Abstract
Arterial stiffness is associated with a higher risk of adverse cardiovascular outcomes. Cardio-metabolic diseases increase the risk and progression of arterial stiffness, and its optimal management along with lifestyle interventions may decrease its impact on the risk of cardiovascular outcomes. In this review, we highlight recent evidence on the impact of cardiometabolic risk factors and their management on arterial stiffness and identify potential areas of opportunity for future research.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide1. Among the many factors contributing to the development and progression of CVD, arterial stiffness (AS) has gained significant attention in recent years due to its strong association with a range of relevant CVD outcomes, including stroke, myocardial infarction, and heart failure2,3. AS is a crucial marker in cardiovascular health, as it reflects the functional state of arteries4, and previous research has shown it may be a useful predictor for increased risk of adverse cardiovascular outcomes in clinical practice5. Therefore, AS can be conceptualized as a cardiovascular risk factor which can be assessed non-invasively in clinical practice, and which can also serve as a marker to monitor the effect of cardiovascular health interventions4,5,6.
Inflammation and vascular aging are key factors involved in large artery stiffening, contributing to atherosclerosis and arteriosclerosis3. AS is often the result of multiple pathophysiological processes which are commonly involved in the development and progression of CVD, including endothelial dysfunction, vascular smooth muscle cell (VSMC) migration and proliferation, vascular calcification, extracellular matrix (ECM) remodeling, oxidative stress and proinflammatory processes7. Furthermore, many of these are also related to the pathophysiology of cardiometabolic diseases, including insulin resistance (IR), adipose tissue dysfunction, dyslipidemia, impaired kidney function, and type 2 diabetes mellitus (T2D), which are all encompassed within the cardiovascular-kidney metabolic syndrome (CKMS)4,6; therefore, it is pivotal to assess the metabolic and aging-related contributions of cardio-metabolic conditions to vascular health5. Moreover, whilst the association between cardiometabolic disease and AS is well-established8, the effect of mitigating cardiometabolic risk factors on AS remains an active area of research. In this narrative review, we explore the pathophysiological links between AS and cardiometabolic diseases, examining evidence on whether managing these diseases and their risk factors influences AS development and progression. Additionally, we highlight potential research opportunities and address unresolved questions in the current literature.
Definition and measurement of arterial stiffness
Definition and relevance of arterial stiffness
AS stems from degeneration of the arterial wall, as a result of arterial thickening and calcification, accompanied by a gradual loss of elasticity9. These changes within AS can be conceptualized as hallmarks of vascular aging; moreover, they can also be related to pathophysiological changes from chronic cardio-metabolic diseases7,9. Healthy, elastic arteries expand to accommodate blood ejected during systolic contraction, generating pulse waves that propagate through the arterial circulation. The velocity of these waves indicates arterial elasticity, with higher velocity reflecting stiffer vessels9. AS increases pulse wave velocity (PWV) during systole, which, combined with reduced reserve function, can elevate blood pressure (BP), particularly systolic BP and pulse pressure. Over time, arterial wall thickening increases stiffness and PWV during systole, consequently impairing vascular hemodynamics4,9. AS also impairs the ability of arteries to adapt to pressure changes during the cardiac cycle4,9,10. Large arteries, such as the aorta, play a crucial role in buffering BP and blood flow fluctuations, protecting the microvasculature of target organs10.
Aging, coupled with environmental, genetic, and social factors, promotes AS, which can lead to isolated systolic hypertension, left ventricular (LV) remodeling, and cardiac dysfunction4,9. These changes have been demonstrated in longitudinal studies were it has been shown that the stiffness of the large arteries leads to a significant increase in BP and systemic arterial hypertension (SAH)11,12,13; similarly, evidence from the Framingham Heart Study suggests that AS is an independent risk factor for the onset of multiple long-term adverse outcomes, including SAH, T2D, chronic kidney disease (CKD), dementia, CVD, and all-cause mortality14. AS also negatively affects cerebrovascular function, contributing to decreased cerebral blood flow and endothelial dysfunction, potentially increasing the risk of stroke15. Notably, the use of medications for common cardiometabolic diseases results in beneficial effects on AS4. Given its well-established relationship with cardiometabolic risk, there is growing interest in using AS as a non-invasive marker of cardiometabolic disease progression and treatment benefit; however, the integration of AS measures into routine clinical practice is not yet common, as discussed in length in later sections16.
Non-invasive measurement of arterial stiffness
AS can be measured at systemic, local, and regional levels. Systemic measurements of AS can only be obtained using circulation models; however, regional and local data can be assessed through non-invasive procedures17. The most commonly used method to measure aortic AS is the determination of PWV using either Magnetic Resonance Imaging (MRI) or tonometry of the right carotid and femoral arteries using the “foot-to-foot” velocity method (carotid-femoral PWV; cf-PWV), or the brachial-ankle PWV (ba-PWV), often with electrocardiogram as a timing marker17,18,19. PWV represents the velocity at which the BP pulse wave travels through the arteries, and it can be calculated using the Bramwell-Hill equation, which defines the relationship between arterial distensibility and PWV20. In clinical practice, PWV is often determined as the ratio of the distance between two measurement sites to the time it takes for the pulse to travel between them17,19. In addition to cf-PWV, ba-PWV, and MRI-based PWV, other non-invasive methods to assess AS include the cardio-ankle vascular index (CAVI), the augmentation index (AIx), and the measurement of carotid intima-media thickness. These methods are briefly described along with their potential advantages and disadvantages in Table 1.
Pathophysiological mechanisms and determinants of arterial stiffness
Pathophysiology of arterial stiffness
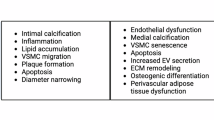
Mechanisms underlying AS include changes in contractility and plasticity of VSMC, arterial wall thickening, degenerative changes in arterial wall elastic fibers, ECM changes, and increased immuno-inflammatory stimuli, all of which induce distinct impacts on specific arterial layers9. Elastic arteries are composed of three layers: 1) the outer layer, also known as tunica adventitia, mainly composed of fibroblasts, collagen-containing matrix tissue, and the external elastic lamina, 2) the middle layer, also known as tunica media, mainly composed of VSMC and elastic fibers, and lastly, 3) the inner layer, also known as tunica intima, mainly composed of a single layer of endothelial cells with a basement membrane or internal elastic lamina19. During vascular aging, collagen deposition in the tunica adventitia and media increases as fibroblast activity rises and elastin degradation accelerates2,7,16. The activation of the renin-angiotensin-aldosterone system (RAAS), overstimulation of angiotensin-II type 1 and mineralocorticoid receptors, and increased expression of α-smooth muscle actin and β1-integrin contribute to VSMC stiffness7,9. Meanwhile, persistent oxidative stress leads to important and irreversible endothelial dysfunction in the tunica intima. Figure 1 summarizes how these processes collectively contribute to the onset of AS. The pathophysiology of AS is conditioned by structural, dynamic, and external components4,21. Structural factors include impairments in collagen and elastin fibers in the intima media, as well as blood vessel calcification. Dynamic factors are characterized by increased stimulation of VSMCs by vasoactive substances, while external components are primarily modifiable risk factors22. The events that contribute to the development of AS are outlined in Table 2.
Changes observed in each layer can occur regardless of the underlying etiology of arterial stiffness. Created in BioRender. Bello Chavolla, O. (2025) https://BioRender.com/o88u142. ECM extracellular matrix RAAS renin-angiotensin-aldosterone system VSMC vascular smooth cells AS arterial stiffness.
Ethnic and genetic variation in arterial stiffness and its impact on CVD risk
AS exhibits significant heritability, likely influenced by a combination of genetic and environmental factors4,9,10. Ethnic and socioeconomic factors also play significant roles in AS. For instance, AS has been shown to be more prevalent and progresses earlier in African and Hispanic compared to Caucasian-descent populations, leading to early vascular aging, and consequently, an increased propensity for AS23. While genetic predisposition contributes to AS, lifestyle factors, socioeconomic adversity, and psychosocial stress further modulate these risks and its prognostic value for AS24,25,26. Socioeconomic status and health behaviors, such as obesity, are also contributors to AS, particularly in communities facing economic adversity23,25. Conversely, metabolomic studies have identified urinary amino acids involved in collagen metabolism and oxidative stress regulation which are protective of AS in African American children against early vascular deterioration27,28. These factors underscore the necessity of addressing social and biological determinants of health to better understand AS as both a clinical entity, and as a CVD risk factor24.
Recent studies have provided insights into the molecular underpinnings of AS, particularly through transcriptional profiling, highlighting the involvement of genes associated with ECM remodeling and VSMC function10. For example, aortic biopsies from patients with AS reveal alterations in genes regulating vascular structure, signaling, and gene expression, including downregulation of PPP1CB and PRKA, which are critical for VSMC contraction, and upregulation of the gene encoding phosphoinositol-3-kinase polypeptide p85α29. Overall, three predominant gene expression patterns have been associated to AS, including 1) genes that promote contractile and relaxant properties as well as intercellular VSMC signaling, 2) genes and inhibitors which regulate VSMC growth, and 3) genes encoding structural proteins like elastin and type III collagen that directly impact AS via ECM remodeling29,30,31. The integration of omics approaches, including genomics, metabolomics, and microbiota composition, offers a promising avenue for identifying molecular pathways and mechanisms of AS in humans9,29. Integration of these approaches could facilitate the development of targeted therapies to mitigate the associated cardiovascular risk related to AS and help characterize the complex interplay of biological, social, and psychosocial factors involved in its pathophysiology32,33.
