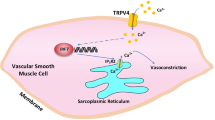
Abstract
Ionic signaling in smooth muscle cells (SMCs) is critical for vascular homeostasis. In this study, we untangled the role of the bifunctional TRPM7 channel kinase (chanzyme) in abdominal aortic aneurysm (AAA) pathogenesis. Comparing SMC-specific, macrophage-specific and endothelial cell–specific Trpm7 knockout, we revealed that SMC-specific Trpm7 deficiency protected mice from AAA in two distinct preclinical models of the disease. We showed that the TRPM7 channel activity increased the Ca2+ and Zn2+ influx and the Ca2+/calcineurin/CRTC2/CREB-dependent and Zn2+/MTF1-dependent Mmp2 transcription. Repurposing the clinical drug FTY720 to prevent and treat AAA resulted in improved aortic phenotypes through inhibition of TRPM7 channel activity. This study highlights the ionic mechanisms underlying AAA, identifies TRPM7 as a potential therapeutic target and suggests that blocking TRPM7 channels could be a viable strategy for treating AAA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings in this study are included in the main article and its associated files. Source data are provided with this paper.
Code availability
No code was used for data analysis in this study.
References
Isselbacher, E. M. et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 146, e334–e482 (2022).
Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J. Vasc. Surg. 35, 72–79 (2002).
Bicknell, C. D. et al. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK). Eur. Heart J. 37, 3213–3221 (2016).
Golledge, J. et al. Efficacy of telmisartan to slow growth of small abdominal aortic aneurysms: a randomized clinical trial. JAMA Cardiol. 5, 1374–1381 (2020).
Golledge, J. et al. Lack of an effective drug therapy for abdominal aortic aneurysm. J. Intern. Med. 288, 6–22 (2020).
Wanhainen, A. & Dalman, R. L. Update on ongoing randomised controlled trials evaluating the protective effect of metformin on abdominal aortic aneurysm progression. Eur. J. Vasc. Endovasc. Surg. 69, 6–8 (2024).
Golledge, J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 16, 225–242 (2019).
Lindeman, J. H. & Matsumura, J. S. Pharmacologic management of aneurysms. Circ. Res. 124, 631–646 (2019).
Wang, M. et al. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation 125, 697–707 (2012).
Wang, M. et al. TRPC3 channel confers cerebrovascular remodelling during hypertension via transactivation of EGF receptor signalling. Cardiovasc. Res. 109, 34–43 (2016).
Antigny, F. et al. Potassium channel subfamily K member 3 (KCNK3) contributes to the development of pulmonary arterial hypertension. Circulation 133, 1371–1385 (2016).
Koivisto, A.-P., Belvisi, M. G., Gaudet, R. & Szallasi, A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug Discov. 21, 41–59 (2022).
Kumar, B. et al. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ. Res. 98, 557–563 (2006).
Chen, Y.-L. et al. Novel smooth muscle Ca2+-signaling nanodomains in blood pressure regulation. Circulation 146, 548–564 (2022).
Zhang, Z. et al. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ. Res. 111, 1137–1146 (2012).
Runnels, L. W., Yue, L. & Clapham, D. E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001).
Nadler, M. J. et al. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 (2001).
Monteilh-Zoller, M. K. et al. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121, 49–60 (2003).
Demeuse, P., Penner, R. & Fleig, A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 127, 421–434 (2006).
Dorovkov, M. V. & Ryazanov, A. G. Phosphorylation of annexin I by TRPM7 channel-kinase. J. Biol. Chem. 279, 50643–50646 (2004).
Krapivinsky, G., Krapivinsky, L., Manasian, Y. & Clapham, D. E. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell 157, 1061–1072 (2014).
Jin, J. et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760 (2008).
Ryazanova, L. V. et al. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 1, 109 (2010).
Sah, R. et al. Timing of myocardial Trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 128, 101–114 (2013).
Sah, R. et al. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl Acad. Sci. USA 110, E3037–E3046 (2013).
Du, J. et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 106, 992–1003 (2010).
