-03654746 (ref. 10), NVP-ADW 742 (ref. 11), NK1 selective antagonists12 and Linsitinib13, translating into a strong need for innovative cyclobutane syntheses (Fig. 1a). However, in contrast to rather well-established bifunctionalization of bicyclo[1.1.0]pentanes (BCPs)14,15,16,17,18 and ring expansions of bicyclo[1.1.0]butanes (BCBs)19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 (Fig. 1b), strategies to access structurally complex cyclobutanes are, unfortunately, scarce. Thus far, these syntheses are limited to radical or nucleophilic additions to BCBs, typically resulting in mono- or disubstituted cyclobutanes42,43,44,45,46,47,48,49, with only few examples for densely substituted cyclobutanes50,51,52,53,54. Furthermore, the aforementioned transformations are largely limited to rather harsh conditions and elements of prefunctionalization on the coupling partner, while the merger of C–C activations55,56,57,58,59,60 of BCBs with remote C–H activation61 has thus far proven elusive. To address these current topical needs, we have now developed the merger of BCBs C–C functionalization with remote C–H functionalization62,63,64,65,66,67,68,69,70,71,72,73 by a single, yet powerful, ruthenium(II) catalyst. Salient features of our findings include (1) versatile 1,3-difunctionalization of BCBs for tri- and tetrasubstituted Fsp3-rich cyclobutanes; (2) a single ruthenium complex for a multi-catalysis manifold including an efficient ruthenacycle-mediated halogen-atom transfer (Ru-XAT) process, C–C scission and meta-C–H functionalization; (3) exceedingly mild reaction conditions and (4) outstanding chemo- and site selectivities (Fig. 1c).
a, Selected drug molecules containing 1,3-difunctionalized cyclobutane skeleton. b, Current strategies50,51,52,53,54 for the synthesis of highly functionalized cyclobutanes via strain release. c, Our hypothesis on the 1,3-difunctionalization of BCBs by remote C−H activation to access valuable 1,1,3-trisubstituted and 1,1,3,3-tetrasubstituted cyclobutanes via a Ru-XAT process. Ar, aryl.; Het, heteroarenes.
Results and discussion
Optimization studies
We initiated our studies on BCBs 1,3-difunctionalization through remote C–H activation, with benzyl-bicyclo[1.1.0]butane-1-carboxylate (1a), 2-phenylpyridine (2a) and ethyl-2-bromo-2,2-difluoroacetate (3a) as the model substrates (Table 1). We were pleased to find that the desired product 4 was efficiently obtained in 75% isolated yield with Ru(O2CMes)2(p-cymene) as the catalyst and P(4-CF3C6H4)3 (Table 1, entry 1). Next, a series of phosphines, as well as alternative ligands such as bipyridine (bpy) and N-heterocyclic carbene (NHC), were tested, and P(4-CF3C6H4)3 was found to be superior (Table 1, entries 2 and 3). Ru(OAc)2(p-cymene) gave the desired product 4 with 52% yield, while [RuCl2(p-cymene)]2 was ineffective (Table 1, entry 4), highlighting the importance of carboxylate assistance in the C–H ruthenation74. [Ru(tBuCN)5(H2O)](BF4)275 as a precatalyst gave inferior results (Table 1, entry 5). Control experiments revealed the crucial role of the ruthenium catalyst and the phosphine ligand for the BCBs C–C cleavage difunctionalization (Table 1, entry 6).
With the optimized reaction conditions in hand, we subsequently evaluated the viable substrate scope for the bifunctionalization of BCBs 1 with differently substituted heteroarenes 2 (Fig. 2). Arenes with distinct electronic features and substituents, such as fluorine, thioether, bromine, ester and keto groups, were fully tolerated by the versatile catalyst (5–29). Transformable pyrazoles, ketimine and oxazolines could be employed to guarantee meta-selectivity (17–24, 30). Drug-relevant motifs, such as diazepam, purines and nucleoside proved to be viable for the ruthenium-catalysed BCBs difunctionalization (26–29).