Arterial stiffness in the pathophysiology of cardiometabolic diseases
AS plays a significant role in the pathophysiology of metabolic diseases, particularly within the framework of the cardiovascular-kidney metabolic syndrome (CKMS), as highlighted by the American Heart Association’s Disease Continuum framework34. CKMS encompasses a variety of related cardiometabolic risk factors, including obesity, elevated BP, IR, abnormal blood lipid levels, and impaired kidney function6,34,35 in patients with or without over CVD. The spectrum of CKMS includes the most relevant cardiometabolic diseases and risk factors and is a useful framework to guide management and policy36. CKMS considers the transition from patients without cardiometabolic risk factors (stage 0), to those with excess or dysfunctional adiposity (stage 1), those with cardiometabolic risk factors including hypertriglyceridemia, SHA, T2D, metabolic syndrome, or CKD (stage 2), those with subclinical CVD, high or very high risk of CVD with additional cardiovascular, kidney or metabolic risk factors (stage 3), and those with clinical CVD in CKMS (stage 4)37. AS arises in CKMS as a result of pathophysiological changes which lead to an imbalance in the elastin-to-collagen ratio, inflammation from ROS, vascular calcification, VSMC stiffening, and endothelial dysfunction resulting from underlying metabolic and inflammatory processes6,34. Notably, AS can occur across CKMS stages 1–4, starting from states of excess of dysfunctional adiposity and in patients with over clinical CVD. Moreover, AS favors the development of CVD through several mechanisms. Pulse waves travel faster in stiffened arteries, combining with the reflected wave in the early stages to increase systolic and decrease diastolic BP, thus increasing pulse pressure18. These BP changes may lead to LV hypertrophy due to increased myocardial strain, diastolic dysfunction and a reduction in coronary artery blood flow, favoring ischemia10,38,39. Furthermore, increased mechanical shear due to increased pulse pressure facilitates lipid accumulation in the arterial wall and weakening of the fibrous cap in atherosclerotic lesions40.
The relationship between cardiometabolic risk factors and long-term patterns in the progression of AS can vary significantly41. Risk factors contributing to elevated PWV include male gender, smoking, and metabolic disruptions in glucose and lipid levels9. Additionally, prolonged exposure to tobacco, excessive alcohol intake, SAH, T2D, hypertriglyceridemia, and hyperuricemia are independently associated with a rapid rise in AS, especially in older individuals41,42. Although AS tends to increase with age, this progression is faster in people with obesity, IR, and diabetes6,34,43,44; furthermore, aging increases the risk of CKMS and its associated comorbidities, further contributing to AS progression43,45, thus increasing the risk of CVD, kidney dysfunction, and stroke. Previous studies showed that an increase of 1 m/s in estimated PWV was associated with ~30% higher risk of CVD outcomes and death46. Currently, the protective factors against this damage remain unclear4. Figure 2 resumes the main pathophysiological mechanisms found in metabolic diseases which increase the risk of AS onset or progression, and which will be described in the following sections. Data from prospective and longitudinal studies have demonstrated the long-term impact of PWV and AS on relevant cardiometabolic markers and outcomes. Notably, AS has been associated with an increased risk of new-onset T2D, SAH, CVD, stroke and dementia, and increased PWV has been consistently associated with worsened management of cardiometabolic risk factors and increases risk of long-term complications for patients living with cardiometabolic diseases. A detailed summary of prospective evidence regarding the relevance of AS and PWV for cardiometabolic diseases from relevant and large-scale studies is summarized in Table 3.
The figure highlights five main etiologic domains which are extensively discussed in the text, including aging, dysglycemia, adipose tissue dysfunction in obesity, dyslipidemia and arterial hypertension. The main mechanisms involved in each etiologic domain are highlighted with the same color. Created in BioRender. Bello Chavolla, O. (2025) https://BioRender.com/p44w501. RAAS renin-angiotensin-aldosterone system VSMC vascular smooth cells AS arterial stiffness SBP systolic blood pressure DBP diastolic blood pressure.
Arterial stiffness and aging
AS has been traditionally associated with increasing chronological age9,44. In early adulthood, cf-PWV increases by 0.2–0.4 m/s per decade, consecutively increasing in average by 1.2–2.4 m/s for individuals 80 years and older47. Notably, these PWV increases are independent of arterial BP, indicating that other factors in addition to chronological aging contribute to this late-life change29,44. These dynamic alterations, which can be conceptualized as hallmarks of vascular aging, are influenced by DNA damage, telomere attrition, genetic alterations, mitochondrial dysfunction, a reduced stem cell pool, and issues in protein processing48. These pathways result in thickening of the intima layer as well as low-grade inflammation (inflammaging) and decreased immune function (immunosenescence), which may also affect the media layer leading to AS, a higher risk of endothelial dysfunction, and subsequent risk of CVD49. Over time, VSMC undergoes phenotypic changes which enhance their proliferation, migration, apoptosis, and senescence29,40,43. Aging causes large elastic arteries, such as the aorta and carotid arteries, to enlarge and stiffen, while medium-sized distal muscular arteries tend to maintain their size and distensibility; however, hypertrophic remodeling occurs universally in these cases44,49. In aged arterial walls, ROS production rises significantly, primarily due to increased NADPH oxidase activity49. Simultaneously, antioxidant proteins in the ECM, such as copper and zinc superoxide dismutase, decrease with age50,51. This imbalance creates a proinflammatory environment which contributes to inflammaging and AS3,44. Aging, coupled with this proinflammatory state, induces structural changes in ECM structure and composition. During this process, telomere shortening and reduced telomerase activity promote DNA damage, which leads to replicative senescence; these senescent cells accumulate in various tissues, particularly affecting fibroblasts, immune system cells, and endothelial cells, contributing to decreased arterial wall elasticity and increased rigidity.
Aging is also associated with increased mental, physical, and environmental stress, as well as a decreased physiological reserve in response to external stimuli52. This results in increased RAAS and sympathetic nervous system activity, which enhances the release of vasoactive substances and promotes the secretion of proinflammatory cytokines and chemokines in the arterial wall leading to sustained vasoconstriction53. These age-related modifications have been proposed to represent an accelerated aging phenotype in the arterial wall in which the biological age of arteries outpaces chronological age, and it may occur at higher rates in individuals with cardiometabolic diseases or in the setting of other age-related diseases54. Previous evidence has shown PWV can be used to predict arterial wall aging and has been correlated with increased vascular age as measures by the Framingham and SCORE 10-year risk of fatal CVD scores55,56; similarly, PWV has been shown to identify subjects with elevated vascular age and has been used as a cardiovascular marker to predict physiological aging rates in humans57,58. Further studies are needed to explore the role of PWV as a measure of biological age or as a component of aging biomarkers to characterize it as a relevant maker of accelerated vascular aging59,60.
Arterial stiffness and arterial hypertension
AS is a pivotal pathophysiological precursor of SAH4. Research indicates that increased cf-PWV often precedes BP rises, and aortic stiffness assessed clinically is linked to faster BP increases and higher risk of incident SAH8. Notably, two major studies reveal that AS progresses independently of baseline BP levels, implying that stiffness itself may play a causative role in the development of sah4,61,62. Isolated systolic hypertension is the most common type of SAH in older adults, and is usually caused by increased aortic AS associated with aging63. Furthermore, AS is frequently present in individuals with SAH and has been associated with a higher risk of IR and diabetes6. In healthy arteries, the stiffness difference between central and peripheral arteries creates backward BP waves, therefore reducing the amount of energy that reaches small blood vessels, providing protection to the microcirculation10; however, this difference decreases in AS, leading to more pressure being transmitted to small peripheral arteries and to the microcirculation, increasing the risk of microvascular damage64. Both high PWV and an enlarged aortic diameter are important contributors of SAH and strong predictors of cardiovascular risk in younger and older adults4,8.
AS reduces diastolic BP due to the premature return of pressure waves to the heart during systole, which diminishes the driving force for coronary blood flow during relaxation65, and may hinder the effectiveness of pharmacological treatments aimed at improving coronary circulation. Furthermore, the accompanying rise in systolic BP increases LV workload and oxygen demand, which also impacts treatment outcomes66. AS also plays a critical role in increasing the risk of SAH-related complications by promoting damage to target organs such as the heart, kidneys, brain, and peripheral arteries66,67. Increased AS worsens cardiovascular prognosis, even in hypertensive patients receiving treatment. While BP-lowering therapy can reduce aortic stiffness in the short term through wall unloading, persistent AS indicates a poor prognosis despite treatment68.
Arterial stiffness and dyslipidemia
The development and progression of AS is closely linked to lipid metabolism69. Standard blood lipids, non-traditional lipid markers, and lipid ratios are all associated with increased AS70. Elevated lipid levels stimulate the release of cytokines and adhesion molecules from leukocytes, which then enable these cells to attach to the vascular endothelium and penetrate the intima, contributing to chronic inflammation and thickening of the arterial wall. Furthermore, lipid accumulation induces oxidative and nitrosative stress, which accelerates arterial stiffening71. Arteriosclerosis, characterized by the thickening and hardening of arterial walls, largely results from structural and functional shifts, such as the replacement of elastic fibers with collagen, damage to muscle fibers, and calcium deposits72. This condition shares risk factors with atherosclerosis, including aging, oxidative and nitrosative stress, high BP, and inflammation69. Multiple studies have shown a significant association between AS as measured by ba-PWV and elevated triglycerides (TG) and total cholesterol levels71,73. While the relationship between PWV and low-density lipoprotein cholesterol (LDL-C) remains unclear, TG are consistently associated with AS and are often involved in early CVD, especially in individuals with low LDL-C levels69. On the other hand, lipoprotein(a) also correlates with PWV, however, its independent association with AIx suggests a more specific impact on the peripheral arterial tree74. When comparing lipid parameters, studies suggest that lipid ratios, such as the TG-to high-density lipoprotein cholesterol (HDL-C), are more strongly linked to high PWV than individual lipid values alone, particularly in those with high TG and low HDL-C levels75,76. Moreover, AS is important for the progression of atherosclerosis in familial hypercholesterolemia (FH), contributing to higher cardiovascular risk and earlier coronary stenosis. Although some studies show increased PWV and other stiffness measures in FH patients, their clinical significance remains unclear. Additional assessments of AS in individuals with FH and familial combined hyperlipidemia (FCHL) revealed important distinctions between the two groups; specifically, patients with FCHL exhibited higher AS compared with FH, which is also in accordance to a higher burden of cardiometabolic risk factors in FCHL compared to FH77. Larger studies are needed to clarify the link between AS, FH, FCHL and other primary dyslipidemias, particularly for improving early detection and guiding treatment approaches for the reduction of cardiovascular risk78.