Rios, F. J. et al. Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis. Cardiovasc. Res. 116, 721–735 (2020).
Thompson, R. W. et al. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann. N. Y. Acad. Sci. 1085, 59–73 (2006).
Daugherty, A., Manning, M. W. & Cassis, L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 105, 1605–1612 (2000).
Qin, X. et al. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br. J. Pharmacol. 168, 1294–1312 (2013).
Schappe, M. S. et al. Chanzyme TRPM7 mediates the Ca2+ influx essential for lipopolysaccharide-induced Toll-like receptor 4 endocytosis and macrophage activation. Immunity 48, 59–74 (2018).
Davis, F. M. et al. Single-cell transcriptomics reveals dynamic role of smooth muscle cells and enrichment of immune cell subsets in human abdominal aortic aneurysms. Ann. Surg. 276, 511–521 (2022).
Hoshina, K. et al. Aortic wall cell proliferation via basic fibroblast growth factor gene transfer limits progression of experimental abdominal aortic aneurysm. J. Vasc. Surg. 40, 512–518 (2004).
Liao, S., Curci, J. A., Kelley, B. J., Sicard, G. A. & Thompson, R. W. Accelerated replicative senescence of medial smooth muscle cells derived from abdominal aortic aneurysms compared to the adjacent inferior mesenteric artery. J. Surg. Res. 92, 85–95 (2000).
Li, Y., Wang, W., Li, L. & Khalil, R. A. MMPs and ADAMs/ADAMTS inhibition therapy of abdominal aortic aneurysm. Life Sci. 253, 117659 (2020).
Hofmann, T. et al. Activation of TRPM7 channels by small molecules under physiological conditions. Pflugers Arch. 466, 2177–2189 (2014).
Longo, G. M. et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Invest. 110, 625–632 (2002).
Satoh, K. et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II–induced aortic aneurysms. Nat. Med. 15, 649–656 (2009).
Fleig, A. & Chubanov, V. TRPM7. Handb. Exp. Pharmacol. 222, 521–546 (2014).
Schmitz, C. et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114, 191–200 (2003).
Desai, B. N. et al. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev. Cell 22, 1149–1162 (2012).
Erdahl, W. L., Chapman, C. J., Wang, E., Taylor, R. W. & Pfeiffer, D. R. Ionophore 4-BrA23187 transports Zn2+ and Mn2+ with high selectivity over Ca2+. Biochemistry 35, 13817–13825 (1996).
Chubanov, V. et al. Natural and synthetic modulators of SK (Kca2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 166, 1357–1376 (2012).
Screaton, R. A. et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119, 61–74 (2004).
Laity, J. H. & Andrews, G. K. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Arch. Biochem. Biophys. 463, 201–210 (2007).
Zierler, S. et al. Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J. Biol. Chem. 286, 39328–39335 (2011).
Doyle, J. J. et al. A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. eLife 4, e08648 (2015).
Bick, A. G. et al. Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter. Proc. Natl Acad. Sci. USA 114, E9096–E9104 (2017).
Chubanov, V., Schäfer, S., Ferioli, S. & Gudermann, T. Natural and synthetic modulators of the TRPM7 channel. Cells 3, 1089–1101 (2014).
Kappos, L. et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 355, 1124–1140 (2006).
O’Connor, P. et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology 72, 73–79 (2009).
Oller, J. et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. 23, 200–212 (2017).
Baxter, B. T. et al. Effect of doxycycline on aneurysm growth among patients with small infrarenal abdominal aortic aneurysms: a randomized clinical trial. JAMA 323, 2029–2038 (2020).
Gopalakrishnan, C. et al. Association of fluoroquinolones with the risk of aortic aneurysm or aortic dissection. JAMA Intern. Med. 180, 1596–1605 (2020).
LeMaire, S. A. et al. Effect of ciprofloxacin on susceptibility to aortic dissection and rupture in mice. JAMA Surg. 153, e181804 (2018).
Altarejos, J. Y. & Montminy, M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151 (2011).