Reaction conditions: 1 (0.3 mmol), 2 (3.0 equiv.), 3 (3.0 equiv.), [Ru(O2CMes)2(p-cymene)] (10 mol%), P(4-CF3C6H4)3 (10 mol%), Na2CO3 (2.0 equiv.), 1,4-dioxane (2.0 ml), 65 °C, 24–48 h. All yields are isolated yields. The ratios of the diastereomers (cis and trans) were determined by 1H-NMR spectroscopy or isolated yield. aWork-up with 3 N HCl and stirring for a further 3 h. Note that the presented structures are the major isomers. Bn, benzyl; Mes, mesityl; Py, pyridyl; Pym, pyrimidinyl.
A series of substituted BCB esters 1 furnished the 1,1,3-trisubstituted cyclobutanes 31–37 (Fig. 3). Thus, BCBs featuring sensitive functional groups, including ester, thiophene, ketone, amide and sulfone (38–41) were efficiently converted to desired products. Furthermore, disubstituted BCBs were identified as amenable substrates (42–47). Here, disubstituted BCBs favoured to form a benzylic radical rather than a tertiary radical, chemo-selectively delivering diarylcyclobutane motifs. Likewise, a wide range of alkyl halides, such as perfluoroalkane halides, fluorine-free alkyl bromide, monofluoroalkyl bromide and difluoroalkyl amides were tolerated to give the desired cyclobutanes 48–57.
Reaction conditions: 1 (0.3 mmol), 2 (3.0 equiv.), 3 (3.0 equiv.), [Ru(O2CMes)2(p-cymene)] (10 mol%), P(4-CF3C6H4)3 (10 mol%), Na2CO3 (2.0 equiv.), 1,4-dioxane (2.0 mL), 65 °C, 24–48 h. All yields are isolated yields. The ratios of the diastereomers were determined by 1H-NMR spectroscopy or isolated yields. Note that the presented structures are the major isomers. In the case of the 1,1,3-trisubstituted cyclobutanes, the cis structure is the major, while for the 1,1,3,3-tetrasubstituted cyclobutanes, the (1r,3r) structure is the major, which was confirmed by X-ray crystallographic analysis (see the X-ray structure of compound 62 in Supplementary Data 6 for details). Naph, 2-naphthyl group.
Gram-scale and late-stage derivatization
To demonstrate the practical utility of our BCBs C–C scission/remote activation strategy, cyclobutane 4 was prepared at gram scale with comparable efficacy (Fig. 4a). The site selectivity ensured that pyrimidine, oxazoline and ketimine (vide infra) as well as pyridine and pyrazole were efficiently diversified, extending the viable portfolio (Fig. 4b). Further, a triple activation manifold proved viable in terms of ruthenium(II)-catalysed C−C/meta-C−H and ortho-photo-induced C−H activation (58 and 59).
a, Gram-scale reaction gave the desired product 4 in high yield. b, Various downstream functionalization of products 4, 42 or 46. Cp*, 1,2,3,4,5-pentamethylcyclopentadienyl; DCE, 1,2-dichloroethane; LED, light emitting diode; MMA, methyl methacrylate; Piv, pivaloyl; PMP, p-methoxyphenyl; r.t., room temperature; THF, tetrahydrofuran.