Arterial stiffness and alterations in glucose metabolism
CKMS and obesity are often linked to IR and hyperinsulinemia, which have been shown to increase the risk of AS in states of impaired glucose metabolism6,79. However, the relationship between AS and IR is complex and not yet fully understood6,80,81. The association of CKMS and elevated AS is also affected by age, which may raise the risk for both AS and T2D82,83. Elevated levels of inflammatory markers such as IL-6 and TNF-α are commonly found in obesity and CKMS, potentially contributing to IR, although the precise mechanisms are still unclear84. Increased pulse pressure during AS may lead to endothelial dysfunction, which can worsen IR85. AS damages capillaries, impacting organs with low resistance like the endocrine pancreas, affecting insulin and glucagon secretion86. Furthermore, microvascular changes in skeletal muscle and liver may impair glucose metabolism and insulin sensitivity, disrupting metabolic balance6,34,63.
Diabetes is recognized as a major risk factor for CVD, kidney damage, and organ dysfunction. Research has shown that PWV is increased in individuals living with prediabetes and T2D compared to individuals with normal fasting plasma blood glucose61,87. Similarly, individuals with abnormal glucose tolerance had stiffer arteries compared to those with normal glucose tolerance. Although the relationship between AS and diabetes might be bidirectional, its temporality remains unclear6,87,88. Individuals living with diabetes may be at a higher risk of developing AS with increasing HbA1c levels, and patients with both diabetes and AS face higher risk of all-cause mortality61,89,90,91; conversely, research has shown that individuals in the highest cf-PWV quartile have three-fold higher risk of developing diabetes compared to those in the first quartile92. A recent longitudinal study found that participants with SAH and AS were at a higher risk of developing T2D compared to those in other groups. Specifically, individuals with SAH but normal AS had the lowest diabetes risk, while those with elevated AS exhibited a substantially higher likelihood of developing T2D74.
HbA1c levels are associated with greater AS both in patients with and without T2D, suggesting a relevant role for chronic hyperglycemia and IR in promoting structural changes in the arterial wall93,94. Hyperglycemia, evaluated by fasting blood glucose, HbA1c, and 2 h blood glucose post-oral glucose tolerance test, independently contributes to the increase in central AS, as measured by cf-PWV95. The presence of multiple altered glycemic markers leads to an even greater rise in cf-PWV, suggesting a cumulative effect on arterial health95. Growing evidence further links increased AS to prediabetic states, such as impaired fasting glucose, glucose intolerance, and IR96,97. In fact, prediabetes is associated with endothelial dysfunction, a key factor in the development of AS98. This dysfunction is largely driven by hyperglycemia-induced oxidative stress, which reduces endothelial nitric oxide synthase expression and impairs NO availability and metabolism, contributing to vascular damage99. Studies have shown that even in the absence of overt diabetes, elevated fasting glucose and HbA1c levels are associated with greater AS94. However, this relationship is not entirely consistent, as some studies have failed to find a significant association between impaired fasting glucose and AS, indicating that further research in this area is needed.
Arterial stiffness and adiposity
AS in individuals with obesity is a significant and independent risk factor for CVD, cognitive decline, and CKD34. In obesity, changes in adipose tissue metabolism cause the release of various bioactive molecules and hormones, including inflammatory cytokines TNF-α, IL-6, angiotensinogen, aldosterone, and leptin. Elevated levels of proinflammatory molecules decreases insulin sensitivity in blood vessels and draw immune and proinflammatory cells to the vascular system, which can lead to AS3. Other elements contributing to this condition are stiffening of endothelial and VSMC, alterations in ECM, inflammation of the adipose tissue surrounding blood vessels, and immune-cell dysfunction34. Recent evidence on arterial distensibility in individuals living with obesity have shown an association between obesity, particularly abdominal adipose tissue, and increased AS100. Adipose tissue distribution plays a key role in cardiovascular risk, with visceral adipose tissue being associated with a higher risk for CVD and AS compared to subcutaneous adiposity, due to higher levels of proinflammatory adipokines produced by visceral adipose tissue depots101. In states of IR, increased lipolysis in visceral adipose tissue results in increased free fatty acid release, which can interfere with insulin’s ability to promote glucose uptake and support metabolic signaling in both adipose and vascular tissues102. This disruption in insulin signaling also lowers NO production, which contributes to endothelial dysfunction and hardening of endothelial and VSMCs34. Premenopausal women typically benefit from the cardiovascular protective effect of estrogens, but this advantage diminishes in the context of obesity, IR, or T2D, and is closely associated with increased AS34. Impaired insulin sensitivity in adipose tissue significantly promotes arterial stiffening by activating RAAS and increasing angiotensin II levels, which contribute to vascular remodeling and AS. This effect is particularly strong in diet-induced obesity, where activation of mineralocorticoid receptors in vascular cells plays a key role in AS, especially in females102,103.
Adiponectin and leptin, two key adipokines produced by white adipose tissue, have opposing effects on AS104,105. Adiponectin helps reduce AS by enhancing NO availability, which regulates vascular tone, and by inhibiting the proliferation and migration of VSMC, thus preventing excessive vascular remodeling106. However, in conditions like obesity, adiponectin levels are often low, hindering endothelial function and contributing to vascular damage and AS, particularly in the presence of low-grade inflammation and arterial hypertension107. In contrast, elevated leptin levels, commonly seen in obesity and SAH, promote VSMC proliferation, endothelial oxidative stress, and reduced NO availability, all of which impair aortic function. Leptin also activates RAAS, increasing BP and worsening endothelial dysfunction, furthering AS105. Therefore, while adiponectin exerts protective effects on vascular health, leptin exacerbates AS, emphasizing the complex role of adipokines in AS progression108.
Evidence of cardiometabolic risk factor management impact on arterial stiffness
As mentioned in earlier sections, AS is recognized as a marker of cardiovascular risk in cardiometabolic conditions, particularly among high-CVD risk groups4. Factors such as obesity, dyslipidemia, inflammation, oxidative stress, and IR are commonly associated with SAH and T2D, and with increased risk of micro and macrovascular complications34,61. Several studies have shown a link between increased AS and inflammation, and research suggests these changes may be reversible if the inflammatory condition is treated. Cardiometabolic risk factors can also accelerate AS progression, but if kept within low, non-disease levels, they may lead to a slower progression rates41. Early detection of vascular stiffening, along with its management through medications and lifestyle modifications, could help minimize future complications. Preventive drugs, including lipid-lowering and antidiabetic medications, have also been shown to impact AS independently of BP4,9. Lifestyle modifications and health interventions such as smoking cessation109, dietary changes, weight loss, physical activity, and adequate metabolic and BP control have been shown to positively impact AS, which highlights the importance of these interventions to manage cardiometabolic risk factors and AS41,110 (Fig. 3).
Created in BioRender. Bello Chavolla, O. (2025) https://BioRender.com/r74b925.
Impact of lifestyle changes on arterial stiffness
Dietary changes
Lifestyle interventions play a fundamental role in AS management. Weight loss regimens based on diet and lifestyle changes have demonstrated benefits on vascular health, and losing an average of 8% of initial body weight through dietary adjustments has been shown to improve PVW79. Some of the most important strategies in dietary interventions which influence AS include caloric restriction (CR), reducing ingestion of saturated fats, simple carbohydrates, and sodium, and incorporating vitamins or antioxidative nutrients like polyphenols111. CR can improve age-related conditions and has demonstrated beneficial effects across various physiological and pathological processes which may help delaying declines in heart function, reducing fibrosis, risk of CVD, and AS79. Research has demonstrated a connection between CR, reduced body weight and improvement in AS112. Reducing carbohydrate intake induces favorable metabolic changes, contributing to decreases in AS, as shown by a reduction in PWV in women113. Both DASH and Mediterranean diets have been shown to positively impact vascular health114,115. The DASH diet is characterized by a high intake of vegetables, fruits, low-fat dairy, and lower sodium intake, which can positively impact AS116,117. A meta-analysis across various populations showed that an average reduction in sodium intake of 89.3 mmol/day (or 5.2 grams of salt per day) is linked to a 2.84% decrease in PWV118. The Mediterranean diet prioritizes vegetables, seeds, cereals, legumes, whole grains (brown rice and oats), lean meats, fish, and olive oil, and a 12-week daily regimen of omega-3 fatty acid supplementation has been shown to reduce PWV in older, but not in healthy young adults119.
Physical activity and arterial stiffness
The benefits of physical activity on AS have been well documented47; however, the impact of resistance training on BP and AS can vary greatly, depending on exercise intensity, chronological age, and overall health levels of an individual110. A systematic review and meta-analysis of randomized controlled trials found that aerobic exercise training resulted in a more prominent decrease in ba-PWV (β = −1.01 m/s) compared to cf-PWV (β = −0.39 m/s)120; furthermore, low-to-moderate intensity resistance training has shown to produce sustained reductions in PWV (β = −0.39 m/s)121, and has been beneficial for AS in patients with kidney disease or established CVD, as well as in normotensive and hypertensive individuals120,121,122. The effect of resistance training on PWV also varies based on the intensity of the exercise. High-intensity resistance training did not significantly impact PWV; in contrast, low-to-moderate intensity training led to significant improvements in PWV (β = −0.34 m/s). In young men and women, combined exercise enhances the benefits of physical activity on AS and reduces carotid artery stiffness, likely due in part to reductions in SBP123. Mechanisms involved in the observed vascular improvements of physical activity likely work synergistically123. Increased bioavailability of NO and reduced inflammation may drive a significant decrease in AS after combined exercise. Because AS increases with chronological age, regular aerobic activity can counter this progression by reducing BP110. This reduction in BP eases mechanical stress, balances metabolic and neurohormonal factors, and decreases inflammation, helping arteries retain their elasticity. In some cases, regular aerobic exercise has been shown to reverse existing stiffness, highlighting its powerful impact on vascular health47.
