Abstract
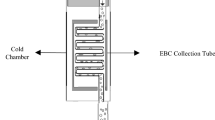
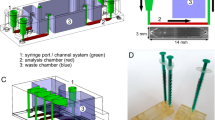
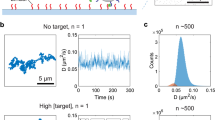
Unlike biomarkers in biofluids, airborne biomarkers are dilute and difficult to trace. Detecting diverse airborne biomarkers with sufficient sensitivity typically relies on bulky and expensive equipment like mass spectrometers that remain inaccessible to the general population. Here we introduce airborne biomarker localization engine (ABLE), a simple, affordable and portable platform that can detect both non-volatile and volatile molecules and particulate biomarkers from open air in about 15 min. ABLE substantially improves the gas detection limits by converting dilute gases into droplets by water condensation, producing concentrated aqueous samples that can be easily tested by existing liquid-sensing platforms. Fundamental studies of multiphase condensation revealed unexpected stability in condensate-trapped biomarkers, making ABLE a reliable, accessible and high-performance system for open-air-based biosensing applications such as non-contact infant healthcare, pathogen detection in public spaces and food safety monitoring.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data required to recreate the figures are available in this article and its Supplementary Information. Source data are provided with this paper.
References
Wu, J., Liu, H., Chen, W., Ma, B. & Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 1, 346–360 (2023).
Liu, W. Y., Cheng, H. Y. & Wang, X. F. Skin-interfaced colorimetric microfluidic devices for on-demand sweat analysis. npj Flex. Electron. 7, 43 (2023).
Ye, Z. L. et al. A breathable, reusable, and zero-power smart face mask for wireless cough and mask-wearing monitoring. ACS Nano 16, 5874–5884 (2022).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Xiang, Y. & Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 3, 697–703 (2011).
Puthussery, J. V. et al. Real-time environmental surveillance of SARS-CoV-2 aerosols. Nat. Commun. 14, 3692 (2023).
Horvath, I. et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur. Resp. J. 26, 523–548 (2005).
Tankasala, D. & Linnes, J. C. Noninvasive glucose detection in exhaled breath condensate. Transl. Res. 213, 1–22 (2019).
Arnold, C. Diagnostics to take your breath away. Nat. Biotechnol. 40, 990–993 (2022).
Zhang, S. L. et al. Rapid measurement of lactate in the exhaled breath condensate: biosensor optimization and in-human proof of concept. ACS Sens. 7, 3809–3816 (2022).
Heng, W. et al. A smart mask for exhaled breath condensate harvesting and analysis. Science 385, 954–961 (2024).
Istif, E. et al. Miniaturized wireless sensor enables real-time monitoring of food spoilage. Nat. Food 4, 427–436 (2023).
Chatterjee, S. G., Chatterjee, S., Ray, A. K. & Chakraborty, A. K. Graphene-metal oxide nanohybrids for toxic gas sensor: a review. Sens. Actuators B 221, 1170–1181 (2015).
Klotz, T., Ibrahim, A., Maddern, G., Caplash, Y. & Wagstaff, M. Devices measuring transepidermal water loss: a systematic review of measurement properties. Skin Res. Technol. 28, 497–539 (2022).
Mainelis, G. Bioaerosol sampling: classical approaches, advances, and perspectives. Aerosol Sci. Technol. 54, 496–519 (2020).
Hering, S. V. & Stolzenburg, M. R. A method for particle size amplification by water condensation in a laminar, thermally diffusive flow. Aerosol Sci. Technol. 39, 428–436 (2005).
Hering, S. V., Spielman, S. R. & Lewis, G. S. Moderated, water-based, condensational particle growth in a laminar flow. Aerosol Sci. Technol. 48, 401–408 (2014).
Asadi, S. et al. Influenza A virus is transmissible via aerosolized fomites. Nat. Commun. 11, 4062 (2020).
Yu, Y. et al. Growth characteristics of submicron particles by water vapor condensation in the multi-section growth tube. Powder Technol. 440, 119797 (2024).
Zervaki, O., Dionysiou, D. D. & Kulkarni, P. A high-throughput, turbulent-mixing, condensation aerosol concentrator for direct aerosol collection as a liquid suspension. J. Aerosol Sci. 182, 106442 (2024).
Gupta, R. et al. Ultrasensitive lateral-flow assays via plasmonically active antibody-conjugated fluorescent nanoparticles. Nat. Biomed. Eng. 7, 1556–1570 (2023).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Xu, C. H. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 7, 168–179 (2024).
Kroger, D. G. & Rohsenow, W. M. Condensation heat transfer in the presence of a non-condensable gas. Int. J. Heat Mass Transfer 11, 15–26 (1968).