DFT calculation and mechanism studies
To gain insights into the reaction mechanism, the site selectivity of radical addition at the two possible BCB sites was probed by means of density functional theory (DFT) calculations (Fig. 5a; see Supplementary Figs. 1, 2, 7 and 8 for details). In the case of monosubstituted BCB 1a, the difluoroalkyl radical preferentially attacked at the unsubstituted carbon, leading to the formation of a more thermodynamically stable tertiary radical. The shorter bridge C−C distance (1.61 Å) and the longer C−CRF distance (2.21 Å) in TS3-s4, compared with TS4-s2, indicated that the radical attack at the unsubstituted site proceeded through an earlier transition state that structurally resembled the starting BCB 1a. In the case of radical addition to the disubstituted BCB 1m, the ester-substituted site is favoured, resulting in the formation of thermodynamically stable benzylic radical. During the cleavage of the σ-bridge bond in disubstituted BCB 1m, TS7-s1 exhibited an earlier transition state characteristic, evidenced by the slightly elongated C–C bridge distance of BCB 1a (1.63 Å in TS7-s1 and 1.64 Å in TS8-s1) and the relatively larger C–C distances between the BCB 1m and the difluoroalkyl radical (2.25 Å in TS7-s1 and 2.21 Å in TS8-s1). The presynthesized p-cymene-free ruthenacycle 66 yielded the desired product 4 in the presence of MesCO2H and phosphine ligand (Fig. 5b). The control experiment without a phosphine ligand as well as with alternative ligands, such as bpy or NHC, in the optimization table failed to provide the desired product, highlighting the essential role of the phosphine ligand assistance76,77,78,79. The key role of the phosphine ligand was further demonstrated by attempting this transformation with carboxylate-free ruthenium(II) phosphine complex 67, yielding the desired product in high yield when MesCO2H was added (Fig. 5c). The carboxylate-free ruthenium(II) phosphine complex 67 could afford the desired product only in 14% yield even in the absence of MesCO₂H (Fig. 5c). Additionally, radical trapping experiments validated the radical mechanism (see Supplementary Data 7.1.1 for details). On the basis of our mechanism and computational findings, a plausible catalytic cycle is put forwards in Fig. 5d, featuring ruthenacycle A in a ruthenacycle-mediated XAT process to furnish ruthenium(III) intermediate B with an energy barrier of 14.9 kcal mol−1. The thus formed alkyl radical 3R attacks the BCB 1a at the unsubstituted site to induce strain-release C–C scission with an energy barrier of 15.9 kcal mol−1, forming the tertiary radical C. Species C reacts at the ruthena(III)cycle B para to the CAr–Ru bond, delivering the stabilized singlet metallacycle D. Here, the formation of the cis product is favoured over the trans product by 1.4 kcal mol−1. The subsequent rearomatization and proto-demetallation releases the desired cyclobutane 4, thereby regenerating the catalytically active ruthenium(II) species F.
a, Computed relative Gibbs free energies (ΔG338.15) in kcal mol−1 for radical attack at the monosubstituted BCB 1a and disubstituted BCB 1m were conducted at the B3LYP-D3BJ/def2-TZVP-SMD(1,4-dioxane)//PBE0-D3BJ/def2-SVP level of theory. b, p-Cymene-free ruthenacycle complex 66 as the catalyst. c, Carboxylate-free ruthenium(II) phosphine complex 67 as the catalyst. d, Proposed catalytic cycle associated with relative Gibbs free energies (ΔG338.15) in kcal mol−1 based on our mechanism studies and DFT calculation. RF, difluoroalkyl; P, P(4-CF3C6H4)3; L, 1,4-dioxane, 2a and so on.
Conclusions
We have achieved the merger of BCBs C–C activation with remote meta-C–H functionalization by a multipotent ruthenium catalyst. The double activation proceeded in a highly chemo- and position-selective fashion and provided access to densely decorated Fsp3-rich cyclobutanes in a single step. Mechanistic studies were suggestive of a Ru-XAT process enabling chemo-selective BCBs opening and meta-diversification.
Methods
General methods for 1,3-difunctionalization of BCBs enabled by ruthenium-catalysed remote C–H activation
The general procedure for 1,3-difunctionalization of BCBs was as follows: [Ru(O2CMes)2(p-cymene)] (16.8 mg, 10.0 mol%), P(4-CF3C6H4)3 (14.0 mg, 10.0 mol%), Na2CO3 (64 mg, 0.6 mmol, 2.0 equiv.), 1,4-dioxane (2.0 ml), 1 (0.3 mmol, 1.0 equiv.), 2 (0.9 mmol, 3.0 equiv.) and 3 (0.9 mmol, 3.0 equiv.) were added into an oven-dried 20 ml pressure tube. The reaction mixture was stirred at 65 °C for 24–48 h. After cooling to ambient temperature, the mixture was purified by column chromatography on silica gel to afford the corresponding cyclobutanes 4–57.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. All other requests for materials and information should be addressed to the corresponding authors. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2344919 (15), 2344920 (11), 2344921 (16), 2344922 (4) and 2344923 (62). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Nicolaou, K. C. et al. Synthesis and biopharmaceutical evaluation of imatinib analogues featuring unusual structural motifs. ChemMedChem 11, 31–37 (2016).