Cardiorespiratory fitness (CRF) is a relevant marker for cardiometabolic health which is inversely and independently associated with risk of all-cause mortality and CVD outcomes124,125,126,127. Notably, the impact of physical activity on CRF is one of the most relevant predictors of its cardiovascular benefits125,126. Higher cf-PWV and ba-PWV values have been associated with decreased CRF, particularly in patients living with metabolic syndrome, obesity, SAH or T2D128,129,130, indicating that AS may lead to lower CRF in patients living with cardiometabolic diseases. Interestingly, aerobic exercise consistently increases CRF and vascular health by increasing aerobic capacity and reducing BP and AS, and in patients with cardiometabolic diseases and individual CKMS components, combined aerobic and resistance training improve CRF in addition to its metabolic benefits131,132,133. A potential obstacle to implementing physical activity as a treatment for AS is the association between high AS and reduced exercise capacity, as it may occur in the setting of cardiometabolic diseases134,135. This limitation arises from two hemodynamic effects; first, AS raises systolic BP, which increases the heart’s workload and its oxygen requirements, and second, it reduces the Windkessel effect in large arteries, leading to less blood being stored in these arteries during diastole and decreasing end-organ perfusion47,110. In the setting of increased oxygen demand during physical activity, these observed hemodynamic changes during AS may decrease exercise capacity and thus limit its utility as a clinical intervention134. Additional research is required to investigate optimal physical activity regimens in patients with reduced CRF in the setting of AS, and its impact on reducing AS in patients with comorbid cardiometabolic diseases.
Impact of cardiometabolic disease management on arterial stiffness
AS accelerates the onset and progression of CVD and serves as a marker of increased CVD risk, particularly in individuals with CKMS, obesity, IR, or diabetes6. In adults, the presence of a high number of CKMS components correlates with elevated ba-PWV and progression of AS41; in fact, evidence suggests that AS may precede the onset of individual CKMS components8,136. Weight loss, reduced salt intake, and exercise are strategies that not only help control BP and cardiometabolic diseases but have also been investigated for their potential to improve AS. Part of the effectiveness of these interventions is linked to improvements in cardiometabolic risk factors68.
Impact of blood pressure management on arterial stiffness
Recent anti-hypertensive drugs offer benefits beyond just lowering BP, and reducing AS with these drugs is expected to provide additional advantages apart from BP reduction4. Evidence from a longitudinal analysis of the Kailuan study showed that antihypertensive medications may have a primary influence in PWV, even before any benefits are observed in BP levels13. Furthermore, ba-PWV prior to treatment initiation in adults with SAH has been shown to influence the BP lowering effects of antihypertensive medications, whereby higher AS was usually indicative of decreased BP response to short-and long-term treatment137. PWV is an important indicator of organ or tissue-specific damage, making it an invaluable tool for identifying arterial injury11. AS measurement can provide a strong rationale for initiating antihypertensive therapy; furthermore, the potential bidirectional causal relationship between SAH and AS highlights the importance of considering the presence of AS as a criterion when deciding to initiate antihypertensive treatment4,66. Notably, uncontrolled systolic and diastolic BP levels in adults with SAH is associated with higher ba-PWV, indicating that AS may be a clinically useful marker for uncontrolled SAH138. The Systolic Blood Pressure Intervention Trial (SPRINT) showed improved survival rates in hypertensive patients whose PWV decreased after treatment, independently of any reduction in SBP139. These results collectively emphasize the role of PWV as a crucial parameter in tailoring antihypertensive strategies to optimize cardiovascular outcomes4.
Specific antihypertensive drug classes may have differential impacts on PWV and AS. For example, angiotensin-converting enzyme inhibitors (ACEI) have been shown to be more effective in reducing PWV compared to placebo, angiotensin-receptor blockers (ARB), calcium-channel blockers (CCB), β-blockers, diuretics, and a combination of ACEI and ARB, with no significant influence of BP changes on their effect on PWV140. Evidence on the impact of ARB on AS suggests similar effects to other antihypertensive agents, largely attributed to their ability to relax VSMCs within primary major elastic arteries, and improve the AIx141,142. The use of CCB and β-blockers have shown heterogeneous effects on AS; while their impact have been beneficial compared to placebo, these antihypertensive drug classes appear to be less effective at reducing PWV compared to ACEI and ARB143,144. The effectiveness of RAAS inhibition on AS management may make ACEI and ARB better alternatives compared to other antihypertensive drug classes in the management of AS.
Impact of weight loss and obesity management on arterial stiffness
Weight loss achieved through dietary and lifestyle changes has a direct impact on reducing AS. For instance, a four-month reduction in body mass significantly improves aortic and carotid stiffness in subjects living with overweight and obesity79,84,108. Even small reductions in weight can enhance glycemic control, lower BP and triglyceride levels, and increase HDL cholesterol, underscoring the importance of weight management in vascular health6. Weight loss with average body weight reductions of 8% may lead to a PWV reduction estimated at −0.32 m/s, with more pronounced (but not statistically significant) reductions observed in ba-PWV compared to cf-PWV (−0.48 m/s vs. −0.35 m/s). Modest reductions ranging 5% to 10% of body weights can lead to substantial improvements in AS particularly through its effect in BP reductions145. Therefore, AS may be managed through the impact of lifestyle interventions on weight loss, particularly in individuals with obesity.
The impact of bariatric surgery on AS in individuals with obesity has also been documented, showing declines of up to −0.65 m/s on PWV post-surgery, independent of baseline BMI and the duration of follow-up146. Notably, the effects of bariatric surgery on AS may be in part due to their impact on other cardiometabolic risk factors, as the positive effect of bariatric surgery on PWV was not observed in metabolically-healthy adults with obesity in a recent study147. Interestingly, individuals with median increases of 104% on GLP-1 levels post-bariatric surgery compared to baseline showed greater decreases in cf-PWV compared to non-responders, indicating that changes in GLP-1 levels after bariatric surgery may be the best predictor of decreases in AS148. This evidence supports a potential role for pharmacological agents, including GLP-1 receptor agonists (GLP-1RA) on improving AS in individuals with obesity, particularly with the strong benefits of GLP-1RA on cardiovascular outcomes149; however, to date no study has shown conclusive evidence of their impact on AS or PWV in adults with obesity but without T2D150.
Management of diabetes and arterial stiffness
In T2D, persistent hyperglycemia and IR accelerate AS by damaging the endothelial glycocalyx and increasing inflammatory activity. This stiffness impairs vascular elasticity, raises PWV, and contributes to hypertension and left ventricular strain65. Management of T2D plays a key role in mitigating AS by reducing advanced glycation end-products and their interaction with their receptors2. When assessed together with glycemic control, individuals with good glycemic control still had a high risk of macrovascular complications when affected by moderate-to-severe AS; conversely, those with poor glycemic control had the highest risk with concomitant severe AS151. Evidence suggests that adequate glycemic control in patients with T2D also reduces PWV and attenuates the impact of AS on T2D152,153.
The role of specific medications for T2D in managing AS has also been investigated. Metformin has shown modest benefits in improving the AIx and reducing aortic PWV, ba-PWV and BP primarily through its effect on peripheral insulin sensitivity, increased adiponectin levels, and weight loss154,155. Pioglitazone and rosiglitazone have also shown improvements in endothelial function and AS by increasing arterial flexibility and causing a secondary amplification of adiponectin levels, and may be superior for improving endothelial function compared to placebo156,157,158. In a recent network meta-analysis of randomized controlled trials, thiazolidinediones, GLP-1RA and SGLT-2 inhibitors were shown to reduce PWV by up to −0.5 m/s compared to placebo, without significant benefits for sulfonylureas, metformin, DPP-4 inhibitors and insulin158. GLP-1RA and SGLT-2 inhibitor therapies can improve the glycocalyx integrity by reducing inflammation and thus enhancing arterial compliance by targeting both metabolic dysfunction and vascular health providing protection against cardiovascular complications65. These drugs offer beneficial effects on BP, body weight, HbA1c levels, and lipid profiles. In a previous study, Ikonomidis et al. showed that combining SGLT-2 inhibitors and GLP-1RA for a 12-month treatment improved glycemic control in individuals with T2D and a steeper reduction in PWV in comparison to insulin and GLP-1RA alone, along with significant reductions in body weight and fat mass159. Despite recent evidence showing a potential benefit of SGLT-2 inhibitors and GLP-1RA on improving AS in patients with T2D, additional studies are required to evaluate whether this effect is conditional on glycemic control or potentially influenced by weight loss160,161.
Management of dyslipidemia and arterial stiffness
Statins confer beneficial properties for vascular function, such as anti-inflammatory and antioxidant effects which may contribute to managing AS5. A meta-analysis of randomized control trials showed that the use of simvastatin, rosuvastatin, lovastatin, fluvastatin, and atorvastatin was associated with slower aortic PWV162. Similarly, high-intensity statin therapies were shown to decrease PWV by −1.17 m/s, compared to −0.80 m/s with moderate-intensity and a non-significant −0.5 m/s for low-intensity statins regimes163. Interestingly, a network meta-analysis which compared the use of statins compared to physical activity for reducing AS showed higher surface under the cumulative ranking curve (SUCRA) for high- and moderate-intensity statin therapies (74% and 67%), but lower SUCRA for low-intensity regimes compared to high-intensity exercise (60% vs. 67%), indicating that physical activity may be considered as an alternative to low-intensity statins for managing AS163. Furthermore, a systematic review and meta-analysis explored the impact of adding PCSK-9 inhibitors to lipid-lowering interventions on arterial stiffness, identifying a reduction of −2.61 m/s in PWV after adding PCSK9 inhibitors to standard statin therapy. Interestingly, the impact of PCSK-9 inhibitor addition on AS showed a greater impact in men and a dependent effect for patients with higher baseline PWV values164.