Wang, R. S., Guo, J. H., Muckleroy, E. A. & Antao, D. S. Robust silane self-assembled monolayer coatings on plasma-engineered copper surfaces promoting dropwise condensation. Int. J. Heat Mass Transfer 194, 123028 (2022).
Cha, H. Y. et al. Nanoscale-agglomerate-mediated heterogeneous nucleation. Nano Lett. 17, 7544–7551 (2017).
Ma, J., Sett, S., Cha, H., Yan, X. & Miljkovic, N. Recent developments, challenges, and pathways to stable dropwise condensation: a perspective. Appl. Phys. Lett. 116, 260501 (2020).
Miljkovic, N., Enright, R. & Wang, E. N. Effect of droplet morphology on growth dynamics and heat transfer during condensation on superhydrophobic nanostructured surfaces. ACS Nano 6, 1776–1785 (2012).
Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 15, 4399–4981 (2015).
Pahlavan, A. A., Yang, L. S., Bain, C. D. & Stone, H. A. Evaporation of binary-mixture liquid droplets: the formation of picoliter pancakelike shapes. Phys. Rev. Lett. 127, 024501 (2021).
Christy, J. R. E., Hamamoto, Y. & Sefiane, K. Flow transition within an evaporating binary mixture sessile drop. Phys. Rev. Lett. 106, 205701 (2011).
Bell, A. K. et al. Concentration gradients in evaporating binary droplets probed by spatially resolved Raman and NMR spectroscopy. Proc. Natl Acad. Sci. USA 119, e2111989119 (2022).
Diddens, C., Li, Y. X. & Lohse, D. Competing Marangoni and Rayleigh convection in evaporating binary droplets. J. Fluid Mech. 914, A23 (2021).
Delgado, J. M. P. Q. Molecular diffusion coefficients of organic compounds in water at different temperatures. J. Phase Equilibria Diffus. 28, 427–432 (2007).
Zhao, P. Y. et al. Multiphysics analysis for unusual heat convection in microwave heating liquid. AIP Adv. 10, 085201 (2020).
Khorshid, M. et al. An efficient low-cost device for sampling exhaled breath condensate EBC. Adv. Sensor Res. 3, 2400020 (2024).
Karyakin, A. A. et al. Non-invasive monitoring of diabetes through analysis of the exhaled breath condensate (aerosol). Electrochem. Commun. 83, 81–84 (2017).
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008).
Richter, L. L. et al. Temporal trends in neonatal mortality and morbidity following spontaneous and clinician-initiated preterm birth in Washington State, USA: a population-based study. BMJ Open 9, e023004 (2019).
Butler, R. B. A. Preterm Birth: Causes, Consequences, and Precention (eds Behram, R. E. & Stith Butler, A.) 313–346 (National Academies Press, 2007).
Lu, L. et al. Transcriptional modulation of intestinal innate defense/inflammation genes by preterm infant microbiota in a humanized gnotobiotic mouse model. PLoS ONE 10, e0124504 (2015).
Lu, J. et al. Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Sci. Rep. 8, 5443 (2018).
Lu, J. et al. Early preterm infant microbiome impacts adult learning. Sci. Rep. 12, 3310 (2022).
Park, S. J. & Im, D. S. Blockage of sphingosine-1-phosphate receptor 2 attenuates allergic asthma in mice. Br. J. Pharmacol. 176, 938–949 (2019).
Patil, M. J., Meeker, S., Bautista, D., Dong, X. & Undem, B. J. Sphingosine-1-phosphate activates mouse vagal airway afferent C-fibres via S1PR3 receptors. J. Physiol. 597, 2007–2019 (2019).
Thebaud, B. et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 5, 78 (2019).
Stevens, N. C. et al. Alteration of glycosphingolipid metabolism by ozone is associated with exacerbation of allergic asthma characteristics in mice. Toxicol. Sci. 191, 79–89 (2023).
Mirhoseini, S. H., Nikaeen, M., Khanahmad, H., Hatamzadeh, M. & Hassanzadeh, A. Monitoring of airborne bacteria and aerosols in different wards of hospitals—particle counting usefulness in investigation of airborne bacteria. Ann. Agric. Environ. Med. 22, 670–673 (2015).
Park, D. U., Yeom, J. K., Lee, W. J. & Lee, K. M. Assessment of the levels of airborne bacteria, Gram-negative bacteria, and fungi in hospital lobbies. Int. J. Environ. Res. Public Health 10, 541–555 (2013).
Buda, R. et al. Dynamics of passive response to a sudden decrease in external osmolarity. Proc. Natl Acad. Sci. USA 113, E5838–E5846 (2016).
Schulte, F., Lingott, J., Panne, U. & Kneipp, J. Chemical characterization and classification of pollen. Anal. Chem. 80, 9551–9556 (2008).