Esteban, V. et al. Regulator of calcineurin 1 mediates pathological vascular wall remodeling. J. Exp. Med. 208, 2125–2139 (2011).
Wu, W. et al. The TRPM7 channel reprograms cellular glycolysis to drive tumorigenesis and angiogenesis. Cell Death Dis. 14, 183 (2023).
Kim, J.-H. et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 156, 730–743 (2014).
Wirth, A. et al. G12-G13–LARG–mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14, 64–68 (2008).
Shelat, H. S. et al. Growth suppression of human coronary vascular smooth muscle cells by gene transfer of the transcription factor E2F-1. Circulation 103, 407–414 (2001).
Zhang, Z. et al. The TRPM6 kinase domain determines the Mg·ATP sensitivity of TRPM7/M6 heteromeric ion channels. J. Biol. Chem. 289, 5217–5227 (2014).
Acknowledgements
We thank Y.-M. Hong (Guangdong Provincial People’s Hospital, Guangdong, China) for providing human samples. We thank Y.-N. Hu (Xiangya School of Pharmaceutical Sciences, Central South University, China) for assistance with the graphical abstract and X. Wang (Xiangya Hospital, Central South University, China) for sharing reagents. We thank other laboratory members for insightful discussion, comments on the draft manuscript and proofreading. The illustrative figures (Fig. 3m and Extended Data Fig. 6a) were created with BioRender. This study was supported by grants from the National Natural Science Foundation of China (82173816, 81973323, 91639114 and 81570429, to Z.Z.; 81500226, to M.W.) and the Provincial Natural Science Foundation of Hunan (2023JJ30739, to Z.Z.; 2019JJ30037, to M.W.).
Author information
Authors and Affiliations
Contributions
Z.Z., X.W. and M.W. conceptualized and designed the experiments. Z.Z. and M.W. supervised, funded and coordinated the project. X.W. conducted animal breeding. X.W., T.-T.Z., Z.-J.Z., S.L. and Z.-Y.S. conducted animal experiments. M.W. conducted primary SMC extraction. M.W. and X.G. conducted cell transfection experiments. Y.-B.T. conducted immunohistochemistry experiments and analysis. N.-N.Z. and Z.-Y.Y. performed compound synthesis. X.W. and S.W. conducted ultrasonic scanning. M.W., Q.W. and C.-P.H. analyzed ultrasonic data. M.W. and Z.Z. conducted cellular electrophysiology experiments and analysis. All authors wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Peer review
Peer review information
Nature Cardiovascular Research thanks Vladimir Chubanov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mouse strains in this study and the design of AAA experiments.
(a) Inducible knockout (iKO) of SMC Trpm7 with tamoxifen (Tam.) treatment. Please note the serendipitous insertion of the Myh11 promoter-driven Cre transgene into the Y chromosome. (b) The protocol for saline or PPE perfusion in SMC-selective Trpm7 null mice (Trpm7sm-iKO). Detailed information on data collection can be found in the Methods section. (c) Inducible knockout of Trpm7 with tamoxifen treatment on Apoe-/- background. (d) The protocol for saline or Ang II infusion in Apoe-/-; Trpm7sm-iKO mice. Please see the Methods section for information on data acquisition.
Extended Data Fig. 2 Validation of cell-selective Trpm7 null mice.