Golfmann, M. & Walker, J. C. L. Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis. Commun. Chem. 6, 9 (2023).
Kelly, C. B., Milligan, J. A., Tilley, L. J. & Sodano, T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci. 13, 11721–11737 (2022).
Bellotti, P. & Glorius, F. Strain-release photocatalysis. J. Am. Chem. Soc. 145, 20716–20732 (2023).
Turkowska, J., Durka, J. & Gryko, D. Strain release—an old tool for new transformations. Chem. Commun. 56, 5718–5734 (2020).
Tsien, J. et al. Three-dimensional saturated C(sp3)-rich bioisosteres for benzene. Nat. Rev. Chem. 8, 605–627 (2024).
Bauer, M. R. et al. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 12, 448–471 (2021).
Wager, T. T. et al. Discovery of two clinical histamine H3 receptor antagonists: trans-N-ethyl-3-fluoro-3-[3-fluoro-4-(pyrrolidinylmethyl)-phenyl]cyclobutanecarboxamide (PF-03654746) and trans-3-fluoro-3-[3-fluoro-4-(pyrrolidin-1-ylmethyl)phenyl]-N-(2-methylpropyl)cyclobutanecarboxamide (PF-03654764). J. Med. Chem. 54, 7602–7620 (2011).
Warshamana-Greene, G. S. et al. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin. Cancer Res. 11, 1563–1571 (2005).
Wrobleski, M. L. et al. Cyclobutane derivatives as potent NK1 selective antagonists. Bioorg. Med. Chem. Lett. 16, 3859–3863 (2006).
Mulvihill, M. J. et al. A selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med. Chem. 1, 1153–1171 (2009).
Dong, W. et al. Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane. Nat. Chem. 14, 1068–1077 (2022).
Huang, W., Keess, S. & Molander, G. A. Dicarbofunctionalization of [1.1.1]propellane enabled by nickel/photoredox dual catalysis: one-step multicomponent strategy for the synthesis of BCP-aryl derivatives. J. Am. Chem. Soc. 144, 12961–12969 (2022).
Yu, I. F. et al. Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes. Nat. Chem. 15, 685–693 (2023).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Nugent, J. et al. Synthesis of all-carbon disubstituted bicyclo[1.1.1]pentanes by iron-catalyzed Kumada cross-coupling. Angew. Chem. Int. Ed. 59, 11866–11870 (2020).
Wang, H. et al. Dearomative ring expansion of thiophenes by bicyclobutane insertion. Science 381, 75–81 (2023).
Liang, Y., Nematswerani, R., Daniliuc, C. G. & Glorius, F. Silver-enabled cycloaddition of bicyclobutanes with isocyanides for the synthesis of polysubstituted 3-azabicyclo[3.1.1]heptanes. Angew. Chem. Int. Ed. 63, e202402730 (2024).
Denisenko, A. et al. 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring. Nat. Chem. 15, 1155–1163 (2023).
Dhake, K. et al. Beyond bioisosteres: divergent synthesis of azabicyclohexanes and cyclobutenyl amines from bicyclobutanes. Angew. Chem. Int. Ed. 61, e202204719 (2022).
Zhang, J., Su, J.-Y., Zheng, H., Li, H. & Deng, W.-P. Eu(OTf)3-catalyzed formal dipolar [4π+2σ] cycloaddition of bicyclo-[1.1.0]butanes with nitrones: access to polysubstituted 2-oxa-3-azabicyclo[3.1.1]heptanes. Angew. Chem. Int. Ed. 63, e202318476 (2024).