Conclusions and perspectives
AS is a risk factor and a relevant marker to evaluate CVD risk. Given the substantial overlap between the pathophysiology of AS, aging, and cardiometabolic diseases, follow-up and management of cardiometabolic diseases must consider their impact on PWV and AS to adequately manage cardiovascular risk in these populations. Individuals living with obesity, particularly abdominal obesity, IR, prediabetes, T2D, SAH, and primary and secondary dyslipidemias must be considered at higher risk of AS and should be evaluated using non-invasive techniques, particularly those without prior history of CVD given the evidence of their utility as a marker of vascular health42. Adequate metabolic and BP control may offer beneficial effects on AS for patients living with cardiometabolic disease; however, it is currently unclear whether serial measurements of PWV may be clinically useful, or whether reductions in PWV after therapeutic interventions may offer benefits relevant to micro or macrovascular outcomes in these patients18,165,166. Further randomized studies are required to evaluate the impact of specific interventions aimed at improving metabolic control on AS and, specifically, on whether these benefits translate to meaningful reductions on the risk of CVD outcomes.
Despite substantial evidence on the impact of lifestyle interventions on AS and PWV, further research is needed to understand the impact of managing specific macro and micronutrients on AS progression, as well as evidence from randomized controlled trials evaluating the impact of dietary interventions on AS and its potential modifiers including but not limited to Mediterranean and DASH diets, CR and intermittent fasting, which has not previously been explored in depth despite some planned trials primarily focused on time restricted eating167. The evidence of physical activity on AS is strong and it may confer stronger benefits for reducing PWV compared to some therapeutic interventions. However, whether the effect of specific physical activity prescription on cardiometabolic diseases may offer additional benefits requires additional studies. Many of the potential benefits of diet and physical activity prescription on AS could be attributed to reductions of between 5–10% of body weight, which may also confer additional benefits in improving other metabolic parameters. Additional research is needed to investigate whether the use of GLP-1RA may be useful to reduce AS in adults living with obesity but without type 2 diabetes.
Specific treatment of cardiometabolic diseases has also shown benefits in managing AS. In patients living with SAH, adequate BP control may require additional management of AS. Evidence suggests that the use of ACEIs and ARBs may provide benefits in reducing PWV and AS even before benefits in BP are observed, and which may be superior to other antihypertensive medications. Monitoring PWV may be useful to assess cardiovascular risk as part of the management of SAH and may lead to greater benefits in cardiovascular outcomes compared to BP monitoring alone; however, this will need to be confirmed in randomized controlled studies. In the case of T2D, the use of SLGT-2 inhibitors and GLP-1RA may be useful to achieve glycemic control, weight loss and reductions in AS and PWV; this may be useful given that AS has been associated with worse outcomes in patients with diabetes independent of glycemic control. Whether management of AS in patients with T2D offers additional benefits for micro and macrovascular complications requires further evidence from prospective observational studies and randomized controlled trials. Finally, moderate and high intensity statin therapy has shown substantial benefits in improving AS and reducing PWV in patients with dyslipidemia and addition of PCSK-9 inhibitors may provide benefit, especially for patients with elevated PWV as it occurs in those living with primary dyslipidemias.
Given the relevance of AS as mechanisms of vascular aging, its influence on cardiovascular health and its utility as a prognostic marker for CVD, PWV should be considered as a potentially useful non-invasive measure of vascular health in patients living with cardiometabolic diseases. Substantial evidence supports the clinical relevance of PWV measurement in investigating CVD, monitoring SAH, assessing the effectiveness of cardiometabolic risk factor management, and even evaluating cerebral blood flow to estimate stroke and dementia risk60. However, its routine use in clinical settings remains limited due to technical and scientific challenges60,168,169. Further research is needed to validate the accuracy and consistency of non-invasive PWV measurements compared to invasive methods and to establish their predictive value for relevant outcomes independent of age and BP levels. Additionally, studies should explore the ability of PWV to detect both overt and subclinical vascular changes, which are may be useful for cardiovascular risk stratification, therapy initiation, and monitoring60,169. As research in this field rapidly evolves, future studies should prioritize assessing the clinical utility of PWV measuring and monitoring, as well as the therapeutic and prognostic implications of AS management in patients with cardiometabolic diseases. Clarifying the role of AS in the pathophysiology of CKMS and its implications for tracking and managing individual cardiometabolic risk factors will further enhance its integration into clinical practice.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACEI :
-
Angiotensin-Converting Enzyme Inhibitors
- AIx :
-
Augmentation Index
- ARB :
-
Angiotensin-Receptor Blockers
- AS :
-
Arterial Stiffness
- ba-PWV :
-
Brachial-Ankle Pulse Wave Velocity
- BP :
-
Blood Pressure
- CAVI :
-
Cardio-Ankle Vascular Index
- CCB :
-
Calcium-Channel Blockers
- cf-PWV :
-
Carotid-Femoral Pulse Wave Velocity
- CKMS :
-
Cardiovascular-Kidney Metabolic Syndrome
- CKD :
-
Chronic Kidney Disease
- CRF :
-
Cardiorespiratory fitness
- CVD :
-
Cardiovascular Disease
- DASH :
-
Dietary Approaches to Stop Hypertension
- DPP-4 inhibitors :
-
Dipeptidyl Peptidase-4 Inhibitors
- ECM :
-
Extracellular Matrix
- FCHL :
-
Familial Combined Hyperlipidemia
- FH :
-
Familial Hypercholesterolemia
- GLP-1 :
-
Glucagon-Like Peptide-1
- GLP-1RA :
-
GLP-1 Receptor Agonists
- IR :
-
Insulin Resistance
- LV :
-
Left Ventricular
- MCP-1 :
-
Monocyte Chemoattractant Protein-1
- MFG-E8 :
-
Milk Fat Globule-Epidermal Growth Factor 8
- MRI :
-
Magnetic Resonance Imaging
- NADPH :
-
Nicotinamide Adenine Dinucleotide Phosphate
- NO :
-
Nitric Oxide
- PCSK-9 inhibitors :
-
Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors
- PPP1CB :
-
Protein Phosphatase 1 Catalytic Subunit Beta
- PRKA :
-
Protein Kinase A
- PWV :
-
Pulse Wave Velocity
- RAAS :
-
Renin-Angiotensin-Aldosterone System
- ROS :
-
Reactive Oxygen Species
- SAH :
-
Systemic Arterial Hypertension
- SGLT2 inhibitors :
-
Sodium-Glucose Cotransporter 2 Inhibitors
- SUCRA :
-
Surface Under the Cumulative Ranking Curve
- T2D :
-
Type 2 Diabetes Mellitus
- TGF-β :
-
Transforming Growth Factor Beta
- TNF-α :
-
Tumor Necrosis Factor Alpha
- VSMC :
-
Vascular Smooth Muscle Cells
References
Mensah, G. A., Fuster, V., Murray, C. J. L., Roth, G. A. & Global Burden of Cardiovascular Diseases and Risks Collaborators Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 82, 2350–2473 (2023).
Castelli, R. et al. Aging of the Arterial System. Int. J. Mol. Sci. 24, 6910 (2023).
Mozos, I. et al. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front. Immunol. 8, 1058 (2017).
Boutouyrie, P., Chowienczyk, P., Humphrey, J. D. & Mitchell, G. F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 128, 864–886 (2021).
Wilkinson, I. B., Mäki-Petäjä, K. M. & Mitchell, G. F. Uses of Arterial Stiffness in Clinical Practice. Arterioscler. Thromb. Vasc. Biol. 40, 1063–1067 (2020).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766 (2021).
Neves, M. F., Cunha, A. R., Cunha, M. R., Gismondi, R. A. & Oigman, W. The Role of Renin-Angiotensin-Aldosterone System and Its New Components in Arterial Stiffness and Vascular Aging. High Blood Press. Cardiovasc. Prev. J. Ital. Soc. Hypertens. 25, 137–145 (2018).
Agbaje, A. O. Arterial stiffness precedes hypertension and metabolic risks in youth: a review. J. Hypertens. 40, 1887–1896 (2022).
Chirinos, J. A., Segers, P., Hughes, T. & Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 74, 1237–1263 (2019).
Regnault, V., Lacolley, P. & Laurent, S. Arterial Stiffness: From Basic Primers to Integrative Physiology. Annu. Rev. Physiol. 86, 99–121 (2024).
Scuteri, A. et al. Arterial stiffness and multiple organ damage: a longitudinal study in population. Aging Clin. Exp. Res. 32, 781–788 (2020).
Sang, Y. et al. Longitudinal association between cardiovascular health and arterial stiffness in the Chinese adult population. J. Int. Med. Res. 49, 300060521998889 (2021).
Wu, S. et al. Arterial stiffness and blood pressure in treated hypertension: a longitudinal study. J. Hypertens. 41, 768–774 (2023).
Vasan, R. S. et al. Arterial Stiffness and Long-Term Risk of Health Outcomes: The Framingham Heart Study.Hypertens. Dallas Tex79, 1045–1056 (2022).
Reeve, E. H., Barnes, J. N., Moir, M. E. & Walker, A. E. Impact of arterial stiffness on cerebrovascular function: a review of evidence from humans and preclincal models. Am. J. Physiol. Heart Circ. Physiol. 326, H689–H704 (2024).
Mitchell, G. F. Arterial Stiffness in Aging: Does It Have a Place in Clinical Practice? Recent Advances in Hypertension.Hypertens. Dallas Tex77, 768–780 (2021).
Oliveira, A. C. et al. Envelhecimento Vascular e Rigidez Arterial. Arq. Bras. Cardiol. 119, 604–615 (2022).
Budoff, M. J. et al. Clinical Applications Measuring Arterial Stiffness: An Expert Consensus for the Application of Cardio-Ankle Vascular Index. Am. J. Hypertens. 35, 441–453 (2022).
DuPont, J. J., Kenney, R. M., Patel, A. R. & Jaffe, I. Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 176, 4208–4225 (2019).
Rasouli, R. et al. Local Arterial Stiffness Assessment: Comparison of Pulse Wave Velocity Assessed by Ultrafast Ultrasound Imaging versus the Bramwell-Hill Equation. J. Am. Soc. Echocardiogr. 35, 1185–1188 (2022).
Alvim, R. et al. Arterial Stiffness: Pathophysiological and Genetic Aspects. Int. J. Cardiovasc. Sci. 30, 433–441 (2017).
Forcada, P., Melgarejo, E. & Echeverri, D. Cuantificación de la rigidez arterial: de lo básico a lo clínico. Rev. Colomb. Cardiol. 22, 69–71 (2015).