(a) Mouse tail genotyping of SMC-selective Trpm7 KO mice. n = 6. (b) PCR of aortic medial genomic DNA with primers flanking exon 17. The Trpm7sm-WT mice were treated with the vehicle (corn oil) or tamoxifen (Tam.). n = 3. (c) RT-qPCR of aortic media-derived mRNA with primers flanking exon 17. Residual band in the KO mice denotes undecayed truncated TRPM7 mutant transcript. n = 3. (d) Immunostaining of TRPM7 protein in abdominal aorta. Colocalization of TRPM7 with SMC as labeled by anti-SM22α was observed in wild type but absent in KO mice. Scale bar = 100μm. n = 3. (e-f) Immunoblots of TRPM7 protein in abdominal aorta from wild type or KO mice. Both full length TRPM7 (TRPM7-FL, ~220 kDa) or cleaved kinase (M7CK, ~65 kDa) were detected in wild type mice but abolished in KO mice. TRPM7-FL and M7CK were immunoblotted separately with anti-TRPM7 antibody commercially from Cayman Chemical and Sigma, respectively. n = 3. (g-h) Whole-cell patch clamping of TRPM7 currents in isolated aortic smooth muscle cells. Current density collected at 100 mV and the representative current traces are shown. n = 8 ~ 10. (i) Mouse tail genotyping of macrophage-selective Trpm7 KO mice. n = 6. (j) Immunofluorescent detection of TRPM7 protein in peritoneal macrophages. Scale bar = 200μm. n = 3. (k) Mouse tail genotyping of endothelial cell-selective KO mice. n = 6. (l) Immunofluorescent staining of TRPM7 in aortic endothelium. Colocalization of TRPM7 with endothelial marker (CD31) was seen in wild type mice but undetectable in KO mice. Scale bar = 100μm. n = 3. Unpaired 2-tailed Student’s t test was performed to compare two groups (Extended Data Figs. 2f, h).
Extended Data Fig. 3 TRPM7 is upregulated in human and mouse AAA.
(a) Immunofluorescence of TRPM7 in infrarenal aorta (InfraAo) collected from brain-dead organ donors (Ctrl) or AAA patients undergoing open surgical repair. EVG staining was simultaneously performed to indicate the identity of human aorta. n = 3. (b) Typical time course of recorded TRPM7 currents in suprarenal aorta (SupraAo) SMC isolated from saline- or Ang II-infused (14 days) Apoe-/- mice. FTY720 (3 μM) was perfused after the currents level off. The two traces are intentionally aligned by the start point of FTY720 application for back-to-back comparison. (c-d) Typical TRPM7 current traces as indicated. (e) Statistics of TRPM7 current density as indicated. n = 8. (f) SupraAo elastic fiber disruption scoring based on EVG staining in saline or Ang II-infused mice (Related to Fig. 1h). n = 6. (g) SupraAo media SM22α-positive cell proportion (Related to Fig. 1h). n = 6. (h) SupraAo CD45-positive cell proportion (Related to Fig. 1h). n = 6. (i) SupraAo DHE-positive cell count (Related to Fig. 1h). n = 6. (j) InfraAo elastic fiber disruption scoring based on EVG staining in saline or PPE-perfused mice (Related to Fig. 1m). n = 6. (k) InfraAo media SM22α-positive cell proportion (Related to Fig. 1m). n = 6. (l) InfraAo CD45-positive cell proportion (Related to Fig. 1m). n = 6. (m) InfraAo DHE-positive cell count (Related to Fig. 1m). n = 6. The differences were analyzed using two-way ANOVA, followed by Tukey’s post-hoc test (Extended Data Figs. 3e–m).
Extended Data Fig. 4 SMC-selective Trpm7 deficiency improves ascending aortopathies.
(a) Echocardiographic images, H&E staining, EVG staining, in situ gelatin zymography of the ascending aorta (AscAo). n = 5. Scale bar=1 mm for Echo. 100μm for staining and 400μm for low-power H&E field. (b) Internal diameter determined with echocardiography at longitudinal image scans. n = 7-13. (c) Medial thickness analyzed from EVG staining. The region between internal and external elastic lamina defines tunica media. n = 5. (d) Elastin breakdowns analyzed from EVG staining. n = 6. (e) Analysis of MMP activity determined with in situ gelatin zymography. n = 3. The differences were analyzed using two-way ANOVA, followed by Tukey’s post-hoc test (Extended Data Figs. 4b–e).
Extended Data Fig. 5 TRPM7 channel function regulates Mmp2 transcription in SMC.