Yang, Y. et al. Programmable late-stage functionalization of bridge-substituted bicyclo[1.1.1]pentane bis-boronates. Nat. Chem. 16, 285–293 (2024).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Bychek, R. M. et al. Difluoro-substituted bicyclo[1.1.1]pentanes for medicinal chemistry: design, synthesis, and characterization. J. Org. Chem. 84, 15106–15117 (2019).
Agasti, S. et al. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Nat. Chem. 15, 535–541 (2023).
Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Guo, R. et al. Strain-release [2π+2σ] cycloadditions for the synthesis of bicyclo[2.1.1]hexanes initiated by energy transfer. J. Am. Chem. Soc. 144, 7988–7994 (2022).
Zheng, Y. et al. Photochemical intermolecular [3σ+2σ]-cycloaddition for the construction of aminobicyclo[3.1.1]heptanes. J. Am. Chem. Soc. 144, 23685–23690 (2022).
Ni, D. et al. Intermolecular formal cycloaddition of indoles with bicyclo[1.1.0]butanes by Lewis acid catalysis. Angew. Chem. Int. Ed. 62, e202308606 (2023).
Radhoff, N., Daniliuc, C. G. & Studer, A. Lewis acid catalyzed formal (3+2)-cycloaddition of bicyclo[1.1.0]butanes with ketenes. Angew. Chem. Int. Ed. 62, e20230477 (2023).
Dutta, S. et al. Double strain-release [2π+2σ]-photocycloaddition. J. Am. Chem. Soc. 146, 5232–5241 (2024).
Dutta, S. et al. Photoredox-enabled dearomative [2π+2σ] cycloaddition of phenols. J. Am. Chem. Soc. 146, 2789–2797 (2024).
de Robichon, M. et al. Enantioselective, intermolecular [π2+σ2] photocycloaddition reactions of 2(1H)-quinolones and bicyclo[1.1.0]butanes. J. Am. Chem. Soc. 145, 24466–24470 (2023).
Tang, L. et al. Silver-catalyzed dearomative [2π+2σ] cycloadditions of indoles with bicyclobutanes: access to indoline fused bicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed. 62, e202310066 (2022).
Liu, Y. et al. Pyridine-boryl radical-catalyzed [2π+2σ] cycloaddition of bicyclo[1.1.0]-butanes with alkenes. ACS Catal. 13, 5096–5103 (2023).
Nguyen, T. V. T., Bossonnet, A., Wodrich, M. D. & Waser, J. Photocatalyzed [2σ+2σ] and [2σ+2π] cycloadditions for the synthesis of bicyclo[3.1.1]heptanes and 5- or 6-membered carbocycles. J. Am. Chem. Soc. 145, 25411–25421 (2023).
Yu, T. et al. Selective [2σ+2σ]-cycloaddition enabled by boronyl radical catalysis: synthesis of highly substituted bicyclo[3.1.1]heptanes. J. Am. Chem. Soc. 145, 4304–4310 (2023).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
Iida, T. et al. Practical and facile access to bicyclo[3.1.1]heptanes: potent bioisosteres of meta-substituted benzenes. J. Am. Chem. Soc. 144, 21848–21852 (2022).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Lopchuk, J. M. et al. Strain-release heteroatom functionalization: development, scope, and stereospecificity. J. Am. Chem. Soc. 139, 3209–3226 (2017).
Ernouf, G., Chirkin, E., Rhyman, L., Ramasami, P. & Cintrat, J. Photochemical strain-release-driven cyclobutylation of C(sp3)-centered radicals. Angew. Chem. Int. Ed. 59, 2618–2622 (2020).
Ociepa, M., Wierzba, A. J., Turkowska, J. & Gryko, D. Polarity-reversal strategy for the functionalization of electrophilic strained molecules via light-driven cobalt catalysis. J. Am. Chem. Soc. 142, 5355–5361 (2020).