Schutte, A. E. et al. Ethnicity and Arterial Stiffness. Arterioscler. Thromb. Vasc. Biol. 40, 1044–1054 (2020).
Lim, W.-H. et al. The Prognostic Value of Arterial Stiffness According to Socioeconomic Status. J. Clin. Med. 12, 6943 (2023).
Kim, H.-L. et al. Relationship of Socioeconomic Status to Arterial Stiffness: Comparison Between Medical Aid Beneficiaries and National Health Insurance Beneficiaries. Am. J. Hypertens. 33, 718–725 (2020).
Faconti, L., Nanino, E., Mills, C. E. & Cruickshank, K. J. Do arterial stiffness and wave reflection underlie cardiovascular risk in ethnic minorities?. JRSM Cardiovasc. Dis. 5, 2048004016661679 (2016).
Craig, A., Kruger, R., Gafane-Matemane, L. F., Louw, R. & Mels, C. M. C. Early vascular ageing phenotypes and urinary targeted metabolomics in children and young adults: the ExAMIN Youth SA and African-PREDICT studies. Amino Acids 55, 1049–1062 (2023).
du Toit, W. L. et al. Markers of arterial stiffness and urinary metabolomics in young adults with early cardiovascular risk: the African-PREDICT study. Metabolomics. J. Metabolomic Soc. 19, 28 (2023).
Lacolley, P., Regnault, V., Segers, P. & Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 97, 1555–1617 (2017).
Laurent, S., Boutouyrie, P. & Lacolley, P. Structural and Genetic Bases of Arterial Stiffness. Hypertension 45, 1050–1055 (2005).
Lacolley, P., Challande, P., Osborne-Pellegrin, M. & Regnault, V. Genetics and pathophysiology of arterial stiffness. Cardiovasc. Res. 81, 637–648 (2009).
Eales, J. M., Romaine, S. P. R., Charchar, F. J. & Tomaszewski, M. A multi-omics glimpse into the biology of arterial stiffness. J. Hypertens. 34, 32–35 (2016).
Cuadrat, R. R. C. et al. Association of the human gut microbiota with vascular stiffness. Sci. Rep. 13, 13348 (2023).
Aroor, A. R., Jia, G. & Sowers, J. R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R387–R398 (2018).
Seifalian, A. M., Filippatos, T. D., Joshi, J. & Mikhailidis, D. P. Obesity and arterial compliance alterations. Curr. Vasc. Pharmacol. 8, 155–168 (2010).
Ndumele, C. E. et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 148, 1606–1635 (2023).
Ndumele, C. E. et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation 148, 1636–1664 (2023).
Mehta, S. et al. Arterial Thickness and Stiffness Are Independently Associated with Left Ventricular Strain. J. Am. Soc. Echocardiogr. Pub. Am. Soc. Echocardiogr. 31, 99–104 (2018).
Cusmà-Piccione, M. et al. How arterial stiffness may affect coronary blood flow: a challenging pathophysiological link. J. Cardiovasc. Med. Hagerstown Md. 15, 797–802 (2014).
Durham, A. L., Speer, M. Y., Scatena, M., Giachelli, C. M. & Shanahan, C. M. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 114, 590–600 (2018).
Lifelong Heterogeneous Contribution of Cardiovascular Risk Factors to Slow and Fast Progression of Arterial Stiffness.Hypertens.DallasTex80, 2159–2168(2023).
Ohkuma, T.et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertens. Dallas Tex 69, 1045–1052(2017).
Sun, Z. Aging, arterial stiffness, and hypertension. Hypertens. Dallas Tex 65,252–256 (2015).
Wang, M., Monticone, R. E. & McGraw, K. R. Proinflammatory Arterial Stiffness Syndrome: A Signature of Large Arterial Aging. J. Vasc. Res. 55, 210–223(2018).
Wang, J., Thornton, J. C., Kolesnik, S. & Pierson, R. N. Anthropometry in body composition. An overview. Ann. NY. Acad. Sci. 904, 317–326 (2000).
Cheng, W. et al. Superior predictive value of estimated pulse wave velocity for all-cause and cardiovascular disease mortality risk in U.S. general adults. BMC Pub. Health 24, 600 (2024).
Mynard, J. P. & Clarke, M. M. Arterial Stiffness, Exercise Capacity and Cardiovascular Risk. Heart Lung Circ. 28, 1609–1611 (2019).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The Hallmarks of Aging. Cell 153, 1194–1217 (2013).
Gkaliagkousi, E. et al. Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging. Int. J. Mol. Sci. 23,8672 (2022).
Gómez-Marcos, M. A. et al. Serum Superoxide Dismutase Is Associated with Vascular Structure and Function in Hypertensive and Diabetic Patients. Oxid. Med. Cell. Longev. 2016, 9124676 (2015).
Wang, J. et al. Improvement of Arterial Stiffness by Reducing Oxidative Stress Damage in Elderly Hypertensive Patients After 6 Months of Atorvastatin Therapy. J. Clin. Hypertens. 14,245–249(2012).
Cesari, M. et al. Early detection of accelerated aging and cellular decline (AACD): A consensus statement. Exp. Gerontol. 146, 111242 (2021).
Della Corte, V. et al. Inflammation, Endothelial Dysfunction and Arterial Stiffness as Therapeutic Targets in Cardiovascular Medicine. Curr. Pharm. Des. 22, 4658–4668 (2016).
Amorim, J. A. et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 18, 243–258 (2022).
Asil, S. et al. Correlation of vascular risk age with pulse wave velocity in young patients with low absolute cardiovascular risk. Turk. Kardiyol. Dernegi Arsivi Turk. Kardiyol. Derneginin Yayin Organidir 49, 214–222 (2021).
Gyöngyösi, H. et al. Comparison of Different Cardiovascular Risk Score and Pulse Wave Velocity-Based Methods for Vascular Age Calculation. Heart Lung Circ. 30, 1744–1751 (2021).
Gyöngyösi, H. et al. Differences between SCORE, Framingham Risk Score, and Estimated Pulse Wave Velocity-Based Vascular Age Calculation Methods Based on Data from the Three Generations Health Program in Hungary. J. Clin. Med. 13, 205 (2023).
Sun, E. D. et al. Predicting physiological aging rates from a range of quantitative traits using machine learning. Aging 13, 23471–23516 (2021).
Oliveira, A. C. et al. Vascular Aging and Arterial Stiffness. Arq. Bras. Cardiol. 119, 604–615 (2022).
Marshall, A. G. et al. Update on the Use of Pulse Wave Velocity to Measure Age-Related Vascular Changes. Curr. Hypertens. Rep. 26, 131–140 (2024).
Tian, X. et al. Hypertension, Arterial Stiffness, and Diabetes: a Prospective Cohort Study. Hypertens. Dallas Tex. 1979 79, 1487–1496 (2022).
Stone, K. et al. Aortic-Femoral Stiffness Gradient and Cardiovascular Risk in Older Adults. Hypertens. Dallas Tex. 1979 81, e185–e196 (2024).
Safar, M. E., Levy, B. I. & Struijker-Boudier, H. Current Perspectives on Arterial Stiffness and Pulse Pressure in Hypertension and Cardiovascular Diseases. Circulation 107, 2864–2869 (2003).
Zhang, Y., Lacolley, P., Protogerou, A. D. & Safar, M. E. Arterial Stiffness in Hypertension and Function of Large Arteries. Am. J. Hypertens. 33, 291–296 (2020).
Ikonomidis, I., Makavos, G. & Lekakis, J. Arterial stiffness and coronary artery disease. Curr. Opin. Cardiol. 30, 422–431 (2015).
Kim, H.-L. Arterial stiffness and hypertension. Clin. Hypertens. 29, 31 (2023).
Vlachopoulos, C., Aznaouridis, K. & Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 55, 1318–1327 (2010).
Sharman, J. E., Boutouyrie, P. & Laurent, S. Arterial (Aortic) Stiffness in Patients with Resistant Hypertension: from Assessment to Treatment. Curr. Hypertens. Rep. 19, 2 (2017).
Baba, M. et al. The Impact of the Blood Lipids Levels on Arterial Stiffness. J. Cardiovasc. Dev. Dis. 10, 127 (2023).
Tomiyama, H. & Shiina, K. State of the Art Review: Brachial-Ankle PWV. J. Atheroscler. Thromb. 27, 621–636 (2020).
Wang, L. et al. Association between lipid profiles and arterial stiffness: A secondary analysis based on a cross-sectional study. J. Int. Med. Res. 48, 300060520938188 (2020).
Mitchell, G. F. & Powell, J. T. Arteriosclerosis: A Primer for ‘In Focus’ Reviews on Arterial Stiffness. Arterioscler. Thromb. Vasc. Biol. 40, 1025–1027 (2020).
Wen, J. et al. Lipoprotein ratios are better than conventional lipid parameters in predicting arterial stiffness in young men. J. Clin. Hypertens. Greenwich Conn. 19, 771–776 (2017).
Brosolo, G. et al. Lipoprotein(a): Just an Innocent Bystander in Arterial Hypertension?. Int. J. Mol. Sci. 24, 13363 (2023).
Wu, Z. et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc. Diabetol. 20, 134 (2021).
Wen, J.-H. et al. Triglyceride to HDL-C ratio and increased arterial stiffness in apparently healthy individuals. Int. J. Clin. Exp. Med. 8, 4342–4348 (2015).
Mehta, R. et al. Evaluation of arterial stiffness in primary atherogenic dyslipidemias. Rev. Mex. Endocrinol. Metab. Nutr. 11, 045–052 (2024).
Kovács, B. et al. The Importance of Arterial Stiffness Assessment in Patients with Familial Hypercholesterolemia. J. Clin. Med. 11, 2872 (2022).
Stanek, A. et al. The Influence of Dietary Interventions on Arterial Stiffness in Overweight and Obese Subjects. Nutrients 15, 1440 (2023).