(a) The statistics of Fig. 3a for MMP activity. n = 6. (b) The statistics of Fig. 3b for MMP activity. n = 6. (c) The statistics of Fig. 3e for MMP2 protein. n = 10. (d-e) The statistics of Fig. 3g for MMP2 protein and activity. n = 6-8 for d, n = 10-11 for e. (f-g) The statistics of Fig. 3i for MMP2 protein and activity. n = 6 for f, n = 6-8 for g. (h) The statistics of Fig. 3o for MMP2 protein levels in M7KO cells. n = 6-8. (i-k) MMP2 mRNA and protein levels in M7KO cells rescued with wild type cleaved kinase (M7CK-WT) or kinase-dead cleaved kinase (M7CK-KD). Expression of M7CK was validated by anti-HA tag. n = 6 for i, n = 4 for j. The differences were analyzed using two-way ANOVA, followed by Tukey’s post-hoc test (Extended Data Fig. 5a–j).
Extended Data Fig. 6 TRPM7-mediated Ca2 + /Zn2+ signaling regulates Mmp2 transcription.
(a) Pharmacology of TRPM7 chanzyme. (b) Gelatin zymography of WT cells pretreated for 1-hour with NS8593 (10 μM), BAPTA-AM (10 μM) or TPEN (10 μM), followed by Ang II stimulation (1 μM, 24 h). n = 3. Scale bar=200μm. (c) MMP14 transcripts in WT cells treated as in Fig. 4d. n = 5-6. (d) MMP14 transcripts in WT cells treated as in Fig. 4g. n = 4-6. (e) MMP2 mRNA in WT cells adenovirally transduced as indicated. Unpaired 2-tailed Student’s t test was performed (n = 9). (f) MMP2 mRNA in WT cells pretreated with PF431396 (10 μM), followed by naltriben (20 μM, 24 h) or Ang II stimulation. n = 6. (g) MMP14 mRNA in WT cells treated as in Fig. 4j. n = 6. (h) Adenoviral transduction of WT cells with WT or CA mutant of CnA. n = 6. (i) Nfatc2 (NFAT1), Nfatc1 (NFAT2), Nfatc4 (NFAT3), Nfatc3 (NFAT4) and Nfat5 (NFAT5) mRNA in aortic media from Trpm7sm-WT mice. n = 3. (j-k) MMP2 mRNA in WT cells. Adenovirus carrying target gene was transduced (MOI: 50) for 48-hours to measure MMP2 mRNA. n = 4-6. (l) Gelatin zymography of WT cells pretreated with NS8593 (10 μM), CsA (1 μM), CREB inhibitor 666-15 (1 μM) or MTF1 inhibitor APTO-253 (0.5 μM), followed by Ang II treatment. n = 3. Scale bar=200μm. (m) The schematic diagram depicting the predicted Mmp2 promoter and the truncated mutants. The 2010 base pairs upstream of Mmp2 start codon were predicted as the promoter. CREB or MTF1 binding sites were predicted using JASPAR (https://jaspar.genereg.net/) & PROMO(https://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) tools. 9 CREB binding sites (the consensus binding sequence is TGACGT(CA)) were retrieved, including #1 (-1905 ~ -1898), #2 (-1795 ~ -1788), #3 (-1471 ~ -1464), #4 (-1351 ~ -1344), #5 (-959 ~ -952), #6 (-635 ~ -628), #7 (-351 ~ -345), #8 (-280 ~ -274), #9 (-37 ~ -30 or -28 ~ -21). No predicted MTF1 binding sites were retrieved. 3 truncated Mmp2 promoter mutants were generated: Δ523bp (-2010 ~ -1488) in mutant-1 to remove #1, 2 binding sites, Δ1025bp (-2010 ~ -986) in mutant-2 to remove #1, 2, 3, 4, or Δ1654bp (-2010 ~ -357) in mutant-3 to remove #1, 2, 3, 4, 5, 6 binding sites. The differences were determined by two-way ANOVA (Figs. 6c,d) or one-way ANOVA (Figs. 6f–h, j), followed by Tukey’s post-hoc test.
Extended Data Fig. 7 SMC-specific Trpm7 deletion suppresses CRTC2/CREB signaling.