Kang, G. et al. Transannular C–H functionalization of cycloalkane carboxylic acids. Nature 618, 519–525 (2023).
Fan, Z., Strassfeld, D. A., Park, H. S., Wu, K. & Yu, J.-Q. Formal γ-C–H functionalization of cyclobutyl ketones: synthesis of cis-1,3-difunctionalized cyclobutanes. Angew. Chem. Int. Ed. 62, e202303948 (2023).
Dasgupta, A. et al. Stereoselective alder-ene reactions of bicyclo[1.1.0]butanes: facile synthesis of cyclopropyl- and aryl-substituted cyclobutenes. J. Am. Chem. Soc. 146, 1196–1203 (2023).
Kraemer, Y. et al. Overcoming a radical polarity mismatch in strain-release pentafluorosulfanylation of [1.1.0]bicyclobutanes: an entryway to sulfone- and carbonyl-containing SF5-cyclobutanes. Angew. Chem. Int. Ed. 63, e202319930 (2024).
Silvi, M. & Aggarwal, V. K. Radical addition to strained σ-bonds enables the stereocontrolled synthesis of cyclobutyl boronic esters. J. Am. Chem. Soc. 141, 9511–9515 (2019).
Fawcett, A., Biberger, T. & Aggarwal, V. K. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem. 11, 117–122 (2019).
Bennett, S. H. et al. Difunctionalization of C–C σ-bonds enabled by the reaction of bicyclo[1.1.0]butyl boronate complexes with electrophiles: reaction development, scope, and stereochemical origins. J. Am. Chem. Soc. 142, 16766–16775 (2020).
Shen, H.-C. et al. Iridium-catalyzed asymmetric difunctionalization of C–C σ-bonds enabled by ring-strained boronate complexes. J. Am. Chem. Soc. 145, 16508–16516 (2023).
Wang, H. et al. Syn-selective difunctionalization of bicyclobutanes enabled by photoredox-mediated C–S σ-bond scission. J. Am. Chem. Soc. 145, 23771–23780 (2023).
Lusi, R. F., Perea, M. A. & Sarpong, R. C–C bond cleavage of α-pinene derivatives prepared from carvone as a general strategy for complex molecule synthesis. Acc. Chem. Res. 55, 746–758 (2022).
Murakami, M. & Ishida, N. Cleavage of carbon–carbon σ-bonds of four-membered rings. Chem. Rev. 121, 264–299 (2021).
Sokolova, O. O. & Bower, J. F. Selective carbon–carbon bond cleavage of cyclopropylamine derivatives. Chem. Rev. 121, 80–109 (2021).
Nairoukh, Z., Cormier, M. & Marek, I. Merging C–H and C–C bond cleavage in organic synthesis. Nat. Rev. Chem. 1, 0035 (2017).
Xu, Y. et al. Deacylative transformations of ketones via aromatization-promoted C–C bond activation. Nature 567, 373–378 (2019).
Murphy, S. K., Park, J.-W., Cruz, F. A. & Dong, V. M. Rh-catalyzed C–C bond cleavage by transfer hydroformylation. Science 347, 56–60 (2015).
Rogge, T. et al. C–H activation. Nat. Rev. Methods Primer 1, 43 (2021).
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Tang, R.-Y., Li, G. & Yu, J.-Q. Conformation-induced remote meta-C–H activation of amines. Nature 507, 215–220 (2014).
Wang, X. C. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015).
Shi, H. et al. Enantioselective remote meta-C–H arylation and alkylation via a chiral transient mediator. Nature 558, 581–585 (2018).
Kuninobu, Y. et al. A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem. 7, 712–717 (2015).
Robbins, D. W. & Hartwig, J. F. Sterically controlled alkylation of arenes through iridium-catalyzed C−H borylation. Angew. Chem. Int. Ed. 52, 933–937 (2013).
Ramadoss, B., Jin, Y., Asako, S. & Ilies, L. Remote steric control for undirected meta-selective C–H activation of arenes. Science 375, 658–663 (2022).