Guedes, A., Ribeiro, H., Fernández-González, M., Aira, M. J. & Abreu, I. Pollen Raman spectra database: application to the identification of airborne pollen. Talanta 119, 473–478 (2014).
Wang, D. J. & White, J. C. Benefit of nano-enabled agrochemicals. Nat. Food 3, 983–984 (2022).
Ho, C. S. et al. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 10, 4927 (2019).
Wang, C. et al. Biomimetic olfactory chips based on large-scale monolithically integrated nanotube sensor arrays. Nat. Electron. 7, 157–167 (2024).
Weisbecker, H. et al. AI-assisted multimodal breath sensing system with semiconductive polymers for accurate monitoring of ammonia biomarkers. Adv. Mater. Technol. 9, 2301884 (2024).
Li, J. et al. Aerodynamics-assisted, efficient and scalable kirigami fog collectors. Nat. Commun. 12, 5484 (2021).
Li, T. et al. Scalable and efficient solar-driven atmospheric water harvesting enabled by bidirectionally aligned and hierarchically structured nanocomposites. Nat. Water 1, 971–981 (2023).
Ma, J. et al. A lipid-inspired highly adhesive interface for durable superhydrophobicity in wet environments and stable jumping droplet condensation. ACS Nano 3, 4251–4262 (2022).
Mustafa, F. & Andreescu, S. Chemical and biological sensors for food-quality monitoring and smart packaging. Foods 7, 168 (2018).
Ho, D. H., Choi, Y. Y., Jo, S. B., Myoung, J. M. & Cho, J. H. Sensing with MXenes: progress and prospects. Adv. Mater. 33, 2005846 (2021).
Huang, L. B. et al. Ultrasensitive, fast-responsive, directional airflow sensing by bioinspired suspended graphene fibers. Nano Lett. 23, 597–605 (2023).
Wang, K. N. et al. A polarity-sensitive ratiometric fluorescence probe for monitoring changes in lipid droplets and nucleus during ferroptosis. Angew. Chem. Int. Ed. 60, 15095–15100 (2021).
Dai, T. J. et al. An AIEgen as an intrinsic antibacterial agent for light-up detection and inactivation of intracellular Gram-positive bacteria. Adv. Healthcare Materials 10, 2100885 (2021).
Zhang, J. J. et al. Ultrasensitive point-of-care biochemical sensor based on metal-AlEgen frameworks. Sci. Adv. 8, eabo1874 (2022).
Thomas, V., Clark, J. & Dore, J. Fecal microbiota analysis: an overview of sample collection methods and sequencing strategies. Future Microbiol. 10, 1485–1504 (2015).
Acknowledgements
We thank K. M. Watters for scientific editing of the manuscript; E. Augustine from the University of Chicago “Engineering + Technical Support Group” for the three-dimensional modeling and fabrication of the ABLE device; and J. Smous, J. Holewczynski and M. Engstrom from the University of Notre Dame Engineering and Design Core Facility for the three-dimensional modeling and fabrication of the ABLE test bed for the dew point measurement. The work is supported by US Army Research Office no. W911NF-24-1-0053 (B.T.), University of Chicago startup grant (B.T.), University of Notre Dame startup grant (J.M.), the Technology Development Fund from the Berthiaume Institute for Precision Health (J.M.), the Grier Prize for Innovation Research in the Biophysical Sciences (P.L.) and the National Institute of Health nos. R01 HD105234 (E.C.C. and J.L.) and R21 NS121432 (E.C.C. and J.L.).
Author information
Authors and Affiliations
Contributions
This study was conceived by J.M. and B.T. J.M. designed the ABLE device. Experimental measurements were performed by J.M., M.L., P.L., Y.M. and A.P.D.R., with input and resources provided by B.T., J.Y., E.C.C., Z.K. and J.M. Theoretical modeling and numerical simulations were performed by J.M. and S.P.P. Animal models for preterm infant disease and the sequential metabolomic analysis was provided by J.L., J.C., Y.Y., K.O. and E.C.C. The paper was prepared by J.M., M.L., P.L., J.L., J.C., Y.M., S.P.P. and B.T., with contributions and final approval from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Can Dincer, Alina Vasilescu and Daniel Orejon for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Texts 1–15, Tables 1–3 and Figs. 1–35.
Supplementary Data 1
Data required to recreate the plots for each figure in the Supplementary Information.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, J., Laune, M., Li, P. et al. Airborne biomarker localization engine for open-air point-of-care detection. Nat Chem Eng 2, 321–333 (2025). https://doi.org/10.1038/s44286-025-00223-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00223-9
This article is cited by
-
A Wearable Platform for Molecular Breath Analysis: Smart Mask Enables Real-Time Exhaled Biomarker Monitoring
Advanced Fiber Materials (2025)