(a-b) Immunoblots of phosphorylated CREB in WT or MKO cells pretreated for 30 mins with TRPM7 blocker (NS8593, 10 μM), Zn2+ chelator (TPEN, 10 μM), Ca2+ chelator (BAPTA-AM, 10 μM), adenylyl cyclase inhibitor (SQ22536, 10 μM), PKA inhibitor (H89, 10 μM), CaMKKβ blocker (STO-609, 1 μM), CaMKII blocker (KN93, 5 μM), Pyk2 blocker (PF431396, 10 μM) or calcineurin inhibitor (CsA, 1 μM), followed by 30-min naltriben stimulation (20 μM). n = 3. (c-d) Statistics of Fig. 5c. n = 6-9 for c, n = 3-5 for d. (e-f) Statistics of Fig. 5d. n = 6-8 for e, n = 3-4 for f. (g-h) Statistics of Fig. 5e. n = 4 for g, n = 6-7 for h. (i) Immunoblots of the denoted protein in M7KO cells rescued with empty vector, M7Ch-WT or M7Ch-PD upon saline or Ang II stimulation. n = 6-8. (j-k) Statistics of Extended Data Fig. 7i. n = 6-8 for j, n = 8 for k. (l) Immunofluorescence of CRTC2 in InfraAo from saline or PPE-perfused Ai9sm-tdT/0 mice. Ai9sm-tdT/0 mice were generated by breeding Ai9(RCL-tdT) mice (JAX 007909) with another SMC Cre driver (Tagln-Cre, JAX 017491). SMC in these mice is labeled by tdTomato for fluorescent visualization. CRTC2 was shown in green, tdTomato autofluorescence in red and DAPI in blue. CRTC2 nuclear localization (white arrows) in SMC was analyzed based on nuclear staining (cyan) in tdTomato-positive cell (red). Scale bar=50μm. n = 3. (m) Immunofluorescence of CRTC2 in InfraAo from saline or PPE-perfused mice. Analysis is identical to Fig. 5f. n = 3. Scale bar=50μm. (n-o) Detection of CRTC2 in extracted cytosolic or nuclear protein from WT or M7KO cells treated with saline or Ang II. n = 3. (p) Immunoblots of CRTC2 in extracted cytosolic or nuclear protein from HEK293 cells transfected with empty vector, M7Ch-WT or M7Ch-PD upon naltriben stimulation. n = 3. (q-r) Statistics of Extended Data Fig. 7p. The differences were determined by two-way ANOVA (Figs. 7c–h,j,kn,o) or one-way ANOVA (Figs. 7b,q,r), followed by Tukey’s post-hoc test.
Extended Data Fig. 8 SMC-specific Trpm7 deletion suppresses MTF1 and KLF4 signaling.
(a) Immunofluorescence of MTF1 in InfraAo from PPE-perfused Ai9sm-tdT/0 mice. MTF1 was shown in green, tdTomato autofluorescence in red and DAPI in blue. MTF1 nuclear localization (white arrows) in SMC was analyzed based on nuclear staining (cyan) in tdTomato positive cell (red). Scale bar=50μm. n = 3. (b) Immunofluorescence of MTF1 in InfraAo from saline or PPE-perfused mice. Analysis is identical to Fig. 5f. n = 3. Scale bar=50μm. (c-d) Detection of MTF1 in extracted cytosolic or nuclear protein from WT or M7KO cells treated with saline or Ang II. n = 3. (e) Immunoblots of MTF1 in extracted cytosolic or nuclear protein from HEK293 cells transfected with empty vector, M7Ch-WT or M7Ch-PD upon naltriben stimulation. (f-g) Statistics of Extended Data Fig. 8e. n = 3. (h) Immunofluorescence of KLF4 in SupraAo from saline or Ang II-infused mice. Scale bar=50μm. n = 3. The differences were determined by two-way ANOVA (Fig. 8c, 8d) or one-way ANOVA (Fig. 8f, 8g), followed by Tukey’s post-hoc test.
Extended Data Fig. 9 FTY720, rather than its metabolites, suppresses Mmp2 transcription.