Saidi, O. et al. Ruthenium-catalyzed meta-sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 133, 19298–19301 (2011).
Hofmann, N. & Ackermann, L. meta-Selective C–H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc. 135, 5877–5884 (2013).
Wang, X.-G. et al. Three-component ruthenium-catalyzed direct meta-selective C–H activation of arenes: a new approach to the alkylarylation of alkenes. J. Am. Chem. Soc. 141, 13914–13922 (2019).
Wang, Y., Chen, S., Chen, X., Zangarelli, A. & Ackermann, L. Photo-induced ruthenium-catalyzed double remote C(sp2)–H/C(sp3)–H functionalizations by radical relay. Angew. Chem. Int. Ed. 61, e202205562 (2022).
Chen, S., Yuan, B., Wang, Y. & Ackermann, L. Ruthenium-catalyzed remote difunctionalization of nonactivated alkenes for double meta-C(sp2)–H/C-6(sp3)–H functionalization. Angew. Chem. Int. Ed. 62, e202301168 (2023).
Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev. 111, 1315–1345 (2011).
McArthur, G. et al. An air- and moisture-stable ruthenium precatalyst for diverse reactivity. Nat. Chem. https://doi.org/10.1038/s41557-024-01481-5 (2024).
Neogi, S. et al. Silylarylation of alkenes via meta-selective C–H activation of arenes under ruthenium/iron cooperative catalysis: mechanistic insights from combined experimental and computational studies. ACS Catal. 14, 4510–4522 (2024).
Ruan, Z. et al. Ruthenium(II)-catalyzed meta C–H mono- and difluoromethylations by phosphine/carboxylate cooperation. Angew. Chem. Int. Ed. 56, 2045–2049 (2017).
Gou, X. Y. et al. Ruthenium-catalyzed radical cyclization/meta-selective C–H alkylation of arenes via σ-activation strategy. ACS Catal. 11, 4263–4270 (2021).
Guillemard, L., Ackermann, L. & Johansson, M. J. Late-stage meta-C–H alkylation of pharmaceuticals to modulate biological properties and expedite molecular optimisation in a single step. Nat. Commun. 15, 3349 (2024).
Acknowledgements
The authors gratefully acknowledge support from the ERC Advanced Grant (no. 101021358) and the DFG (Gottfried Wilhelm Leibniz award to L.A.). The CSC scholarship (B.Y. and Z.X) are gratefully acknowledged. The authors thank C. Golz (University of Göttingen) for the assistance with the X-ray diffraction analysis, H. Frauendorf for mass spectrometry and M. John for help with NMR studies.
Funding
Open access funding provided by Georg-August-Universität Göttingen.
Author information
Authors and Affiliations
Contributions
L.A. conceived the project. S.C. designed the experiments and performed initial screening studies. S.C., Z.X. and X.-Y.G. performed synthetic experiments. B.Y. carried out DFT theoretical studies. S.C., B.Y. and L.A. wrote the paper. L.A. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Milan Gembicky, Masanobu Uchiyama and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, Tables 1–7 and experimental procedures, products characterization, late-stage derivatization, mechanism studies, computational details and NMR spectra.
Supplementary Data 1
Cartesian coordinates for all the calculated structures.
Supplementary Data 2
Crystallographic data for compound 4 (CCDC 2344922).
Supplementary Data 3
Crystallographic data for compound 11 (CCDC 2344920).
Supplementary Data 4
Crystallographic data for compound 15 (CCDC 2344919).
Supplementary Data 5
Crystallographic data for compound 16 (CCDC 2344921).
Supplementary Data 6
Crystallographic data for compound 62 (CCDC 2344923).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S., Xu, Z., Yuan, B. et al. Difunctionalization of bicyclo[1.1.0]butanes enabled by merging C−C cleavage and ruthenium-catalysed remote C−H activation. Nat. Synth 4, 655–663 (2025). https://doi.org/10.1038/s44160-025-00745-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00745-3