Bello-Chavolla, O. Y. et al. Prediction of incident hypertension and arterial stiffness using the non-insulin-based metabolic score for insulin resistance (METS-IR) index. J. Clin. Hypertens. Greenwich Conn. 21, 1063–1070 (2019).
Mehta, R. et al. Association between insulin resistance and arterial stiffness in Mexican patients without type 2 diabetes. Gac. Med. Mex. 157, 522–530 (2021).
Dregan, A. et al. Associations Between Depression, Arterial Stiffness, and Metabolic Syndrome Among Adults in the UK Biobank Population Study: A Mediation Analysis. JAMA Psychiatry 77, 598–606 (2020).
Wang, M. et al. BMI-based metabolic syndrome severity score and arterial stiffness in a cohort Chinese study. Nutr. Metab. Cardiovasc. Dis. NMCD 34, 1761–1768 (2024).
Para, I., Albu, A. & Porojan, M. D. Adipokines and Arterial Stiffness in Obesity. Med. Kaunas. Lith. 57, 653 (2021).
Kodama, S. et al. Meta-analysis of the quantitative relation between pulse pressure and mean arterial pressure and cardiovascular risk in patients with diabetes mellitus. Am. J. Cardiol. 113, 1058–1065 (2014).
Sung, K.-C. Arterial Stiffness and Incident Diabetes. Pulse Basel Switz. 12, 12–18 (2024).
Zheng, M. et al. Arterial Stiffness Preceding Diabetes: A Longitudinal Study. Circ. Res. 127, 1491–1498 (2020).
Muhammad, I. F. et al. Arterial Stiffness and Incidence of Diabetes: A Population-Based Cohort Study. Diab. Care 40, 1739–1745 (2017).
Antonopoulos, A. S. et al. Arterial stiffness and microvascular disease in type 2 diabetes. Eur. J. Clin. Invest. 51, e13380 (2021).
Tynjälä, A. et al. Arterial Stiffness Predicts Mortality in Individuals With Type 1 Diabetes. Diab. Care 43, 2266–2271 (2020).
Sun, J. et al. Estimated glucose disposal rate and risk of arterial stiffness and long-term all-acuse mortality: a 10-year prospective study. J. Epidemiol. Community Health 78, jech-2023-220664 (2023).
Bao, W. et al. Association between estimated pulse wave velocity and risk of diabetes: A large sample size cohort study. Nutr. Metab. Cardiovasc. Dis. NMCD 33, 1716–1724 (2023).
Patoulias, D. et al. Prognostic value of arterial stiffness measurements in cardiovascular disease, diabetes, and its complications: The potential role of sodium-glucose co-transporter-2 inhibitors. J. Clin. Hypertens. Greenwich Conn. 22, 562–571 (2020).
Martagón, A. J. et al. Arterial Stiffness and HbA1c: Association Mediated by Insulin Resistance in Hispanic Adults. Int. J. Environ. Res. Public. Health 19, 11017 (2022).
Pereira, W. D. S. et al. Fasting Glucose, Glycated Hemoglobin, and 2h Post-load Blood Glucose Are Independently Associated With Arterial Stiffness in Diabetes: The ELSA-Brasil Study. Angiology 75, 635–644 (2024).
Prenner, S. B. & Chirinos, J. A. Arterial stiffness in diabetes mellitus. Atherosclerosis 238, 370–379 (2015).
Cederqvist, J. et al. Arterial stiffness and subclinical atherosclerosis in the coronary arteries at different stages of dysglycaemia. Diabet. Med. J. Br. Diabet. Assoc. 40, e15102 (2023).
Shah, A. S., Gao, Z., Urbina, E. M., Kimball, T. R. & Dolan, L. M. Prediabetes: the effects on arterial thickness and stiffness in obese youth. J. Clin. Endocrinol. Metab. 99, 1037–1043 (2014).
Yu, J. et al. Association Between Glucose Metabolism And Vascular Aging In Chinese Adults: A Cross-Sectional Analysis In The Tianning Cohort Study. Clin. Interv. Aging 14, 1937–1946 (2019).
Antonio-Villa, N. E. et al. Increased visceral fat accumulation modifies the effect of insulin resistance on arterial stiffness and hypertension risk. Nutr. Metab. Cardiovasc. Dis. NMCD 31, 506–517 (2021).
Koenen, M., Hill, M. A., Cohen, P. & Sowers, J. R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 128, 951–968 (2021).
Jia, G. et al. Vascular stiffness in insulin resistance and obesity. Front. Physiol. 6, 231 (2015).
Aroor, A. R. et al. The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front. Endocrinol. 4, 161 (2013).
Youn, J.-C. et al. Adiponectin and progression of arterial stiffness in hypertensive patients. Int. J. Cardiol. 163, 316–319 (2013).
D’Elia, L., Giaquinto, A., De Luca, F., Strazzullo, P. & Galletti, F. Relationship between circulating leptin levels and arterial stiffness: a systematic review and meta-analysis of observational studies. High. Blood Press. Cardiovasc. Prev. J. Ital. Soc. Hypertens. 27, 505–513 (2020).
Kovesdy, C. P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int. Suppl. 12, 7–11 (2022).
Bielecka-Dabrowa, A., Bartlomiejczyk, M. A., Sakowicz, A., Maciejewski, M. & Banach, M. The Role of Adipokines in the Development of Arterial Stiffness and Hypertension. Angiology 71, 754–761 (2020).
Sabbatini, A. R., Fontana, V., Laurent, S. & Moreno, H. An update on the role of adipokines in arterial stiffness and hypertension. J. Hypertens. 33, 435–444 (2015).
Yu-Jie, W., Hui-Liang, L., Bing, L., Lu, Z. & Zhi-Geng, J. Impact of smoking and smoking cessation on arterial stiffness in healthy participants. Angiology 64, 273–280 (2013).
Yang, J., Chen, X., Chen, X. & Li, L. Physical Activity and Arterial Stiffness: A Narrative Review. J. Clin. Hypertens. Greenwich Conn. https://doi.org/10.1111/jch.14941 (2024).
Nicoll, R. & Henein, M. Y. Caloric Restriction and Its Effect on Blood Pressure, Heart Rate Variability and Arterial Stiffness and Dilatation: A Review of the Evidence. Int. J. Mol. Sci. 19, 751 (2018).
Jefferson, M. E. et al. Effects of Resistance Training With and Without Caloric Restriction on Arterial Stiffness in Overweight and Obese Older Adults. Am. J. Hypertens. 29, 494–500 (2016).
Syed-Abdul, M. M. et al. Effect of carbohydrate restriction-induced weight loss on aortic pulse wave velocity in overweight men and women. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 43, 1247–1256 (2018).
Jeong, S. Y. et al. Effects of Diet on 10-Year Atherosclerotic Cardiovascular Disease Risk (from the DASH Trial). Am. J. Cardiol. 187, 10–17 (2023).
Panagiotakos, D. B., Pitsavos, C. & Stefanadis, C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. NMCD 16, 559–568 (2006).
Zou, Y. et al. Effect of low-sodium salt applied to Chinese modified DASH diet on arterial stiffness in older patients with hypertension and type 2 diabetes. Nutr. Hosp. 40, 967–974 (2023).
Agbo, L.-D. et al. Dietary inflammatory potential and arterial stiffness in a French cohort: Insights from the STANISLAS study. Nutr. Metab. Cardiovasc. Dis. NMCD 34, 1959–1967 (2024).
D’Elia, L., Galletti, F., La Fata, E., Sabino, P. & Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 36, 734–743 (2018).
Monahan, K. D., Feehan, R. P., Blaha, C. & McLaughlin, D. J. Effect of omega-3 polyunsaturated fatty acid supplementation on central arterial stiffness and arterial wave reflections in young and older healthy adults. Physiol. Rep. 3, e12438 (2015).
Ashor, A. W., Lara, J., Siervo, M., Celis-Morales, C. & Mathers, J. C. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PloS One 9, e110034 (2014).
Liu, H., Shivgulam, M. E., Schwartz, B. D., Kimmerly, D. S. & O’Brien, M. W. Impact of exercise training on pulse wave velocity in healthy and clinical populations: a systematic review of systematic reviews. Am. J. Physiol. Heart Circ. Physiol. 325, H933–H948 (2023).
Lopes, S. et al. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J. Hypertens. 39, 214–222 (2021).
Park, W. et al. Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study. Int. J. Environ. Res. Pub. Health 17, 7233 (2020).
Laukkanen, J. A., Isiozor, N. M. & Kunutsor, S. K. Objectively Assessed Cardiorespiratory Fitness and All-Cause Mortality Risk: An Updated Meta-analysis of 37 Cohort Studies Involving 2,258,029 Participants. Mayo Clin. Proc. 97, 1054–1073 (2022).
Kaminsky, L. A. et al. 2023 update: The importance of cardiorespiratory fitness in the United States. Prog. Cardiovasc. Dis. 83, 3–9 (2024).
Ross, R., Arena, R., Myers, J., Kokkinos, P. & Kaminsky, L. A. Update to the 2016 American Heart Association cardiorespiratory fitness statement. Prog. Cardiovasc. Dis. 83, 10–15 (2024).
Ozemek, C., Arena, R. & Lavie, C. J. Predicting the Future in Primary Care Patients Through Graded Exercise Testing. Mayo Clin. Proc. 98, 1270–1272 (2023).
Jae, S. Y. et al. Association Between Cardiorespiratory Fitness and Trend of Age-Related Rise in Arterial Stiffness in Individuals With and Without Hypertension or Diabetes. Am. J. Hypertens. 38, 46–54 (2024).
Nayor, M. et al. Arterial Stiffness and Cardiorespiratory Fitness Impairment in the Community. J. Am. Heart Assoc. 12, e029619 (2023).
Jae, S. Y. et al. Association between cardiorespiratory fitness and arterial stiffness in men with the metabolic syndrome. Diab. Res. Clin. Pract. 90, 326–332 (2010).
Al-Mhanna, S. B. et al. Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: a systematic review and meta-analysis. PeerJ 12, e17525 (2024).
Ferreira, L., Abrantes, C., Alves, M. E., Moreira, C. & Moreira, H. Effects of exercise programs on cardiorespiratory fitness and arterial stiffness on postmenopausal women: A systematic review study. Maturitas 181, 107917 (2024).