(a) MMP2 transcripts in Ang II-stimulated SMC pretreated for 1 h with vehicle, FTY720 (3 μM) and its metabolites FTY720 (S)-P (3 μM) or FTY720 (R)-P (3 μM). n = 4-6. (b-c) Immunoblots of MMP2 in cells treated as indicated. n = 6-9. (d) MMP2 transcripts in saline or Ang II-infused mice treated with FTY720 (6 mg/kg/day) or FTY720 (S)-P (6 mg/kg/day). n = 6. (e-f) Immunoblots of MMP2 in mice treated as indicated. n = 5-6. (g) MMP2 transcripts in saline or PPE-perfused mice treated with FTY720 (6 mg/kg/day) or FTY720 (S)-P (6 mg/kg/day). n = 6. (h-i) Immunoblots of MMP2 in saline or PPE mice treated as indicated. n = 5-7. The differences were analyzed using two-way ANOVA, followed by Tukey’s post-hoc test (Extended Data Fig. 9a,b,d,e,g,h).
Extended Data Fig. 10 FTY720 mitigates AAA through TRPM7 block in PPE mice.
(a) SupraAo elastic fiber disruption scoring in mice prophylactically treated with vehicle, FTY720 or FTY720 (S)-P (Related to Fig. 6f). n = 6. (b) SupraAo media active MMP proportion in mice prophylactically treated (Related to Fig. 6f). n = 6. (c) SupraAo elastic fiber disruption scoring in mice therapeutically treated with vehicle or FTY720 (Related to Fig. 6o). n = 6. (d) SupraAo medial active MMP proportion in mice therapeutically treated with vehicle or FTY720 (Related to Fig. 6o). n = 6. (e) The protocol for FTY720 prophylactic dosing in C57BL/6 J or Trpm7sm-iKO mice perfused with saline or PPE. (f) Micrograph of the InfraAo from saline or PPE-perfused mice prophylactically treated with vehicle, FTY720 (6 mg/kg/day) or FTY720 (S)-P (6 mg/kg/day). Scale bar=1 mm. (g) Growth of InfraAo external diameter within 14 days. The diameter measured in situ on day 14 prior to circulatory arrest was compared with the baseline diameter of individual mouse. n = 6 ~ 12. (h) Growth of InfraAo internal diameter on day 3, 7, 14 after saline or PPE perfusion. * P = 0.0080 (PPE-perfused FTY720-treated vs Veh.-treated mice on day 7). † P < 0.0001 (PPE-perfused FTY720-treated vs Veh.-treated mice on day 14). n = 6-15. (i) H&E, EVG staining and in situ gelatin zymography of InfraAo in saline or PPE-perfused mice treated as indicated. n = 6. Scale bar=100μm except for scale bar=400μm for low-power H&E field. (j) InfraAo elastic fiber disruption scoring in saline or PPE-perfused mice prophylactically treated with vehicle, FTY720 or FTY720 (S)-P. n = 6. (k) InfraAo media active MMP proportion in mice prophylactically treated with vehicle, FTY720 or FTY720 (S)-P. n = 6. (l) Immunofluorescence of CRTC2 or MTF1 in InfraAo from saline or PPE-perfused mice with the indicated treatments. Analysis is identical to Fig. 5f. n = 3. Scale bar=50μm. (m) CREB ChIP in InfraAo. n = 6-7. (n) MTF1 ChIP in InfraAo. n = 5-7. (o-p) SupraAo external diameter or growth of internal diameter in mice with 50 ~ 70% dilatation at randomization. The differences were determined by two-way ANOVA (Fig. 10a, 10b, 10g, 10h, 10j, 10k, 10m, 10n) or one-way ANOVA (Fig. 10c, 10d), followed by Tukey’s post-hoc test.
Source data
Source Data Figs. 1–6 and Source Data Extended Data Figs. 2–10
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Wang, M., Zhu, TT. et al. The TRPM7 chanzyme in smooth muscle cells drives abdominal aortic aneurysm in mice. Nat Cardiovasc Res 4, 216–234 (2025). https://doi.org/10.1038/s44161-025-00613-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-025-00613-5