Tanaka, H. Peak Cardiorespiratory Fitness and Destiffening of Arteries. Am. J. Hypertens. 38, 7–8 (2024).
Kontsas, K. et al. Delayed blood pressure recovery ratio might indicate increased arterial stiffness in hypertensive patients with reduced aerobic exercise capacity. Blood Press. 22, 290–296 (2013).
Wang, S. et al. Exercise-Induced Oxygen Desaturation Increases Arterial Stiffness in Patients with COPD During the 6WMT. Int. J. Chron. Obstruct. Pulmon. Dis. 19, 1479–1489 (2024).
Starzak, M., Stanek, A., Jakubiak, G. K., Cholewka, A. & Cieślar, G. Arterial Stiffness Assessment by Pulse Wave Velocity in Patients with Metabolic Syndrome and Its Components: Is It a Useful Tool in Clinical Practice?. Int. J. Environ. Res. Pub. Health 19, 10368 (2022).
Fan, Y. et al. Effect of the baseline pulse wave velocity on short term and long term blood pressure control in primary hypertension. Int. J. Cardiol. 317, 193–199 (2020).
Hu, L. et al. Associations between Blood Pressure Indices and Brachial-ankle Pulse Wave Velocity in Treated Hypertensive Adults: results from the China Stroke Primary Prevention Trial (CSPPT). Sci. Rep. 9, 8178 (2019).
Vlachopoulos, C. et al. Association of Estimated Pulse Wave Velocity With Survival: A Secondary Analysis of SPRINT. JAMA Netw. Open 2, e1912831 (2019).
Shahin, Y., Khan, J. A. & Chetter, I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis 221, 18–33 (2012).
Yen, C.-H. et al. Angiotensin Receptor Blockades Effect on Peripheral Muscular and Central Aortic Arterial Stiffness: A Meta-Analysis of Randomized Controlled Trials and Systematic Review. Acta Cardiol. Sin. 30, 98–107 (2014).
Chen, X., Huang, B., Liu, M. & Li, X. Effects of different types of antihypertensive agents on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J. Thorac. Dis. 7, 2339–2347 (2015).
Niu, W. & Qi, Y. A meta-analysis of randomized controlled trials assessing the impact of beta-blockers on arterial stiffness, peripheral blood pressure and heart rate. Int. J. Cardiol. 218, 109–117 (2016).
Schettini, I. V. G. et al. Use of Antihypertensive Drugs and Arterial Stiffness in the Longitudinal Study of Adult Health (ELSA-Brasil). Cardiovasc. Drugs Ther. 39, 287–296 (2025).
Petersen, K. S., Blanch, N., Keogh, J. B. & Clifton, P. M. Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 35, 243–252 (2015).
Jamialahmadi, T. et al. Impact of Bariatric Surgery on Pulse Wave Velocity as a Measure of Arterial Stiffness: a Systematic Review and Meta-analysis. Obes. Surg. 31, 4461–4469 (2021).
Zhang, Y., Xue, J., Li, S., Yang, H. & Kang, C. Impact of bariatric surgery on carotid intima-media thickness and arterial stiffness in metabolically healthy obesity: a prospective study. Horm. Athens Greece 23, 467–475 (2024).
Moriconi, D. et al. Role of endogenous GLP-1 on arterial stiffness and renal haemodynamics following bariatric surgery. Eur. J. Clin. Invest. 54, e14256 (2024).
Lincoff, A. M. et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 389, 2221–2232 (2023).
Rizos, E. C. et al. The effect of SGLT2 inhibitors and GLP1 receptor agonists on arterial stiffness: A meta-analysis of randomized controlled trials. J. Diab. Complications 38, 108781 (2024).
Wu, Z. et al. Combined evaluation of arterial stiffness, glycemic control and hypertension for macrovascular complications in type 2 diabetes. Cardiovasc. Diabetol. 21, 262 (2022).
Ibata, J. et al. Increased arterial stiffness is closely associated with hyperglycemia and improved by glycemic control in diabetic patients. J. Diab. Investig. 4, 82–87 (2013).
Cui, C. et al. Diabetes, glycemic control and arterial stiffness: a real-world cohort study in the context of predictive, preventive, and personalized medicine. EPMA J. 14, 663–672 (2023).
Agarwal, N. et al. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J. Clin. Endocrinol. Metab. 95, 722–730 (2010).
Panagiotopoulou, O. et al. Metformin use in obese mothers is associated with improved cardiovascular profile in the offspring. Am. J. Obstet. Gynecol. 223, 246.e1–246.e10 (2020).
Harashima, K., Hayashi, J., Miwa, T. & Tsunoda, T. Long-term pioglitazone therapy improves arterial stiffness in patients with type 2 diabetes mellitus. Metabolism 58, 739–745 (2009).
Ryan, K. E., McCance, D. R., Powell, L., McMahon, R. & Trimble, E. R. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis 194, e123–e130 (2007).
Kim, H. et al. Comparative effects of glucose-lowering agents on endothelial function and arterial stiffness in patients with type 2 diabetes: A network meta-analysis. Atherosclerosis 391, 117490 (2024).
Ikonomidis, I. et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients With Type 2 Diabetes Mellitus After 12-Month Treatment. J. Am. Heart Assoc. 9, e015716 (2020).
Batzias, K. et al. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Diab. Res. 2018, 1232583 (2018).
Bekki, M. et al. Switching Dipeptidyl Peptidase-4 Inhibitors to Tofogliflozin, a Selective Inhibitor of Sodium-Glucose Cotransporter 2 Improve Arterial Stiffness Evaluated by Cardio-Ankle Vascular Index in Patients with Type 2 Diabetes: A Pilot Study. Curr. Vasc. Pharmacol. 17, 411–420 (2019).
Upala, S., Wirunsawanya, K., Jaruvongvanich, V. & Sanguankeo, A. Effects of statin therapy on arterial stiffness: A systematic review and meta-analysis of randomized controlled trial. Int. J. Cardiol. 227, 338–341 (2017).
Cavero-Redondo, I. et al. Comparative effect of physical exercise versus statins on improving arterial stiffness in patients with high cardiometabolic risk: A network meta-analysis. PLoS Med. 18, e1003543 (2021).
Cavero-Redondo, I. et al. Effect of adding PCSK9 inhibitors to lipid-lowering interventions on arterial stiffness: A systematic review and meta-analysis. Eur. J. Clin. Invest. 54, e14269 (2024).
Park, J. B. et al. Expert Consensus on the Clinical Use of Pulse Wave Velocity in Asia. Pulse 10, 1–18 (2022).
Pilz, N. et al. Pulse Wave Velocity: Methodology, Clinical Applications, and Interplay with Heart Rate Variability. Rev. Cardiovasc. Med. 25, 266 (2024).
Ghannadzadeh Yazdi, A. et al. The effect of time-restricted eating on arterial stiffness indices in men with metabolic syndrome: study protocol for a randomized controlled trial. Trials 25, 497 (2024).
Milan, A. et al. Current assessment of pulse wave velocity: comprehensive review of validation studies. J. Hypertens. 37, 1547 (2019).
Spronck, B. et al. 2024 Recommendations for Validation of Noninvasive Arterial Pulse Wave Velocity Measurement Devices. Hypertens. Dallas Tex. 81, 183–192 (2024).
Wang, M., Huang, J., Wu, T. & Qi, L. Arterial Stiffness, Genetic Risk, and Type 2 Diabetes: A Prospective Cohort Study. Diab. Care 45, 957–964 (2022).
Cohen, J. B. et al. Arterial Stiffness and Diabetes Risk in Framingham Heart Study and UK Biobank. Circ. Res. 131, 545–554 (2022).
Shaikh, A. Y. et al. Relations of Arterial Stiffness and Brachial Flow-Mediated Dilation With New-Onset Atrial Fibrillation: The Framingham Heart Study. Hypertens. Dallas Tex. 68, 590–596 (2016).
Niiranen, T. J. et al. Relative Contributions of Arterial Stiffness and Hypertension to Cardiovascular Disease: The Framingham Heart Study. J. Am. Heart Assoc. 5, e004271 (2016).
Niiranen, T. J. et al. Aortic-Brachial Arterial Stiffness Gradient and Cardiovascular Risk in the Community: The Framingham Heart Study. Hypertens. Dallas Tex. 69, 1022–1028 (2017).
Bäck, M. et al. Cardio-ankle vascular index for predicting cardiovascular morbimortality and determinants for its progression in the prospective advanced approach to arterial stiffness (TRIPLE-A-Stiffness) study. EBioMedicine 103, 105107 (2024).
Pewowaruk, R. J. et al. Methods of arterial stiffness calculation and cardiovascular disease events: the multiethnic study of atherosclerosis. J. Hypertens. 41, 486–493 (2023).
Hartz, J. et al. Lipoprotein Particle Predictors of Arterial Stiffness after 17 Years of Follow Up: The Malmö Diet and Cancer Study. Int. J. Vasc. Med. 2020, 4219180 (2020).
Cui, C. et al. Arterial Stiffness and Obesity as Predictors of Diabetes: Longitudinal Cohort Study. JMIR Pub. Health Surveill. 10, e46088 (2024).
Author information
Authors and Affiliations
Contributions
Conceptualization: O.Y.B.C., N.E.A.V., C.A.F.M. Writing and Review of Manuscript: N.S.M.B., M.L.A., C.P.A., K.P.Z.M., O.Y.B.C. Editing: O.Y.B.C., N.E.A.V., C.A.F.M. Supervision: O.Y.BC. Each author contributed important intellectual content during manuscript drafting or revision and accepted accountability for the overall work by ensuring that questions about the accuracy or integrity of any portion of the work were appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De la Maza-Bustindui, N.S., León-Álvarez, M., Ponce-Acosta, C. et al. Impact of cardiometabolic risk factors and its management on the reversion and progression of arterial stiffness. npj Cardiovasc Health 2, 36 (2025). https://doi.org/10.1038/s44325-025-00074-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44325-025-00074-6